Abstract
The level of relative humidity is one of the key parameters in evaluating indoor air quality and comfort. In principle, humidity can be kept more uniform over time by use of materials that adsorb moisture from the air reversibly. This study was conducted to investigate the effect of loess treatment and carbonization on the hygric performance of medium-density fiberboard (MDF). The loess treatment was conducted with different sizes of loess particle prepared by a high-pressure homogenizer. After loess treatment on the surface of the MDF, it was carbonized at high temperature (600 °C). Loess is an abundant mineral high in Si content, which has high moisture absorption capacity, which remained after the carbonization process. The study also found that the loess treatment positively affected the hygric performance of carbonized MDF (c-MDF). The hygric performance of c-MDF almost doubled after the loess treatment compared with the non-treated c-MDF. However, the nano conversion of loess did not influence the hygric performance. Loess-treated carbonized MDF could be used as a humidity controller in buildings.
Download PDF
Full Article
Effect of Loess Treatment and Carbonization on the Hygric Performance of Medium-density Fiberboard
Min Lee, Jaehyuk Jang, Sangmin Lee, and Sangbum Park*
The level of relative humidity is one of the key parameters in evaluating indoor air quality and comfort. In principle, humidity can be kept more uniform over time by use of materials that adsorb moisture from the air reversibly. This study was conducted to investigate the effect of loess treatment and carbonization on the hygric performance of medium-density fiberboard (MDF). The loess treatment was conducted with different sizes of loess particle prepared by a high-pressure homogenizer. After loess treatment on the surface of the MDF, it was carbonized at high temperature (600 °C). Loess is an abundant mineral high in Si content, which has high moisture absorption capacity, which remained after the carbonization process. The study also found that the loess treatment positively affected the hygric performance of carbonized MDF (c-MDF). The hygric performance of c-MDF almost doubled after the loess treatment compared with the non-treated c-MDF. However, the nano conversion of loess did not influence the hygric performance. Loess-treated carbonized MDF could be used as a humidity controller in buildings.
Keywords: Carbonization; Carbonized board; Loess; Hygric performance; Water absorption and desorption
Contact information: Department of Forest Products, National Institute of Forest Science, Seoul 02455, Republic of Korea; *Corresponding author: parksb57@naver.com
INTRODUCTION
Since the 1970s the development of composite materials that have low environmental impact has been pursued and improved rapidly worldwide. Their use has also expanded in a variety of ways such as in aerospace, aircraft, marine, and civil engineering structural applications. Composite materials are often applied to humid environments or used as humidity controllers in curtain spaces (Jedidi et al. 2005). With the increase of public interest in environmental welfare, there has been more research on sustainable architecture, environmentally friendly architecture, and ecological architecture. Modern people have more opportunities to come in contact with hazardous substances because they spend most of their time in indoor areas such as office buildings, public facilities, transportation systems, and houses. Therefore, indoor air quality is significantly correlated with human health.
The level of relative humidity is one of the key parameters in evaluating indoor air quality and comfort. According to the World Health Organization (WHO), respiratory comfort, skin humidity, and perceived indoor air quality can be affected by relative humidity (RH%)(WHO 2010). Moreover, complaints of dry noses, dry mouths, dry eyes, dry skin, and respiratory illnesses can occur at lower values of RH% (Lechner 2000). Some European countries have introduced a thick layer of insulating material in the walls and roofs of the building structure envelope to reduce energy consumption and gas emission. Unfortunately, thick layers of insulating material in walls and roofs has led to an increase in indoor RH% levels in occupied buildings due to low air permeability (Cerolini 2009). Energy consumption may increase if electronic devices are used to reduce indoor RH%. Consequently, the problems persist.
Porous carbon materials can be used for a number of applications including filters, adsorbents, catalyst supports, and indoor construction. Monolithic porous carbon materials can be obtained by carbonizing wood and wood-based materials in a controlled thermal decomposition process (Kercher and Nagle 2002). The carbonization process does not require any chemical additions, and the cellular anatomical features of the wood are retained in the new carbon material (Treusch et al. 2004). In the authors’ previous study, carbonized wood-based materials were introduced for use as environmentally friendly indoor construction materials, because they had excellent flame retardant qualities, sound absorption, electrical resistance, and hygric performance (Kwon et al. 2013; Lee et al. 2014; Park et al. 2014). In particular, Lee et al. (2014) reported that the moisture absorption and desorption capacity of medium-density fiberboard (MDF) were significantly improved by carbonization, and that the highest value was obtained at 600 °C.
Loess, which is widely distributed in Asia and an abundant natural resource, is a sediment largely composed of silt-sized particles that has been transported and deposited by wind (Muhs 2007). The nontoxic material of loess has outstanding properties of adsorption, deodorization, and emits far-infrared radiation when heated; thus it has been widely used in Asian culture as a material for construction and purification (Darling et al. 2012; Jang et al. 2016). Loess consists of mostly of silica, aluminum, calcium, and iron. Additionally, the silica content, which relates to the moisture and adsorption properties, was reported to be approximately 45% to 80% (Taylor et al. 1983; Muhs 2007). Some studies have reported that loess not only improves indoor air quality, but also has moisture purifying properties (Sun et al. 2004; Lee et al. 2013).
In this study, carbonization and nano conversion techniques were combined to develop a new interior construction material with functionalities such as toxic chemical adsorption and moisture control. Different sizes of untreated loess powder were applied to medium-density fiberboard (MDF), and then loess-treated MDF was carbonized at 600 °C. The hygric performance of prepared loess-treated c-MDFs was evaluated.
EXPERIMENTAL
Materials
Medium-density fiberboard (MDF, 12 mm-thick, Sunchang Corp., Incheon, South Korea) was used as the mother material for the carbonized board and cut into 260 mm (length) × 130 mm (width) pieces. Loess, which has similar characteristics to ultisols or red clay, was purchased from GreenBio Inc. (Incheon, South Korea). The size of the loess powder was such that it could fit through a 2000-mesh (10 μm) sieve.
Methods
Homogenization of loess
The nano conversion techniques can be improved the hygric performance of materials due to their increased specific surface area. Loess powder (red clay, 1 g) was added to 200 mL of water and homogenized with a high-pressure homogenizer (M-110EH, Microfluidizer® Processors, Westwood, MA, USA). The loess emulsion was passed at a rate of 100 mL/min through two nozzle sizes (200 µm and 87 µm) under a pressure of 1000 bar as described in literature (Lee et al. 2011). Different repetitions (2, 4, 6, 8, and 10 times) of homogenization were conducted for the loess emulsion preparations. A 20 mL quantity of homogenized loess emulsion was brushed onto one side of the cut MDF. The final total weight of loess was approximately 0.1 g on each MDF sample.
Carbonization of medium density fiberboard
The loess-treated MDF was first wrapped with paper and then wrapped again with aluminum foil in order to prevent oxygen contact. Carbonization of MDF and loess-treated MDF was conducted by stacking the MDF between two graphite sheets (10 mm thick) in an electric vacuum furnace under a nitrogen gas flow (200 mL/min). The temperature in the electric vacuum furnace was raised by 50 °C/h until reaching the target temperature of 600 °C, and was then maintained continuously for 2 h. After carbonization, carbonized products were cooled under ambient conditions. Schematic of sample preparation was described in Fig. 1.
Fig. 1. Schematic and appearances of loess treated c-MDF
Elemental and morphological analysis
The elemental composition of loess was investigated via X-ray fluorescence spectrometry (XRF) (S4 pioneer, Bruker Corp., Karlsruhe, Germany). The XRF analysis was conducted in MultiRes Vac34 mode with a 35-mm collimator under helium as the carrier gas. Additionally, to investigate the crystal form of loess-treated carbonized MDF, an X-ray diffraction (XRD) analysis was performed using a X-ray diffractometer (D/MAX-2500, Rigaku Corp., Tokyo, Japan) by scanning in the 2θ = 5° to 80° range at a scan rate of 2°/s. To determine the loess size and surface of specimens, scanning electron microscope (SEM) images were taken with an EM-30 (Coxem Ltd. Daejeon, South Korea) with gold ion coating (5 mA, 120 s) using an ion-coater (KIC-1A, Coxem Ltd., Daejeon, South Korea).
Moisture buffering capacity
The moisture absorption and desorption properties of specimens were examined in a sealed chamber according to ISO 24353 (2008), KS F 2611 (2009), and JIS A 1470-1 (2014). The test chamber included an electronic balance, thermostat, temperature sensor, humidity sensor, airflow sensor, and humidifier (UNT-HEAT-01, Unitech, Siheung, South Korea). The data from the sensors were collected by computer every minute for 24 h. Each sample was cut into 250 mm × 120 mm pieces and the thickness varied by sample types. All surfaces of the prepared samples, except for the loess-treated surface, were covered by aluminum tape.
The samples were cured at the temperature of 23 °C ± 0.5 °C and 50% ± 1% relative humidity (RH) until the weight was stable (below 0.01 g of variation) for 24 h. The moisture absorption and desorption properties were determined under middle humidity level conditions as follows: preconditioning of a specimen for 50% RH; moisture absorption process for 75% RH, 12 h and moisture desorption process for 50% RH, 12 h. The moisture absorption and desorption contents were calculated according to ISO 24353 (2008).
RESULTS AND DISCUSSION
Elemental Compositions
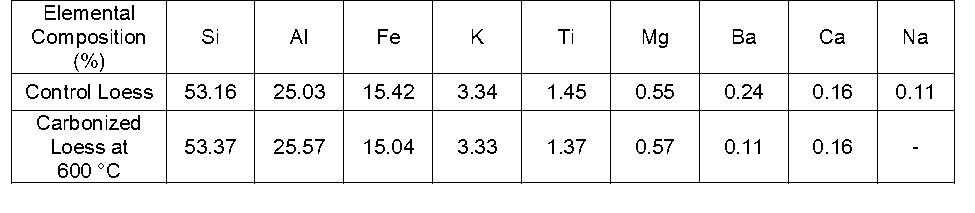
Table 1 shows the results of the XRF analysis for the control (natural raw material without any treatment) and carbonized loess at 600 °C to investigate the thermal degradation of elements in loess due to the carbonization process. The loess consisted of mainly silicon (53.16%), aluminum (25.03%), and iron (15.42%). However, there were no elemental composition changes even after carbonization treatment at high temperature. Silicon is the second most abundant material in Earth’s crust and is usually found in the form of silicon dioxide (silica, SiO2). Moreover, silicon is present in crystalline forms such as quartz, agate amethyst, rock crystal, chalcedony, flint, jasper, and opal. The structure of silicon and oxygen is a network-solid in three-dimensional crystals. The abundant silicon in loess can be transformed into silicon dioxide, which has a higher moisture absorbing capacity (Angst and Simmons 1991).
Table 1. Effect of Carbonization on the Elemental Composition Change of Loess
Crystal Structures
The crystal habit of control and carbonized loess at 600 °C was also investigated by XRD as shown in Fig. 2. Based on the results, both original loess and carbonized loess at 600 °C represented 4 types of crystal habit, which were quartz (SiO2), kaolinite (Al2Si2O5(OH)4), halloysite (Al2Si2O5(OH)4), and gibbsite (Al2O3HaO). The majority of the peaks represented quartz and trace peaks represented other crystals. As expected, loess mainly consisted of quartz, which has a continuous structure of SiO4, giving an overall formula of SiO2. However, in the case of carbonized loess at 600 °C, some of the halloysite and kaolinite peaks disappeared at 13°, 36°, and 25°, respectively. It is known that kaolinite begins to become transformed into disordered metakaolin (Al2Si2O7) through endothermic dehydroxylation or dehydration (Redfern 1987).
Figure 3 shows the X-ray diffractograms of carbonized-MDF (c-MDF) with different homogenization repeated loess treatments. The c-MDF without loess treatment showed the typical diffraction patterns of carbonized cellulosic materials in the disappearance of the peaks at 2θ = 16.2° and 2θ = 22.6°. It is well-known that cellulose crystalline substances becomes non-crystallized when the cellulosic materials are carbonized above 350 °C (Kwon et al. 2009). X-ray diffractograms of the loess-treated c-MDFs showed a sharp peak at 2θ = 26.5° caused by SiO2, as described in Fig. 2.
Fig. 2. X-ray diffractograms of control and carbonized loess at 600 °C
Fig. 3. X-ray diffractograms of c-MDF with different homogenization repeated loess treatments
Morphology
The SEM images of c-MDF with different homogenization repeated loess treatments are presented in Fig. 4. The SEM images revealed that the loess covered the surface of MDF well with the carbonization treatment, whereas c-MDF without the loess treatment showed relatively large porosities between wood fibers. However, the pores of c-MDF were filled by loess particles, and this phenomenon was more clearly detected with increased repetitions of homogenization. The loess particles that were homogenized 8 to 10 times were packed more intimately, thereby reducing the pore volume in MDF. No difference between non-homogenized loess and the loess that was homogenized 2 to 4 times was observed. The non-homogenized loess powder already had a small size of 10 μm and the c-MDF had abundant porosities that were larger than10 μm. Therefore, the packing of c-MDF’s pores could affect moisture absorption and desorption properties. The size of loess should be considered for controlling or influencing functionality.
Fig. 4. SEM images of c-MDF with different homogenization repeated loess treatments
(a) without loess c-MDF; (b) non-homogenization loess-treated c-MDF; (c) 2 repeated homogenization loess-treated c-MDF; (d) 4 repeated-; (e) 8 repeated-; (f) 10 repeated-
Moisture Buffering Capacity
Figure 5 shows the moisture absorption and desorption capacities of c-MDF with different numbers of repeated homogenization loess treatments. The c-MDF showed approximately 1.8 times higher moisture buffering capacity than the MDF without carbonization treatment. Carbonized wood-based materials, including c-MDF, can be considered disordered carbon and can greatly affect moisture-absorbing capacity (Kwon et al. 2013). In this study, loess-treated c-MDF showed a 100% increase in moisture absorbing and desorbing properties. The 8 times homogenized loess-treated c-MDF had approximately 88 g/m2 in the moisture absorbing region. However, the non-homogenized loess-treated c-MDF and 2 times homogenized loess-treated c-MDF did not show a difference. This revealed that repeating homogenization 2 times did not reduce the loess particle size. The 10 times homogenized loess-treated c-MDF showed lower moisture absorbing and desorbing properties than the other amounts of repeated homogenization, which meant that the loess particle size was reduced to cover all micro and macro pores of c-MDF. Lee et al. (2014) explained that not only chemical reactions of materials including wood, wood-based board, and c-MDF influence absorption and desorption, but also physical reactions between materials, which can be influenced by moisture. Furthermore, moisture buffering in porous surfaces was found to have a large influence on the humidity distribution of the dwelling. In this experiment, the authors found that the c-MDF material known as charcoal had a slow moisture absorption property but a large moisture holding capacity. These characteristics indicated that charcoal could be an outstanding moisture control material after loess treatment due to high silica content. In theory, the moisture molecule first reacted with or attached to silica due to its hydrophilic property (chemical and physical reaction), and then transferred to the pores of the c-MDF. The increase in the total amount of moisture could be explained by the authors’ theory.
Fig. 5. Water absorption and desorption of c-MDF with different homogenization repeated loess treatments
CONCLUSIONS
- Loess was found to contain about 53% silica, and the silica was still remained after a carbonization process.
- Homogenization process did not improve the hygric performance of c-MDF.
- The moisture absorption and desorption of MDF was improved 100% by carbonization and additionally 100% improved by the loess treatment. Altogether, loess treated c-MDF achieved 88 g/m2 in capability to absorb and desorb moisture.
- Loess treated c-MDF could be used as moisture buffering material in high dry or humid area.
REFERENCES CITED
Angst, D. L., and Simmons, G. W. (1991). “Moisture absorption characteristics of organosiloxane self-assembled monolayers,” Langmuir 7(10), 2236-2242. DOI: 10.1021/la00058a043
Cerolini, S., D’Orazio, M., Perna, C. D., and Stazi, A. (2009). “Moisture buffering capacity of highly absorbing materials,” Energ. Buildings 41(2), 164-168. DOI: 10.1016/j.enbuild.2008.08.006
Darling, E. K., Cros, C. J., Wargocki, P., Kolarik, J., Morrison, G. C., and Corsi, R. L. (2012). “Impacts of a clay plaster on indoor air quality assessed using chemical and sensory measurements,” Build. Environ. 57, 370-376. DOI: 10.1016/j.buildenv.2012.06.004
ISO 24353 (2008). “Hygrothermal performance of building materials and products – Determination of moisture adsorption/desorption properties in response to humidity variation,” International Organization for Standardization, Geneva, Switzerland.
Jang, H., Kang, H., and So, S. (2016). “Humidity-controlling and deodorizing effects of yellow loess plastering as finishing materials,” Int. J. Appl. Eng. Res. 11(4), 2865-2871.
Jedidi, J., Jacquemin, F., and Vautrin, A. (2005). “Design of accelerated hygrothermal cycles on polymer matrix composites in the case of a supersonic aircraft,” Compos. Struct. 68(4), 429-437. DOI: 10.1016/j.compstruct.2004.04.009.
JIS A 1470-1 (2014). “Determination of water vapour adsorption/desorption properties for building materials – Part 1: Response to humidity variation,” Japanese Standards Association, Tokyo, Japan.
Kercher, A. K., and Nagle, D. C. (2002). “Evaluation of carbonized medium-density fiberboard for electrical applications,” Carbon 40(8), 1321-1330. DOI: 10.1016/S0008-6223(01)00299-8
KS F 2611 (2009). “Hygrothermal performance of building materials and products – Determination of moisture adsorption/desorption properties in response to humidity variation,” Korean Agency for Technology and Standards, Seoul, Republic of Korea.
Kwon, J. H., Park, S. B., Ayrilmis, N., Oh, S. W., and Kim, N. H. (2013). “Effect of carbonization temperature on electrical resistivity and physical properties of wood and wood-based composites,” Compos. Part B-Eng. 46, 102-107. DOI: 10.1016/j.compositesb.2012.10.012.
Kwon, S. M., Kim, N. H., and Cha, D. S. (2009). “An investigation on the transition characteristics of the wood cell walls during carbonization,” Wood Sci. Technol. 43(5), 487-498. DOI: 10.1007/s00226-009-0245-6
Lechner, N. (2000). Heating, Cooling, Lighting, Design Methods for Architects, John Wiley & Sons, Inc. Hoboken, NJ, USA.
Lee, M., Park, S. B., and Lee, S. M. (2014). “Effect of carbonization temperature on hygric performance of carbonized fiberboards,” J. Korean Wood Sci. Technol. 42(5), 615-623. DOI: 10.5658/WOOD.2014.42.5.615
Lee, S. Y., Chun, S. J., Doh, G. H., Lee, S., Kim, B. H., Min, K. S., Kim, S. C., and Huh, Y. S. (2011). “Preparation of cellulose nanofibrils and their applications: High strength nanopapers and polymer composite films,” J. Korean Wood Sci. Technol. 39(3), 197-205. DOI: 10.5658/WOOD.2011.39.3.197
Lee, S. Y., Oh, H. Y., and Kim, O. B. (2013). “Isolation and characterization of volatile organic compounds-degrading Bacillus strains from loess,” J. Environ. Prot. 4(4), 50-57. DOI: 10.4236/jep.2013.44A007
Muhs, D. R. (2007). “Loess deposits, origins, and properties,” in: Encyclopedia of Quaternary Science, S. A. Elias (ed.), Elsevier, Amsterdam, Netherlands. DOI: 10.1016/B0-44-452747-8/00158-7
Park, S. B., Lee, M., Son, D. W., Lee, S. M., and Kim, J. I. (2014). “Fire performance of carbonized medium density fiberboard manufactured at different temperatures,” J. Wood Sci.60(1), 74-79. DOI: 10.1007/s10086-013-1379-6
Redfern, S. A. T. (1987). “The kinetics of dehydroxylation of kaolinite,” Clay Minerals 22, 447-456.
Sun, X. X., Lee, Y. J., Choi, J. K., and Kim, E. K. (2004). “Synergistic effect of sophorolipid and loess combination in harmful algal blooms mitigation,” Mar. Pollut. Bull. 48(9-10), 863-872. DOI: 10.1016/j.marpolbul.2003.11.002
Taylor, S. R., McLennan, S. M., and McCulloch, M. T. (1983). “Geochemistry of loess, continental crustal composition and crustal model ages,” Geochim. Cosmochim. Ac. 47(11), 1987-1905. DOI: 10.1016/0016-7037(83)90206-5
Treusch, O., Hofenauer, A., Tröger, F., Fromm, J., and Wegener, G. (2004). “Basic properties of specific wood-based materials carbonised in a nitrogen atmosphere,” Wood Sci. Technol.38(5), 323-333. DOI: 10.1007/s00226-004-0245-5
World Health Organization (WHO) (2010). “WHO guidelines for indoor air quality: Selected pollutants,” World Health Organization, Copenhagen, Denmark, (http://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf), Accessed 5 April 2015.
Article submitted: April 6, 2017; Peer review completed: June 29, 2017; Revised version received and accepted: July 10, 2017; Published: July 18, 2017.
DOI: 10.15376/biores.12.3.6418-6426
