Abstract
Download PDF
Full Article
Microwave-assisted Depolymerization of Lignin with Metal Chloride in a Hydrochloric Acid and Formic Acid System
Rongge Zou,a,b Yunfeng Zhao,a,b Yunpu Wang,a,b,* Dengle Duan,a,bLiangliang Fan,a,b Leilei Dai,a,b Yuhuan Liu,a,b,* and Rongsheng Ruan a,b,c
A microwave-assisted depolymerization method of lignin with various metal chloride catalysts (MgCl2, AlCl3, FeCl3, ZnCl2, and MnCl2) in a formic acid and hydrochloric acid system under mild conditions (160 °C for 30 min) was studied. The resulting bio-oil was identified by a gas chromatography-mass spectrometer, and the solid residue was analyzed by Fourier transform infrared spectroscopy (FT-IR). Furthermore, the molecular weight change of lignin after the reaction was measured by gel permeation chromatography. The MnCl2 catalyzed lignin to produce most aromatic monomers, including approximately 23.0% G-type, 11.9% S-type, and 14.8% H-type monomer compounds. Different metal chloride catalysts had different effects on the depolymerization of lignin, which were embodied in the type and content of the products. It was also worth noting that the catalytic effects of transition metal chlorides on lignin may have been related to each chloride’s cation radius.
Keywords: Organosolv lignin; Microwave-assisted; Metal chloride; Hydrochloric and formic acid system
Contact information: a: Nanchang University, State Key Laboratory of Food Science and Technology, Nanchang 330047, China; b: Nanchang University, Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang 330047, China; c: Center for Biorefining and Department of Bioproducts and Biosystems Engineering, University of Minnesota, 1390 Eckles Ave., St. Paul, MN 55108, USA; *Corresponding authors: wangyunpu@ncu.edu.cn; liuyuhuan@ncu.edu.cn
INTRODUCTION
With the rapid depletion of fossil resources, there is an urgency to find alternative renewable energy resources. Lignocellulosic biomass (made up of cellulose, hemicellulose, and lignin) is considered to be an alternative organic renewable resource for the production of biofuels and aromatic chemicals (Li et al. 2015). In theory, lignin, based on its chemical structure, is a feasible raw material for the production of phenols (Higuchi 1990). Also, the efficient degradation of paper in composting plants means that biodegradation of lignin is also needed (Tuomela et al. 2000). However, its complex three-dimensional (3D) structure and diverse chemical bonds limit the direct uses of lignin. Thus, the efficient depolymerization of lignin into more phenolic compounds has become a hot spot in research (Asina et al. 2017).
As an emerging thermochemical method, microwave heating has the advantages of fast speed and high efficiency (Liu et al. 2017). Its magnetic field is capable not only of producing thermal effects, but also generating non-thermal effects, causing the violent vibration of some chemical bonds and bond breaking (Toledano et al. 2013). Therefore, microwave heating can degrade lignin under mild conditions. Dhar and Vinu (2017) investigated the microwave-assisted degradation of alkali lignin in the presence of different organic solvents. They showed that a significant yield of phenolics (20 wt%) containing acetosyringone, guaiacol, syringaldehyde, anisole, and lignin dimers (m/z 306, 322 Da) were produced at 100 °C in dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO), while at 140 °C, 11 wt% phenolics were obtained in ethylene glycol (EG). Dong et al. (2014) studied the microwave-assisted degradation of black-liquor lignin with formic acid, showing that the liquid product consisted of bio-oil 1 (phenolic monomeric compounds) and bio-oil 2 (oligomers), and achieved a maximal yield of 64.1% at 160 °C after 30 min (the yield for bio-oil 1 was 9.7% and 54.4% for bio-oil 2). Toledano et al. (2014) studied lignin depolymerization into simple aromatics with different metal nanoparticles on mesoporous Al-SBA-15 using a mild microwave-assisted approach. The results showed that the catalyst containing 10 wt% nickel achieved the highest degree of lignin depolymerization, with a maximum yield of 30% bio-oil after a short reaction time (typically 30 min of microwave irradiation).
Formic acid used as a catalyst and hydrogen donor in the depolymerization of lignin has been shown to be effective by many experiments. Ouyang et al. (2015) established that formic acid, as a hydrogen donor, provides in situ hydrogen, and its hydrogen supply ability is better than that of an external hydrogen source. Formic acid is also a readily available green reagent in biorefineries (Xu et al. 2012). Furthermore, HCl as a typical protonic acid can effectively promote the fracture of the ether bond in lignin and promote depolymerization (Zhang et al. 2014).
Moreover, recently scientists have found that Lewis acids, such as aluminum chloride (AlCl3) and manganese chloride (MnCl2), etc., are excellent hydrogen bonding acceptors and nucleophilic reagents (Güvenatam et al. 2015). Many experiments have studied the depolymerization of lignin catalyzed by different chlorides. Maldhure and Ekhe (2013) have studied the pyrolysis of purified kraft lignin in the presence of AlCl3 and ZnCl2 at 300 °C to 400 °C, and found that the presence of ZnCl2 yielded comparatively more liquid products, whereas the presence of AlCl3 yielded comparatively more gaseous products. Pan et al. (2015) investigated the degradation of lignin model compounds with metal salts assisted by microwave. The results showed that chromium chloride (CrCl3) and MnCl2 were the most effective in the degradation of the lignin model compounds. Du et al. (2017) concluded that either polyoxometalate (POM) or ferric chloride (FeCl3) was used as the catalyst and charge transfer agent in anode. However, the study by Jia et al. (2016) proved that FeCl3, copper chloride (CuCl2), and AlCl3 were effective for the β-O-4 bond cleavage of the phenolic lignin model compound guaiacyl glycerol-β-guaiacyl ether (GG). Furthermore, the HCl formed in situ by the hydrolysis of metal chlorides catalyzed the β-O-4 bond cleavage. According to Kinrade et al. (1999), the addition of KCl, CaCl2, and magnesium chloride (MgCl2) promoted the decomposition of the experimental sample and reduced the coke yield of lignin.
This paper is based on related previous research conducted in the authors’ laboratory. According to Duan et al. (2017), the best conditions for the low-power microwave-assisted depolymerization of organosolv lignin is a temperature of 160 °C and a duration of 30 min. Compared with a water, methanol, and isopropanol system, ethanol was the only effective solvent for the depolymerization process (Jing et al. 2016). According to the previous work by the author and colleagues of the same laboratory, the effects of different mixed acid systems (HCl-HCOOH system, H3PO4-HCOOH system, and H2SO4-HCOOH system) were studied. The weight-average molecular mass (Mw) and number-average molecular mass (Mn) of lignin depolymerization products reached the minimum when the hydrochloric acid and formic acid system was present. The optimal ratio for ethanol, formic acid, and hydrochloric acid is 2:1:1. This study used ethanol as a swelling agent, a formic acid and hydrochloric acid system as hydrogen donors, metal chloride as a catalyst, and was conducted using a microwave at 160 °C for 30 min. The resulting products were separated into oil product and solid residue and analyzed using the following techniques: high-performance liquid chromatography (HPLC), Fourier transform infrared spectroscopy (FT-IR), and gas chromatography-mass spectrometer (GC-MS). The catalytic abilities of different metal chlorides are discussed under these circumstances. In this paper, the authors explored the catalytic ability of metal chlorides in the depolymerization of lignin into aromatic monomers under mild reaction conditions, aiming to provide a theoretical basis for lignin depolymerization.
EXPERIMENTAL
Materials
Ethanol (99%), formic acid (analytical grade), ethyl acetate, and tetrahydrofuran (HPLC grade) were purchased from Da Mao Reagent Co. (Tianjin, China). The AlCl3, FeCl3, MnCl2, ZnCl2, and MgCl2were provided by Xi Long Scientific Co. (Guangdong, China). Hydrochloric acid was obtained from Solarbio (Beijing, China).
Methods
Alcohol-soluble lignin extraction
Lignin was extracted using an adaptation of the method of Fan et al. (2016). Dried bamboo was first cut into pieces, sieved, extracted with methylbenzene/ethanol (2:1, v/v), and then air-dried to acquire dewaxed bamboo powder. The bamboo powder (150 g) was dissolved in 50% ethanol aqueous solution (8:1, v/v) and then loaded into the autoclave set at 180 °C for 3h. After the reaction, the mixture was vacuum-filtered and dried to obtain the lignin powder.
Low-power microwave depolymerization
The experimental materials were mixed according to Table A1. Briefly, 1 g lignin, 0.005 mol metal chloride, 10 mL hydrochloric acid, and 10 mL formic acid (1:1, v/v) system was dissolved into 20 mL ethanol.
Lignin depolymerization was performed in the MDS-6G microwave digestion system (Sineo, Shanghai, China). The reaction temperature was set at 160 °C, after which, according to the previous study of Duan et al. (2017), the depolymerization reaction played the dominant role in the lignin degradation process, with a reaction time of 30 min.
Product separation and analysis method
After reaction, the reactants were cooled to room temperature prior to filtration. Then the mixture was filtered to separate the liquid part from the solid part. The pH of the liquid part was regulated to 6 using 2 mol/L sodium hydroxide solution (NaOH). It was then extracted with ethyl acetate to obtain the aqueous and organic phases. The organic phase was evaporated and the bio-oil was obtained.
The bio-oil was detected by GC-MS. The solid part was cleaned with deionized water three times and dried at 60 °C in the vacuum-drying box for 1 h. A portion taken from the dry solid was dissolved with tetrahydrofuran, and analyzed by GPC-HPLC. Another portion taken from the dry solid was analyzed by FT-IR. The specific experimental treatment process is shown in Fig. 1.
Fig. 1. Schematic process of lignin depolymerization and separation of the reaction products
The selectivity of monophenolic compounds (YMC)
The method for calculating the selectivity of monophenolic compounds is as follows, with Eqs. 1 and 2,
WO= WL – WR (1)
YMC=∑WP / Wo (2)
where WO is the mass of the bio-oil (g), WL is the mass of lignin (g), WR is the mass of residual lignin (g), YMC is the selectivity of monophenolic compounds (%), and ∑Wp is the weight (g) of the sum of identified monophenolic compounds via GC-MS.
Analysis method of bio-oil
The oil samples obtained in Fig. 1 were first dissolved in methyl alcohol for detection. In this experiment, the authors used a gas chromatograph-mass spectrometer (GC-MS). The GC-MS analysis was performed using a GC-7890B, MS-7000D from Agilent Technologies (Santa Clara, CA, USA) equipped with a thermal conductivity detector (TCD) and an Agilent capillary column (HP-PONA, 50 m × 200 μm × 0.5 μm). The column temperature program started at 50 °C for 2 min and then was raised to 120 °C at a rate of 10 °C/min (held for 5 min at this temperature), increased to 280 °C at a rate of 10 °C/min (held for 8 min at this temperature), and then increased to 300 °C at a rate of 10 °C/min (held for 10 min at this temperature). The injection sample volume was 2 μL, with a split ratio of 50:1. The ion source temperature was 230 °C for the mass selective detector. Helium was employed as the carrier gas, and the flow rate was 1.0 mL/min. The compounds were identified by comparing the spectrograms with those in the NIST14 data library. The relative content of each compound in the products was determined by semi-quantitative methods according to the chromatographic percentage.
Analysis method of lignin structure
For residual lignin, FT-IR spectroscopy (Nicolet iS5, Thermo Fisher, Waltham, MA, USA) was used to characterize the functional groups of the raw material and depolymerization products. The samples were ground and mixed with KBr and the spectra were scanned in the range of 4000 cm–1 to 700 cm–1 with 32 scans at a 4-cm–1 spectral resolution per sample. Each sample was scanned three times to eliminate operation error.
Analytical method for the molecular weight of residual lignin
The changes in the molecular weight of residual lignin were studied by GPC (Mono GPC-100, 7.8 mm × 300 mm, Sepax Technologies, Suzhou, China). The HPLC can detect changes in compounds’ composition in a rapid, efficient, and simple way. Therefore, in addition to identification by GC-MS, the authors also conducted a HPLC analysis for quantitative analysis of the compounds. All of the samples were first dissolved in tetrahydrofuran (THF) for analysis. Then, they were filtered by a 0.45-μm membrane with a syringe filter.
The gel permeation chromatography-high-performance liquid chromatography (GPC-HPLC) instrument (G1312B binary pump; Agilent Technologies, Santa Clara, USA; UV detector at 254 nm; GPC column at a column temperature of 30 °C) was used to determine the molecular weight of the product. The mobile phase was THF at a flow rate of 0.5 mL/min. A polystyrene standard calibration curve was adopted to calibrate the number average molecular weight (Mn) and weight average molecular weight (Mw) of the samples. The authors applied this method to estimate the degree of lignin depolymerization as well.
RESULTS AND DISCUSSION
Yield of Depolymerization Products Bio-oil
Wan et al. (2009) found that MgCl2 could increase the bio-oil yield by either suppressing charcoal yield or gas yield or both during microwave-assisted pyrolysis of corn stover and aspen wood. Its catalysts may function as a microwave absorbent to speed up heating. Toledano et al. (2016) adopted base catalysts to depolymerize organosolv lignin of olive tree pruning in a batch reactor and found that the yield of oil reached 20% at 300 °C for 40 min. Liu et al. (2017) investigated lignin degradation in isopropanol under mild microwave-assisted heating, showing that the highest yield of liquid product obtained was 45.4% using a temperature of 120 °C with a reaction time of 30 min, together with a char yield of 38.6% and 14.7% residual lignin. Also, Wang et al. (2017) studied microwave-assisted depolymerization of black-liquor lignin in formic acid, concentrating on the yield of liquid fractions as bio-oil 1 (mainly aromatic monomers) and bio-oil 2 (mainly aromatic oligomers) and the distribution of the specific compositions. Bio-oil 1 (9.69%) and bio-oil 2 (54.39%) achieved their maximum yields under 160 °C with the reaction time of 30 min.
According to Table 1, it was apparent that the metal chloride improved the yield of bio-oil compared with no catalyst. Among all the catalysts, MnCl2 showed the best catalytic effect. Compared with the results by Wang et al. (2017), the yield of oil improved. Moreover, it was found that the yield of oil catalyzed by ZnCl2 and MnCl2 reached more than 70%.
Table 1. Yield of Bio-oil (Wo)
Compounds of Depolymerization Products Identified by GC-MS
Lignin depolymerization products are diverse, including mainly guaiacyl (G-type, 2-methoxy-4-propylphenol), syringyl (S-type, 1,3-dimethoxy-2,5-dimethylbenzene), and p-hydroxy phenyl (H-type, 4-propylphenol) species (Grabber et al. 1997).
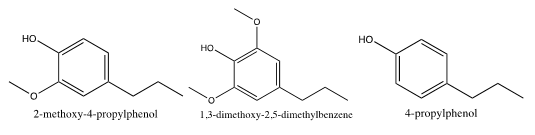
Fig. 2. Three basic phenyl propane structure units of lignin
From Figs. 2 and 3, it was evident that catalysts improved the total yield of monophenols compared to that without catalysts. This may be because metal chlorides used in this catalytic system are hydrolyzed, causing the production of Brønsted acid and the HCl formed in situ by the hydrolysis of metal chlorides catalysts (Davoudzadeh et al. 1985).
Fig. 3. The percentages of total monomer content of lignin depolymerization generated by different catalysts
Therefore, oxonium salt can be formed when H+ attacks the etheric O atom, which activates the C-O bond, favoring the cleavage of the β-O-4 bond (Zhang et al. 2014). For example, ZnCl2, during the cleavage of β-O-4, Zn2+ coordinated with the O atom positioned in the β-O-4, which could have weakened the bond of Cα– Cβ. The weakened Cα– Cβ bond was then cleaved, resulting in the formation of vanillin (Fig. 4) (Lim et al. 2013).
The total amount of the phenolic monomer gradually increased in the following order: No catalyst < MgCl2 < AlCl3 < FeCl3 < ZnCl2 < MnCl2. Among all of the catalysts, MnCl2 showed the highest catalytic effect, with the highest total yield of mono-phenols. With MgCl2, the lignin produced the least mono-phenol compounds. For MgCl2 and AlCl3, this may have been caused by the effect of Lewis acid. A stronger acidity of the metal chloride resulted in a potentially stronger catalytic effect. However, this trend may not be suitable for FeCl3, ZnCl2, and MnCl2. It is known that magnesium is an alkali-earth metal, but zinc, iron, and manganese are transitional metals. This different characteristic also causes them to produce different effects on the reaction mixture, thus producing different products. According to Xianwu et al. (2007), the catalytic mechanism of alkali/alkaline earth metal chlorides is distinct from that of transition metal chlorides. All transition metal chlorides had stronger catalytic effects than alkaline earth metal chlorides in this experiment. As opposed to alkaline earth metal chlorides, the accurate catalytic mechanism of transition metal chlorides has not been reported. One possible explanation is that transition metal chlorides always have a larger cation radius, which may influence their catalytic effect (Zhdanov and Brown 1965). These results were also in agreement with the study of Shu et al. (2015). For transition metal chlorides, a larger cation radius resulted in a stronger catalytic effect.
Fig. 4. The proportion of different types of phenolic monomer in all monomers of the lignin depolymerization products
Specific types of phenolic monomers are shown in Fig. 4. In the presence of no catalyst, the yield of each species of monomers was similar. However, in the presence of catalyst, the production of each monomer was different, which fully reflects the different effects of each catalyst.
It was shown that transition metal chlorides MnCl2, ZnCl2, and FeCl3catalyzed the depolymerization of lignin to produce a variety of products. However, alkaline earth metal chlorides MgCl2 and AlCl3were more effective at producing certain products. Both MgCl2 and AlCl3 had a weak effect on producing H-type phenolic compounds. It was also noteworthy that when MgCl2 was used as a catalyst, G-type monomer compounds were hardly produced either. This may be because under low temperatures, the depolymerization of lignin is considerable through the cleavage of inter-unit linkages (such as α-O-4 and β-O-4 bonds) on account of their relatively low bond dissociation energy (Hu et al. 2013), and the cleavage of bond that helps to produce H-type compounds requires more energy. Compared with MnCl2 or ZnCl2, the existence of FeCl3 produced a lower proportion of other species of monophenols. This indicated that FeCl3was more effective at producing G-type, H-type, and S-type monomer compounds.
Table 2. Main Monomers in the Products of Lignin Depolymerization Under the Catalysis of Different Catalysts
Table 2 shows the different monomers in the products of lignin depolymerization. As shown, some of the products yielded only with the presence of a specific catalyst. For example, vanillin (4-hydroxy-3-methoxybenzaldehyde), as the major flavor constituent of vanilla, has a wide range of applications in the food industry and in perfumery (Araújo et al. 2010). Vanillin is also very useful in the synthesis of several pharmaceutical chemicals (Bjoersvik and Liguori 2002). Vanillin almost did not appear with MgCl2 or AlCl3, but it reached the highest yield when there was 2.78% MnCl2 present. Vanillin production could be associated with the release of guaiacyl intermediates (Guerra et al. 2006). The important precursor to producing vanillin is 4-(1,3-dihydroxypropyl)-2-methoxyphenol through H abstraction. This is a result of β-O-4cleavage (Lin et al. 1997). The authors speculate that transition metal chlorides are more likely to induce the precursor to producing vanillin.
Aspidinol [1-(2,6-dihydroxy-4-methoxy-3-methylphenyl)butan-1-one], which is a compound comprising a C4 chain, is considered to be among the intermediates leading to the production of phthalates (Pineda et al. 2011). It only appeared in the presence of FeCl3. In contrast, desaspidinol (Thring and Breau 1996) appeared when lignin was catalyzed by catalysts other than FeCl3. The difference between aspidinol and desaspidinol is that the former has one more methyl group than the latter, which demonstrates that the catalyst FeCl3 helps to maintain the presence of methyl.
According to the study made by Shu et al. (2018), a metal chloride catalyst such as ZnCl2 could promote the occurrence of etherification remarkably. The Lewis acid sites in metalchloride were also conducive to reduce the dissociation energy of β-O-4 bond. Transition metal chlorides catalysts such as CrCl3 could interact with the oxygen electron pair and promote the cleavage of the methoxyl group, which resulted in the rapid conversion of guaiacols to phenols. The occurrence of alkylation was also promoted, which improved the stability of the products. High reaction temperature and long reaction time both could promote the occurrence of demethoxylation and alkylation, producing a lot of alkylphenols.
FT-IR Spectra of the Original and Regenerated Lignin
The FT-IR spectra of liquid products were recorded in the range of 4000 cm–1 to 700 cm–1, and the results are shown in Fig. 5.
The main peaks of the chemical functional groups present in lignin before and after depolymerization were found at 3430 cm–1, 2985 cm–1, 2829 cm–1, 1610 cm–1, 1514 cm–1, 1491 cm–1, 1369 cm–1, 1261 cm–1, 1216 cm–1, 1172 cm–1, 1117 cm–1, 1008 cm–1, 832 cm–1, 798 cm–1, and 773 cm–1. The infrared spectra of lignin before and after depolymerization were different, which demonstrated that the lignin components changed during the depolymerization process. The infrared spectra of lignin with or without catalysts were different, which meant that the catalyst played an important role in lignin depolymerization. The infrared spectra of lignin with different catalysts were various, which indicated that different catalysts or, precisely, different metal cations played different catalytic roles in lignin depolymerization.
Fig. 5. FT-IR spectra of the lignin before and after depolymerization with various metal chloride catalysts
Among these peaks, the broad peak at approximately 3430 cm–1 was related to the –OH stretching absorption of the aliphatic hydroxyl and phenolic hydroxyl groups. Figure 5 shows that in addition to being catalyzed by AlCl3 or MnCl2, the hydroxyl content of lignin depolymerization products was reduced. Combined with GC-MS, the authors inferred that lignin depolymerization catalyzed by AlCl3 and MnCl2 may have produced more products that contained phenolic hydroxyl groups.
The wavenumbers at approximately 2985 cm–1 and 2829 cm–1, assigned to the C-H vibration in methyl, were weaker in the lignin depolymerization products compared with the raw lignin. Therefore, a decrease in the methyl groups was indicated. It is worth mentioning that when MnCl2 or ZnCl2 was present, the C-H group may have had a certain displacement and both 2985 cm–1peaks and 2829 cm–1 peaks shifted to the middle. This may have been caused by changes in the C-H group during the process of depolymerization, such as hydrogenation or hydrogen reduction.
There are four main vibrations of C=C double bonds in mononuclear aromatic hydrocarbons, which appear in the range of 1620 cm-1 to 1450 cm–1 (Xie et al. 2015). As shown in Fig. 5, the peaks appeared around 1610 cm-1 and 1491 cm-1, regardless of which catalyst was used. However, the peaks at 1514 cm–1 disappeared in the catalytic products after depolymerization. This may have been caused by a series of substitutions of the groups on the aromatic ring during depolymerization. Additionally, the absorption peak at 1514 cm–1was weakened after depolymerization, which may indicate that the aromatic rings were partially opened.
In addition, the peaks near 1032 cm–1, which are associated with the stretching vibration of the C–O single bond in the esters, aldehydes, and ketones, disappeared in all the depolymerization products. It was speculated that esters, aldehydes, and ketones decreased in all products.
GPC-HPLC Analysis of Residual Lignin Degradation Products
Table 2 shows that the Mw and Mn of processed lignin decreased compared with the original lignin. This meant that through the reaction with the catalysts, lignin was degraded into smaller molecules. It was evident that the Mw or Mn of lignin depolymerization products with different catalysts had nothing in common. This also demonstrated that the effect of each metal cation on lignin depolymerization was distinct.
For the Mw of lignin depolymerization products, the number ranged from 1389 (when it was catalyzed by FeCl3) to 1270 (when it was catalyzed by AlCl3). This data roughly reflected the average molecular weight of depolymerization products. It was concluded that AlCl3 showed favorable catalytic activity in the experiment. As for Mn, the number ranged from 822 (when it was catalyzed by MgCl2) to 610 (when it was catalyzed by MnCl2). This may have been because depolymerization products include more high molecular weight products in the presence of MgCl2. However, when MnCl2was used as a catalyst, lignin depolymerization products included more low molecular weight molecules. In the authors’ statistical data, Mw/Mn was used to measure the range of molecular weight. Table 3 shows that the molecular weight distribution of depolymerization products was the most extensive when AlCl3 was used to catalyze depolymerization.
Table 3. Weight-average Molecular Mass (Mw), Number-average Molecular Mass (Mn), and Distributions (Mw/Mn) of Depolymerization Products
CONCLUSIONS
- The Formic acid and Lewis acid system effectively catalyzed the depolymerization of lignin. In this experiment, the total amount of the phenolic monomer catalyzed by different metal chlorides gradually increased in the following order: No catalyst < MgCl2 < AlCl3 < FeCl3 < ZnCl2 < MnCl2. The MnCl2 had the best catalytic effect. The highest total monomer yield was 48.7%, including approximately 23.0% G-type, 11.9% S-type, and 14.8% H-type monomer compounds.
- Different catalysts had different effects on the depolymerization of lignin, which were embodied not only in the type but also in content of the products. Transition metal chlorides MnCl2, ZnCl2, and FeCl3 catalyzed the depolymerization of lignin to produce a variety of products. However, alkaline earth metal chlorides, MgCl2 and AlCl3, were more effective at producing certain products.
- Under the conditions of this experiment, lignin produced the most vanillin (2.78%) in MnCl2 depolymerization.
ACKNOWLEDGMENTS
This work was financially supported by the International Science and Technology Cooperation Program of China (2-14DFA61040) and the Program of the Natural Science Foundation of China (21466022).Thanks also goes to Sepax Technologies, Inc. for the use of their equipment.
REFERENCES CITED
Araújo, J. D. P., Grande, C. A., and Rodrigues, A. E. (2010). “Vanillin production from lignin oxidation in a batch reactor,” Chem. Eng. Res. Des. 88(8), 1024-1032. DOI: 10.1016/j.cherd.2010.01.021
Asina, F., Brzonova, I., Kozliak, E., Kubátová, A., and Ji, Y. (2017). “Microbial treatment of industrial lignin: Successes, problems and challenges,” Renew. Sust. Energ. Rev. 77, 1179-1205. DOI: 10.1016/j.rser.2017.03.098
Bjoersvik, H. R., and Liguori, L. (2002). “Organic processes to pharmaceutical chemicals based on fine chemicals from lignosulfonates,” Cheminform 33(47), 831-832. DOI: 10.1021/op010087o
Davoudzadeh, F., Smith, B., Avni, E., Coughlin, R. W., Avni, E., and Coughlin, R. W. (1985). “Depolymerization of lignin at low pressure using Lewis acid catalysts and under high pressure using hydrogen donor solvents,” Holzforschung 39(3), 159-166.
Dhar, P., and Vinu, R. (2017). “Understanding lignin depolymerization to phenols via microwave-assisted solvolysis process,” J. Environ. Chem. Eng. 5(5), 4759-4768. DOI: 10.1016/j.jece.2017.08.031
Dong, C., Feng, C., Qian, L., Shen, D., and Rui, X. (2014). “Mechanism on microwave-assisted acidic solvolysis of black-liquor lignin,” Bioresource Technol. 162(6), 136-141. DOI: 10.1016/j.biortech.2014.03.060
Du, X., Liu, W., Zhang, Z., Mulyadi, A., Brittain, A., and Gong, J. (2017). “Low-energy catalytic electrolysis for simultaneous hydrogen evolution and lignin depolymerization,” ChemSusChem 10(5), 847-854. DOI: 10.1002/cssc.201601685
Duan, D., Zhao, Y., Fan, L., Dai, L., Lv, J., and Ruan, R. (2017). “Low-power microwave radiation-assisted depolymerization of ethanol organosolv lignin in ethanol/formic acid mixtures,” BioResources 12(3), 5308-5320. DOI: 10.15376/biores.12.3.5308-5320
Fan, L., Ruan, R., Liu, Y., Wang, Y., and Tu, C. (2016). “Effects of extraction conditions on the characteristics of ethanol organosolv lignin from bamboo (Phyllostachys pubescensmazel),” BioResources 10(4), 7998-8013. DOI: 10.15376/biores.10.4.7998-8013
Grabber, J. H., Ralph, J., Hatfield, R. D., and Quideau, S. (1997). “P-hydroxyphenyl, guaiacyl, and syringyl lignins have similar inhibitory effects on wall degradability,” J. Agr. Food Chem. 45(7), 2530-2532. DOI: 10.1021/jf970029v
Güvenatam, B., Heeres, E. H. J., Pidko, E. A., and Hensen, E. J. M. (2015). “Decomposition of lignin model compounds by Lewis acid catalysts in water and ethanol,” J. Mol. Catal. A- Chem. 410, 89-99. DOI: 10.1016/j.molcata.2015.09.007
Guerra, A., Filpponen, I., Lucia, L. A., and Argyropoulos, D. S. (2006). “Comparative evaluation of three lignin isolation protocols for various wood species,” J. Agr. Food Chem. 54(26), 9696-9705. DOI: 10.1021/jf062433c
Higuchi, T. (1990). “Lignin biochemistry: Biosynthesis and biodegradation,” Wood Sci. Technol. 24(1), 23-63. DOI: 10.1007/BF00225306
Hu, J., Shen, D., Xiao, R., Wu, S., and Zhang, H. (2013). “Free-radical analysis on thermochemical transformation of lignin to phenolic compounds,” Int. J. Remote Sens. 27(1), 285-293. DOI: 10.1021/ef3016602
Jia, S., Cox, B. J., Guo, X., Zhang, Z. C., and Ekerdt, J. G. (2016). “Hydrolytic cleavage of β-o-4 ether bonds of lignin model compounds in an ionic liquid with metal chlorides,” Ind. Eng. Chem. Res. 50(50), 78-82.
Jing, Y., Liang, Z., Liu, S., Wang, Y., and Dai, L. (2016). “High-quality bio-oil from one-pot catalytic hydrocracking of kraft lignin over supported noble metal catalysts in isopropanol system,” Bioresource Technol. 212, 302-310.
Kinrade, S. D., Nin, J. W. D., Schach, A. S., Sloan, T. A., Wilson, K. L., and Knight, C. T. G. (1999). “Stable five- and six-coordinated silicate anions in aqueous solution,” Science 285(5433), 1542-1545. DOI: 10.1126/science.285.5433.1542
Li, C., Zhao, X., Wang, A., Huber, G. W., and Zhang, T. (2015). “Catalytic transformation of lignin for the production of chemicals and fuels,” Chem. Rev. 115(21), 11559-11624. DOI: 10.1021/acs.chemrev.5b00155
Lim, S. H., Nahm, K., Ra, C. S., Cho, D. W., Yoon, U. C., and Latham, J. A. (2013). “Effects of alkoxy groups on arene rings of lignin β-o-4 model compounds on the efficiencies of single electron transfer-promoted photochemical and enzymatic c–c bond cleavage reactions,” J. Org. Chem. 78(18), 9431-9443. DOI: 10.1021/jo401680z
Lin, L., Yoshioka, M., Yao, Y., and Shiraishi, N. (1997). “Liquefaction mechanism of lignin in the presence of phenol at elevated temperature without catalysts. Studies on ß-0-4 lignin model compound. II. Reaction pathway,” Holzforschung 51(4), 325-332. DOI: 10.1515/hfsg.1997.51.4.325
Liu, Q., Li, P., Liu, N., and Shen, D. (2017). “Lignin depolymerization to aromatic monomers and oligomers in isopropanol assisted by microwave heating,” Polym. Degrad. Stabil. 135, 54-60. DOI: 10.1016/j.polymdegradstab.2016.11.016
Maldhure, A. V., and Ekhe, J. D. (2013). “Pyrolysis of purified kraft lignin in the presence of AlCl3, and ZnCl2,” J. Environ. Chem. Eng. 1(4), 844-849. DOI: 10.1016/j.jece.2013.07.026
Ouyang, X., Huang, X., Zhu, Y., and Qiu, X. (2015). “Ethanol-enhanced liquefaction of lignin with formic acid as an in situ hydrogen donor,” Energ. Fuel. 29(9), 5835-5840. DOI: 10.1021/acs.energyfuels.5b01127
Pan, J., Fu, J., and Lu, X. (2015). “Microwave-assisted oxidative degradation of lignin model compounds with metal salts,” Energ. Fuel. 29(7), 4503-4509. DOI: 10.1021/acs.energyfuels.5b00735
Pineda, A., Balu, A. M., Campelo, J. M., Romero, A. A., Carmona, D., and Balas, F. (2011). “A dry milling approach for the synthesis of highly active nanoparticles supported on porous materials,” ChemSusChem 4(11), 1561-1565. DOI: 10.1002/cssc.201100265
Shu, R., Long, J., Yuan, Z., Qi, Z., Wang, T., and Wang, C. (2015). “Efficient and product-controlled depolymerization of lignin oriented by metal chloride cooperated with pd/c,” Bioresource Technol. 179, 84-90. DOI: 10.1016/j.biortech.2014.12.021
Shu, R., Xu, Y., Ma, L., Zhang, Q., Wang, C., and Chen, Y. (2018). “Controllable production of guaiacols and phenols from lignin depolymerization using Pd/C catalyst cooperated with metal chloride,” Chemical Engineering Journal. 338, 457-464. DOI: 10.1016/j.cej.2018.01.002
Thring, R. W., and Breau, J. (1996). “Hydrocracking of solvolysis lignin in a batch reactor,” Fuel 75(7), 795-800. DOI: 10.1016/0016-2361(96)00036-1
Toledano, A., Serrano, L., Labidi, J., Pineda, A., Balu, A. M., and Luque, R. (2013). “Heterogeneously catalysed mild hydrogenolytic depolymerisation of lignin under microwave irradiation with hydrogen‐donating solvents,” ChemCatChem 5(4), 977-985. DOI: 10.1002/cctc.201200616
Toledano, A., Serrano, L., Pineda, A., Romero, A. A., Luque, R., and Labidi, J. (2014). “Microwave-assisted depolymerisation of organosolv lignin via mild hydrogen-free hydrogenolysis: Catalyst screening,” Appl. Catal. B-Environ. 145(2), 43-55. DOI: 10.1016/j.apcatb.2012.10.015
Tuomela, M., Hatakka, A., and Itavaara, M. V. M. (2000). “Biodegradation of lignin in a compost environment: A review,” Bioresource Technol. 72(2), 169-183.
Wang, Q., Guan, S., and Shen, D. (2017). “Experimental and kinetic study on lignin depolymerization in water/formic acid system,” International Journal of Molecular Sciences. 18(10), 2082.
Wan, Y., Chen, P., Zhang, B., Yang, C., Liu, Y., Lin, X., and Ruan, R. (2009). “Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity,” Journal of Analytical & Applied Pyrolysis, 86(1), 161-167.
Xianwu, Z., Jianzhong, Y., Xuemin, Y., Wenli, S., and Weigang, L. (2007). “Catalytic effects of metal chlorides on the pyrolysis of lignite,” Energ. Fuel. 21(2), 619-624. DOI: 10.1021/ef060477h
Xie, J., Qi, J., Hse, C., and Shupe, T. F. (2015). “Optimization for microwave-assisted direct liquefaction of bamboo residue in glycerol/methanol mixtures,” J. Forestry Res. 26(1), 261-265. DOI: 10.1007/s11676-015-0032-1
Xu, W., Miller, S. J., Agrawal, P. K., and Jones, C. W. (2012). “Depolymerization and hydrodeoxygenation of switchgrass lignin with formic acid,” ChemSusChem 5(4), 667-675. DOI: 10.1002/cssc.201100695
Zhang, X., Zhang, Q., Long, J., Xu, Y., Wang, T., and Ma, L. (2014). “Phenolics production through catalytic depolymerization of alkali lignin with metal chlorides,” BioResources 9(2), 3347-3360. DOI: 10.15376/biores.9.2.3347-3360
Zhdanov, G. S., and Brown, A. F. (1965). Crystal Physics, Oliver & Boyd, London, UK.
Article submitted: January 7, 2018; Peer review completed: March 5, 2018; Revised version received and accepted: March 23, 2018; Published: March 30, 2018.
DOI: 10.15376/biores.13.2.3704-3719
APPENDIX
Table A1. List of Reactants
