Abstract
5-Hydroxymethyl furfural (5-HMF) was prepared using microcrystalline cellulose as the raw material, an ionic liquid as the solvent, and ethyl butyrate as the co-solvent. The decomposition of microcrystalline cellulose to 5-HMF in an ionic liquid/organic solvent (ethyl butyrate) biphasic system was investigated. The optimum conditions were an ionic liquid:organic solvent ratio of 1:4, reaction temperature of 130 °C, and reaction time of 3 h, which resulted in yields of 5-HMF and reducing sugar of 40.95% and 41.7%, respectively. The ionic liquid was re-utilized. The re-utilization process of the biphasic system was also studied. The solvent [BMIM]Cl could be reused twice. The primary recovery rate of [BMIM]Cl was 75.6%, and the yield of 5-HMF was 32.6%. The second recovery rate of [BMIM]Cl was 46.8%, and the yield of 5-HMF was 17.6%.
Download PDF
Full Article
Preparation of 5-Hydroxymethylfurfural Based on the Biphasic System of Ionic Liquid/Ethyl Butyrate
Heng Zhang,a,b* Zhe Wang,a and Hongkun Gao a
5-Hydroxymethyl furfural (5-HMF) was prepared using microcrystalline cellulose as the raw material, an ionic liquid as the solvent, and ethyl butyrate as the co-solvent. The decomposition of microcrystalline cellulose to 5-HMF in an ionic liquid/organic solvent (ethyl butyrate) biphasic system was investigated. The optimum conditions were an ionic liquid:organic solvent ratio of 1:4, reaction temperature of 130 °C, and reaction time of 3 h, which resulted in yields of 5-HMF and reducing sugar of 40.95% and 41.7%, respectively. The ionic liquid was re-utilized. The re-utilization process of the biphasic system was also studied. The solvent [BMIM]Cl could be reused twice. The primary recovery rate of [BMIM]Cl was 75.6%, and the yield of 5-HMF was 32.6%. The second recovery rate of [BMIM]Cl was 46.8%, and the yield of 5-HMF was 17.6%.
Keywords: Cellulose; Ionic liquid; Ethyl butyrate; 5-HMF; Biphasic system
Contact information: a: College of Marine Science and Biological Engineering, Qingdao University of Science & Technology, Qingdao, Shandong 266042 China; b: Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control, Nanning 530004;*Corresponding author:hgzhang@sina.com
INTRODUCTION
In recent years, 5-hydroxymethyl furfural (5-HMF) has been recognized as a new compound (Roman-Leshkov et al. 2007) to produce furan, alkanes, and levulinic acid (LA) ester liquid fuel (Huber et al. 2005; Roman-Leshkov et al. 2007), as well as solvents, pharmaceutical intermediates, and polyester materials (Chheda et al. 2007; Yadav and Borkar 2008; Yan et al. 2009; Lilga et al. 2010). Therefore, 5-HMF has broad market potential. At present, monosaccharides, such as fructose, are generally used as raw material to prepare 5-HMF (Yan et al. 2009). Cellulose, which is cheap and widely distributed, is more promising and economical than fructose. Therefore, an increasing number of researchers have studied the preparation of 5-HMF directly from cellulose in recent years. In the preparation of 5-HMF from cellulose, an important factor affecting the yield is the selection of suitable reaction solvents that are not only green but also improve the solubility of cellulose to help in increasing the 5-HMF yield. Moreover, it should be easy to reuse.
In the catalytic conversion of cellulose to 5-HMF, ionic liquids have the advantages of facile dissolution of cellulose and short reaction time (Hu et al. 2012). Hsu et al. (2011) studied the reaction of cellulose to 5-HMF in different ionic liquids, ionic liquids act as both solvents and catalysts. The effects of different ionic liquids, reactants, water and cellulose ratio, solution temperature, solution time, and reaction time on the conversion were investigated. The reaction conditions were water:cellulose, 10:1, dissolution temperature of 120 °C, reaction temperature of 120 °C, dissolution time of 0.5 h, and reaction time of 3 h. Under these conditions the yield of 5-HMF was 21%. However, 5-HMF was highly unstable under the single-phase reaction conditions and easy to hydrolyze into by-products, such as LA. Simultaneously, 5-HMF was also difficult to separate and extract (Holladay et al. 2007; Kim et al. 2013), resulting in declined yield and selectivity. Thus, more researchers have begun to focus on the preparation of 5-HMF under two-phase or multiphase conditions. For example, indium chloride (InCl3), a type of Lewis acid compatible with water, can be used to promote the catalytic degradation of microcrystalline cellulose and improve the production of 5-HMF in the aqueous two-phase system composed of tetrahydrofuran. Additional sodium chloride (NaCl) can also increase the yield of 5-HMF and inhibit the LA production. Shen et al. (2014) used Indium chloride (InCl3) to catalyze the degradation of microcrystalline cellulose, and the yield of 5-HMF was 39.7% in the NaCl-H2O/THF system under 2 h reaction at 200 °C. Sun et al. (2015) studied the preparation of 5-HMF from lignocellulosic biomass via catalytic degradation under microwave heating with solid organic acid as a catalyst in the H2O/THF two-phase system, and the yield of 5-HMF reached 52.2% with bamboo fiber treated using NH2SO3H as a catalyst at 180 °C for 40 min with 500 Hz microwave heating. Although the above methods improved the yield of 5-HMF, organic solvents are poisonous, harmful, and difficult to recycle and reuse. Therefore, an environmentally friendly reaction system is needed to improve the feasibility of the process.
In this study, the preparation of 5-HMF in the biphasic system of ionic liquid-butyric acid was studied using microcrystalline cellulose as the raw material, an ionic liquid as the solvent, and ethyl butyrate as the co-solvent. The reaction system was carried out in the lower layer of ionic liquid, and the generated 5-HMF was soluble in the upper layer of ethyl butyrate. In the two-phase system, the partial product 5-HMF was separated from the ionic liquid, which improved the yield and selectivity. Butyric acid ethyl ester, which is classified as an environmentally friendly solvent, is not harmful to the human body. This study aimed to provide novel ideas and possibilities for the direct preparation of 5-HMF from biomass resources using environmentally friendly solvents.
EXPERIMENTAL
Materials
Medicinal-grade microcrystalline cellulose was provided by Chengdu Kelong Chemical Co., Ltd. (Chengdu, China). The 1-butyl-3-methyl-imidazolium chloride (99%) was provided by Shanghai Cheng Jie Chemical Co., Ltd. (Shanghai, China). The 5-HMF was high performance liquid chromatography (HPLC)-grade, which was provided by Shanghai Song Chemical Co., Ltd. (Shanghai, China). Chromium chloride (CrCl3 ∙ 6H2O) was provided by Shanghai Aibi Chemical Reagent Co., Ltd. (Shanghai, China). Glucose was purchased from Beijing Combi Technology Co., Ltd. (Beijing, China). Ethyl butyrate was supplied by Sinopharm Chemical Reagent Co., Ltd. (Qingdao, China). The three aforementioned chemicals underwent pure analysis. Deionized water was prepared in the laboratory.
A TU-1810-type ultraviolet visible spectrophotometer was purchased from the Beijing Purkinje General Instrument factory (Beijing, China). X-ray diffraction (XRD) was conducted using a DX-2700 by Brook Spectrum Instrument Co., Ltd. (Ettlingen, Germany). A T27-type FT-IR infrared instrument was provided by Bruker Spectrum Instrument Co., Ltd. (Bruker, Germany).
Experimental steps
A certain amount of ionic liquid 1-butyl-3-methyl-imidazolium chloride was added to four flasks filled with nitrogen, dissolved in 100 °C, and added with microcrystalline cellulose and a specified amount of chromium chloride as the catalyst and organic solvent. After the reaction, a certain amount of liquid (5-HMF and reducing sugar) in the upper and lower layers was obtained, and the yields of reducing sugar and 5-HMF were calculated at 540 nm and 284 nm, respectively.
Production yield calculation
Figure 1, parts (a) through (c), shows the calibration for determination of glucose, 5-HMF in water, and 5-HMF in organic solvent, respectively, based on absorbance results.
Fig. 1(a). Calibration curves of glucose content
Fig. 1(b). Calibration curves of 5-HMF content in water
Fig. 1(c). Calibration curves of 5-HMF content in organic solvent
The glucose yield was calculated according to Eq. 1
(1)
where Cglucose is mass concentration of glucose (mg/mL), V is the bulk after constant volume of reaction solution (mL), and m is the mass of microcrystalline cellulose in the reaction liquid (mg).
The yield calculation formula of 5-HMF, the yield of 5-HMF in an ionic liquid layer and organic solvent layer was calculated using Eq. 2,
(2)
where C5-HMF is the mass concentration of 5-HMF (mg/mL), V is the bulk after constant volume of reaction solution (mL), and m is the mass of microcrystalline cellulose in the reaction liquid (mg).
The total yield of 5-HMF was calculated using Eq. 3,
(3)
where M1 is the yield of 5-HMF in the lower ionic liquid system (mg), M2 is the yield of 5-HMF in upper butyric ester system (mg), and M is the mass of microcrystalline cellulose as the raw material (mg).
Performance testing- XRD characterization
Approximately 20 g of ionic liquid (chlorinated 1-butyl-3-methyl-imidazole) was added to the four flasks, and 2 g of microcrystalline cellulose was added after dissolution. After reacting for 80 min, the microcrystalline cellulose was fully soluble in the ionic liquids. The reaction liquid was cooled after being dissolved in a 500-mL beaker. The dissolved microcrystalline cellulose was filtered and oven-dried. The samples were then characterized by a DX-2700-type X-ray diffractometer (Ettlingen, Germany) in the range of 5° to 50° (2θ).
Infrared characterization
After the reaction, the reaction liquid was divided into two layers. The upper liquid was ethyl butyrate, and the lower layer was ionic liquid. The lower liquid was extracted three times with ethyl acetate. After extraction, the liquid was washed with deionized water. After washing, the sample was distilled by a rotary evaporator (Shanghai, China) at 60 °C and then vacuum-dried in a drying box at 70 °C. The sample was obtained using the tablet method (KBr), and the infrared spectrum of the sample was tested by an FT-IR spectrometer (Bruker, Germany).
RESULTS AND DISCUSSION
Effects of Organic Solvents on Catalytic Degradation
In the catalytic degradation of microcrystalline cellulose to produce 5-HMF, the addition of an organic solvent has an important influence on the dissolution of microcrystalline cellulose. In this study, the XRD spectra of microcrystalline cellulose dissolved in ionic liquid 1-butyl-3-methyl-imidazolium chloride ([BMIM]Cl), and a mixture of [BMIM]Cl and organic solvent were measured, and the spectra are depicted in Figs. 2 and 3.
Fig. 2. XRD spectra of microcrystalline cellulose (a) and regenerated cellulose in [BMIM]Cl (b)
Figures 2 and 3 show the XRD spectra of microcrystalline cellulose dissolved in ionic liquid [BMIM]Cl and a mixture of [BMIM]Cl and organic solvent. In the interaction of ionic liquid, [BMIM]Cl, and organic solvent, cellulose underwent crystalline changes. This finding showed that the hydrogen bonds between the molecules in cellulose decreased, and the crystallinity also decreased. These phenomena are prerequisites for the dissolution of cellulose.
Fig. 3. XRD spectra of microcrystalline cellulose (a) and regenerated cellulose in a mixture of [BMIM]Cl and organic solvent (b)
The diffraction peak of microcrystalline cellulose intensity was low, and the peak was also relatively wide after the dissolution in [BMIM]Cl and organic solvent in comparison with that after the dissolution in [BMIM]Cl. This result showed that the combination of [BMIM]Cl and organic solvent was more effective in the dissolution of microcrystalline cellulose, with a better solubility effect. Organic solvents can promote the dissolution of microcrystalline cellulose through ionic liquids; the organic solvent not only had a swelling effect on microcrystalline cellulose but also reduced the crystallinity of microcrystalline cellulose. In addition, the organic solvent can also decrease the viscosity of ionic liquid and increase the intensity of ion movement, which is beneficial to the dissolution of microcrystalline cellulose in ionic liquid.
Effect of Reaction Temperature on Catalytic Degradation
The reaction conditions were as follows: microcrystalline cellulose:ionic liquid:butyric acid ethyl ester = 1:10:40; CrCl3 content was 0.2g; temperature of 140 °C, 130 °C, 120 °C, and 110 °C.
When the temperature is 110 °C and 120 °C, the yield of reducing sugar and 5-HMF increases with the increase of time, but the yield of sugar and 5-HMF is low relative to 130 °C. When the temperature was 130 °C, the yield of reducing sugar increased with increased time. When the reaction time was 3 h, the yield of reducing sugar peaked. Subsequently, the reduced sugar yield decreased because the reduced sugars were converted into 5-HMF. When the reaction time was 3 h, the 5-HMF yield peaked but then decreased slightly. When the temperature is 140 °C, the yield of reducing sugar and 5-HMF is lower than 130 °C.
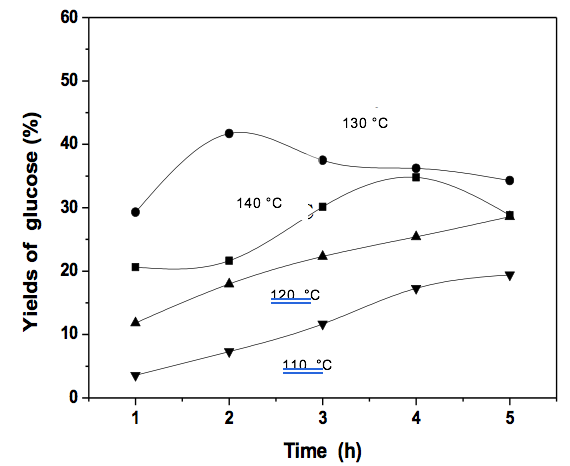
Fig. 4. Effect of the temperature on glucose yield
Fig. 5. Effect of the temperature on 5-HMF yield
The reaction temperature played an important role on the yields of reducing sugar and 5-HMF during the preparation of 5-HMF via the catalytic degradation of cellulose, and the yield increased with increased reaction temperature. Therefore, the optimum reaction temperature was 130 °C in the two-phase system of [BMIM]Cl-butyric acid ethyl ester with the reaction time of 3 h, and the yields of 5-HMF and reducing sugar were 40.95% and 41.7%, respectively.
According to the Arrhenius equation, the reaction rate constant was related to the reaction temperature. The maximum concentration of glucose formation increased with the increased temperature, and the reaction time also became shorter with the increase in temperature. In this research, glucose and 5-HMF were the intermediate products of the continuous reaction, and the maximum value was 130 °C. With the increase in time, both glucose and 5-HMF initially increased and then decreased beyond 120 °C. This finding was due to the amorphous areas in cellulose that hydrolyzed quickly under mild conditions during the hydrolysis reaction. The degradation rate of glucose was lower than that of the formation rate under a short reaction time, and the glucose concentration and yield decreased with the temperature increase and the formation of small molecules. Furthermore, cellulose did not dissolve completely in the ionic liquid within a short time, and a long reaction time allowed for the self-polymerization of 5-HMF, thereby decreasing the yield. Based on the above results, 180 min was considered as the optimum reaction time.
Influences of the Amount of Organic Solvents on the Catalytic Effect
The reaction conditions included microcrystalline cellulose:ionic liquid =1:10 and content of CrCl3 of 0.2 g. The ratio of ionic liquid:butyric acid ethyl ester was 1:5.5, 1:4.0, and 1:2.0, and the yield of reducing sugar and 5-HMF at 130 °C was determined. The experimental results are shown below.
Fig. 6. Effect of the ratio of ionic liquid to ethyl butyrate on yield of glucose
Fig. 7. Effect of the ratio of ionic liquid to ethyl butyrate on yield of 5-HMF
As shown in Fig. 6, before 3 h, the yield of reducing sugar increased with the increase in ethyl butyrate in the early stage of reaction, and the reducing sugar yield was minimal at the ratio of 1:2.0. The maximum yield of reducing sugar was not very different between 1:4.0 and 1:5.5. After 3 h, the reduction rate of reducing sugar was accelerated at 1:5.5. Considering the economic, environmental, and conservation factors, thus, the authors chose the ethyl butyrate:ionic liquid ratio of 1:4.
As shown in Fig. 7, the yield of 5-HMF increased with time when the ratio of the ionic liquid to butyric acid was 1:2.0. However, the yield of 5-HMF was low in comparison to when the ratios of reagents were 1:4.0 and 1:5.5. When the ratio of ionic liquid to butyric acid was 1:5.5, the yield of 5-HMF was almost the same as that of 1:4.0. Furthermore, at the ratio of 1:5.5, the advantage of the 5-HMF yield was very small, possibly because the temperature was much higher than the boiling point of the organic solvent at the reaction temperature of 130 °C. With the reaction time extension, the organic solvent and 5-HMF were increasingly volatile and unable to return to the reactor by the condensing equipment, which resulted in product loss and a high amount of volatile organic solvent. After considering economy and environmental protection, the ratio of butyric acid to ionic liquid was 1:4.0.
In summary, this study determined the following conditions for the synthesis of 5-HMF: [BMIM]Cl:organic solvent ratio of 1:4.0 and temperature of 130 °C. The yields of glucose and 5-HMF under these conditions were 41.7% and 40.95%, respectively.
When impurities were present, the solubility of ionic liquids to cellulose was affected. The studies of Nishiyama et al. (2003) revealed that the presence of water greatly reduced the solubility of cellulose in ionic liquids. Water was easily combined with the hydroxyl groups on the cellulose molecule to form hydrogen bonds relative to the ionic liquid, thereby breaking off the hydrogen bonds already formed between the ionic liquid and cellulose. The cellulose that was precipitated from the ionic liquid reduced the reaction efficiency. Therefore, the absorption and dissolution of ionic liquid should be prevented as much as possible to reduce the effect of water on the experimental results. However, some researchers believe that the amount of water can improve the yield of 5-HMF. Zhang et al. (2010) reported that the successful degradation of cellulose was achieved with CrCl3 as a catalyst and 89% 5-HMF conversion was obtained with [EMIM]Cl and water. The researchers believed that the high yield of 5-HMF was due to the high purity of the ionic liquids used, as well as the common effect of water. This phenomenon will be further investigated in future studies.
RECOVERY OF IONIC LIQUIDS
Feasibility of Ionic Liquids Recovery
The ionic liquid was first recovered in this study. The reaction conditions were as follows: microcrystalline cellulose:ionic liquid:ethyl butyrate = 1:10:40, temperature of 130 °C, CrCl3 content of 0.2 g, and reaction time of 5 h. After the reaction was completed, the reaction solution was separated. The lower layer was extracted three times with ethyl acetate, and the extract was washed with deionized water after filtration.
After washing, the mixture was distilled at 60 °C using a rotary evaporator and then vacuum-dried in an oven at a drying temperature of 70 °C. The KBr was used to compress the sample, and the infrared spectrum was tested. The spectrum is as follows:
Fig. 8. IR spectrum of [BMIM]Cl before (a) and after the reaction (b)
Figure 8 shows that the ionic liquid [BMIM]Cl displayed characteristic absorption peaks at 2958.6 cm–1, 2875.7 cm–1, 1635.6 cm–1, 1568.0 cm–1, and 1170.7 cm–1 prior to the reaction.
These absorption peaks represented the characteristic absorption of the H–H bond on the telescopic vibrational phase, the vibration characteristic absorption of the imidazole ring skeleton, the absorption characteristic of the carbon and nitrogen bond expansion, and the vibration characteristic absorption of the hydrogen bond on the imidazole ring plane. The peak at 854.8 cm–1 represented the C–H in-plane oscillatory vibration in the imidazole ring, and that at 756.1 cm–1 was the C–H external oscillatory vibration in the imidazole ring.
After a reaction time of 5 h, some characteristic absorption peaks indicated that Cl displacement was observed in the reaction ionic liquid [BMIM]. The displacement was small, and the change that occurred in the structure did not belong to the scope. This result indicated that the structure of the ionic liquid [BMIM]Cl did not change after the reaction. The structure was stable and could be recovered and applied to the next experiment.
Each substance in the reaction system underwent the recycling process. After the completion of the reaction, the supernatant liquids, including the organic solvent layer, were concentrated to produce dried 5-HMF and organic solvent, which could be used for the next experiment. After the addition of a certain amount of distilled water to the lower reaction liquid, the solid and solution were separated by centrifugation.
c)
d)
Fig. 9. IR spectrum of c) first-recycled [BMIM]Cl and d) second-recycled[BMIM]Cl
As shown in Fig. 9, the primary and secondary recovery of [BMIM]Cl were consistent with the characteristic absorption peaks of pure [BMIM]Cl sample. The results also showed that the recovered [BMIM]Cl did not change structurally. In this work, the primary recovery rate of [BMIM]Cl was as high as 75.6%, the secondary recovery rate of [BMIM]Cl was 46.8%, and the tertiary recovery of [BMIM]Cl was virtually devoid of [BMIM]C because of the loss caused by the recovery process.
Recovery of Ionic Liquid for the Preparation of 5-HMF
Microcrystalline cellulose was directly added to the reacting solution that has been extracted from 5-HMF, which was used to prepare 5-HMF. The reaction conditions were as follows: the reaction temperature was 130 C, the reaction time was 3 h, the final yield of 5-HMF was 32.6%, and the yield of 5-HMF obtained by two recovery was 17.6%. The yield of two times recovery was too low. The two recovery rate was too low. The reason is that after two times of reaction, the caramel substances in the reaction fluid increase, and the viscosity of the system increases, which makes the mobile ions in [BMIM]Cl difficult to move, and the yield is low.
CONCLUSIONS
- In this study, 5-HMF was prepared via the catalytic degradation of microcrystalline cellulose in an ionic liquid-organic solvent (ethyl butyrate) system. The optimum reaction conditions were an ionic liquid:organic solvent ratio of 1:4.0, reaction temperature of 130 °C, and reaction time of 3 h. Under these conditions, the 5-HMF yield was 41.0%, and the yield of reducing sugar was 41.7%.
- The reusability of the reaction system was studied, and the recycling of ionic liquids was found to be feasible. The structure of recovered [BMIM]Cl was consistent with the structure of pure [BMIM]Cl sample after primary and secondary recoveries, indicating that [BMIM]Cl after two reactions could still be used for cellulose degradation.
- The primary recovery rate of [BMIM]Cl was 75.6%, and the yield of 5-HMF was 32.6%. The second recovery rate of [BMIM]Cl was 46.8%, and the yield of 5-HMF was 17.6% .
ACKNOWLEDGMENTS
This work was supported by Shandong Provincial Natural Science Foundation of China (ZR2017MC032), and the National Natural Science Foundation of China (No. 21406123), and the Science and Technology Major Project (Emerging Industries) of Shandong Province (2015ZDXX0403B03), and Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control (KF201705).
REFERENCES CITED
Chheda, J. N., Huber, G. W., and Dumesic, J. A. (2007). “Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals,” Angewandte Chemie International Edition 46(38), 7164-7183. DOI: 10.1002/anie.200604274
Holladay, J. E., Bozell, J. J., White, J. F., and Johnson, D. (2007). Top Value-Added Chemicals from Biomass-Volume II, Results of Screening for Potential Candidates from Biorefinery Lignin (Report No. PNNL-16983), U.S. Department of Energy-Energy Efficiency Renewable Energy, Pacific Northwest National Laboratory, Oak Ridae, TN.
Hsu, W. H., Lee, Y. Y., Peng, W. H., and Wu, K. C. W. (2011). “Cellulosic conversion in ionic liquids (ILs): Effects of H2O/cellulose molar ratios, temperatures, times, and different ILs on the production of monosaccharides and 5-hydroxymethylfurfural (HMF),” Catalysis Today 174(l), 65-69. DOI: 10.1016/j.cattod.2011.03.020
Huber, G. W., Chheda, J. N., Barrett, C. J., and Dumesic, J. A. (2005). “Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates,” Science 308(5727), 1446-1450. DOI: 10.1126/science.1111166
Hu, L., Sun, Y., and Lin, L. (2012). “Ionic liquids-mediated formation of 5-hydroxymethylfurfural,” Progress in Chemistry 24(4), 483-491.
Kim, J. H., Na, J. G., Yang, J. W., and Chang, Y. K. (2013). “Separation of galactose, 5-hydroxymethylfurfural and levulinic acid in acid hydrolysate of agarose by nanofiltration and electrodialysis,” Bioresource Technology 140, 64-72. DOI: 10.1016/j.biortech.2013.04.068
Lilga, M. A., Hallen, R. T., and Gray, M. (2010). “Production of oxidized derivatives of 5-hydroxymethylfurfural (HMF),” Topics in Catalysis 53(15-18), 1264-1269. DOI: 10.1007/s11244-010-9579-4
Román-Leshkov, Y., Barrett, C. J., Liu, Z. Y., and Dumesic, J. A. (2007). “Production of dimetbylfuran for liquid fuels from biomass-derived carbohydrates,” Nature 447(7147), 982-985. DOI: 10.1038/nature05923
Shen, Y., Sun, J., Yi, Y., Li, M., Wang, B., Xu, F., and Sun, R. (2014). “InCl3-catalyzed conversion of carbohydrates into 5-hydroxymethylfurfural in biphasic system,” Bioresource Technology 172, 457-460. DOI: 10.1016/j.biortech.2014.09.077
Sun, J., Yuan, X., Shen, Y., Yi, Y., Wang, B., Xu, F., and Sun, R. (2015). “Conversion of bamboo fiber into 5-hydroxymethylfurfural catalyzed by sulfamic acid with microwave assistance in biphasic system,” Industrial Crops & Products 70, 266-271. DOI: 10.1016/j.indcrop.2015.03.044
Yadav, G. D., and Borkar, I. V. (2008). “Kinetic modeling of immobilized lipase catalysis in synthesis of n-butyl levulinate,” Industrial and Engineering Chemistry Research 47(10), 3358-3363. DOI: 10.1021/ie800193f
Yan, H., Yang, Y., Tong, D., Xiang, X., and Hu, C. (2009). “Catalytic conversion of glucose to 5-hydroxymethylfurfural over SO42-/ZrO2 and SO42-/ZrO2-Al2O3 solid acid catalysts,” Catalysis Communications 10(11), 1558-1563. DOI: 10.1016/j.catcom.2009.04.020
Zhang, Y., Du, H., Qian, X., and Chen, E. Y. X. (2010). “Ionic liquid−water mixtures: Enhanced Kwfor efficient cellulosic biomass conversion,” Energy & Fuels 24(4), 2410-2417. DOI: 10.1021/ef1000198
Article submitted: July 5, 2017; Peer review completed: November 24, 2017; Revised version received: December 14, 2017; Accepted: December 15, 2017; Published: December 19, 2017.
DOI: 10.15376/biores.13.1.1189-1201
