Abstract
Biochar (BC) is a carbonaceous and porous product generated from the incomplete combustion of biomass and has been recognized as an efficient adsorbent. This study evaluated the ability of BC to sorb atrazine pesticide in tropical soil, and explored potential environmental values of BC on mitigating organic micro-pollutants. BC was produced from cassava waste via pyrolyzation under oxygen-limiting conditions at 350, 550, and 750 °C (MS350, MS550, and MS750, respectively). Three biochars were characterized and investigated as sorbents for the removal atrazine from tropical soil. BC pyrolyzed at higher temperatures more quickly reached equilibrium. The pseudo-second-order model perfectly simulated the sorption kinetics for atrazine with the coefficients R2 above 0.996, and the sorption amount at equilibrium (qe) was 0.016 mg/g for MS350, 0.025 mg/g for MS550 and 0.050 mg/g for MS750. The isotherms of MS350 displayed relatively linear behavior, whereas the sorption of atrazine on MS550 and MS750 followed a nonlinear isotherm. The sorption data were well described by the Freundlich model with logKF of 0.476 for MS350, 0.771 for MS550, 1.865 for MS750. A thermodynamic study indicated that the sorption of atrazine in BC-added soil was a spontaneous and endothermic process and was primarily controlled by physisorption. In addition, lower pH was conducive to the sorption of atrazine in BC-added soil.
Download PDF
Full Article
Sorption of Atrazine in Tropical Soil by Biochar Prepared from Cassava Waste
Hui Deng,a, b Huamei Yu,c Miao Chen,d and Chengjun Ge a,b,*
Biochar (BC) is a carbonaceous and porous product generated from the incomplete combustion of biomass and has been recognized as an efficient adsorbent. This study evaluated the ability of BC to sorb atrazine pesticide in tropical soil, and explored potential environmental values of BC on mitigating organic micro-pollutants. BC was produced from cassava waste via pyrolyzation under oxygen-limiting conditions at 350, 550, and 750 °C (MS350, MS550, and MS750, respectively). Three biochars were characterized and investigated as sorbents for the removal atrazine from tropical soil. BC pyrolyzed at higher temperatures more quickly reached equilibrium. The pseudo-second-order model perfectly simulated the sorption kinetics for atrazine with the coefficients R2 above 0.996, and the sorption amount at equilibrium (qe) was 0.016 mg/g for MS350, 0.025 mg/g for MS550 and 0.050 mg/g for MS750. The isotherms of MS350 displayed relatively linear behavior, whereas the sorption of atrazine on MS550 and MS750 followed a nonlinear isotherm. The sorption data were well described by the Freundlich model with logKF of 0.476 for MS350, 0.771 for MS550, 1.865 for MS750. A thermodynamic study indicated that the sorption of atrazine in BC-added soil was a spontaneous and endothermic process and was primarily controlled by physisorption. In addition, lower pH was conducive to the sorption of atrazine in BC-added soil.
Keywords: Sorption; Biochars; Cassava wastes; Atrazine; Paddy soil
Contact information: a: Key Laboratory of Protection and Development Utilization of Tropical Crop Germplasm Resources (Hainan University), Ministry of Education, Haikou 570228, China; b: Department of Environmental Science, Hainan University, Renmin Road, Haikou 570228, China; c: School of Chemical and Environmental Engineering, China University of Mining and Technology, Beijing 100083, China; d: Institute of Environment and Plant Protection, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, China; *Corresponding author: cjge3007@163.com
INTRODUCTION
Atrazine (AT), 2-chloro-4-ethylamino-6-isopropylamino-s-triazine, is one of the most widely applied pesticides for the control of annual grasses and broadleaf weeds in cereals, orchards, and plantations; it is effective because it inhibits photosynthesis (Mudhoo and Garg 2011). Because of its widespread application, AT and its metabolites have been frequently detected in the environment beyond safe concentrations (Jin and Ke 2002; Sun et al. 2010; Lima et al. 2011; Lin and Chu 2011). Recently, it has been recognized as an endocrine disruptor and a possible carcinogen by the EPA (Lin and Chu 2011), as it has adverse effects on the central nervous, endocrine, and immune systems of mammals and aquatic life (Hayes et al. 2002; 2006; 2010). Therefore, widespread occurrence of AT and its adverse health effects have led to extensive environmental studies. Sorption is one of the major processes for the removal of AT. Several studies have evaluated the removal efficiency of AT in soils and water bodies by adding different sorbents, such as organic matter fractions, humics, carbonate soil, activated carbon, and minerals (Salvestrini et al. 2010; Shi et al. 2010; Sun et al. 2010; Guzman-Perez et al. 2011; Jamil et al. 2011; Kovaios et al. 2011; Kasozi et al. 2012).
At present, a number of waste materials from agricultural products have been exploited as alternatives to activated carbon to remove hazardous chemicals (Mittal et al. 2005; Gupta et al. 2011). Biochar (BC) has attracted much attention in environmental and agricultural applications as an efficient, versatile, and environment-friendly material. It is also of interest for its potential for in situ environmental remediation of organic pollutants due to its specific properties (Cao et al. 2009). BC is a carbon-rich material generated from oxygen-limited pyrolysis of biomass and has high aromaticity. Its sources, such as plant pruning (Zheng et al. 2010), straw (Nag et al. 2011), woodchips, livestock, and sludge, are widely available. BC plays a crucial role in controlling the environmental effects of pollutants and risks to the ecosystem with a porous structure and special physicochemical properties. Studies dealing with the sorption of pollutants, such as heavy metals (Chen et al. 2011), antibiotics (Teixido et al. 2011), and pesticides (Mesa and Spokas 2011), on BC suggest that BC may be an effective material for pre-concentration and removal of contaminants. The sorption of AT on BC from aqueous solution has been reported by Zheng et al. (2010), Zhang et al. (2011), Zhang et al. (2013), and Zhao et al. (2013). They found that BC demonstrated high efficiency in improving the sorption affinity for AT. However, little is known concerning the sorption of AT in BC-added soil, especially in tropical areas. Understanding of sorption kinetics and thermodynamics is also critical for AT removal from the environment.
Cassava is a common food crop in tropical area and is mainly used to produce starch and alcohol. However, cassava processing results in a large amount of waste. It is reported that the output is more than 15 million tons per year, with an annual growth rate of over 30% in China (Wang et al. 2012). In this study, cassava waste was chosen as the source to produce BC, which is valuable for waste resource utilizations and BC application to agriculture.
The objective of the study was to obtain a wide and deep understanding of the influence of BC on pesticides. Three BC samples were generated from cassava waste at different pyrolysis temperatures, and their characteristics were compared. Atrazine was selected as the model agricultural chemical. The sorption behavior of AT was investigated in tropical soil with BC amendment, and the sorption mechanism was determined.
EXPERIMENT
Materials
Chemicals
Atrazine (99.9% purity) was purchased from Dr. Ehrenstofer company (German). Atrazine is a weakly basic pesticide with a pKa value of 1.7, and its solubility in water is 28 mg/L (20 °C) with a logkow of 2.74 (Chen et al. 2009). Methanol was supplied by Sinopharm Chemical Reagent Co. Ltd. (China). Calcium chloride and sodium azide were of analytical grade. Deionized water (18.25 MΩ) was obtained from a Spring-S60i+PALL system (Research Scientific Instruments Co. Ltd. China).
Properties of soil
Paddy soils (0 to 20 cm) used for sorption experiments were collected from Qiongzhong in Hainan Province, China. Posthole diggers washed with deionized water between samples were used to collect the soils, which were air-dried slowly under sheets of paper at room temperature until the moisture content reached about 3%. The soil samples were then sifted through a 40-mesh sieve for the analysis of their chemical and physical properties and a 1-mm sieve for sorption experiments. Characteristics of the soils were determined according to routine methods (Institute of Soil Science 1978) and are listed in Table 1.
Table 1. Physicochemical Properties of Soil
a cation exchange capacity.
b adding soil to deionized water in a mass ratio of 1:20.
Methods
Production and characterization of biochar
Cassava waste was collected from Danzhou City, Hainan Province, China. The waste was air dried, cut into small pieces, and mashed into powder with a crusher. The powder was stacked in porcelain crucibles with lids and placed in a muffle furnace under O2-limited conditions at 200 °C for 2 h, then subsequently heated at 350/550/750 °C for 3 h to carbonize. The BC was ground to pass through 0.3-mm (60-mesh) sieves and stored in hermetic bags. Based on the temperature at which they were carbonized, the BC was denoted as MS350, MS550, and MS750.
The physical and chemical properties of the BC were investigated. Yields of BC were calculated by mass difference between source material and BC. Ash content was measured by combusting the BC at 750 °C for 4 h. The pH value of the BC was measured by adding the BC to deionized water in a mass ratio of 1:20. The cation exchange capacity of the samples was analyzed according to the BaCl2-H2SO4forcing exchange method (Zhang et al. 2014). The abundance of elemental C, H, N, and S was determined using a Vario EL analyzer (German). The O content was estimated by mass difference (100%-C, H, N, and ash %). Scanning electron microscopy (SEM) analysis of the samples was performed on a Zeiss EVO18 operating at an accelerating voltage of 20 kV. The Brunauer-Emmett-Teller (BET) surface area and the pore volume of the samples were measured using a JW-BK224 surface area analyzer (JWGB Sci. & Tech. Co. Ltd., China) and calculated from the BET equation. The functional groups of the samples were detected using Fourier transform infrared (FTIR) spectroscopy (Nicolet iS10, Thermo Fisher Scientific), recording the spectral region from 4000 to 400 cm-1 with a resolution of 4 cm-1.
Sorption experiments
Sorption experiments were conducted using a batch equilibration method. Briefly, 10 mL of AT solution that contained 0.01 M CaCl2to maintain a constant ionic strength and 0.2 g/L of NaN3 to inhibit microbial activities and an appropriate amount of sample (BC/(soil + BC), w/w: 1%) was placed in centrifuge tubes. The samples were designated as CK (without BC), MS350, MS550, and MS750 based on their types. All tubes were agitated in the dark at 200 rpm at 25 °C. Preliminary experiments confirmed that 24 h was suitable to reach equilibrium. After equilibrium, the samples were centrifuged for 5 min at 4000 rpm, and supernatants were filtered through a 0.45-µm membrane using a syringe. To determine the desorption behavior, the remaining supernatant was replaced by fresh background solution without AT.
To analyze the sorption kinetics, sampling times were set at 35, 180, 360, 540, 1290, 1440, and 2880 min. AT initial concentration was 5 mg/L. To evaluate the impact of the solution temperature on the isothermal sorption and compare the sorption capacities of AT on the four sorbents, sorption equilibrium experiments were carried out at 15, 25, and 35 °C, at initial concentrations of 0.5, 1, 5, 10, and 20 mg/L. The effect of solution pH on AT sorption was investigated using 0.1 M HCl and 0.1 M NaOH to adjust the pH of the suspensions to values of 3, 5, 7, and 9. During the experimental process, the solution pH was checked frequently to verify that it remained unchanged. All experiments were carried out in triplicate. Preliminary experiments showed that loss during the tests was negligible, and no interference was found. Origin 8.0 software was used to analyze kinetics and isotherms.
The filtrates were analyzed using a Waters 2695 Separations Module HPLC (U.S.A.) equipped with a 2487 UV detector. Solutes were separated using a Gemini C18 analytical column (150 mm × 4.0 mm; ID, 5 µm) at a set temperature of 35 °C. The mobile phase consisted of methanol/water (70:30, v/v), the flow rate was 1.0 mL/min, and the detector wavelength was 220 nm. The retention time of AT was 4.8 min.
RESULTS AND DISCUSSION
Characterization of Biochar
The physicochemical properties of the BC are presented in Table 2. With increasing pyrolysis temperature, the BC yields decreased from 29.81% for MS350 to 18.79% for MS750. The total number of basic groups was increasing due to pyrolysis of biomass, and the pH values were in the order of MS750 > MS550 > MS350. Chen et al. (2011) indicated that alkali salts begin to separate from the organic matrix during biomass pyrolysis, resulting in an increase of the pH of the BC. The high pH of the BC suggested that it can have potential for tropical soil remediation. Ash is known to be an important factor to influence the sorption behavior of hydrophobic organic contaminants (HOCs). Some organic sorption sites in BC can be blocked by ash or become difficult to access due to their interactions with inorganic moieties (Zhang et al. 2013). As the charring temperature increased, the ash content of BC also increased, e.g., 5.03% for cassava waste and 30.56% for MS750.
With increasing pyrolysis temperature, the C content increased, while the H and N contents decreased. Chen et al. (2008) demonstrated that the C content increased with increasing charring temperature. However, the C content of MS750 (62.38%) was lower than that of MS550 (68.93%), which indicated that some amorphous carbon was decomposed into CO2 at 750 °C; the ash content also increased. The elemental ratio of H/C was used to evaluate the carbonization degree of the BC. Thus, an H/C ratio of 0.024 for MS750 suggested its high degree of carbonization. The decrease in the O/C and (N+O)/C ratios indicated an increase in aromaticity and a reduction in polarity. Hydrophobic carbon can provide more sorption domains for HOCs, and aromaticity and pore-filling are positively correlated with aromatic carbon contents (Teixido et al. 2011; Yang et al. 2011).
Scanning electron microscopy (SEM) was used to examine the specific surface area changes of BC and to determine the elemental composition of the surface (Fig. 1). The surface structure of cassava waste was complete and smooth before heating. However, after pyrolysis, its structure became rough, and multiple holes were formed, which increased the surface area of the BC. The surface properties of the biochars are given in Table 3. The specific surface area (SSA) of the BC increased as the charring temperature increased, from 48.19 m2/g for MS350 to 430.37 m2/g for MS750, and some micropores could be detected in MS750 (0.144 cm3/g). Total pore volume (TPV) and micropore volume mostly depend on the charring temperature (Cao and Harris 2010). Increasing pyrolysis temperature contributed to the increase in aromatic C content by the progressive destruction of -OH, ester C=O, aliphatic CH2, and C-O groups.
Fig. 1. Scanning electron micrographs: (a) cassava waste, (b) MS350, (c) MS550, and (d) MS7500
The FTIR spectra of the three BCs were similar for most of the wavenumbers (Fig. 2). As pyrolysis temperature increased, the band intensities at 3400 cm-1, the stretching vibration of hydroxyl groups (Chen et al. 2011), declined, suggesting that cellulose and lignin were transformed into aromatic carbon. The disappearance of the bands at 2940 and 2904 cm-1, the -CH2 and -CH3 groups of long-chain aliphatic carbons, respectively (Das et al. 2009), implied the destruction of the original lignin structure at 350 °C. The bands near 1730 cm-1 and at 1650 cm-1 were appointed to the stretching vibrations of ester carbonyl groups and C=O stretching vibrations of amides, respectively (Das et al. 2009).
Table 2. Properties of Biochar and Cassava Waste
a estimated by mass difference (100%-C, H, N, and ash %)
b presents “not been analyzed”
Table 3. Specific Surface Areas and Pore Structure Parameters of Biochar
Fig. 2. FTIR spectra of cassava waste biochar
Sorption Kinetics
Sorption kinetics is an important factor affecting pollutants sorption behavior. Pseudo-second-order kinetics (Eq. 1) and the Elovich model (Eq. 2) were used to study the sorption kinetics of AT in soil with amended BC. The equations can be expressed as,
(1)
(2)
where qt (mg/g) and qe (mg/g) are the amounts of AT adsorbed at time t and at equilibrium, respectively; k2 (g/mg/min) is a second-order kinetics sorption rate constant; a is an initial sorption rate constant; and b is a sorption activation energy constant.
Biochar amendment into soil not only could increase the amount of AT sorption but also change the physicochemical properties of paddy soil, for instance, increasing pH values and CEC of soil, promoting soil water-holding ability. According to Fig. 3, the kinetics of AT in BC-added soil was completed in two steps: a fast step and a slow step. At the early stage, the sorption rate of AT increased quickly, and 80 to 90% of the ultimate sorption occurred in the first 180 min. Once BC was added to the soil solution, approximate AT amounts of 17.2%, 21.54%, 35.12%, and 86.64% were removed by CK, MS350, MS550, and MS750, respectively, in 180 min. With increasing contact time, the sorption rate gradually decreased until the maximum sorption of the BC was reached. Atrazine was absorbed first at the readily accessible sites around the pores; it then diffused to the interior of the BC. The time at which AT reached equilibrium may be related to the carbonization temperature of the BC; MS750 was found to require less time to achieve equilibrium than other sorbents.
Fig. 3. Sorption kinetics of AT by adding biochar into soil. The initial concentration of AT was 5 mg/L, the amount of biochar was 1%, and the volume of pesticide solution was 10 mL
The highly hydrophobic surface and special functional groups of BC may be conducive to the initial rapid removal of AT. The surface areas of BC at low temperatures were too small to accommodate higher amounts of AT. Generally speaking, the sorption behavior of HOCs is primarily driven by hydrophobic interactions (Zhou et al. 2010). However, once coming into contact with water, the structure and composition of the BC would be altered by hydration (Chun et al. 2004), so the sorption of AT was a gradual process and controlled by intra-particle diffusion mechanisms before approaching equilibrium (Pan et al. 2008; Valderrama et al. 2008).
Pseudo-second-order and Elovich models were employed to study the AT sorption mechanism (Table 4). The R2 value for the pseudo-second-order model was near 1, higher than that of the Elovich equation (0.801~0.954), which indicated that the pseudo-second-order model provided a good fit to the kinetics of AT.
Table 4. Kinetics Constants for AT Sorption in BC-amended Soil
a Bold letters represent the highest R2 values
Sorption Isotherm
To examine the AT sorption intensity and understand its mechanism, the Langmuir (Eq. 3) (Langmuir 1918), Freundlich (Eq. 4) (Freundlich 1906), and Tekmin (Eq. 5) (Yang 2014) models were used to fit the experimental data,
(3)
(4)
(5)
where ce (mg/L) is the equilibrium concentration of AT; qmax (mg/g) is the maximum sorption amount of adsorbent; n is the unitless Freundlich exponent; KL and KF are Langmuir and Freundlich coefficients, respectively; and A and B are the coefficients of the Tekmin model.
Sorption isotherms were used to describe the partitioning of AT between the soil solution and solid surface and to help in understanding the nature of interactions between AT and the soil matrix (Kasozi et al. 2012). The BC comprised carbonized and non-carbonized fractions, which demonstrated that BC was heterogeneous (Chen et al. 2008; Cao et al. 2009). The sorption for AT by BC had two principal types of sorption domains; one was a soft carbon domain analogous to rubbery polymers and characterized by a partition mechanism exhibiting a linear sorption behavior, and the other was a hard carbon domain analogous to glassy polymers and characterized by a pore-filling mechanism; its sorption behavior was nonlinear (Xia and Pignatello 2001). Sorption isotherms of AT in BC-added soil were linear/nonlinear (Fig. 4). The isotherms of CK and MS350 displayed relatively linear behavior, and partition was a dominant mechanism. By contrast, because of presence of the glassy domain, the sorption of AT with added MS550 and MS750 followed a pore-filling mechanism, which can be described by nonlinear isotherms. Pore-filling was the primary mechanism for AT sorption in MS750 and contributed more at low solute concentrations. According to the H/C, O/C, and (O+N)/C ratios, the aromaticity increased in the order of MS750 > MS550 > MS350, so the affinity of the BC for AT should increase strongly and nonlinearity should be higher in the same order. This suggested that the organic carbon of BC produced at different temperatures had different sorption capacities.
Fig. 4. Sorption isotherms of AT in BC-amended soil. The initial concentrations of AT were 0.5, 1.0, 5.0, 10.0, and 20.0 mg/L. The volume of AT solution was 10 mL, and the amount of biochar was 1% in soil
Isotherm parameters of the three AT models are listed in Table 5. The Freundlich fitting was found to give relatively higher values of coefficients of determination (0.944 to 0.984) than the Langmuir (0.770 to 0.933) and Tekmin (0.872 to 0.958) equations, which suggested that the surface of the BC was heterogeneous and supplied indeterminate sorption sites. Higher surface areas and aromaticity of MS750 led to a linearly decreasing adsorption heat as the adsorption quantity decreased. The sorption capacity of AT had a positive correlation with the charring temperature of the BC, which was illustrated by the values of qmax, 1/n, and log KF. For instance, the maximal sorption value on MS750 was approximately 13.5 times larger than that of MS350. Based on these results, the nonlinearity coefficient 1/n value decreased from 0.715 for MS350 to 0.400 for MS750, indicating that the isotherms changed from linear to strongly nonlinear. Compared with CK (1/n > 1), 1/n of adding BC was lower, since BC had more condensed sorption domain to enhance affinity for AT.
Table 5. Isotherms Parameters for AT Sorption in BC-Amended Soila
a The initial concentrations of AT were 0.5,1.0,5.0,10.0, and 20.0 mg/L. The volume of AT solution was 10 mL, and the amount of biochar in soil was 1%
b Bold letters represent the highest R2 values
The mechanism of sorption of AT as a result of adding BC into soil is complicated because many factors can influence the sorption behavior, such as polarity, aromaticity, surface area, and pore size. In this study, MS350 contained more amorphous carbons and lacked micropores, leading to a weak interaction mechanism. Biochar pyrolyzed at high temperatures contained crystalline carbons with specific interactions, which could provide sorption sites. The HOCs on micro-porous and meso-porous BC usually undergo specific interactions, including hydrophobic effects, pore-filling, and electron donor-acceptor interaction (Zhang et al. 2011). Atrazine not only is an H-bonding donor for N atoms of heterocyclic rings but also is an acceptor of H atoms of amino groups (Sun et al. 2010); thus, charge-transfer interactions could occur between aromatic C, an electron acceptor on the BC surface, and the AT ring electron donor (Zhang et al. 2011; Zhao et al. 2013). In addition, the hydrophobic effects, dispersion, weak dipolar forces, and micropore diffusion could greatly influence the sorption reaction (Chen et al. 2009). Therefore, specific interactions between AT molecules and the BC surfaces could contribute to high sorption affinity.
Sorption Thermodynamics
Thermodynamic parameters were determined using the following equations,
(6)
(7)
(8)
where T (K) is the absolute temperature, R is the universal gas constant (8.314 J/molK), and Kd is the distribution constant. It is assumed here that n= 1, Kd can be calculated as Kd=KF (Zhang et al. 2008; Liu 2009); and the parameter △Go (kJ/mol) is the standard free energy change. The values of enthalpy (△Ho) and entropy change (△So) can be obtained from the slope and intercept of a plot of △Goversus T.
The thermodynamic parameters (△Go, △Ho, △So) for the sorption process are given in Table 6. All of the △Go values were negative, suggesting that the process was spontaneous and thermodynamically favorable. The absolute values of △Go increased with increasing sorption temperature, i.e., △Go 308 K >△Go 298 K >△Go 288 K. Higher temperatures can provide more energy to overcome the diffuse double layer and adsorb into the interior structure of the BC (Liu and Zhang 2009). In addition, adding BC to soil was beneficial to AT sorption under the same experimental conditions. In general, the △Go value for physical sorption is in the range of 0 to -20 kJ/mol, and that for chemisorption is between -80 and -400 kJ/mol (Zhang et al. 2014). In this study, the values of △Go were in the range of -0.4 to -11.3 kJ/mol, which suggested that the sorption process may be mainly controlled by physisorption.
Table 6. Thermodynamic Parameters for the AT Sorption Processa
a The initial concentrations of AT were 0.5, 1.0, 5.0,10.0, and 20.0 mg/L. The volume of AT solution was 10 mL, and the amount of biochar was 1% in soil. The sorption equilibrium experiments were carried out at 15, 25, and 35 °C
A negative △Ho value suggests the sorption process is exothermic. Based on the results, all of the △Ho values were positive, which indicated that the sorption process was endothermic and that increasing temperature could be mostly favorable for sorption. In addition, the absolute value of △Ho was lower than 40 kJ/mol, which indicated that physisorption was mainly adsorption mechanism (Fenget al. 2013). In this work, the △Ho values were between 20 and 50 kJ/mol, confirming that AT sorption was primarily physisorption. According to the sorption energy of different forces (Table 7) (von Oepen et al. 1991), the sorption mechanism of AT on CK may be an H-bonding and dipole bond force, and that on MS350, MS550, and MS750 may involve H-bonding and ion exchange. Atrazine is a weak base, and its pKa value is 1.7. At the pH in the experiment, AT existed as neutral molecules and formed weak H-bonds with clay surfaces and carboxyl groups because of their heterocyclic nitrogen atoms (Zhang et al. 2013).
The △So values were all positive, indicating that the entropy increased because of the increase in molecule randomness in the soil solutions.
Table 7. Energy of Sorption by Different Forces (kJ/mol)
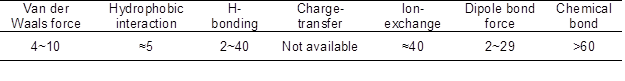
Effect of pH
The pH value is an important factor for AT sorption-desorption and determines the sorption capacity of BC. Figure 5 shows that the effect of pH value on the sorption of AT exhibited a similar tendency in the studied pH range (3 to 9) for all samples. The sorbed amount of AT decreased with an increase in the pH value, indicating that the sorption of AT was favored by a lower pH. For example, the sorption capacities of CK were 11.97, 8.24, 7.38, and 6.81 mg/kg at pH 3, 5, 7, and 9, respectively. In addition, the sorption ability of different sorbates was different and the sorption level for pesticides was in the order of MS750 > MS550 > MS350 > CK, which showed that BC addition could improve the removal of AT in paddy soil.
Fig. 5. Effect of pH on AT sorption in biochar-amended soil. The initial concentration of AT was 5 mg/L, the amount of biochar was 1%, and the volume of AT solution was 10 mL. Error bars represent standard deviation of triplicate samples
The results were primarily caused by the interaction between BC and AT through charge forces in aqueous environments (Zhao et al. 2013). The pKa of AT is 1.7, indicating a weakly basic pesticide. Biochar exists mostly in protonated form in acidic media and in deprotonated form in alkaline media (Oliveira et al. 2001). Low pH solutions would be favorable for the formation of AT cations, which would combine with the negatively charged surfaces of BC through electrostatic attraction in acidic media to improve the sorption affinity of BC for AT. At higher pH values, AT would combine with the negatively charged BC surface with a weak electronic interaction.
The effects of pH on desorption are displayed in Fig. 6. Higher pH values were more conducive to enhancing desorption for AT, i.e., pH 9 > pH 7 > pH 5 > pH 3. For MS750, the desorption rate at pH 9 was approximately 2.65 times higher than at pH 3. Atrazine desorbed more, but less from absorbed soil with increasing BC pyrolysis temperature. For instance, the desorption rate of MS750 was 20.01 times less than that of CK at pH 3, which showed that BC addition could reduce the mobility of AT in paddy soil.
Fig. 6. Effect of solution pH on the desorption capacity of amended biochars for AT. The initial concentration of AT was 5 mg/L, the amount of biochar was 1%, and the volume of AT solution was 10 mL. Error bars represent standard deviation of triplicate samples
CONCLUSIONS
1. The sorption kinetics showed that the removal rate of atrazine (AT) depends on the carbonization temperatures of biochar. Biochar carbonized at high temperatures may reach equilibrium in shorter amounts of time. The pseudo-second-order model gives the best fit to the sorption kinetics of AT.
2. The isotherms of CK and MS350 displayed relatively linear behavior, and partitioning was the dominant mechanism, whereas the sorption of AT by MS550 and MS750 occurred via a pore-filling mechanism, which can be described by nonlinear isotherms. The Freundlich model can be used to describe the sorption isotherm of AT in biochar-amended soil.
3. A thermodynamic study indicated that the sorption of AT in biochar-added soil is a spontaneous endothermic process.
4. Acidic solutions are more conducive to the sorption of AT in biochar-added soil.
ACKNOWLEDGMENTS
This study was partially supported by the National Natural Science Foundation of China (No. 21367011), the Open Fund Program of the Key Laboratory of Protection and Development Utilization of Tropical Crop Germplasm Resources (Hainan University), the Ministry of Education (No. 2012hckled-5), the Natural Science Fund Program of Hainan Province (No. 413123), and Midwest University Project (MWECSP-RT08,ZXBJH-XK004 and ZXBJH-XK005). The authors thank the anonymous referees for their comments on this manuscript.
REFERENCES CITED
Cao, X., Ma, L., Gao, B., and Harris, W. (2009). “Dairy-manure derived biochar effectively sorbs lead and atrazine,” Environ. Sci. Technol. 43(9), 3285-3291. DOI: 10.1021/es803092k
Cao, X. D., and Harris, W. (2010). “Properties of dairy-manure-derived biochar pertinent to its potential use in remediation,” Bioresour. Technol. 101(14), 5222-5228. DOI: 10.1016/j.biortech.2010.02.052
Chen, B. L., Zhou, D. D., and Zhu, L. Z. (2008). “Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures,” Environ. Sci. Technol. 42(14), 5137-5143. DOI: 10.1021/es8002684
Chen, G. C., Shan, X. Q., Zhou, Y. Q., Shen, X., Huang, H. L., and Khan, S. U. (2009). “Adsorption kinetics, isotherms and thermodynamics of atrazine on surface oxidized multiwalled carbon nanotubes,” J. Hazard. Mater. 169(1-3), 912-918. DOI: 10.1016/j.jhazmat.2009.04.034
Chen, X. C., Chen, G. C., Chen, Y. X., Lehmann, J., McBride, M. B., and Hay, A. G. (2011). “Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and straw in aqueous solution,” Bioresour. Technol. 102(19), 8877-8884. DOI: 10.1016/j.biortech.2011.06.078
Chun, Y., Sheng, G., Chiou, C. T., and Xing, B. (2004). “Compositions and sorptive properties of crop residue-derived chars,” Environ. Sci. Technol. 38(17), 4649-4655. DOI: 10.1021/es035034w
Das, D. D., Schnitzer, M. I., Monreal, C. M., and Mayer, P. (2009). “Chemical composition of acid-base fractions separated from biooil derived by fast pyrolysis of chicken manure,” Bioresour. Technol.100(24), 6524-6532. DOI: 10.1016/j.biortech.2009.06.104
Feng, Y. F., Dionysiou, D. D., Wu, Y. H., Zhou, H., Xue, L. H., He, S. Y., and Yang, L. Z. (2013). “Adsorption of dyestuff from aqueous solutions through oxalic acid-modified swede rape straw: Adsorption process and disposal methodology of depleted bioabsorbents,”Bioresour. Technol. 138, 191-197. DOI: 10.1016/j.biortech.2013.03.146
Freundlich, H. (1906). “Über die Adsorption in Lösungen (Adsorption in solution),” Z. Phys. Chem. 57, 384-470.
Gupta, V. K., Gupta, B., Rastogi, A., Agarwal, S., and Nayak, A. (2011). “A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113,” J. Hazard. Mater. 186(1), 891-901. DOI: 10.1016/j.jhazmat.2010.11.091
Guzman-Perez, C. A., Soltan, J., and Robertson, J. (2011). “Kinetics of catalytic ozonation of atrazine in the presence of activated carbon,” Sep. Purif. Technol. 79(1), 8-14. DOI: 10.1016/j.seppur.2011.02.035
Hayes, T. B., Collins, A., Lee, M., Mendoza, M., Noriega, N., Stuart, A. A., and Vonk, A. (2002). “Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses,” Proc. Natl. Acad. Sci. U. S. A. 99(8), 5476-5480. DOI: 10.1073/pnas.082121499
Hayes, T. B., Stuart, A. A., Mendoza, M., Collins, A., Noriega, N., Vonk, A., Johnston, G., Liu, R., and Kpodzo, D. (2006). “Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17beta-estradiol): Support for the demasculinization/feminization hypothesis,” Environ. Health. Persp.114(s-1), 134-141. DOI: 10.1289/ehp.8067
Hayes, T. B., Khoury, V., Narayan, A., Nazir, M., Park, A., Brown, T., Adame, L., Chan, E., Buchholz, D., Stueve, T., and Gallipeau, S. (2010). “Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis),” Proc. Natl. Acad. Sci. U. S. A. 107(10), 4612-4617. DOI: 10.1073/pnas.0909519107
Institute of Soil Science, Chinese Academy of Science (1978). Soil Physical and Chemical Analyses. Shanghai, China.
Jamil, T. S., Gad-Allaha, T. A., Ibrahim, H. S., and Saleh, T. S. (2011). “Adsorption and isothermal models of atrazine by zeolite prepared from Egyptian kaolin,” Solid State Sci. 13(1), 198-203. DOI: 10.1016/j.solidstatesciences.2010.11.014
Jin, R., and Ke, J. (2002). “Impact of atrazine disposal on the water resources of the Yang River in Zhangjiakou area in China,” Bull. Environ. Contam. Toxicol. 68(6), 893-900. DOI: 10.1007/s00128-002-0038-1
Kasozi, G. N., Nkedi-Kizza, P., Li, Y., and Zimmerman, A. R. (2012). “Sorption of atrazine and ametryn by carbonatic and non-carbonatic soils of varied origin,” Environ. Pollut. 169, 12-19. DOI: 10.1016/j.envpol.2012.05.002
Kovaios, I. D., Paraskeva, C. A., and Koutsoukos, P. G. (2011). “Adsorption of atrazine from aqueous electrolyte solutions on humic acid and silica,” J. Colloid Interf. Sci. 356(1), 277-285. DOI: 10.1016/j.jcis.2011.01.002
Langmuir, I. (1918). “The adsorption of gases on plane surfaces of glass, mica and platinum,” J. Am. Chem. Soc. 40, 1361-1403.
Lima, D. L. D., Silva, C. P., Schneider, R. J., and Esteves, V. I. (2011). “Development of an ELISA procedure to study sorption of atrazine onto a sewage sludge-amended luvisol soil,” Talanta 85(3), 1494-1499. DOI: 10.1016/j.talanta.2011.06.024
Lin, K. Y., and Chu, W. (2011). “Simulation and quantification of the natural decay of a typical endocrine disrupting chemical atrazine in an aquatic system,” J. Hazard. Mater. 192(3), 1260-1266. DOI: 10.1016/j.jhazmat.2011.06.042
Liu, Y. (2009). “Is the free energy change of adsorption correctly calculated?” J. Chem. Eng. Data 54(7), 1981-1985. DOI: 10.1021/je800661q
Liu, Z., and Zhang, F. S. (2009). “Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass,” J. Hazard. Mater. 167(1-3), 933-939. DOI: 10.1016/j.jhazmat.2009.01.085
Mesa, A. C., and Spokas, K. (2011). “Impacts of biochar (black carbon) additions on the sorption and efficacy of herbicides,” in: Herbicides and Environment, Kortekamp, A. (ed.), InTech, pp. 315-340.
Mittal, A., Kurup, L., and Gupta, V. K. (2005). “Use of waste materials—Bottom ash and de-oiled soya, as potential adsorbents for the removal of amaranth from aqueous solutions,” J. Hazard. Mater.117, 171-178. DOI: 10.1016/j.jhazmat.2004.09.016
Mudhoo, A., and Garg, V. K. (2011). “Sorption, transport and transformation of atrazine in soils, minerals and composts: A review,” Pedosphere 21(1), 11-25. DOI: 10.1016/S1002-0160(10)60074-4
Nag, S. K., Kookana, R., Smith, L., Krull, E., Macdonald, L. M., and Gill, G. (2011). “Poor efficacy of herbicides in biochar-amended soils as affected by their chemistry and mode of action,” Chemosphere84(11), 1572-1577. DOI: 10.1016/j.chemosphere.2011.05.052
Oliveira Jr., R. S., Koskinen, W. C., and Ferreira, F. A. (2001). “Sorption and leaching potential of herbicides on Brazilian soil,” Weed Res. 41(2), 97-110. DOI: 10.1046/j.1365-3180.2001.00219.x
Pan, B. J., Zhang, W. M., Pan, B. C., Qiu, H., Zhang, Q. R., Zhang, Q. X., and Zheng, S. R. (2008). “Efficient removal of aromatic sulfonates from wastewater by a recyclable polymer: 2-naphthalene sulfonate as a representative pollutant,” Environ. Sci. Technol. 42(19), 7411-7416. DOI: 10.1021/es801370n
Salvestrini, S., Sagliano, P., Iovino, P., Capasso, S., and Colella, C. (2010). “Atrazine adsorption by acid-activated zeolite-rich tuffs,” Appl. Clay Sci. 49(3), 330-335. DOI: 10.1016/j.clay.2010.04.008
Shi, B. Y., Zhuang, X. Y., Yan, X. M., Lu, J. J., and Tang, H. X. (2010). “Adsorption of atrazine by natural organic matter and surfactant dispersed carbon nanotubes,” J. Environ. Sci. 22(8), 1195-1202. DOI: 10.1016/S1001-0742(09)60238-2
Sun, K., Gao, B., Zhang, Z. Y., Zhang, G. X., Zhao, Y., and Xing, B. S. (2010). “Sorption of atrazine and phenanthrene by organic matter fractions in soil and sediment,” Environ. Pollut. 158(12), 3520-3526. DOI: 10.1016/j.envpol.2010.08.022
Teixido, M., Pignatello, J. J., Beltran, J. L., Granados, M., and Peccia, J. (2011). “Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar),” Environ. Sci. Technol. 45(23), 10020-10027. DOI: 10.1021/es202487h
Valderrama, C., Cortina, J. L., Farran, A., Gamisans, X., and de las Heras, F. X. (2008). “Kinetics study of acid red “dye” removal by activated carbon and hyper-cross-linked polymeric sorbents Macronet Hypersol MN200 and MN300,” React. Funct. Polym. 68(3), 718-731. DOI: 10.1016/j.reactfunctpolym.2007.11.013
von Oepen, B., Kördel, W., and Klein, W. (1991). “Sorption of nonpolar and polar compounds to soils: Processes, measurements and experience with the applicability of the modified OECD-Guideline 106,” Chemosphere 22(3-4), 285-304. DOI: 10.1016/0045-6535(91)90318-8
Wang, Y. R., Wang, H. S., and Zhang, H. T. (2012). “Research progress in animal application of cassava wastes,” Development and Utilization of Feed Resource 12, 42-47.
Xia, G., and Pignatello, J. J. (2001). “Detailed sorption isotherms of polar and apolar compounds in a high-organic soil,” Environ. Sci. Technol. 35, 84-94. DOI: 10.1021/es001320l
Yang, X. S. (2014). “Studies in effects of kaolinite and acidic-kaolinite on Cr, NH3-N and F adsorption,” Chin. Agri. Sci. Bull.30(3), 168-172.
Yang, Y., Shu, L., Wang, X. L., Xing, B. S., and Tao, S. (2011). “Impact of de-ashing humic acid and humin on organic matter structural properties and sorption mechanisms of phenanthrene,” Environ. Sci. Technol. 45(9), 3996-4002. DOI: 10.1021/es2003149
Zhang, G. X., Zhang, Q., Sun, K., Liu, X. T., Zheng, W. J., and Zhao, Y. (2011). “Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures,” Environ. Pollut. 159(10), 2594-2601. DOI: 10.1016/j.envpol.2011.06.012
Zhang, J. Q., and Dong, Y. H. (2008). “Thermodynamics and kinetics of norfloxacin adsorption in typical soils of China,” Acta Pedologica Sinica. 45(5), 978-986.
Zhang, J. Y., Wu, C. D., Jia, A. Y., and Hu, B. (2014). “Kinetics, equilibrium, and thermodynamics of the sorption of p-nitrophenol on two variable charge soils of Southern China,” Appl. Surf. Sci. 298, 95-101.
Zhang, P., Sun, H. W., Yu, L., and Sun, T. H. (2013). “Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars,” J. Hazard. Mater. 244-245, 217-224. DOI: 10.1016/j.jhazmat.2012.11.046
Zhang, W., Wang, L., and Sun, H. W. (2011). “Modifications of black carbons and their influence on pyrene sorption,” Chemosphere 85(8), 1306-1311. DOI: 10.1016/j.chemosphere.2011.07.042
Zhao, X. C., Hao, F. H., Lin, C. Y., Wang, F. L., Han, S., and Geng, X. J. (2013). “Properties comparison of biochars from corn straw with different pretreatment and sorption behavior of atrazine,” Bioresour. Technol. 147, 338-344. DOI: 10.1016/j.biortech.2013.08.042
Zheng, W., Guo, M. X., Chow, T., Bennett, D. N., and Rajagopalan, N. (2010). “Sorption properties of greenwaste biochar for two triazine pesticides,” J. Hazard. Mater. 181(1-3), 121-126. DOI: 10.1016/j.jhazmat.2010.04.103
Zhou, Z. L., Lu, Y., and Sun, H. W. (2010). “Sorption kinetics and isotherms of phenanthrene in charcoals with different properties,” J. Agro-Enviro. Sci. 29(3), 476-480.
Article submitted: June 12, 2014; Peer review completed: August 9, 2014; Revised version received and accepted: September 3, 2014; Published: September 15, 2014.
