Abstract
Several different methods for the extraction, separation, and purification of wood constituents were combined in this work as a unified process with the purpose of achieving a high overall efficiency of material extraction and utilization. This study aimed to present a laboratory-scale demonstrator biorefinery that illustrated how the different wood constituents could be separated from the wood matrix for later use in the production of new bio-based materials and chemicals by combining several approaches. This study builds on several publications and ongoing activities within the Wallenberg Wood Science Center (WWSC) in Sweden on the theme “From wood to material components.” Combining the approaches developed in these WWSC projects – including mild steam explosion, membrane and chromatographic separation, enzymatic treatment and leaching, ionic liquid extraction, and fractionation together with Kraft pulping – formed an outline for a complete materials-biorefinery. The process steps involved were tested as integral steps in a linked process. The scale of operations ranged from the kilogram-scale to the gram-scale. The feasibility and efficiency of these process steps in a biorefinery system were assessed, based on the data, beginning with whole wood.
Download PDF
Full Article
The Development of a Wood-based Materials-biorefinery
Tuve Mattsson,a Shoaib Azhar,b Susanna Eriksson,a Mikaela Helander,b Gunnar Henriksson,b Kerstin Jedvert,a Martin Lawoko,b Mikael E. Lindström,b Lauren S. McKee,b,c Petri Oinonen,b Olena Sevastyanova,b Niklas Westerberg,a and Hans Theliander a*
Several different methods for the extraction, separation, and purification of wood constituents were combined in this work as a unified process with the purpose of achieving a high overall efficiency of material extraction and utilization. This study aimed to present a laboratory-scale demonstrator biorefinery that illustrated how the different wood constituents could be separated from the wood matrix for later use in the production of new bio-based materials and chemicals by combining several approaches. This study builds on several publications and ongoing activities within the Wallenberg Wood Science Center (WWSC) in Sweden on the theme “From wood to material components.” Combining the approaches developed in these WWSC projects – including mild steam explosion, membrane and chromatographic separation, enzymatic treatment and leaching, ionic liquid extraction, and fractionation together with Kraft pulping – formed an outline for a complete materials-biorefinery. The process steps involved were tested as integral steps in a linked process. The scale of operations ranged from the kilogram-scale to the gram-scale. The feasibility and efficiency of these process steps in a biorefinery system were assessed, based on the data, beginning with whole wood.
Keywords: Biorefinery; Wood components; Separation; Demonstrator
Contact information: a: Wallenberg Wood Science Center, Chalmers University of Technology, SE-412 96 Gothenburg, Sweden; b: Wallenberg Wood Science Center, School of Chemical Science and Engineering, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden; c: Division of Glycoscience, School of Biotechnology, Royal Institute of Technology (KTH), AlbaNova University Centre, SE-106 91 Stockholm, Sweden; *Corresponding author: Hanst@chalmers.se
INTRODUCTION
There is an urgent need to develop sustainable alternatives to fossil-based materials, chemicals, and fuels. Wood, the most abundant lignocellulosic raw material, is a key potential feedstock for these bio-based products (Ragauskas et al. 2006). Wood, as a starting material for the production of chemicals, is a well-established resource, including the use of wood to produce ethanol for chemical production during turbulent times in the petroleum trade. Ethanol production still continues in sulphite mills that can also produce lignosulfonates, lignin-based chemicals (e.g. vanillin) (Tarabanko et al. 1995), and cellulose pulps.
Globally, many research and development activities are focused on the development of bio-based replacements for petrochemicals and petroleum-derived products. Recent developments range from simple commodities, such as fuel pellets made of waste wood material and transportation fuels such as ethanol, to more advanced materials such as nanocellulose and carbon fibres (Gellerstedt et al. 2010; Lee et al. 2014). The focus of such research has often been on maximising process efficiency and product yield (e.g., Galbe and Zacchi 2002; Abdul Khalil et al. 2014). In the case of ethanol production from lignocellulosic material, the theoretical maximum yield of ethanol (for spruce) is less than 40 wt.% (Galbe and Zacchi 2007). However, for industrial processes the yield can be expected to be considerably lower. This is because the yields for both pretreatment steps to extract the sugars from the wood material and subsequent fermentation need to be considered. Based on a theoretical ethanol yield from hexoses, 425 L/ton spruce (Galbe and Zacchi 2007), and assuming yields for the pretreatment and fermentation steps between 85% to 90%, this would result in an ethanol yield of approximately 25 wt.% given the density of ethanol. In more energy efficient cases, hydrolysate lignin may also be a recoverable product (Lora and Glasser 2002), nonetheless, the overall product yield will be less than 40 wt.%. Compared to an oil-refinery or a kraft pulping process for paper and board, this is a very low yield and efficiency (Maples 2000; Ragnar et al. 2013).
This research is essential for finding out what is actually possible to achieve in terms of yields and properties. However, an important next step in this area of research is maximising the overall yield of a more complete process for recovering and utilising each of the different wood components. Several suggested pathways for the extraction of wood components for fuel, chemical, and material production can be found in previous literature (Galbe and Zacchi 2002; Öhman et al. 2008; Alvira et al. 2010; Li et al. 2012).
Wood constituents (mainly hemicelluloses but also partly lignin fragments) can be extracted via hot water (Örså et al. 1997). This extraction includes several steps: dissolution of the components as well as transport out to the bulk solution both through the cell wall itself and further through the overall wood structure. With respect to the macroscopic dimensions of the wood material, extraction has been found to be facilitated by grinding the wood material (Song et al. 2008), suggesting that shorter transport distances is beneficial for extraction. It has also been shown that even during alkaline cooking conditions (i.e. swollen fibres), sorption and mass transport in the fibre wall plays a central role for the removal of macromolecules from the fibre (Mattsson et al. 2017). This suggests that fracturing of the fibre wall may also be an important step to facilitate extraction.
Traditional thermomechanical pulping liquid can be considered as a hot water extract and values of 11 kg hemicelluloses per ton of produced pulp and 8 kg aromatic compounds per ton of produced pulp have been reported (Persson et al. 2010). Another approach found in previous literature is steam explosion (STEX), which was first introduced for the treatment of wood in the early twentieth century (Mason 1928). This conversion method is based on a sudden drop in pressure of steam-saturated wood at elevated temperatures, leading to an expansion of the steam in the wood material and a resulting mechanical defibrillation of the fibres. However, the elevated temperature during STEX and hot water extraction triggers the release of acetic acid, resulting in an autohydrolysis that also affects the material and the extracted components, for instance reducing the molecular weight of the extracted hemicelluloses (Marchessault 1988; Carrasco 1992).
The additional recovery of wood components from different streams in the kraft pulping process has also been suggested and investigated. One approach is to recover kraft lignin by precipitation of the spent cooking liquor through a lowering of the pH with CO2 (Alén et al. 1979). With the recently commercialised LignoBoost® process, a highly pure kraft lignin can be isolated from the spent cooking liquor by precipitation with an improved dewatering and washing procedure (Öhman et al. 2008; Ziesig et al. 2015). In addition, ultrafiltration with ceramic membranes can be used to extract and fractionate kraft lignin from spent cooking liquor (Wallberg et al. 2003; Brodin et al. 2009; Arkell et al. 2014). Ultrafiltration requires less added chemical for pH adjustment and is less sensitive to temperature and liquor concentration. Lignin extraction by ultrafiltration directly from the recycled cooking liquors during continuous digesting has also been suggested (Wallberg and Jönsson 2006). The membrane technique can also be used for the subsequent upgrading of lignin through molecular weight fractionation at desired molecular weight cut-offs. A subsequent acidic precipitation is required to isolate lignin in a solid form. A direct precipitation approach is often more cost efficient (Uloth and Wearing 1989), but a membrane approach has the advantage of producing more homogeneous lignin material with a narrow molecular weight distribution. The use of this method may make lignin a more attractive raw material for various applications.
One challenge in achieving a high overall wood component utilization is the recovery of the non-crystalline polysaccharides hemicellulose and pectin, which are largely degraded during traditional kraft pulping. However, a chemical pretreatment, such as reduction or oxidation, can diminish this degradation of the hemicelluloses. The polysaccharide degradation products in kraft pulping are mainly sugar acids that, in contrast to the neutral monosaccharides released during acidic sulphite pulping, are difficult to separate and cannot generally be used for fermentation. As such, they have a low commercial value. For this reason, it would be advantageous to extract a maximum proportion of the hemicelluloses before alkaline pulping. However, the yield of hemicelluloses directly extracted from untreated wood is generally low, which is likely due to the extensive crosslinking of the plant cell wall matrix where polysaccharides and lignin interact covalently (Lawoko et al. 2006).
The Wallenberg Wood Science Center (WWSC) is working on processes for the extraction and valorisation of hemicellulose polymers, which can be combined with a pulping process for the production of conventional paper-grade pulp, dissolving pulp, and/or cellulose nanocrystals or cellulose nanofibrils. The aim has been to retain the polymeric structure of the wood component as much as possible, thereby avoiding the losses and costs associated with rebuilding the polymeric units needed for subsequent material production. So far, individual process steps and combinations of a select few of these steps have been investigated in a series of recent publications (Azhar et al. 2011; Jedvert et al. 2012; Westerberg et al. 2012; Helander et al. 2013; Oinonen et al. 2013; Bylin et al. 2014; Sevastynova et al. 2014; Azhar et al. 2015; Giummarella and Lawoko 2017; Wojtasz-Mucha et al. 2017).
The purpose of the present study is to combine the different process steps developed during the authors’ research activities to achieve a high overall material utilisation. The suggested process concept is simulated on a laboratory scale, linking together each operation so that the products from each step are used as raw material in the subsequent step, with flows ranging from a kilogram scale to a gram scale. The emphasis of this study is on the overall yield of the process and on the quality of the extracted components and the remaining fibre.
EXPERIMENTAL
The principal approach in developing the demonstrator was to utilize several primary separation steps to extract wood components from the wood matrix that subsequently can serve as raw material for the production of materials and chemicals. The extracted soluble material was purified and fractionated, and the solid remains were processed either by conventional pulping methods or with the aid of ionic liquids. From this general outline, a process concept for a wood-based biorefinery was proposed in Fig. 1. The numbering of the streams indicated in the figure is consistently used throughout this work and the figure can also be used as a guide for the appended data.
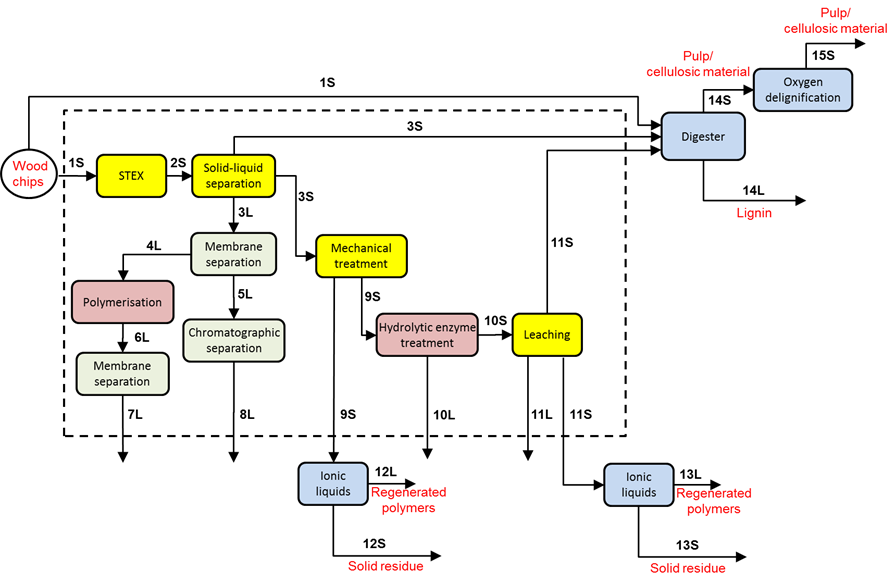
Fig. 1. An overview of the demonstrator biorefinery process; the dotted area encloses the various primary separation steps utilised; digester: cooking with the sulphate pulping method
Materials
Industrially chipped softwood sourced from a pulp mill in the southwestern part of Sweden, containing mainly Norway spruce (Picea abies) with some pine (Pinus sylvestris), was used as the raw material. A full description of this starting material and the methods and conditions used in each process step can be found in the supplementary information. A brief outline of the process and some key conditions are given below.
Methods
In this demonstrator process a mild (7 bar) steam explosion (STEX) was used for a first extraction of hemicelluloses, yielding hemicelluloses with a relatively high molecular weight (Stream 3L). Conditions were varied in the initial STEX step, where a 7 bar 20 min treatment and a 4 bar 10 min treatment were both investigated. Throughout this manuscript, these treatments are referred to as STEX-4 and STEX-7, and are indicated in the stream numbering with (a) denoting 4 bar treatment and (b) denoting 7 bar treatment. The phase of the stream is indicated by (S) for solids and (L) for liquids. Not all process steps were investigated using the 4 bar STEX-treated material. Because less of this material was produced, the yields of certain process steps could not be ascertained for the 4 bar experiments.
After a membrane fractionation step, the extracted hemicellulose-rich fraction was either treated with laccase, yielding a crosslinking of lignin functionalities on the hemicelluloses (Stream 6L), or further fractionated with chromatographic methods, yielding, e.g., a Lignin-carbohydrate-complex (LCC)-rich fraction (Stream 8L).
The remaining wood chips, residual solid material from the STEX treatment, were either directly digested by the sulphate pulping method or treated for further extraction. Before subsequent extractions, the chips were mechanically-treated and refined down to a matchstick size (Stream 9S). This fraction was then treated with a commercial hemicellulose-degrading enzyme cocktail, Gamanase™, and subjected to a leaching process with a mixture of methanol and alkali, enabling other hemicellulose-rich fractions to be recovered (Streams 10L and 11L). Ionic liquids were applied on both the mechanically-treated fraction (Stream 9S) and the residual solids from the enzyme-assisted leaching process (Stream 11S) to achieve further polymer fractionation (Streams 12 and 13).
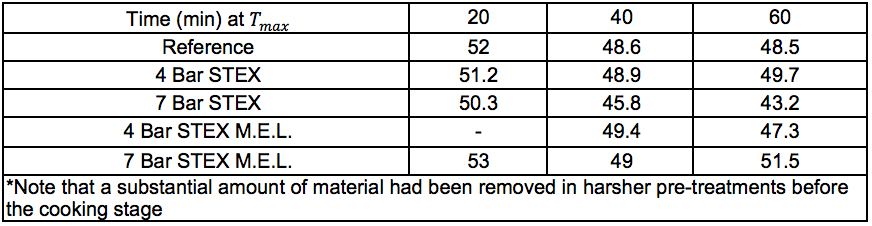
Solid materials from the STEX-treated wood and residual solids from the enzyme-assisted leaching process (Streams 3S and 11S, respectively) were subjected to cooking using the sulphate pulping method (producing Stream 14S) and a subsequent oxygen delignification (producing Stream 15S). As a reference, a portion of the wood chips from the original starting material were also digested and oxygen-delignified without any prior treatment (producing a reference material for Streams 14S and 15S). In the cooking steps described above, three different cooking times of 20 min, 40 min, and 60 min were investigated at the maximum temperature (170 °C).
RESULTS AND DISCUSSION
Yields
The yields based on the total mass for the softwood chips treated with the 7 bar STEX method and a 60-min digestion step are shown in Fig. 2. A total of 5 kg of dry wood chips was used as the raw material for this process. A more detailed account of the individual yields and of the varied cooking conditions can be found in the supplementary data.
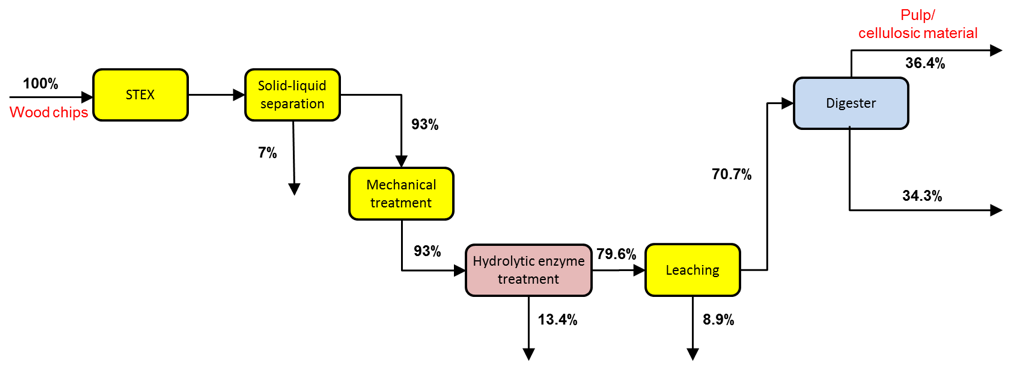
Fig. 2. Cumulative yields for a 7 bar STEX pre-treatment followed by a cooking step (60 min at Tmax = 170 °C). No loss of solid material has been considered during the mechanical treatment.
After pretreatment with a mild steam explosion at 7 bar followed by enzyme-assisted leaching steps, a total of approximately 29 wt.% of the wood was extracted before digesting. The effect of each process step on the material and composition of the streams is reported below or in the supplementary material (see the Appendix).
Mild Steam Explosion
The mild STEX treatment had a dual purpose: it made the wood structure more open and more accessible to chemicals and enzymes in subsequent process steps and it was a hydrothermal treatment during which some of the wood components were released into the aqueous solution after the condensation of steam. These components originated mainly from the hemicelluloses, but some fatty acids from wood extractives as well as some aromatic structures from lignin and/or lignin carbohydrate complexes (LCC) were also present due to autohydrolysis. Based on a sugar analysis of the samples taken, the wood components released during the steam explosion were mainly derived from (galacto) glucomannan and arabinose that was likely derived from glucuronoarabinoxylan. This arabinose was unlikely to have been derived from pectin because the primary cell wall was a very minor component of the wood chips utilized, which were composed largely of secondary cell wall material.
As mentioned above, morphological changes to the wood structure occurred during the steam explosion (see “Chip structure after mild steam explosion” in supplementary material). The expansion of the steam when the pressure was released and the impact of the wood chips against the walls of the equipment during the discharge led to mechanical damage, such as the formation of cracks in the wood tissue.
The porosity measurements using mercury porosimetry (Fig. 3) revealed that there was a decrease in the intrusion volume associated with pores with diameters in the range from 0.1 μm to 0.5 μm, and an increase in volume associated with the pores with diameters between 0.5 μm and 10 μm, which indicated that the steam explosion treatment opened up the structure and increased the pore size.
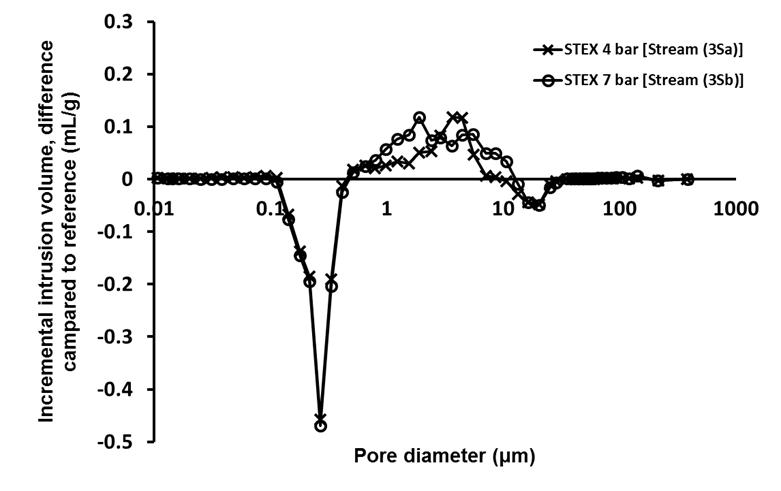
Fig. 3. Difference in incremental intrusion volume for STEX-treated chips (Stream 3S); Stream 1S material was used as a reference for these analyses
Pore diameters of 1 μm to 4 μm corresponded to pits in the cell wall of tracheids (Usta and Hale 2005). Therefore, these results suggested that some pits in the cell wall were opened or ruptured during the steam explosion, which made the structure of the wood more open and accessible.
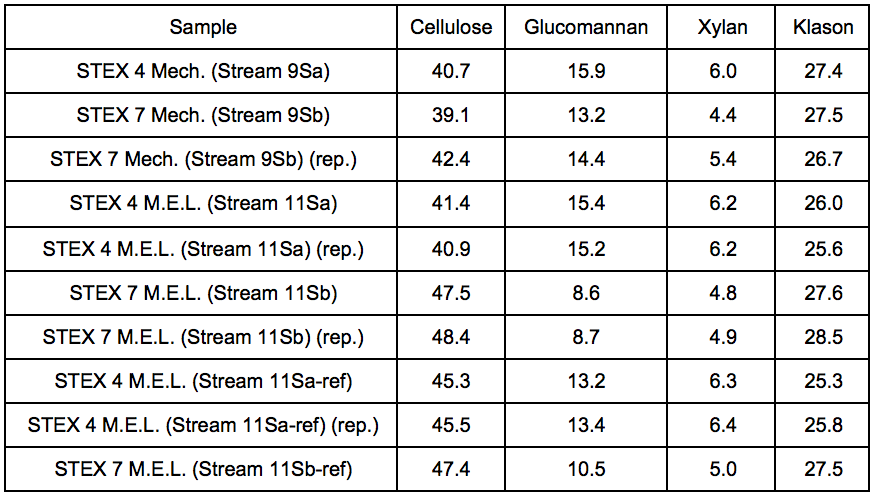
The results of the carbohydrate analysis after STEX treatment (Table 1) showed that there was a pronounced decrease in the hemicellulose content in the sample treated at 7 bar (20 min), which indicated a substantial release of hemicelluloses from the wood into the liquid fraction (Stream 3L). The content of (galacto)glucomannan was almost half that of the original material, and the content of glucuronoarabinoxylan decreased from 6.9% to 4.8% of the sample weight. The small apparent increase in cellulose and Klason lignin content in the sample treated at 7 bar was due to the proportional decrease in hemicellulose content in the steam exploded wood.
Table 1. Lignocellulosic Content of Wood Material Before and After STEX 7 Bar Treatment (wt.%) *
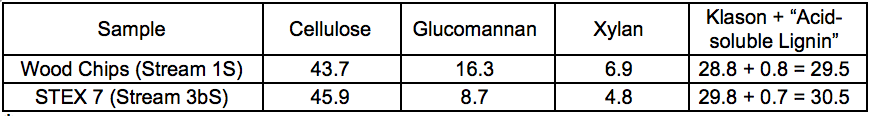
* Contents are given as relative composition of the respective streams.
Enzymatic Treatment and Leaching Steps
To extract more wood components in the form of macromolecules, an enzymatic degradation step combined with a leaching step was utilized. The enzymatic treatment using the mannanase-rich mixture Gamanase™ required the use of a buffer at pH 5.0 and heating overnight at 60 °C. For reference, the material was incubated under these conditions without enzymatic treatment (reference samples are marked ‘–ref’).
A considerable amount of material was washed out after the enzymatic incubation stage prior to leaching (Stream 10L). The relative yields from enzymatic treatment and leaching are shown in Tables 2a and 2b.
Table 2a. Material Yields for 4 Bar STEX Pre-Treatment and Enzymatic Treatment (wt.%)
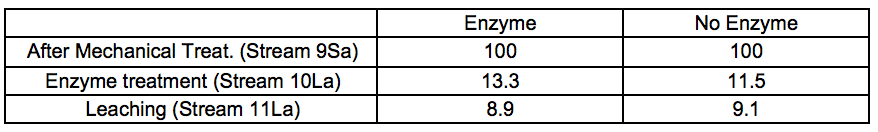
Table 2b. Material Yields for 7 bar STEX Pre-treatment and Enzymatic Treatment (wt.%)
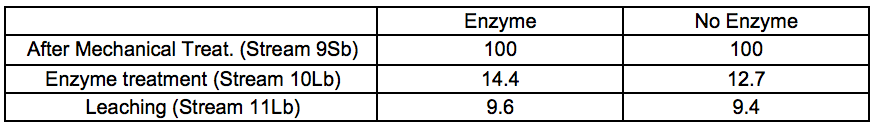
In general, the amount of extracted material increased under more severe conditions. It also appeared that incubation in these conditions of temperature and pH, regardless of the presence or absence of hydrolytic enzymes, was the major factor associated with the removal of wood constituents during the incubation stage.
For the leached residues, a substantial decrease in the relative glucomannan content was found in the samples that had been pre-treated with a STEX 7 treatment (Stream 11Sb). For the STEX 4 pretreatment the relative composition of the different wood constituents were very similar to their composition prior to the enzymatic and leaching treatments (Stream 9); see Table S8.
Analyses of extracted material after enzymatic treatment and leaching
A series of purified (monocomponent) enzymes of specific hydrolytic activity were used to probe the carbohydrates of Stream 11L for the presence of specific polysaccharides. The relative changes in reducing sugar levels between Streams 11L-ref and 11L indicated that the Gamanase™ treatment increased the amount of polysaccharides available for enzymatic hydrolysis, as all of the monocomponent enzymes tested (xyloglucanase, xylanase, and mannanase) were able to release substantially more reducing sugar from Stream 11L than from Stream 11L-ref. The difference was most striking for the STEX-7 samples (Tables 3 and 4).
Table 3. Amount (g) of Reducing Sugar Released by the Enzyme per kg Total Dissolved Solids in the Sample, 4 Bar STEX Pre-treatment
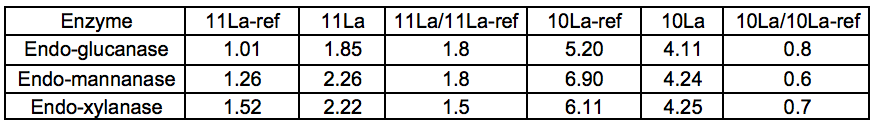
Table 4. Amount (g) of Reducing Sugar Released by the Enzyme per kg Total Dissolved Solids in the Sample, 7 Bar STEX Pre-treatment
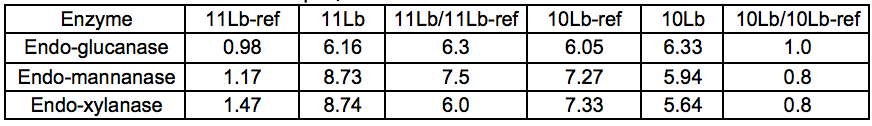
This increased response to the monocomponent enzymes could be explained by an increase in the concentration of polysaccharides in Stream 11L compared to 11L-ref, or simply by an increased polysaccharide accessibility. A general loosening of the lignocellulosic network via chain cutting of the glucomannan may have increased the accessibility of polysaccharides within the LCC network. The Gamanase™ pretreatment was not specific for inter-network connections but had mostly endo–type activities (cleaving polysaccharides at points along the backbone), so it was likely that cell wall polysaccharides were made more soluble by a reduction in chain length (degree of polymerisation) by the degradative activity of the Gamanase™. This would lead to an increased concentration of shorter, dissolved oligosaccharides in these samples of leaching water, which would serve as effective substrates for the monocomponent enzymes utilized in this analytical step.
Wood components in the material leached from STEX-4 (Stream 11La) had a higher molar mass than the corresponding STEX-7 Stream 11Lb or either of the reference samples (Streams 11La-ref, 11Lb-ref) (Fig. 4). This and the fact that the STEX-7 leached material contained a higher proportion of reducing end groups indicated that there was a large number of short oligosaccharides in the STEX-7 leached material. A possible interpretation of this result was that the Gamanase™ treatment made it possible to leach relatively large molar mass material from the STEX-4 sample that was not possible to leach from the reference samples, and that the high molar mass leachable material in STEX-7 was likely degraded and dissolved during steam explosion.
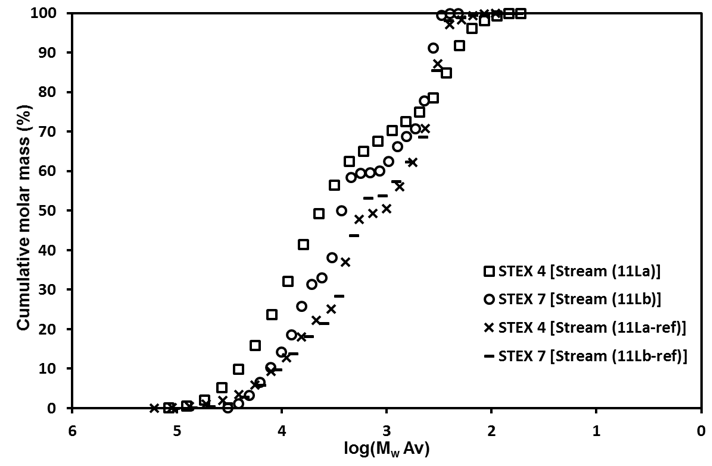
Fig. 4. Molecular weight distributions for the components in the leached liquor (Stream 11L)
Pulp Production
To evaluate the quality of the pulp produced from the solid wood material remaining after the pre-treatment and extraction steps, sulphate pulping and subsequent oxygen delignification were performed.
Pulping
The pulping (producing Stream 14S) followed reasonable trends with lower Kappa numbers and lower intrinsic viscosities after longer pulping times at the cooking temperature, even after the more intensive STEX-7 treatment (see “Pulp and oxygen-delignified samples; yields and characteristics” in supplementary material). The STEX-4 treatment resulted in values similar to those of the untreated reference material, which indicated that the wood material was not substantially changed after treatment under this condition. Material subjected to mechanical treatment followed by enzyme-assisted leaching treatment (Stream 11S) was also subjected to cooking, which led to low pulp viscosities with no great difference between the STEX 4 or STEX 7 pretreatments.
The mechanical, enzymatic, and leaching process steps affected the material more than the steam explosion pre-treatment. It would be possible to optimize the pulping conditions, i.e. lower the charge of cooking chemicals for the STEX, mechanical, enzymatic, and leaching treated material, and/or reduce the cooking time to increase the pulp viscosity. Applying the different treatments alone or in combination with each other and controlling the intensity of the steps more carefully provided a toolbox where pulp grades that ranged from a product closely resembling conventional kraft pulp, to a product approaching dissolving pulp, was achieved.
Oxygen delignification
The pulp properties investigated after oxygen delignification (producing Stream 15S) followed the same trends as those of the unbleached pulps (see “Pulp and oxygen-delignified samples; yields and characteristics” in supplementary material). The STEX-4 resulted in values similar to those of the reference untreated wood material, whereas STEX-7 gave pulps with lower Kappa numbers, lower viscosities, and higher brightness than the reference material. The mechanical, enzymatic, and leaching treatments led to pulps with lower viscosities and higher brightness values but the Kappa numbers were comparatively high. Hemicelluloses have been found to protect the cellulose chain from alkaline hydrolysis (Lindström and Teder 1995) and this may be a reason for the lower viscosities in this study, because much of the hemicellulose was removed during the previous process steps, yet much of the lignin still remained.
Fractionation of Mild STEX Extracted Components
The wood components released from the mild STEX treatment (Stream 3L) were, after being frozen for transport, fractionated by membrane and chromatographic separation. The yields of the different fractions based on the original wood material are shown in Fig. 5. It may be noted that a substantial amount of the material fell into the fraction of 0.45 μm to 5 kDa, which indicated the presence of hemicellulose with a considerable degree of polymerisation.
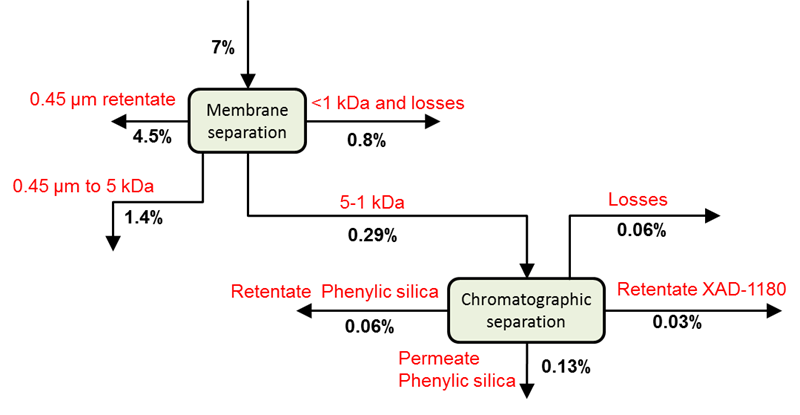
Fig. 5. Cumulative yields after fractionation of material released from a STEX 7 bar pre-treatment (Stream 3L)
Membrane fractionation of mild-STEX extracted components
The sugar composition of the different fractions recovered after membrane fractionation is shown in Table 5. These results showed that arabinose was found mainly in the low molecular weight fractions. As a side chain of xylan, arabinose can be easily cleaved from the polysaccharide backbone by autohydrolysis under harsher conditions and therefore can be expected to be enriched in the low molecular weight fractions. This debranching is also expected to decrease the solubility of xylan and may thus affect the extractability of that component. The data reported in Table 5 also showed that the largest amount of galactoglucomannan (GGM) was found as higher molecular weight material in the > 5 kDa fraction.
The Klason lignin and the acid-soluble lignin content in the membrane-fractionated samples revealed that the amount of lignin was greater in the fraction with a lower cut-off, which indicated that the lignin extracted during hot water treatment was of lower molecular weight relative to the hemicelluloses.
Table 5. Anhydrosugar Composition and Lignin Content of Membrane-fraction-ated Samples of the Extracted Liquid After STEX 7 Bar Treatment (wt.%) *
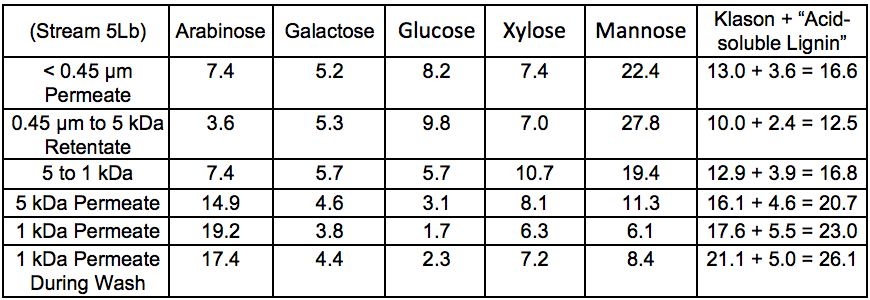
* Values are based on the total mass (dry basis) of each stream
Fractionation by chromatography
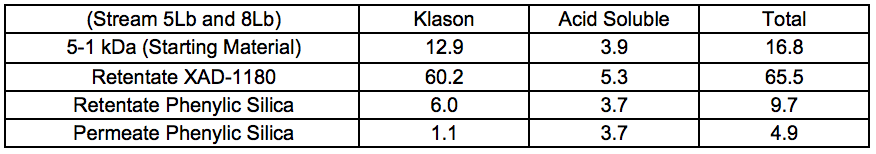
Chromatographic separation was used to divide the 5 to 1 kDa fraction of Stream 5Lb into three fractions, which displayed increasing aromatic affinity in the order of: permeate phenylic silica, retentate phenylic silica, and retentate XAD-1180.
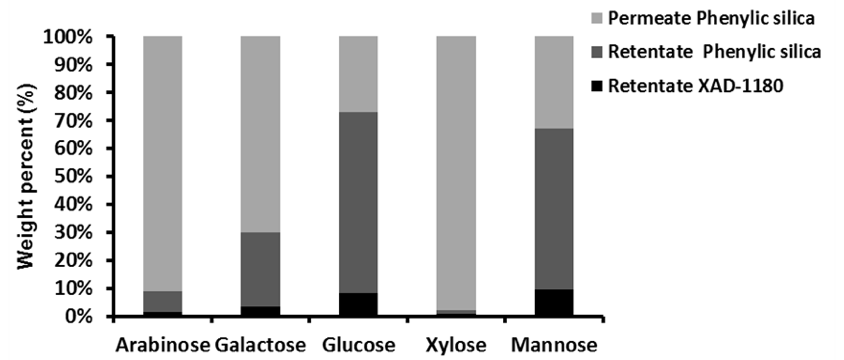
Fig. 6. The partitioning properties of the sugars towards each of the three chromatographically-separated fractions (Stream 8Lb)
The Π-Π interactions between the phenylic resins or the XAD resins were believed to be responsible for the retention. Hence, because the carbohydrate fractions lack Π electrons, the retained carbohydrate fractions were probably linked to lignin by chemical bonds.
Figure 6 shows the distribution of sugars in the three fractions and the detailed values can be found in the supplementary material (Table S7). The sugar distribution suggested that the hemicelluloses consisted mainly of galactoglucomannan, xylan, and arabinogalactans. Because only very small amounts of xylose and arabinose were found in the retentate fractions, it was here suggested that, if the retained material consisted of LCC, then galactoglucomannan-LCC predominates.
Table 6 shows that acid-soluble lignin was rather evenly distributed in the fractions, and that it could not be removed with the hydrophobic sorption procedure used.
Table 6. Lignin Content of Chromatographically-separated Samples (Membrane-filtered Liquid After STEX 7 Bar Treatment) (wt.%)
Enzymatic Crosslinking
To further upgrade the hemicelluloses that were recovered after the STEX treatment, enzymatic crosslinking according to the Ecohelix technology described by Oinonen et al. (2013) was employed to produce Stream 7L. After a prefiltration (0.2 µm nominal pore size) and a one-step membrane fractionation, the 5 kDa retentate (Stream 4L) with a high concentration of hemicelluloses, was treated with enzymes as described in “Enzymatic treatment and leaching” in the supplementary material. The polymer samples were successfully crosslinked using an oxidative enzyme treatment as shown by the size exclusion chromatography (SEC) data (Table 7). The table also gives an indication of the degree of polymerisation of the 5 kDa retentate STEX extract and suggests that the hemicelluloses released in this fraction had an average degree of polymerisation of approximately 30 sugar monomer units. The crosslinked sample was separated into two fractions of comparatively high and low average molecular weight. The sugar compositions of the fractions are given in Table 8.
Table 7. Molecular Weight Distributions of Fractions Before and After Crosslinking
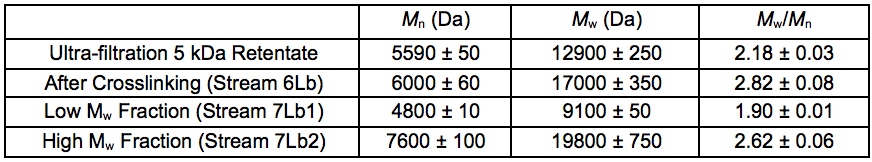
Table 8. Anhydrosugar Composition of Crosslinked Samples (wt.%)
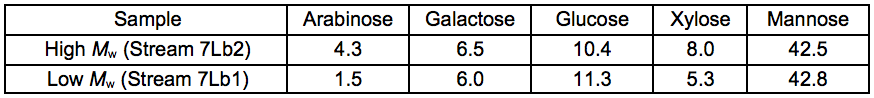
Therefore, the Ecohelix technology seems promising for the crosslinking and upgrading of hemicelluloses derived from the steam explosion process.
Ionic Liquid Treatment
As a fractionation step, a partial Ionic Liquid (IL) solubilisation of the residual wood material after different routes of pre-treatment and hemicellulose extraction was performed. The IL treatment was either performed on STEX-treated wood immediately after mechanical treatment or on the solid residue left after the enzyme-assisted leaching process.
Yields
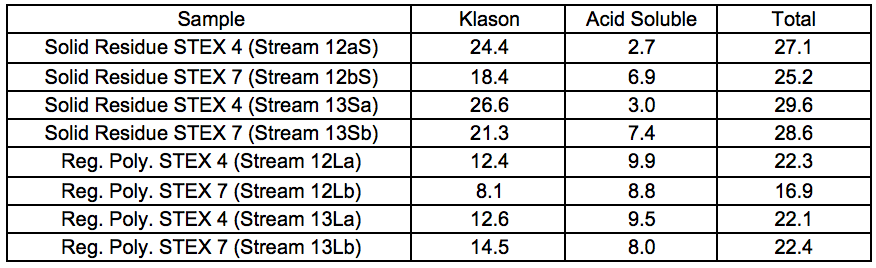
The relative yields, considering both the solid residues and the regenerated polymers, and the composition of the streams are shown in Fig. 7. The relative yields for the regenerated polymers are also summarised in Table S5. In addition to the recovered streams, some wood material was also lost in the separation of the ethanol used to regenerate the polymers, but the amount could not be quantified. For all pretreatments, the yield of regenerated polymers was higher for the STEX 7 material than for the STEX 4 material (Fig. 7; 12Lb compared to 12La and 13Lb compared to 13La). The difference in yields between the STEX 4 and the STEX 7 regenerated polymers was reduced if the samples were treated with enzymes followed by leaching prior to the IL-extraction.
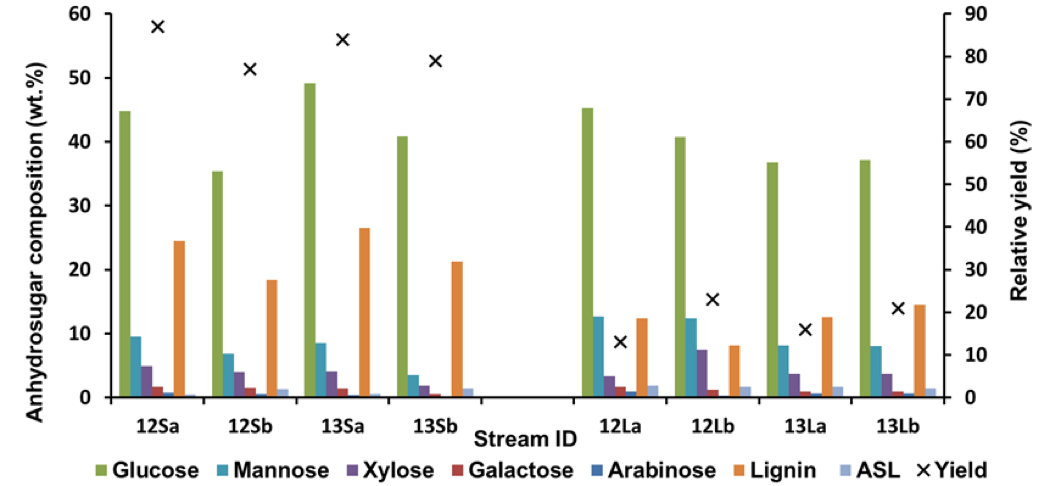
Fig. 7. Anhydrosugar and lignin compositions of the solid residues and of the regenerated materials, × indicating relative yields; the relative yield (wt.%) of the streams 12Xy and 13Xy are defined as 12Xy / (12Ly + 12Sy) × 100 and 13Xy / (13Ly + 13Sy) × 100, respectively, where X is S or L and y is a or b
Components
The solid residue streams from the less processed feeds (Streams 12S) were generally more enriched with mannose than their more processed counterparts (Streams 13S; Fig. 7), especially for the STEX 7 samples. This was in line with the decreased glucomannan content in the material after the leaching and enzymatic treatment steps (Table S8). The xylan content was approximately 4% to 5% in all of the residual materials except for the residual material from Stream 13Sb, where the content was about 2% (Fig. 7). The content of hemicellulose sugars was particularly low for this stream, as was expected from the more extensive pre-treatment followed by IL extraction of a material containing accessible sugars.
General Discussion
This work has demonstrated how wood components can be separated and purified in a process where hemicelluloses, lignin, and cellulose fractions are recovered. However, can this process be economically feasible? First, it must be emphasised that all of the methods used in this work can be scaled up, and several of the individual stages have long been used industrially. The enzymes and other chemicals used were of industrial grade. Currently, the hemicelluloses and lignins produced in this process are of grades that have limited commercial value but the value of such compounds is expected to increase as the availability of non-renewable resources decreases. The most valuable product of the process today and in the foreseeable future is the cellulose fibre. The properties of the fibre range from that for normal paper grades to that for dissolving grades but the cellulose can also be used for the production of other cellulose-based products, such as for example cellulose nanocrystals. In the current conventional processes for production of these products the yield losses are relatively high. With the concepts proposed in this work, additional products based on hemicelluloses and/or lignin are obtained, increasing the potential overall profit. However, it must be kept in mind that this is a first attempt to develop a novel industrial process and that it could be optimized in several ways. Additionally, the process design used here is flexible and open to future modifications and additions.
CONCLUSIONS
- This work demonstrated the feasibility of combining multiple different methods of biomass treatment, extraction, separation, and modification to create a palette of different fractions– a cellulose fraction with different qualities and fractions of solubilised hemicelluloses and lignins.
- It was found that by applying different treatments alone or in combination with each other and controlling the intensity of the steps the pulp grades could be produced that ranged from a product closely resembling conventional kraft pulp, to a product approaching dissolving pulp.
- The yields of the respective fractions varied with process conditions, but up to approximately 29 wt.% of the wood could be extracted, as streams containing high fractions of hemicelluloses and lignin, before digesting, using mild steam explosion (7 bar) and enzyme-assisted leaching steps.
- The extracted components could be fractionated and purified using membrane and chromatographic methods, and the molecular weight could, in some cases, be increased by enzyme-initiated radical coupling.
ACKNOWLEDGEMENTS
The authors are grateful to the Knut and Alice Wallenberg Foundation for their financial support. The skilful experimental work of Mr. Tommy Friberg and Ms. Lena Fogelquist is also gratefully acknowledged. Part of the work included in this article has also been published in the form of a short preceding for the 6th Nordic Wood Biorefinery Conference.
REFERENCES CITED
Abdul Khalil, H. P. S., Davoudpour, Y., Islam, M. N., Mustapha, A., Sudesh, K., Dungani, R., and Jawaid, M. (2014). “Production and modification of nanofibrillated cellulose using various mechanical processes: A review,” Carbohydrate Polymers 99, 649-665. DOI: 10.1016/j.carbpol.2013.08.069
Alén, R., Patja, P., and Sjöström, E. (1979). “Carbon dioxide precipitation of lignin from pine kraft black liquor,” TAPPI Journal 62(11), 108-110.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresource Technology 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093
Arkell, A., Olsson, J., and Wallberg, O., (2014). “Process performance in lignin separation from softwood black liquor by membrane filtration,” Chemical Engineering Research and Design, 92(9), 1792-1800. DOI: 10.1016/j.cherd.2013.12.018
Azhar, S., Wang, Y., Lawoko, M., Henriksson, G., and Lindström, M. E. (2011). “Extraction of polymers from enzyme-treated softwood,” BioResources 6(4), 4606-4614.
Azhar, S., Henriksson, G., Theliander, H., and Lindström, M. E. (2015). “Extraction of hemicelluloses from fiberized spruce wood,” Carbohydrate Polymers 117, 19-24. DOI: 10.1016/j.carbpol.2014.09.050
Brodin, I., Sjöholm, E., and Gellerstedt, G. (2009). “Kraft lignin as feedstock for chemical products: The effects of membrane filtration,” Holzforschung 63(3), 290-297. DOI: 10.1515/HF.2009.049
Bylin, S., Olsson, C., Westman, G., and Theliander, H. (2014). “Solvation behavior of cellulose and xylan in the MIM/EMIMAc ionic liquid solvent system: Parameters for small scale solvation,” BioResources 9(1), 1038-1054. DOI: 10.15376/biores.9.1.1038-1054
Carrasco, F. (1992). “Thermo-mechano-chemical pretreatment of wood in a process development unit,” Wood Science and Technology 26(6), 413-428. DOI: 10.1007/BF00229246
Galbe, M., and Zacchi, G. (2002). “A review of the production of ethanol from softwood,” Applied Microbiology and Biotechnology 59(6), 618-628. DOI: 10.1007/s00253-002-1058-9
Galbe, M., and Zacchi, G. (2007). “Pretreatment of lignocellulosic materials for efficient bioethanol production,” Advances in Biochemical Engineering/Biotechnology 108, 41-65. DOI: 10.1007/10_2007_070
Gellerstedt, G., Sjöholm, E., and Brodin, I. (2010). “The wood-based biorefinery: A source of carbon fiber?,” The Open Agricultural Journal 11(4), 119-124. DOI: 10.2174/1874331501004010119
Giummarella, N., and Lawoko, M. (2017). “Structural insights on recalcitrance during hydrothermal hemicellulose extraction from wood,” ACS Sustainable Chemistry and Engineering 5(6), 5156-5165. DOI: 10.1021/acssuschemeng.7b00511
Helander, M., Theliander, H., Lawoko, M., Henriksson, G., Zhang, L., and Lindström, M. E. (2013). ”Fractionation of technical lignin: Molecular mass and pH effects,” BioResources 8(2), 2270-2282. DOI: 10.15376/biores.8.2.2270-2282
Jedvert, K., Wang, Y., Saltberg, A., Henriksson, G., Lindström, M. E., and Theliander, H. (2012). “Mild steam explosion: A way to activate wood for enzymatic treatment, chemical pulping and biorefinery processes,” Nordic Pulp and Paper Research Journal 27(5), 828-835. DOI: 10.3183/NPPRJ-2012-27-05-p828-835
Lawoko, M., Henriksson, G., and Gellerstedt, G. (2006). “Characterisation of lignin-carbohydrate complexes (LCCs) of spruce wood (Picea abies L.) isolated with two methods,” Holzforchung 60(2), 156-161. DOI: 10.1515/HF.2006.025
Lee, H. V., Hamid, B. A., and Zain S. K. (2014). “Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process,” The Scientific World Journal V 2014. DOI: 10.1155/2014/631013
Lindström, M., and Teder, A. (1995). “The effect of polysulfide pretreatment when kraft pulping to very low Kappa numbers,” Nordic Pulp and Paper Research Journal 10(1), 8-11. DOI: 10.3183/NPPRJ-1995-10-01-p008-011
Li, M. -F., Sun, S. -N., Xu, F., and Sun, R. -C. (2012). “Organosolv fractionation of lignocelluloses for fuels, chemicals and materials: A biorefinery processing perspective,” in: Biomass Conversion, C. Baskar, S. Baskar, and R. S. Dhillon (Eds.), Springer Berlin Heidelberg, Berlin, Germany. DOI: 10.1007/978-3-642-28418-2_11
Lora, J. H., and Glasser, W. G. (2002). “Recent industrial applications of lignin: A sustainable alternative to nonrenewable material,” Journal of Polymers and the Environment 10(1), 39-44. DOI: 10.1023/A:1021070006895
Marchessault, R. H. (1998). “Steam explosion: A refining process for lignocellulosics,” in: Steam Explosion Techniques, Fundamentals and Applications, B. Focher, A. Marzetti, and V. Crescenzi (eds.), Gordon and Breach Science Publishers, Milan, Italy.
Maples, R. E. (2000). Petroleum Refinery Process Economics, PennWell Cooperation, Tulsa, OK, USA.
Mason, W. H. (1928). “Process and apparatus for disintegration of fibrous material,” U.S. Patent No. 1824221.
Mattsson, C., Hasani, M., Dang, B., Mayzel, M., and Theliander, H. (2017). “About structural changes of lignin during kraft cooking and the kinetics of delignification,” Holzforschung 71(7-8), 545-553. DOI: 10.1515/hf-2016-0190
Öhman, F., Theliander, H., Tomani, P., and Axegård, P. (2008). “Method for separating lignin from black liquor,” U.S. Patent No. 0047674.
Oinonen, P., Areskogh, D., and Henriksson, G. (2013). “Enzyme catalyzed cross-linking of spruce galactoglucomannan improves its applicability in barrier films,” Carbohydrate Polymers 95(2), 690-696. DOI: 10.1016/j.carbpol.2013.03.016
Örså, F., Holmbom, B., and Thornton, J. (1997). “Dissolution and dispersion of spruce wood components into hot water,” Wood Science and Technology 31(4), 279–290. DOI: 10.1007/BF00702615
Persson, T., Krawczyk, H., Nordin, A. -K., and Jönsson, A. -S. (2010). “Fractionation of process water in thermomechanical pulp mills,” Bioresource Technology 101(11), 3884-3892. DOI: 10.1016/j.biortech.2009.12.142
Ragnar, M., Henriksson, G., Lindström, M. E., Wimby, M., and Süttinger, R. (2013). “Pulp,” in: Ullman Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Berlin, Germany, pp. 3-89. DOI: 10.1002/14356007.a18_545.pub4
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., Frederick Jr., W. J., Hallett, J. P., Leak, D. J., Liotta, C. L., et al. (2006). “The path forward for biofuels and biomaterials,” Science 311(5760), 484-489. DOI: 10.1126/science.1114736
Sevastynova, O., Helander, M., Chowdhury, S., Lange, H., Wedin, H., Chang, L., Ek, M., Kadla, J. F., Crestini, C., and Lindström, M. E. (2014). “Tailoring the molecular and thermo-mechanical properties of kraft lignin by ultrafiltration,” Journal of Applied Polymer Science 131(18), 40799(1-11). DOI: 10.1002/app.40799
Song, T., Pranovich, A., Sumerskiy, I., and Holmbom, B. (2008). “Extraction of galactoglucomannan from spruce wood with pressurised hot water,” Holzforschung 62, 659-666. DOI: 10.1515/HF.2008.131
Tarabanko, V. E., Fomova, N. A., Kuznetsov, B. N., Ivanchenko, N. M., and Kudryashev, A. V. (1995). “On the mechanism of vanillin formation in the catalytic oxidation of lignin with oxygen,” Reaction Kinetics Catalysis Letters 55(1), 161-170. DOI: 10.1007/BF02075847
Uloth, V. C., and Wearing, J. T. (1989). “Kraft lignin recovery: Acid precipitation versus ultrafiltration. Part II: Technology and economics,” Pulp and Paper Canada 90(10), 34-37.
Usta, I., and Hale, M. D. (2005). “Comparison of the bordered pits of the two species of spruce (Pinaceae) in a green and kiln-dried condition and their effects on fluid flow in the stem wood in relation to wood preservation,” Forestry 79(4), 467-475. DOI: 10.1093/forestry/cpl011
Wallberg, O., Jönsson, A. -S., and Wimmerstedt, R. (2003). “Fractionation and concentration of kraft black liquor lignin with ultrafiltration,” Desalination 154(2), 187-199. DOI: 10.1016/S0011-9164(03)80019-X
Wallberg, O., and Jönsson, A. -S. (2006). “Separation of lignin in kraft cooking liquor from a continuous digester by ultrafiltration at temperatures above 100 °C,” Desalination 195(1-3), 187-200. DOI: 10.1016/j.desal.2005.11.011
Westerberg, N., Sunner, H., Helander, M., Henriksson, G., Lawoko, M., and Rasmuson, A. (2012). “Separation of galactoglucomannans, lignin, and lignin-carbohydrate complexes from hot-water-extracted Norway spruce by cross-flow filtration and adsorption chromatography,” BioResources 7(4), 4501-4516. DOI: 10.15376/biores.7.4.4501-4516
Wojtasz-Mucha, J., Hasani, M., and Theliander, H. (2017). “Hydrothermal pretreatment of wood by mild steam explosion and hot water extraction,” Bioresource Technology 241, 120-126. DOI: 10.1016/j.biortech.2017.05.061
Ziesig, R., Sedin, M., Tomani, P., and Theliander H. (2015). “Production of a pure lignin product, Part 3: Distrubution and removal of inorganics from softwood lignin,” Nordic Pulp & Paper Research Journal 30(2), 199-206. DOI: 10.3183/NPPRJ-2015-30-02-p199-205
Article submitted: June 21, 2017; Peer review completed: Sept. 17, 2017; Revised version received and accepted: October 6, 2017; Published: October 17, 2017.
DOI: 10.15376/biores.12.4.9152-9182
APPENDIX
Abbreviations
IL Ionic liquid
LCC Lignin-carbohydrate Complex
M.E.L. Sample after mechanical, enzymatic, and leaching treatment
STEX Steam explosion
WWSC Wallenberg Wood Science Center
SUPPLEMENTARY EXPERIMENTAL MATERIAL
Materials
Industrially chipped softwood sourced from a pulp mill in the southwestern part of Sweden, mainly Norway spruce (Picea abies) with some pine (Pinus sylvestris), was dried and sieved. A fraction that passed through holes with 30-mm diameter but was retained on sieves with 3-mm holes was used for the subsequent process steps.
Methods
Mild steam explosion
The mild steam STEX equipment has been described by Jedvert et al. (2013). For each run, 500 g (dry basis) of wood chips (Stream 1S) were used. Prior to the steam treatment, the chips were impregnated with de-ionised water at a liquor-to-wood ratio of 10:1. A vacuum was applied for 5 min followed by pressurising to 5 bars with nitrogen gas to improve impregnation and the chips were left under pressure overnight. The chips were then drained and placed in the steam explosion reactor. They were heated with saturated steam (operating steam pressure of 16 bar) until a desired pressure was reached. The pressure was maintained for 10 min at 4 bar or for 20 min at 7 bar. The treatment was terminated by a sudden release of the pressure (i.e. the actual “explosion”) and the wood chips and the condensed steam were discharged. Directly after the STEX treatment, the condensed steam was recovered by filtration through a Büchner funnel and the steam explosion liquor was then frozen (Stream 3L). The steam-exploded wood chips were rinsed with water and stored at 5 °C. The procedure was repeated until several kg of wood chips had been processed at each pressure level.
Mechanical treatment
Steam-exploded wood chips (Stream 3S) were subjected to a mechanical treatment in a laboratory-scale 12” disc refiner (Sprout-Waldron, Muncy, USA). The chips were subjected to atmospheric steam for 15 min and treated in the refiner in a single pass mode, where an energy input of approximately 250 kWh/dry ton was used.
Enzymatic treatment and leaching
The solid material in Stream 9S was subjected to enzymatic treatment prior to leaching, using Gamanase™ from Novo Nordisk. 50 mM acetate buffer at pH 5.0 was added to the wood to reach a final wood consistency of 3 wt.%. Next, 20 µL of Gamanase™ (activity: 1000 VHCU/mL) was added to the mixture for each gram of dry wood. After 24 h of incubation at 60 °C, samples were filtered through a fibre cloth “Monodur” (Clear Edge, Minneapolis, USA) with a mesh opening of 71 microns and 86 mesh count per cm and washed with boiling water to inactivate the enzymes. The stream was recovered as Stream 10L. The Gamanase™ enzyme cocktail utilised in the extraction experiments was not a pure mannan-degrading enzyme, but was contaminated with hydrolytic enzymes of various activities and with quantifiable oligosaccharides of unknown structure.
The enzyme-treated material was then leached with a mixture of 50% wt.% methanol and water containing a 5% alkali charge on wood. The liquor-to-wood ratio was 10:1. Leaching was performed in 2-L stainless steel autoclaves rotating in a vessel filled with polyethylene glycol as the heating medium for 2 h at 130 °C. Thereafter, the autoclaves were quenched in a cold-water bath for 30 min. Liquid containing dissolved solids were separated from the wood material by vacuum filtration using a wire cloth (mesh size: 71 microns) followed by washing with deionised water. The separated liquid was collected in Stream 11L.
The enzymatic-treatment step was also performed without active enzymes and a reference wash (Stream 10L-ref) and leached material (Stream 11L-ref) were recovered.
Pulping
In the cooking process, two different cooking liquids and loads were used depending on the physical size of the wood material.
For wood chips (Stream 1S and 3S), 100 g (dry basis) of chips were placed in stainless steel autoclaves for each batch. The cooking chemicals, added as NaOH, Na2S, and Na2CO3, were added to a liquor-to-wood ratio of 4.5:1 (kg/kg). The effective alkali (EA) charge was 22% (on wood), the sulphidity was 35%, and the concentration of carbonate in the white liquor was 0.1 M.
For the pulping of the mechanically-treated wood material, 50 g (dry basis) of the material was used for each batch and the cooking chemicals were charged to a liquor-to-wood ratio of 9:1 (kg/kg). The EA charge was 29% (on wood), the sulphidity was 35%, and the concentration of carbonate in the white liquor was 0.1 M.
The autoclaves were placed in a pre-heated polyethylene glycol bath at 80 °C for 20 min. The temperature was then increased to 170 °C at 0.8 °C/min and was maintained for 20 min, 40 min, or 60 min. The autoclaves were cooled and the cooked chips were separated from the black liquor, which was re-filtered once. Then, the filter cake was displacement washed with 20 L of de-ionised water. This was followed by disintegration in a laboratory defibrator (L&W Noram, Stockholm, Sweden) at 3000 rpm and the filtrate was re-filtered once. The pulp was then subjected to a second wash with 15 L of de-ionised water.
Oxygen delignification
For oxygen delignification, 35 g (dry basis) of each pulp was mixed with MgSO4 (equivalent to 1 kg magnesium/ton dry pulp), NaOH (25 kg/ton dry pulp), and de-ionised water to a pulp concentration of 12 wt.%. The suspensions were placed in autoclaves and were pressurised with oxygen gas at 6 bar for 2 min. The autoclaves were then placed in a pre-heated polyethylene glycol bath at a temperature of 105 °C for 1 h. The first 15 min were considered a heating-up period until a temperature of 105 °C was reached in all parts of the material inside the autoclaves. The autoclaves were then cooled and the overpressure was released. The pulps were washed in a series of steps: first the filtrate was re-filtered once and the pulp was then displacement washed with 1 L of de-ionised water. Subsequently, the pulp was re-suspended in 1 L of de-ionised water for 10 min. The water was then filtered and re-filtered once. Finally, the pulp was washed with an additional litre of de-ionised water and collected, forming Steam 15S.
Membrane filtration
Cross-flow filtration was performed using pilot-scale membrane equipment. The system consisted of a 30-L tank equipped with a stirrer, heating element, gear pump, and Kerasep membrane unit (Novasep, Pompay, France). The membranes used were TiO2/ZrO2 ceramic membranes with an area of 816 cm2.
Extraction liquid (Stream 3Lb) from STEX at 7 bar and 20 min were thawed and filtered through a 0.45 μm wire in a Büchner funnel. All filtrations were performed at room temperature. For the first step of cross-flow filtration, 12.5 L was filtered through a 0.45 μm membrane, which resulted in 10.6 L of permeate. In the second step, 9.4 L of 0.45 μm permeate were filtered through a 5 kDa membrane, which resulted in 7.3 L of permeate. In the third step, 7 L of 5 kDa permeate were filtered through a 1 kDa membrane, which resulted in approximately 2 L of retentate. The 2 L of retentate was washed by adding 4 L of deionised water and filtered through a 1 kDa membrane until the permeate reached approximately 4 L. The 2 L of retentate consisting of a 5 to 1 kDa fraction was frozen and further processed by chromatography (Stream 5L).
Chromatographic separation
A description of the chromatographic separation apparatus is given by Westerberg et al. (2012). Two sorbents with different hydrophobic aromatic sorption were used: Amberlite XAD-1180 (Rohm & Haas, Philadelphia, USA) and granular phenylic silica, 68 µm (Sorbtech, Norcross, USA), respectively (Table S1).
Table S1. Properties of the two Sorbents
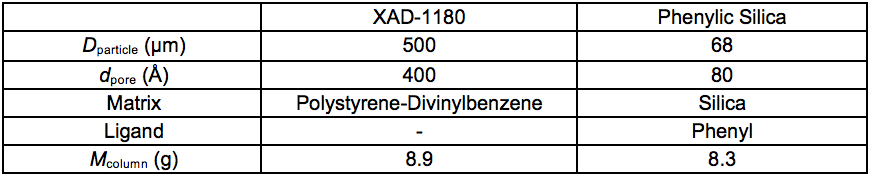
Prior to separation, it was noticed that the thawed sample, Stream 5L, contained substantial amounts of particulate matter. To avoid column contamination and aborted operation, a 32 mm, 0.2 µm filter (Pall) was fitted in the plastic tubing between the glass flask and the pump. The filter had to be exchanged every 50 mL to 150 mL in the loading sequence due to restricted flow. The particulate matter probably consisted of lignin residues that were precipitated in the freezing following the membrane filtration procedure. The recovered filters were coloured dark brown. Approximately 1300 mL of the sample passed through the XAD-1180 resin followed by a 100 mL wash in deionised water. The column was then eluted, first with 28 mL 1:1 MeOH/H2O and then with 58 mL MeOH. The eluate was coloured dark brown at first and the final 30 mL was fully transparent. The permeate of the XAD-1180 resin was then passed through the phenylic silica resin in the same set-up without the Pall-filter. The sorptive procedure was performed once on the XAD1180 resin and twice on the phenylic silica resin. The smaller content of aromatic constituents entering the phenylic silica column reduced the necessary elution volume. The total volume of eluate from the phenylic silica column was approximately 80 mL.
Enzymatic crosslinking
A schematic representation of the enzymatic crosslinking process is shown in Fig. S1. The extraction liquid, Stream 3Lb, after the STEX 7-bar treatment, was first filtered in a Büchner funnel using microfiltration with a 0.2 µm regenerated cellulose membrane (RC 58, Membrane filters, regenerated cellulose, Whatman, Maidstone, UK) to achieve a particle-free sample. The hemicelluloses were then isolated by ultrafiltration (Solvent-resistant Stirred Cell, Millipore, Billerica, USA) under a nitrogen atmosphere (3 bar) with constant stirring employing a regenerated cellulose membrane with a molecular weight cut-off of 5 kDa (Ultracel PLCC07610, Millipore, Billerica, USA). The ultrafiltration began with a volume of 1 L and ended after a volume reduction of 98% was achieved. The sample, a 5 kDa retentate, was thereafter freeze-dried and used for the crosslinking experiments.
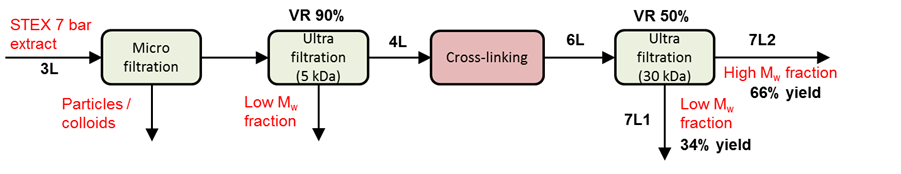
Fig. S1. A schematic representation of the enzymatic crosslinking process
For the oxidative enzyme treatment, the sample was dissolved in distilled water to a concentration of 50 g/L and treated with a fungal laccase (E.C. 1.10.3.2, Sigma 53739, Sigma-Aldrich, Saint Louis, USA) without further purification. The conditions for the treatment were as follows: enzyme dosage of 14 U/g of hydrolysate (~1.4 mg/g substrate), pH of 5.0, reaction temperature of 40 °C, reaction time of 3 h and hydrolysate concentration of 50 g/L. Pure oxygen was introduced to the samples during the reaction.
The cross-linked samples were fractionated to high and low molecular weight fractions (denoted high Mw and low Mw, respectively) by ultrafiltration (Solvent-resistant Stirred Cell, Millipore, Billerica, USA) employing a cellulose membrane with a molecular weight cut-off of 30 kDa (PLTK07610, Millipore, Billerica, USA). Prior to ultra-filtration, the samples were diluted ten times. Ultra-filtration was conducted under a nitrogen atmosphere (3 bar) with constant stirring and continued until a retentate/permeate volume ratio of 1/1 was achieved.
Ionic liquid extraction and fractionation
Pre-treated wood material from Streams 9S and 11S, steam-exploded at 4 or 7 bars, was used as a starting material for an ionic liquid (IL) treatment. The ionic liquid 1-ethyl-3-methylimidazolium acetate (EMIMAc) and the co-solvent 1-methylimidazole (MIM) were obtained commercially from BASF and Sigma-Aldrich, respectively (Bylin et al. 2014). The solvents were kept under an inert atmosphere and were used without further processing.
Wood material (3.5 wt.% of final total weight) was weighed in and added to a glass reactor (250-mL). The material was wetted in co-solvent MIM (30 wt.% of liquid) under an inert atmosphere and subjected to impregnation at 70 °C with a stirring speed of 95 rpm. After 30 min, EMIMAc (70 wt.% of liquid) was added from the top of the reactor in a single batch. After 16 h, the solid was separated from the liquid using vacuum filtration through a polyester filament filter cloth with a mesh opening of 5 µm. The material was dried at 45 °C resulting in solid residues, (Streams 12aS, 12bS, 13Sa, and 13Sb). The dissolved wood material was regenerated in warm ethanol overnight, after which it was separated using vacuum filtration. Drying at room temperature for 24 h resulted in regenerated materials, Streams 12La, 12Lb, 13La, and 13Lb.
Analytical methods
Klason lignin, acid soluble lignin, and carbohydrate content were determined by the method described in Jedvert et al. (2012), except for the data reported in Tables 8 and S8, where the lignocellulosic composition presented was determined using TAPPI UM 250 (1991), TAPPI T 222 (2002), or SCAN-CM 71:09 (2009) with slight modifications. The intrusion volume was investigated using mercury porosimetry (Auto pore IV, micromeritics, Norcross, USA). The ISO brightness, viscosity, and Kappa number were determined according to SCAN-C11:75 (1975), SCAN-C15:62 (1962), and SCAN-C1:77 (1977), respectively. Molar masses were determined using 10 mM NaOH as a mobile phase. More details of the molar mass determination can be found in Azhar et al. (2015).
SUPPLEMENTARY RESULTS
Yields
Table S2. Solid Contents and Yields for Membrane Separated Fractions Recovered After 7 Bar STEX (wt.%)
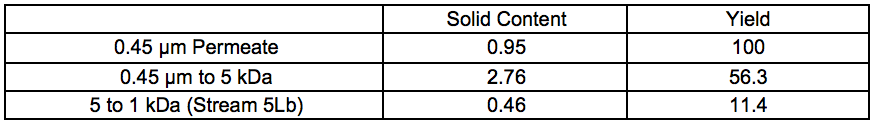
Table S3. Solid Contents and Yields Before and After Enzymatic Crosslinking of Fractions Recovered After 7 Bar STEX (wt.%)
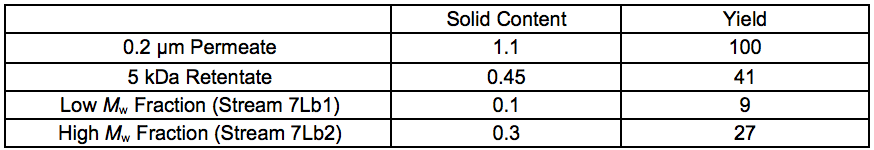
The yield after chromatographic separation was estimated from the freeze-dried weight of the solids after evaporation of the fractions. The fraction 5 to 1 kDa, after STEX 7 bar was used (Table S4). It was reasonable to believe that the missing 21.2 wt.% was removed by pre-filtration using a 0.2 µm filter.
Table S4. Yields of Fractions After Chromatographic Separation (wt.%), Stream 8Lb
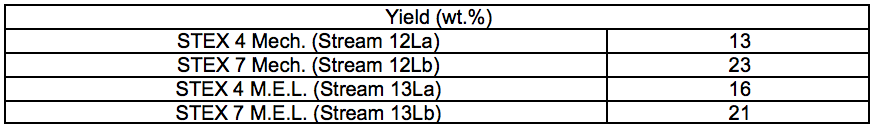
Table S5. Relative Yields of Regenerated Polymers after IL Treatment (wt.%)
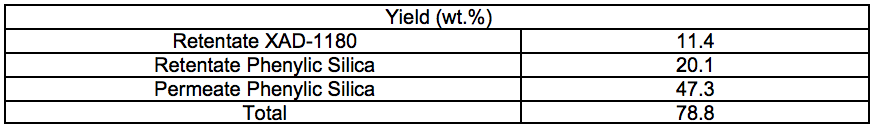
Table S6. Cooking Yields for Different Pre-treatments and Cooking Times (wt.%)
Chip structure after mild steam explosion
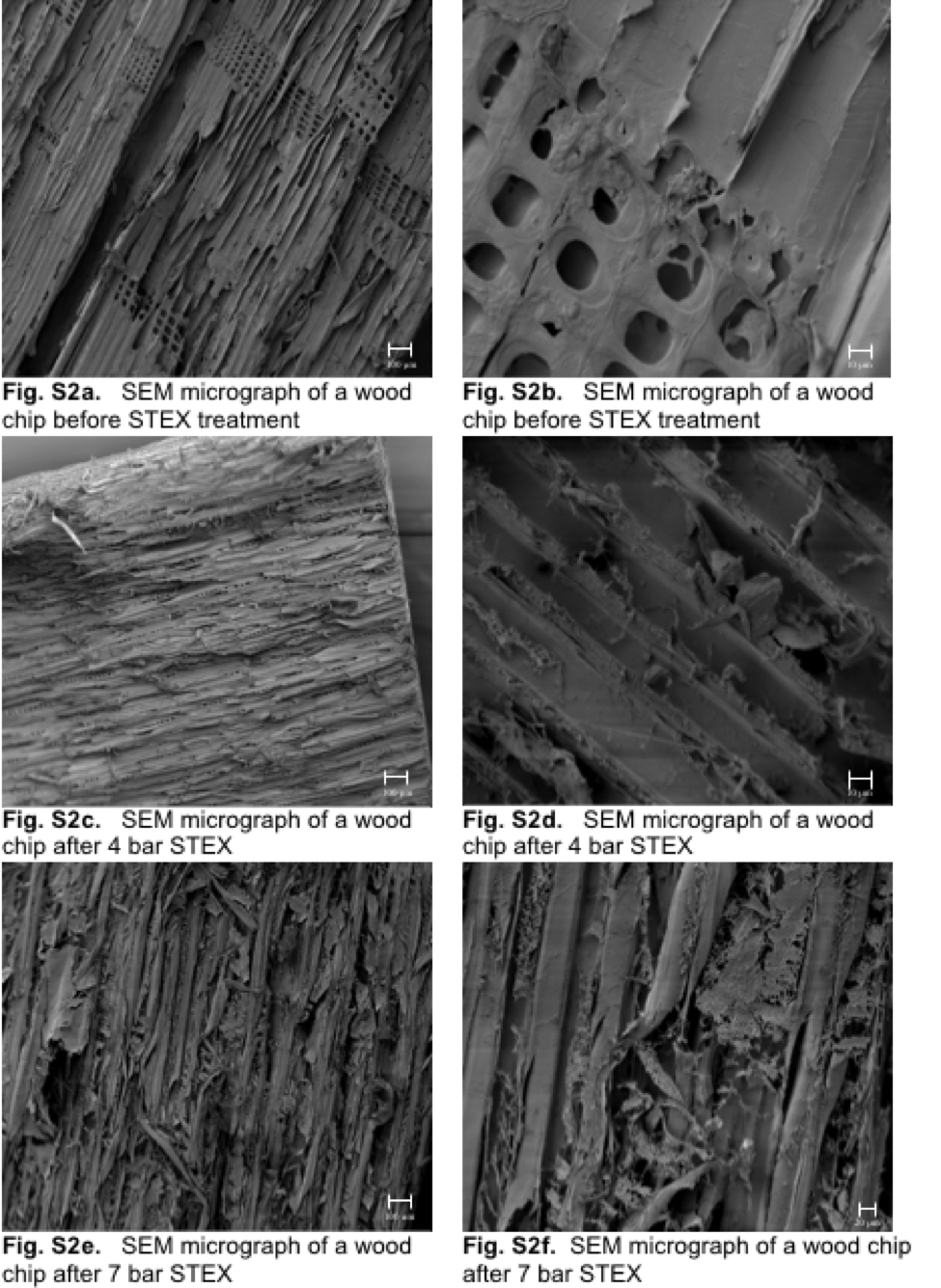
The physical structure of the wood chips after the STEX treatment was investigated using an SEM (EVO HD15, Zeiss, Oberkochen, Germany). The images are of gold sputtered (JFC-1100E, Jeol, Akishima, Japan) samples (Fig. S2).
Observed with the naked eye, the wood chips treated at 7 bar clearly had more breakages and cracks. The images in Fig. S2 also showed that the morphological changes were more severe for the samples subjected to the 7 bar treatment than for the samples subjected to the 4 bar treatment. The surfaces of the samples after the 7 bar treatment were rougher and some damaged wood cells were observed. However, due to differences in the surfaces of the samples from the industrial chipping and the small number of samples investigated, it was difficult to draw definite conclusions from these images. The samples were probably also affected to some extent by the drying prior to the SEM-analysis. A spider-web-like structure was observed in the SEM-images on th
e surfaces of several of the wood chip samples. These structures were seen more prominently on the samples after the 7 bar treatment. Chemical analysis indicated that this material consisted mainly of re-precipitated hemicelluloses, such as galactoglucomannan.
Composition of Chromatographically Separated Samples
Table S7. Anhydro-sugar Composition, Chromatographically Separated, and Membrane-filtered Samples After STEX 7 Bar (wt.%)
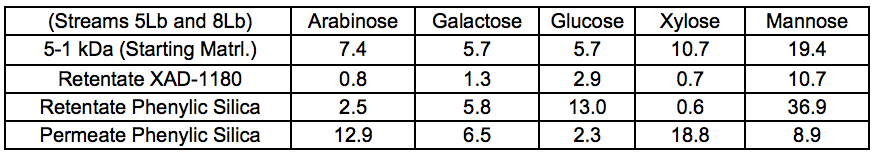
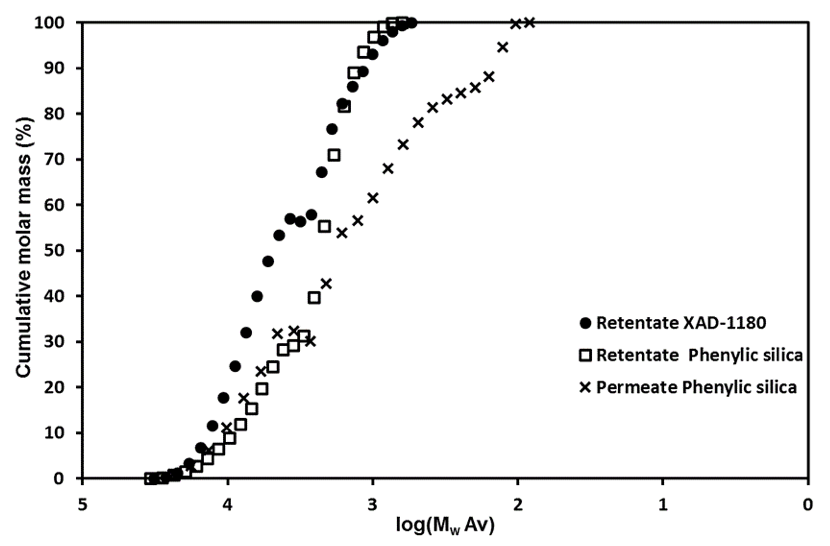
Fig. S3. Molecular weight distributions for the three fractions from the chromatographic separation (Stream 8Lb)
Influence of the Enzymatic/leaching Treatment on the Physical Structure of the Wood
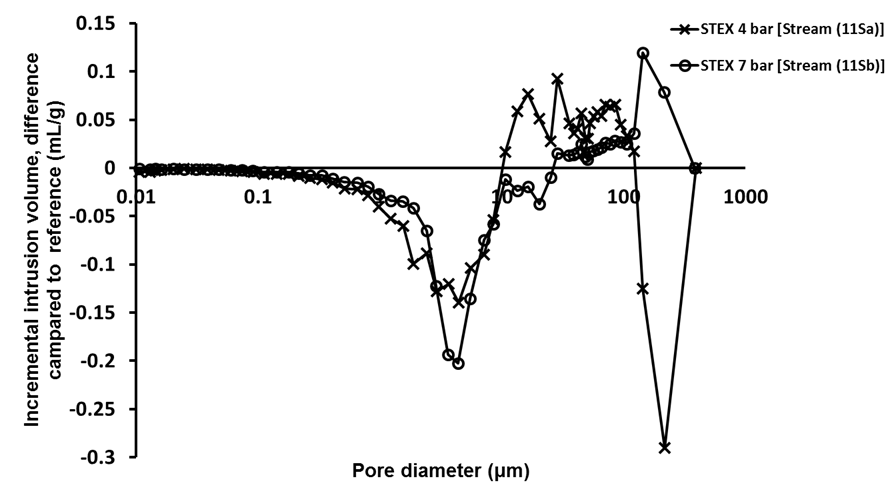
Fig. S4. Difference in incremental intrusion volume for M.E.L-treated solid residuals; comparing Stream 11S, obtained from enzyme-assisted leaching of mechanically-treated STEX-4 and STEX-7 samples, with Stream 9S, which has been mechanically treated but not subjected to enzymatic hydrolysis or leaching
Chemical Composition: Leached Samples
Table S8. Lignocellulosic Composition After Mechanical Treatment, Enzymatic Treatment, and Leaching (M. E. L.) Treatment, Respectively (wt.%)
Chemical Composition: Ionic Liquid Treatment
Table S9. Lignin Content of Ionic-liquid-treated Samples (wt.%)
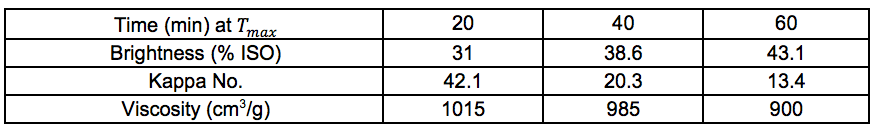
 Pulp and oxygen-delignified samples; yields and characteristics
Pulp and oxygen-delignified samples; yields and characteristics
Tables S10 through S14 in this section refer to Stream 14S. Tables S15 through S19 in this section refer to Stream 15S.
Table S10. No Pre-treatment
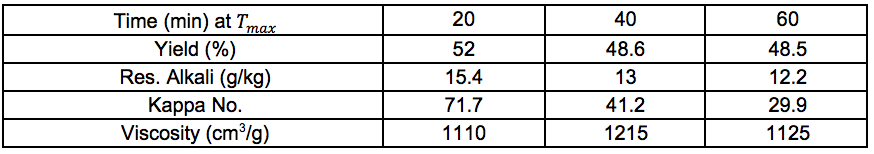
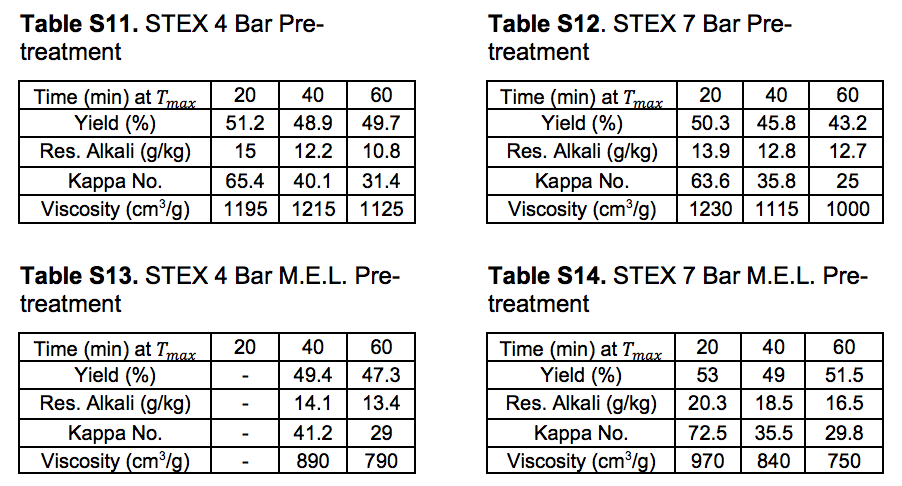
Table S15. No Pre-treatment
Chemical Composition: Pulp and Oxygen Delignified Samples
Table S20. Lignocellulosic Composition of Pulps (wt.%)
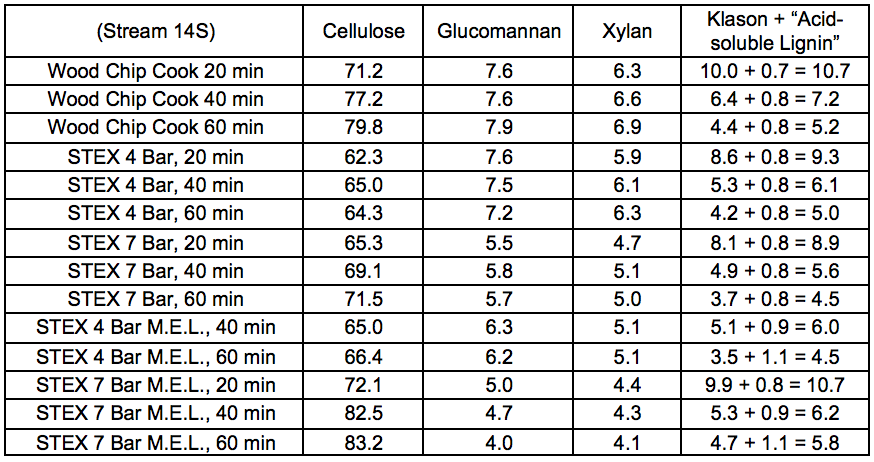
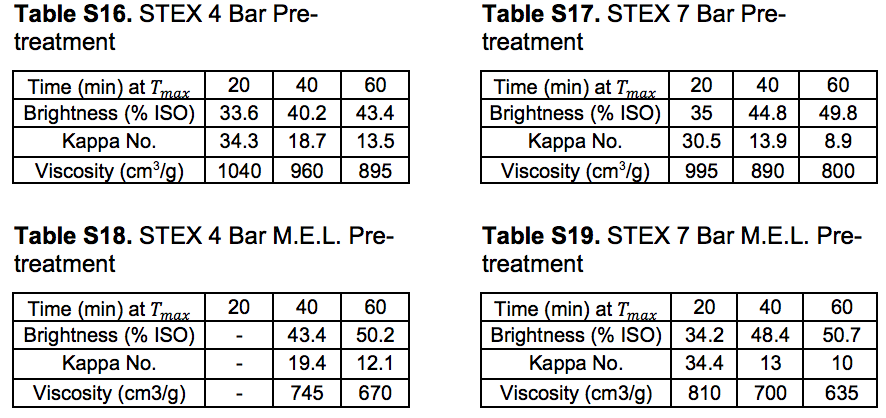 Table S21. Lignocellulosic Composition of Oxygen Delignified Pulps (wt.%)
Table S21. Lignocellulosic Composition of Oxygen Delignified Pulps (wt.%)
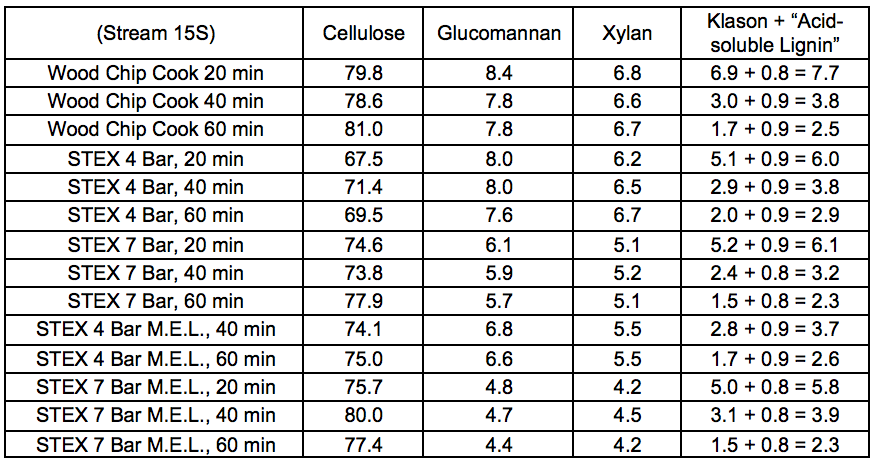
REFERENCES CITED (SUPPLEMENTARY MATERIAL)
Azhar, S., Henriksson, G., Theliander, H., and Lindström, M. (2015). “Extraction of hemicelluloses from fiberized spruce wood,” Carbohydrate Polymers 117, 19-24. DOI: 10.1016/j.carbpol.2014.09.050
Bylin, S., Olsson, C., Westman, G., and Theliander, H. (2014). “Solvation behavior of cellulose and xylan in the MIM/EMIMAc ionic liquid solvent system: Parameters for small scale solvation,” BioResources 9(1), 1038-1054. DOI: 10.15376/biores.9.1.1038-1054
Jedvert, K., Wang, Y., Saltberg, A., Henriksson, G., Lindström, M. E., and Theliander, H. (2012). ”Mild steam explosion: A way to activate wood for enzymatic treatment, chemical pulping and biorefinery processes,” Nordic Pulp and Paper Research Journal 27(5), 828-835. DOI: 10.3183/NPPRJ-2012-27-05-p828-835
Jedvert, K., Saltberg, A., and Theliander, H. (2013). “Mild steam explosion followed by kraft cooking and oxygen delignification of spruce (Picea abies),” Appita Journal 66(4), 322-330.
SCAN-C11:75 (1975). “Massa ISO-ljushet,” Scandinavian Pulp, Paper and Board Testing Committee, Stockholm, Sweden.
SCAN-C15:62 (1962). “Viskositet hos cellulosa i kopparetylendiamin lösning (CED) [Viscosity of the cellulose solution in kopparetylendiamin],” Scandinavian Pulp, Paper and Board Testing Committee, Stockholm, Sweden.
SCAN-C1:77 (1977). “Massa Kappatal,” Scandinavian Pulp, Paper and Board Testing Committee, Stockholm, Sweden.
SCAN-CM71:09 (2009). “Pulps – Carbohydrate content,” Scandinavian Pulp, Paper and Board Testing Committee, Stockholm, Sweden.
TAPPI T222 om-02 (2002). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI UM 250 (1991). “Acid-soluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
Westerberg, N., Sunner, H., Helander, M., Henriksson, G., Lawoko, M., and Rasmuson, A. (2012). “Separation of galactoglucomannans, lignin, and lignin-carbohydrate complexes from hot-water-extracted Norway spruce by cross-flow filtration and adsorption chromatography,” BioResources 7(4), 4501-4516. DOI: 10.15376/biores.7.4.4501-4516
DOI: 10.15376/biores.12.4.9152-9182