Abstract
Montmorillonite clay particles that had been prepared with an alklyl-ammonium surfactant were used to modify the moisture-sensitivity of bleached softwood kraft fibers through solvent exchange and adsorption methods. Moisture absorption and water uptake of the wood pulp fibers were significantly lower after the organo-nanoclay treatment. Thermal stability, surface energy, and surface morphology of the treated fibers were characterized using Thermogravimetric Analysis (TGA), Inverse Gas Chromatography (IGC), Scanning Electron Microscopy-Energy Dispersive X-ray Analysis (SEM-EDX), and Transmission Electron Microscopy (TEM) imaging. The Fourier Transform Infrared (FT-IR) spectral characteristics of the treated fibers were obtained to better understand the modified surface functional groups of the treated fibers. The treated bio-fibers had nano-scale surface roughness and a much reduced surface energy. The contact angle of water on the treated fiber mat was found to be higher than 160º. The thermal stability of the treated fibers was not affected by the modification.
Download PDF
Full Article
Adsorption of TOLUENE ONTO bleached eucalyptus pulp treated with Ultrasound
Iñaki Urruzola, Maria Angeles Andrés, Luis Serrano, and Jalel Labidi*
Bleached kraft semichemical eucalyptus pulp was used as raw material to adsorb an organic compound, toluene, from aqueous solution. The pulp was sonicated with different powers and different times to obtain smaller cellulose fibers. The adsorption capacity for toluene of sonicated fibers and bleached eucalyptus pulp was measured by ultraviolet spectroscopy. The absorption capacity for toluene was increased considerably when cellulose nanofibres were obtained. The adsorption capacity of bleached eucalyptus pulp was 36 μmol/g, while sonicated fibres at 30 W and 20 hours increased the adsorption by 47% and at 50 W and 20 h increased it by 67% compared with untreated fibres. Visual examination and optical microscopy were used to observe the reduction of fibers width and the dispersion increase. Contact angle measurements were used to analyze the variation of hydrophilic character of cellulose. Fourier transform infrared spectroscopy was used to study variations introduced by the ultrasound treatments on the chemical structure of the samples. The adsorption capacity studies showed that the treatment with ultrasound improved the retention capacity of the fibres, increasing considerably the adsorption capacity when the fiber width approached the nanoscale.
Keywords: Cellulose; Ultrasound; Adsorption; Toluene; Nanofibers
Contact information: Chemical and Environmental Engineering Department, University of the Basque Country, Plaza de Europa 1, 20018, Donostia-San Sebastián, Spain.
* Corresponding author: jalel.labidi@ehu.es
INTRODUCTION
Cellulose is one of the most important biopolymers that can be found in nature due to its natural character, biocompatibility, biodegradability, and low price. It is a polysaccharide composed exclusively of glucose molecules, is rigid, insoluble in water, and contains several hundred to several thousand units of β-glucose. It is the most abundant organic biomolecule, as it forms the bulk of terrestrial biomass (Fratzl 2003; Vincent 1999; Bidlack et al. 1992).
In recent years, the study of cellulose has been increased due to its high avail-ability; annually about 100 million tons of cellulose are produced by living plants around the world (Samir et al. 2005).
Cellulose has various applications in industry. One important aspect is the conversion of cellulose into ethanol for the production of alternative biofuels, which offers the potential for low environmental impacts (Andren et al. 1976). It is also used in the manufacture of explosives – the most famous of which is nitrocellulose, artificial silk, varnish, as well as thermal and acoustic insulation. The principal application of cellulose is the production of pulp and paper, using different bleached wood pulps obtained from different raw materials. Industrially, bleached cellulose pulp is obtained through two process stages, the pulping and bleaching. The objective of pulping is to remove the lignin to release cellulose fibers, thus separating the cellulose from the other components of wood by using mechanical or chemical processes. Moreover, lignin produces a brown discoloration in the final paper. The bleaching is carried out using different stages. Some of the main chemical reagents used are elemental chlorine (Cl2), chlorine dioxide (ClO2), and hydrogen peroxide (H2O2).
The adsorption capacity of cellulose is an aspect that has attracted considerable interest in recent years (Brochier et al. 2005; Bel-Hassen et al. 2008; Alila et al. 2005). This is because of the possibility of removal of organic matter by adsorption onto waste materials at low cost (Xu et al. 2007; Aloulou et al. 2004a-c; Bras et al. 1999). Several studies have been performed to evaluate the toluene retention capacity on cellulose, which demonstrates the ability of cellulose to adsorb aromatic compounds (Voronova and Zakharov 2009; Xing et al. 1994; da Silva Perez et al. 2004). Also, the ability of cellulose to fix metal ions by adsorption has been demonstrated (Chakravarty et al. 2008; Al-Ghouti et al. 2010; Gerente et al. 2000; Marshall and Campagne 1995). However, the characteristics of adsorption of native cellulose are not constant and vary depending on the origin of the cellulose and the preliminary treatments. Native cellulose has a relatively low adsorption capacity that can be increased by chemical functionalization of the fibres, where the adsorption capacity of modified cellulose fibres may be increased by as much as 10 times in comparison to the same fibres without any treatments. This can be achieved by the special structure of cellulose, introducing chemical groups that exhibit a high affinity for chemical species in aqueous solution such as acrylamide and acrylic acid to adsorb water, chitin, to adsorb heavy metals, or sorption of Cu2+ ions by cellulose graft copolymers (Zhou et al. 2004; Chauhan et al. 2000; Chauhan and Lal 2003). Numerous studies on this subject (Alila et al. 2011; Aloulou et al. 2006; Boufi and Belgacem 2006) have shown that the retention capacity can reach 300 to 600 mol/g substrate.
Different studies have shown that there are several factors that influence the adsorption capacity of cellulose. Two important factors are the hydrophobicity and their water solubilty of organic solute. In addition, the hydrodynamic volume, the shape of the molecule, and the interaction potential between the adsorbent and adsorbate are likely to play an important role (Alila and Boufi 2009).
The individualization of cellulose nanofibres from renewable sources has gained more attention in recent years because of their exceptional mechanical properties (high specific strength and modulus), large specific surface area, low coefficient of thermal expansion, high aspect ratio, environmental benefits, and low cost (Nishino et al. 2004). Suitable applications of cellulose nanofibres, such as reinforcement components in flexible display panels (Iwamoto et al. 2007), and oxygen-barrier layers (Fukuzum et al. 2009) have been proposed as well. Different steps of chemical treatments, such as mercerization, acetylating, hydrolysis, or organosolv pulping have been used for the elimination of non-cellulosic components and then mechanical treatments to obtain cellulose nanofibres.
Mechanical treatments such as high pressure homogenization and ultrasound techniques have been used to reduce the size of the cellulose fibers to the nano size scale, where the properties of the fibers vary considerably. Recently, the ultrasonic technique has been sufficiently described as an emerging method to reduce cellulose fibre size (Cheng et al. 2007, 2009, 2010). Ultrasound energy is transferred to cellulose chains through a process called cavitation, which refers to the formation, growth, and violent collapse of cavities in water. The energy provided by cavitation in this so-called sonochemistry is approximately 10 to 100 kJ/mol, which is within the hydrogen bond energy scale. Thus, the ultrasonic impact can gradually disintegrate the micron-sized cellulose fibres.
In this work, bleached eucalyptus pulp has been used as a raw material for the adsorption of toluene. The pulp has been submitted to a sonication process at different times and powers with the aim to increase the adsorption capacity of bleached pulp.
EXPERIMENTAL
Materials
Semi-chemical kraft bleached eucalyptus pulp, with a fiber length about 1 mm and fiber width about 20 μm, used in this work was kindly supplied by Papelera Guipuzcoana de Zicuñaga, S.A. from Hernani (Spain). Table 1 shows the composition of these fibres, which was determined according to standard methods (TAPPI Standards 2007) and published procedures (Rowell 1983). This pulp presented a low lignin content (0.2 %) and high holocellulose content (93.9%).
Table 1. Composition of the Raw Material
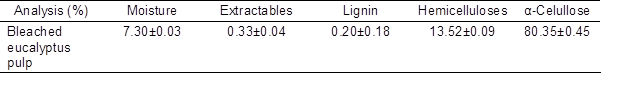
Sonication was carried out with a Bandelin Sonopuls ultrasonic homogeniser with the purpose of reducing the size of the fibers to nanoscale. Then 0.2 g of bleached eucalyptus pulp in 100 mL of distilled water were used with two different powers: 30 W and 50 W (20 to 25 KHz of frequency) and three time durations (5 h, 10 h, and 20 h) to study the effect of the treatment on the fibre size.Sonication of Bleached Eucalyptus Pulp
The fixed frequency was due to the ultrasonic homogeniser limitations, whereas the powers and times were selected in order to obtain the minimum fibre size. An optimized study will be carried out in future works to measure the energy consumption.
Visual Examination
The changes in dispersion of the bleached pulp suspension after ultrasonic treatment were observed trough visual examination of the pulp-water flask. For this purpose, 0.02 g of fibre were introduced in 10 mL distilled water.
Optical Microscopy
In order to observe the variation of fibre size of the eucalyptus bleached pulp microscopically, the samples were prepared by adding 0.05 grams of bleached eucalyptus pulp in 25 mL of distilled water to obtain a good dispersion of fibers in solution. Optical images of the bleached eucalyptus pulp fibers were taken, before and after sonication treatments, using an optical microscope (Nikon Eclipse E600) at appropriate magnifications (200 x, and 500 x).
Contact Angle Measurements
Contact angle measurements were also carried out with water in order to determine changes in the hydrophilic character of each sample, before and after the ultrasound treatment. These measurements were performed using a Dataphysics Contact-angle system OCA 20. Uniform bleached pulp pellets were used for this propose.
Fourier Transforms Infrared Spectroscopy (FT-IR)
The FTIR spectra were recorded on a Perkin-Elmer 16PC instrument, by direct transmittance with an MKII Golden Gate SPEACAC accessory in the range of 400 to 4000 cm−1 with a resolution of 8 cm−1 and 20 scans.
Batch Adsorption Studies
Solute adsorption experiments in batch mode were performed by adding toluene at 1×10-4 mol toluene/L concentration using a micro-syringe to a water solution containing 0.05 mg of bleached eucalyptus pulp before and after ultrasonic treatments. This low concentration permits the partial dissolution of toluene in water (solubility of toluene in water 0.052% at 25 ºC).
Toluene adsorption experiments were performed in a 50 mL flask with a contact time of 12 hours. Toluene concentration was determined at several times using a UV-visible spectrophotometer Jasco V-630 and wavelength of 208 nm.
RESULTS AND DISCUSSION
Visual Examination
Aqueous suspensions of the original fibre, before and after sonication treatment, at 30 W and 50 W during 5, 10, and 20 hours were placed in a flask to obtain pictures, such as that shown in Fig. 1. Bleached eucalyptus pulp without treatment was precipitated at the bottom of the glass bottle. A substantial increase in the dispersion of the fibre suspensions was observed after sonication treatment. The dispersion of the fibres increased substantially when the treatment time was increased. This dispersion was not very significant at 5 hours, but when the treatment increased to 10 hours, the dispersion increased and the dispersion was considerably higher at 20 hours. The spread with the different powers also had a significant effect, because increasing the power to 50 W resulted in a greater dispersion of the fibres. Treatment at this power level for the time period 20 hours resulted in the highest dispersion, and the material was converted into a highly viscous suspensions.
This study indicated that there was an improvement in the degree of fibrillation on the fibres, and more surface area on the fibres was exposed as the sonication output power increased.
Fig. 1. Dispersion state of the a) bleached eucalyptus pulp, b) 30 W 5 h, c) 30 W 10 h, d)30 W 20 h, e) 50 W 5 h, f) 50 W 10 h, and g) 50 W 20 h
Optical Microscopy
Images of fibres were obtained by light microscopy to analyze the variation of fibres size with a concrete power as a function of time.
Bleached eucalyptus pulp fibres before the ultrasound treatment had an average width of 10 to 12 microns, as shown in Fig. 2.
Fig. 2. Optical images of the bleached eucalyptus pulp fibres before sonication treatments
When ultrasound treatment is applied to bleached eucalyptus pulp, the reduction in size of the fibres can be attributed to the effect of acoustic cavitation of high frequency (20 to 25 kHz) ultrasound in the formation, expansion, and implosion of microbubbles in the aqueous solution. The violent collapse that occurs causes microjets and shock waves on the surface of cellulose fibres, causing erosion of the surface of the fibres and splitting along the axial direction. The impact of sonication can break relatively weak hydrogen bonds of the fibres. Thus, the ultrasonic treatment gradually disintegrates the cellulosic fibres, reducing their width to a few microns and even to nano scale (Tischer et al. 2010; Wang and Cheng 2009; Zhao et al.2007).
Figures 3 and 4 show the evolution of the fibres dimensions for the different treatments. It can be observed as the increase of time reduces the size of the fibres.
Fig. 3. Optical images of bleached eucalyptus fibres sonicated at 30W: a) 5h, b) 10 h, and c) 20 h
No significant decrease of size could be observed when 30 W power was applied for up to 10 hours. In Fig. 3-A it can be observed that there were no appreciable size changes of most of the fibres, but also that a few fibres had decreased in width by up to 5 μm. When the sonication time was 10 hours, a greater decrease in the fibres width became apparent. In Fig. 3-B, it can be seen that the solid material had been reduced in size to 1 micron, although many of the solids still had sizes approximately corresponding to a fibre width of 10 μm. Finally, a higher dispersion of the fibres was observed (Fig. 3-C) with a sonication time of 20 hours. The fibre width decreased considerably to an average width of 1 micron, but there were still fibres with a 10 μm width due to the low power used in sonication treatment.
Fig. 4. Optical images of bleached eucalyptus fibres sonicated at 50W: a) 5h, b) 10 h, and c) 20 h
With a sonication time of 5 hours at 50 W, the reduction of fibre width was greater than the treatment at the same time at 30 W power. In Fig. 4-A, the widths of most fibres had been reduced to around 5 μm. At the sonication time of 10 hours, there was an increase in the dispersion and a significant decrease of the fibres width (Fig. 4-B). The observed widths of fibres were around 2 μm, where the smaller fibres were 1 micron and the largest fibres were 3 μm. Finally, Fig. 4-C shows the highest decrease in the size of cellulose material and also obtained a considerable dispersion. The obtained material showed an average size of 0.58 microns, and it can be observed that there were a very large amount of smaller particles. With these results, it can be concluded that the sonication power and time are important aspects to reducing the size of the fibres, and high powers made it possible to obtain improved results, eventually yielding nanofibers.
Contact Angle Measurements
Because the hydroxyl groups of the monomers of cellulose, the hydrophilic character of this biopolymer is a known issue and has been confirmed in different works (Pasquini et al. 2006; Ly et al. 2010). These hydroxyl groups (OH) form hydrogen bonds with water, which helps the water absorption and makes the molecule hydrophilic.
Making the contact angle measurements, there is rapid absorption of the water drop, indicating a high degree of hydrophilic character in the bleaching of cellulose pulp. Analyzing the results of the contact angle before and after being treated with ultrasound, it follows that the mechanical method of ultrasound does not affect the hydrophilic character of cellulose, maintaining nearly constant contact angle with a value of about θ = 38.
Fourier Transform Infrared Spectroscopy (FT-IR)
FTIR spectroscopy is an appropriate technique because it helps to observe the variations introduced by different treatments on the chemical structure of the samples.
In Fig. 5, a FTIR spectra comparison of bleached eucalyptus pulp before and after ultrasonic treatments can be observed. The signals at 3325 cm-1, 2900 cm-1, and 2860 cm-1 are characteristic of stretching vibrations of OH and CH groups, respectively. The signal of 2900 cm-1 corresponds to CH3 groups, and the signal at 2860 cm-1 corresponds to CH2 groups. The peak at 1645 cm-1 can be attributed to the bending mode of the absorbed water in carbohydrates. The band at 1436 cm-1 corresponds to CH2 bending, and the one at 1213 cm-1 is originated from the OH in plane-bending cellulose (Sun et al. 2004a,b). The adsorption band at 1162 cm1 can be attributed to C-O antisymetric bridge stretching. Finally, the peak at 894 cm-1 is characteristic of β-glycosidic linkages between the glucose units (Buschle-Diller et al. 2005).
Fig. 5. FT-IR spectra of the (a) bleached eucalyptus pulp and bleached eucalyptus pulp fibers sonicated at 30W: (b) 5h, (c) 10h, (d) 20h, and sonicated at 50W: (e) 5h, (f) 10h, (g) 20h
No differences were found between the spectra of bleached cellulose pulp obtained under different ultrasonic output powers. This result suggests that the molecular structures of cellulose were unchanged in the case of ultrasonic treatment (Chen et al. 2011).
Batch Adsorption Studies
The variation of the sample concentration over time can be analyzed by an ultraviolet spectrophotometer. For this purpose, a calibration curve was prepared at 208 nm and the variation in absorbance was studied, obtaining different concentration values, based on the law of Beer-Lambert,
A = ε l C (1)
where A is the absorbance, ε is the molar absorptivity coefficient (L mol-1 cm-1), l is the optical path length (cm), and C is the concentration of the absorbing substance (mol L-1).
Ultrasound treatments reduce the eucalyptus fibres size to nanoscale, and when the fiber size is smaller, there is a greater retention capacity on the fibres. This may be due to the fact that the ultrasonic treatments do not change the chemical properties of the fibres. This reduction in size increases the contact with the contaminant, and the sorption rate and the retention capacity is higher.
It could be observed that the adsorption capacity was higher when longer sonication time was applied to the fibres. However, the adsorption capacity was only increased by 3% after 5 hours treatment. An increase of 10% was observed following 10 hours treatment, and finally the adsorption capacity increased by 47% with a treatment of 20 hours to values of 55 μmol/g to 12 hours (Fig. 6).
Fig. 6. Kinetic data of toluene adsorption onto fibres after treatment at sonication power of 30W
The adsorption capacity exhibited the same behaviour when using a sonication power of 50W, as observed before for sonication power of 30 W. As the times of sonication were increased, a smaller size of fibres was obtained, increasing the adsorption capacity. Thus, the adsorption capacity was increased by 17% in 5 hours (42 μmol/g), by 33% in 10 hours, and by 67% in 20 hours to values of 61 μmol/g to 12 hours in contact with toluene (Fig. 7).
In order to study the specific rate constant of solute adsorption on modified cellulose fibres, the pseudo-first and pseudo-second-order equations were used. The pseudo-first-order rate expression of Lagergreen (Periasamy and Namasvayam 1994) is given as,
(2)
where K1 (min-1) is the rate constant for the pseudo-first-order adsorption, and Qe and Qt are the amount of solute adsorbed at equilibrium and at time t, respectively.
Fig. 7. Kinetic data of toluene adsorption onto fibres after treatment at 50 W power
The pseudo-second-order kinetic model (McKay and Ho 1999) can be expressed as,
(3)
where K2 (g μmol−1 min−1) is the rate constant for the pseudo-second-order adsorption, and Qe and Qt are the amount of solute adsorbed at equilibrium and at time t, respectively. The plot of log(Qe −Qt) vs t did not display a linear behaviour over the whole range of contact time, indicating that the first-order model is only suitable over the initial stage of the adsorption process.
The straight lines in a plot of t/Qt versus t (Fig. 8) showed a good behaviour for the experimental data relative to the pseudo-second-order kinetic model for the different solutes. The results obtained from the pseudo-second-order model indicated that Qe values obtained were close to the experimental values (Table 2).
With these results, it can be concluded that the adsorption capacity and the retention rate of the fibre is increased with the decrease in the fibres size and best results are when the fibre size approaches to the nanoscale.
Fig. 8. Plot of pseudo second-order model to adsorption of toluene onto different fibres
Table 2. Kinetic Parameters of Toluene Adsorption onto Different Fibres Using Pseudo-First-Order and Pseudo-Second-Order Models
The impact of pH and temperature on the adsorption capacity has been examined. The adsorption capacity of toluene was increased with the temperature using fibers with the greater retention capacity (20 hours at 50 W) (Fig. 9). Moreover, an increase in the adsorption capacity of toluene has been observed only at acid pH using the fibers with the greater retention capacity (20 hours at 50 W) (Fig. 10).
Fig. 9. Kinetic data of toluene adsorption, varying temperature, onto bleached eucalyptus pulp after treatment with 20 h and 50 W
Fig. 10. Kinetic data of toluene adsorption, varying pH, onto bleached eucalyptus pulp after treatment with 20 h and 50 W
Finally to determine and to compare the ability of the sonicated fibres to trap organic compound from aqueous solutions, adsorption isotherm of Langmuir was established (Fig. 11 and Fig. 12).
Fig. 11. Langmuir isotherm of toluene onto sonicated fibres at 30 W
Fig. 12. Langmuir isotherm of toluene onto sonicated fibres at 50 W
The experiments were carried out with a contact time of 24 h, and it was observed that the adsorption grew rapidly to the value of concentration of 0.05 mmol L-1 and continued to grow more slowly until a solute concentration of about 0.1 mmol L-1 to finally reach the equilibrium concentration.
The experimental results were confirmed by the linear curve of Csol/Qads ratio as a function of Csol, where Qads, and Csol are, respectively, the adsorbed amount, the equilibrium solute concentration, and the maximum adsorbed amount per unit mass of the substrate. K is the Langmuir equilibrium constant related to the energy of adsorption. The experimental values of the Langmuir constants were determined using the linear form of the equation:
(4)
The adsorption equilibrium constant K, as well as the maximum concentration of the solute uptake are summarized in Table 3.
Table 3. Langmuir Constants of Toluene Adsorption onto Different Sonicated Fibres
The steep rise of the adsorption isotherm and the increment of the values of Langmuir constants denote the affinity of the substrate toward the efficient trapping of the dissolved organic compound in aqueous solutions when the fibres are treated with ultrasound.
CONCLUSIONS
- The mechanical treatment with ultrasound is effective for reducing the size of the fibres of the bleached eucalyptus pulp. This was confirmed with optical microscopy, by which it was observed that the fibre size reduction was significant when applying 50 W and 20 hours of ultrasound treatment. Thus, the greater the power of ultrasound and the longer the sonication time, the greater will be the reduction of the fibre size.
- Visual examination showed that with a longer sonication time, the dispersion was higher due to the size reduction of the fibre. The contact angle measurements showed that the hydrophilic character of the cellulose did not vary with mechanical treatments of ultrasound. Fourier transform infrared spectroscopy (FT-IR) showed that there was no change in the chemical structure of cellulose with ultrasound treatment.
- The adsorption capacity studies showed that the ultrasound treatment increases the retention capacity of toluene, considerably increasing the adsorption capacity when the fibre size approaches to nanoscale. The pseudo-second-order kinetic model was successfully applied to describe the progress of the adsorption.
ACKNOWLEDGMENTS
The authors would like to thank to Spanish Ministry of Economy and Competiveness for financially supporting this research (CTQ2010-19844-C02-02 and Juan de la Cierva contract JCI-2011-09399).
REFERENCES CITED
Al-Ghouti, M. A., Li, J., Salamh, Y., Al-Laqtah, N., Walker, G., and Ahmad, N. M. (2010). “Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent,” J. Hazard. Mater. 176, 510-520.
Alila, S., Boufi, S., Belgacem, M. N., and Beneventi, D. (2005). “Adsorption of a cationic surfactant onto cellulosic fibers. I. Surface charge effects,” Langmuir 21, 8106-8113.
Alila, S., and Boufi, S. (2009). “Removal of organic pollutants from water by modified cellulose fibres,” Ind. Crops Prod. 30, 93-104.
Alila, S., Aloulou, F., Thielemans, W., and Boufi, S. (2011). “Sorption potential of modified nanocrystals for the removal of aromatic organic pollutant from aqueous solution,” Ind. Crops Prod. 33, 350-357.
Aloulou, F., Boufi, S., Belgacem, M. N., and Gandini, A. (2004a). “Adsorption of cationic surfactants and subsequent adsolubilization of organic compounds onto cellulosic fibers,” Colloid Polym. Sci. 283, 344-350.
Aloulou, F., Boufi, S., and Beneventi, D. (2004b). “Adsorption of organic compound onto poyelectrolyte immobilized-surfactant aggregates onto cellulosic fibres,” J. Colloid Interface Sci.280, 350-358.
Aloulou, F., Boufi, S., and Chachouk, M. (2004c). “Adsorption of octadecyltrimethyl-ammonium chloride and adsolubilization onto cellulosic fibres,” Colloid Polym. Sci. 282, 699-707.
Aloulou, F., Boufi, S., and Labidi, J. (2006). “Modified cellulose fibres for adsorption of organic compound in aqueous solution,” Sep. Purif. Technol. 52, 332-342.
Andren, R., Mandels, N., and Modeiros, J. (1976). “Production of sugars from waste cellulose by enzymatic hydrolysis,” Process Biochem. 11, 2-11.
Bel-Hassen, R., Boufi, S., Brochier, M. C., Abdelmouleh, M., and Belgacem, M. N. (2008). “Adsorption of silane onto cellulose fibers. II. The effect of pH on silane hydrolysis, condensation and adsorption behaviour,” J. Appl. Polym. Sci. 108, 1958-1968.
Bidlack, J., Malone, M., and Benson, R. (1992). “Molecular structure and component integration of secondary cell walls in plants,” Proc. Oklahoma Acad. Sci. 72, 51-56.
Boufi, S., and Belgacem, M. N. (2006). “Modified cellulose fibres for adsorption of dissolved organic solutes,” Cellulose 13, 81-94.
Bras, I. P., Santos, L., and Alves, A. (1999). “Organochlorine pesticides removal by pinus bark sorption,” Env. Sci. Techn. 33, 631-634.
Brochier, M. C., Abdelmouleh, M., Boufi, S., Belgacem, M. N., and Gandini, A. (2005). “Silane adsorption onto cellulose fibers: Hydrolysis and condensation reactions,” J. Colloid Interface Sci. 289, 249-261.
Buschle-Diller, G., Inglesby, M. K., and Wua, Y. (2005). “Physicochemical properties of chemically and enzymatically modified cellulosic surfaces,” Colloids Surf. A 260, 63-70.
Chakravarty, S., Pimple, S., Chaturvedi, H. T., Singh, S., and Gupta, K. K. (2008). “Removal of copper from aqueous solution using newspaper pulp as an adsorbent,” J. Hazard. Mater.159, 396-403.
Chauhan, G. S., Mahajan, S., and Guleria, L. K. (2000). “Polymer from renewable resources: Sorption of Cu2+ ions by cellulose graft copolymers,” Desalination 16, 331-334.
Chauhan, G. S., and Lal, H. (2003). “Novel grafted cellulose-based hydrogel for water technologies,” Desalination 159, 131-138.
Cheng, Q., Wang, S., Rials, T., and Lee, S. (2007). “Physical and mechanical properties of polyvinyl alcohol and polypropylene composite materials reinforced with fibril aggregates isolated from regenerated cellulose fibers,” Cellulose 14, 593-602.
Cheng, Q., Wang, S., and Rials, T. G. (2009). “Poly (vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication,” Compos. Part A Appl. Sci. Manuf. 40, 218-224.
Cheng, Q., Wang, S., and Han, Q. (2010). “Novel process for isolating fibrils from cellulose fibers by high-intensity ultrasonication. II. Fibril characterization,” J. Appl. Polym. Sci. 115, 2756-2762.
Chen, W., Yu, H., Liu, Y., Chen, P., Zhang, M., and Hai, Y. (2011). “Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments,” Carbohydr. Polym. 83, 1804-1811.
Da Silva Perez, D., Ruggiero, R., Morais, L. C., Machado, A. E. H., and Mazeau, K. (2004). “Theoretical and experimental studies on the adsorption of aromatic compounds onto cellulose” Langmuir 20, 3151-3158.
Fratzl, P. (2003). “Cellulose and collagen: From fibres to tissues,” Curr. Opin. Colloid. Interface Sci. 8, 32-39.
Fukuzum, H., Saito, T., Iwata, T., Kumamoto, Y., and Isogai, A. (2009). “Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation,” Biomacromolecules 10, 162-165.
Gerente, C., Mesnil, P. C., Andres, Y., Thibault, J. F., and Le Cloirec, P. (2000). “Removal of metal ions from aqueous solution on low cost natural polysaccharides sorption mechanism approach,” React. Funct. Polym. 46, 135-144.
Iwamoto, S., Nakagaito, A. N., and Yano, H. (2007). “Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites,” Appl. Phys. A Mater. Sci. Process 89, 461-466.
Marshall, W. E., and Campagne, E. T. (1995). “Agricultural byproducts as adsorbent for metal ions in laboratory prepared solution and in manufacturing waste water,” J. Env. Sci. Health A30, 241-261.
Ly, E. B., Bras, J., Sadocco, P., Belgacem, M. N., Dufresne, A., and Thielemans, W. (2010). “Surface functionalization of cellulose by grafting oligoether chains,” Mater. Chem. Phys. 120,438-445.
McKay, G., and Ho, Y. (1999). “Pseudo-second order model for sorption processes,” Process. Biochem. 34, 451-465.
Nishino, T., Matsuda, I., and Hirao, K. (2004). “All-cellulose composite,” Macromolecules 37, 7683-7687.
Pasquini, D., Belgacem, M. N., Gandini, A., and Curvelo, A. A. (2006). “Surface esterification of cellulose fibers: Characterization by DRIFT and contact angle measurements,” J. Colloid Interface Sci. 295, 79-83.
Periasamy, K., and Namasvayam, C. (1994). “Process development for removal and recovery of cadmium from waste water by a low-cost adsorbent: adsorption rates and equilibrium studies,” Ind. Eng. Chem. Res. 33, 317-320.
Rowell, R. (1983). The Chemistry of Solid Wood, In: Advances in Chemistry Series, American Chemical Society, Washington, DC, pp. 70-72.
Samir, M., Alloin, F., and Dufresne, A. (2005). “Review of recent research into cellulosic whisker, ther propieties and ther application nanocomposites field,” Biomacromolecules 6, 612-626.
Sun, J. X., Sun, X. F., Zhao, H., and Sun, R. C. (2004). “Isolation and characterization of cellulose from sugarcane bagasse,” Polym. Degrad. Stab. 84, 331-339.
Sun, X. F., Sun, R. C., Fowler, P., and Baird, M. S. (2004). “Isolation and characterisation of cellulose obtained by a two-stage treatment with organosolv and cyanamide activated hydrogen peroxide from wheat straw,” Carbohydr. Polym. 55, 379-391.
TAPPI Standards (2007). TAPPI Test Methods, Atlanta, GA, USA.
Tischer, P., Sierakowski, M. R., Westfahl, H., and Tischer, C. A. (2010). “Nanostructural reorganization of bacterial cellulose by ultrasonic treatment,” Biomacromolecules 11, 1217-1224.
Vincent, J. F. (1999). “From cellulose to cell,” J. Exp. Biol. 202, 3263-3268.
Voronova M. I. and Zakharov A. G. (2009). “Adsorption of Phenol and Toluene from the Gas Phase and Aqueous Solutions on Cellulose” Russian Journal of Applied Chemistry. 82, 402-405.
Wang, S., and Cheng, Q. (2009). “A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication. Part 1: Process optimization,” J. Appl. Polym. Sci. 113, 1270-1275.
Xing, B., McGill, W. B., and Dudas, M. J. (1994). “Sorption of benzene, toluene, and o-xylene by collagen compared with non-protein organic sorbents,” Can. J. Soil. Sci. 74, 465-469.
Xu, M., Wang, Q., and Hao, Y. (2007). “Removal of organic carbon from wastepaper pulp effluent by lab-scale solar photo Fenton process,” J. Hazard. Mater. 148, 103-109.
Zhao, H. P., Feng, X. Q., and Gao, H. (2007). “Ultrasonic technique for extracting nanofibers from nature materials,” Appl. Phys. Lett. 90, 073112.
Zhou, D., Zhang, L., Zhou, J., and Guo, S. (2004). “Cellulose/chitin beads for adsorption of heavy metals in aqueous solution,” Water Res. 38, 2643-2650.
Article submitted: April 19, 2012; Peer review completed: May 28, 2012; Revised version received and accepted: July 10, 2012; Published: July 13, 2012.
