Abstract
The formation of oxalate during oxygen delignification causes a number of operational problems in pulp and paper mills. In this work, the oxygen delignification of wheat straw pulp was carried out under various conditions and the concentration of resulting oxalate in the effluent was determined. The experimental results show that the amount of oxalate in the effluent was closely related to the reaction conditions, specifically reaction temperature, oxygen pressure, and alkali charge. Raising reaction temperature and/or oxygen pressure could promoted oxalate formation. The oxalate concentration increased linearly with the consumption of alkali but logarithmically with reduction of kappa number. An empirical model for describing the oxalate formation in the oxygen delignification of wheat straw pulp was generated with a reasonably high correlation coefficient (R2=0.909), which can provide useful guidance for control of oxalate formation during oxygen delignification through adjustment of process parameters.
Download PDF
Full Article
Experimental Determination and Empirical Modeling of Oxalate Formation During Oxygen Delignification of Wheat Straw Kraft Pulp
Shilin Cao,a,* Xiaojuan Ma,a Xiaolin Luo,a Fang Huang,b Liulian Huang,a and Lihui Chen a
The formation of oxalate during oxygen delignification causes a number of operational problems in pulp and paper mills. In this work, the oxygen delignification of wheat straw pulp was carried out under various conditions and the concentration of resulting oxalate in the effluent was determined. The experimental results show that the amount of oxalate in the effluent was closely related to the reaction conditions, specifically reaction temperature, oxygen pressure, and alkali charge. Raising reaction temperature and/or oxygen pressure could promoted oxalate formation. The oxalate concentration increased linearly with the consumption of alkali but logarithmically with reduction of kappa number. An empirical model for describing the oxalate formation in the oxygen delignification of wheat straw pulp was generated with a reasonably high correlation coefficient (R2=0.909), which can provide useful guidance for control of oxalate formation during oxygen delignification through adjustment of process parameters.
Keyword: Wheat straw; Oxygen delignification; Oxalate formation; Empirical model
Contact information: a: College of Material Engineering, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; b: School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332, United States; *Corresponding author: scutcsl@gmail.com
INTRODUCTION
To remove lignin in brownstock pulps and further reduce the pollution load and toxicity of the effluents originating from subsequent bleaching stages, oxygen delignifi-cation (OD), an environmental friendly process, has been successfully applied in the paper industry (van Heiningen et al. 2003; Ghosh 2006). Due to its similarity in chemical composition to that of black liquor, the effluent produced in the OD process is used for washing the pulps from pulping process and then the filtrate (mixed with black liquor) is piped to evaporators for concentrating in an alkali recovery system.
Because of the presence of a significant amount of scale-forming ions (associated with calcium), e.g. carbonate and oxalate that are formed during OD, a difficult scale problem is created that reduces the heat exchange efficiency during black liquor evaporation (Elsander et al. 2000; Ulmgren and Rådeström 2001; He et al. 2003; DeMartini and Verrill 2005; Yu and Ni 2006; Verrill and DeMartini 2006). Therefore, it is important to have a better understanding of oxalate formation and its relationship to the process conditions in order for OD to be conducted in such a way as to minimize the concentration of these scaling-forming ions.
There are many studies on the origin, formation, and removal of oxalate during the bleaching processes. It has been reported that oxalate is formed mainly from the oxidation of lignin (Krasowski and Marton 1983). Hexenuronic groups in xylan can also give rise to oxalate (Nilvebrant and Reimann 1996; Sjöde et al. 2005; Sjöde et al. 2008). However, the amount of oxalic acid formed from carbohydrate bleaching is negligible (Elsander et al. 2000; Krasowski and Marton 1983).
The formation of oxalate is affected by many factors, such as wood species (softwood or hardwood) (Sjöde et al. 2005; He et al. 2008; Yu and Ni 2005), the types of bleaching agent (molecular oxygen, hydrogen peroxide, chlorine dioxide, peracetic acid and ozone) (Elsander et al. 2000), bleaching additives (MgSO4, Mg(OH)2) (He et al. 2003, 2008; Yu et al. 2004; Yu and Ni 2006, 2007), and bleaching parameters (chemical charges, pulp consistency, retention times, and temperatures) (He et al. 2003; Sjöde et al. 2005; He et al. 2008; Yu and Ni 2005; Yu and Ni 2007; Zhang et al. 2006).
Several control methods have been investigated to remove oxalate in the pulping and papermaking process through the introduction of some chemicals and enzymes. For example, chemical additives have been used for minimizing the scale deposit build-up (Shevchenko and Duggirala 2010), while oxalate oxidase and oxalate decarboxylase have been used for degradation of the oxalic acid (Nilvebrant et al. 2002; Cassland et al. 2010). However, there is still a lack of work reported on the model of oxalate formation during the OD process, although Li et al. (2012) recently reported on empirical models to describe the formation of oxalate during alkaline pulping processes.
The formation of oxalate in the OD process is caused by the oxidation of both lignin and hemicellulose components in the pulp phase and reaction liquid medium. Therefore, the further study of the formation of oxalate and its relationship to the major parameters in the OD process will be necessary, which can control the oxalate-related scaling problem in pulp mill operation. Moreover, the study on the formation of oxalate is also helpful in understanding the major pathways of oxalate formation during both the delignification of pulps and the reactions of the dissolved lignin or hemicelluloses in liquid phase.
In this work, we report an investigation of oxalate formation during the OD process of wheat straw kraft pulp. The goal was to characterize the relationship between the oxalate formation and the corresponding pulp kappa number (proportional to the residual lignin), from which an empirical model could be developed to predict the amount of oxalate in the OD effluent.
Such a model would be highly useful in developing a control strategy for optimizing the OD process, improving the product quality, while, at the same time, also controlling scaling during the oxygen delignification effluent and black liquor evaporation. That control strategy could also provide guidance in other bleaching, chemical pulping and biorefinery operations.
EXPERIMENTAL
Materials
Wheat straw kraft pulp with an initial kappa number of 10.9 and an intrinsic viscosity of 1045 mL/g was provided by a pulp mill in Shandong Province, China. The brownstock was washed and screened (mesh size = 0.15mm) to remove oversized, troublesome particles. All of the chemicals used in this study were of reagent grade and used as received.
Methods
Oxygen Delignification (OD)
The OD was carried out in a Parr reactor (Model 4843, Parr Instrument Company, Illinois, U.S.A.). Based on previous studies (Fu et al. 2012), the process variables shown in Table 1 were used. Pulp consistency was maintained at 10% in all experiments. At the end of the OD experiment, the effluent was collected and filtered through a filter paper (Whatman No. 4, Fisher Scientific, U.S.) to remove suspended particles.
Table 1. Oxygen Delignification Conditions Used for Wheat Straw Kraft Pulp
a based on o.d. pulp.
Analysis
The kappa number of pulp, the intrinsic viscosity of pulp, and the residual alkali content in the effluent were determined, respectively, by TAPPI standard methods T 236 om-06, SCAN-C 15:62, and T 624 cm-00.
Oxalate was quantified by the headspace gas chromatographic method, which was developed by Chai et al. (2006). A small volume (50 to 100 μL) of liquor sample was introduced into a sampling vial that contained 1.0 mL of 2 mol/L sulfuric acid. After removal of carbon dioxide (generated from carbonate in the acidic medium) by heating, the sample was mixed with a 0.5 mL of 0.02 mol/L potassium permanganate solution in a closed testing vial. At an elevated temperature (70 oC) the oxalate in the sample was rapidly converted to carbon dioxide by reacting with permanganate. The carbon dioxide in the headspace was measured by gas chromatography with a thermal conductive detector.
All experiments were duplicated and the reported values for oxalate concentration, pulp viscosity, kappa number and residual alkali content were the average of duplicate samples.
RESULTS AND DISCUSSION
Variation with Reaction Time and Alkali Charge
Previous studies (Li et al. 2012; Elsander et al. 2000; Yu and Ni 2005) have reported that the operational variables of alkaline pulping, including reaction temperature, alkali charge, sulfidity and reaction time, and the variables of peroxide bleaching, such as alkali charge and peroxide charge, affected the oxalate formation. In this study, the effects of similar variables on oxalate formation during OD of wheat straw kraft pulp were also investigated. The dependencies of oxalate formation with reaction time and alkali charge are shown in Figs. 1 and 2.
Figure 1 shows the amount of oxalate formed during OD at various alkali charges. The amount of oxalate increased rapidly with the reaction time in the early stage of OD, but it increased more slowly and leveled off after a reaction time of 45 min. Also, the amount of oxalate rose as the alkali charge was increased.
Fig. 1. Effect of reaction time and alkali charge on oxalate formation
Fig. 2. Effect of NaOH consumption on the oxalate formation
Earlier work has shown that lignin oxidation and carbohydrate degradation are two principal pathways for oxalate formation during bleaching (He et al. 2003). Oxalate formation is directly related to the delignification process: a rapid initial lignin removal, followed by a slower delignification phase (Gullichsen and Paulapuro1999; Agarwal et al. 1999; Tao et al. 2011). Lignin can be attacked directly by the oxygen-based radicals (superoxide anion and hydroxyl radicals, et al.) (Gierer et al. 1997, 2001) that firstly open aromatic rings to form muconic acid, which is then further oxidized to oxalate (Kempf and Dence 1980).
Figure 2 shows the variation of the oxalate formation with alkali consumption for different alkali charges. In each case, the oxalate concentration in the effluent increased linearly with the consumption of alkali. Also, the data for the oxalate formation with an alkali charge of 2.5% and 3.5% of NaOH fall onto the same curve, while the data for the oxalate formation with an alkali charge of 1.5% of NaOH formed a separate, but parallel, curve with the same slope but a different intercept. This behavior can be understood as follows. First, OD is the oxidation reaction of lignin derivatives by various oxygen species, and the alkali charge is not directly involved in the lignin oxidation reaction. Second, the oxalate formation mainly depended on the amount of oxygen-based reactants, such as superoxide anion radicals (O2·–) and hydroxyl radicals (HO·). That is, the phenolates react with oxygen at the beginning of the OD process, and the resulting phenoxyl radicals react with superoxide anion radicals or oxygen to produce quinone and muconic acid intermediates (Rovio et al. 2011), with the latter leading to oxalate formation (He et al. 2003, 2008). Third, the generation of these oxygen-based reactants are significantly influenced by the concentration of alkali, so that the concentration of oxalate in the effluent is dependent on the concentration of alkali.
The same alkali consumption resulted in the different amounts of oxalate formation when different alkali charges were used. For example, the consumption of NaOH for a low (1.5%) alkali charge and a high (2.5% or 3.5%) alkali charge was approximately 1.20% and 0.92%, respectively, and 80 mg/L oxalate was formed in the effluent. The final pH value is 9.95 after OD with 1.5% alkali charge but 11.71 and 11.90, respectively, with 2.5% and 3.5% alkali charge. Therefore, the pH is too low for the 1.5% alkaki dosage to ionize the phenolics, which retards the rate determining step, the formation of the phenolic radical anion. Without the phenolic radical anion, the subsequent reaction of ring opening to form muconic acid and its degradation to oxalic acid is inhibited. As a result, at the same rate at lower initial alkali charge, the alkali was insufficient to initiate the oxidation reaction. As a result, the oxalate concentration was lower.
Effect of Reaction Temperature and Oxygen Pressure on Oxalate Formation
The formation of oxalate versus the temperature of OD is plotted in Fig. 3a when the oxygen pressure and the alkali charge are held constant (0.7MPa and 2.5%, respectively). Data at reaction times of 30 min and 60 min are displayed to illustrate the relationship between oxalate concentration and reaction temperature. The concentration of oxalate in the effluent increased exponentially with increasing reaction temperature. Due to different reactivity of the lignin in kraft pulp with high kappa number, the rate of lignin dissolution during OD was generally expressed as two stages (the fast early stage and the slow late stage) (Olm and Teder 1981).
Fig. 3. Effect of reaction temperature (A) and oxygen pressure (B) on oxalate formation
To fully describe the whole delignification process, the kinetics of OD was therefore derived into two expressions with different activation energy and reaction order (Hsu and Hsieh 1988; Perng and Oloman 1994). Unlike two-phase kinetics of lignin dissolution during OD, however, in this study the similarity of the slopes of the two curves in Fig. 3a suggests that a single kinetic expression was enough to describe the formation rate of oxalate during OD. This is mainly contributed from low initial kappa number of wheat straw kraft pulp and a relatively narrow range of delignification (seen in Fig. 4), which probably stayed in a certain delignification stage.
The formation of oxalate versus the pressure of OD is plotted in Fig. 3b with the temperature of 100 oC and the alkali charge of 2.5%. Since oxygen pressure determines the concentration of dissolved oxygen in the reaction system, raising oxygen pressure accelerated the formation of oxalate, as shown in Fig. 3b. However, oxalate increase at the low pressures (0.3 to 0.7 MPa) were small, in contrast to the large increases observed at higher oxygen pressures. An exponential function can be expected to describe this relationship.
Empirical Modeling of Oxalate Formation
Although the oxalate formation is mainly attributed to the lignin oxidation (Krasowski and Marton 1983), the mathematical model of oxalate formation during OD has not been reported. Based on the previous studies, it is known that (1) hexenuronic acid concentration is unchanged in the OD process and (2) the oxalate concentration is closely correlated with the lignin content in oxygen delignified pulps (Krasowski and Marton 1983). Therefore, one of the effective means for predicting the amount of oxalate in the effluent is to identify the relationship between oxalate concentration and the kappa number of oxygen-delignified pulp.
Fig. 4. Relationship between oxalate concentration and kappa number
From Fig. 4, it is notable that oxalate concentration increased logarithmically with reduction in the kappa number. Therefore, the relationship between the oxalate concentration and the kappa number of wheat straw pulp can be expressed as,
(1)
where A and C are constants, and K is the kappa number.
In order to develop a kinetic model to describe this empirical observation, a general-order expression was used to describe the lignin removal (Schoon 1982; Iribarne and Schroeder 1997; Gendron et al. 2002; Violette 2003):
(2)
(3)
where L is the lignin content of pulp (%); t is the time of reaction (min); kL is the rate constant (min-1); k0 is the frequency factor ((min-1)·(g/L)-γ·(MPa)-β); EL is the activation energy (J/g·mol); T is the absolute temperature of reaction (K); R is the gas constant (8.314J/g·mol·K); α is the general order of the reaction; β is the parameter showing oxygen pressure dependence (unitless), and γ is the parameter showing alkali dependence (unitless); [OH-] is the initial alkali concentration (g/L); and [PO2] is the oxygen partial pressure (MPa).
The relationship between the kappa number of unbleached kraft pulp and the lignin content has been previously reported as (Tasman and Berzins 1959),
(4)
where L and K are, respectively, the lignin content and the kappa number of unbleached kraft pulp.
We assume there is the same relationship between the kappa number of oxygen-delignified pulp and the lignin content as the unbleached kraft pulp according to van Heiningen and Ji (2012), although the value is rough estimate and the relationship between kappa number and lignin (i.e. the slope) changes when the lignin is oxidized. Therefore, combining Equations (2) and (4) results in Eq. 5,
(5)
where
(6)
Integrating Eq. 5 and applying the initial conditions (at t=0, K=K0) gives the following equation,
(7)
where .
Taking the logarithm of Eq. 7 leads to the following expression:
(8)
After substituting Eq. 8 into Eq. 1, the expression for the oxalate concentration in the effluent is:
(9)
Equation 9 can be rearranged as,
(10)
where, ,
Of course, no oxalate has yet been formed at the beginning of the reaction ( ,
), so the integration constant
can be evaluated from these initial conditions to give:
(11)
Since , Eq. 11 can be rewritten as:
(12)
Equation 12 constitutes a general kinetic model developed for oxalate formation in the OD effluent of wheat straw. To use this model for prediction of oxalate concentration, the variables in Equation (12) need to be estimated. For a fixed ,
is constant because the reaction order and the activation energy in Eq. 12 are constants. Taking
and
, Eq. 12 becomes:
(13)
The constants in Eq. 13 were determined by the least square curve fitting method incorporated in the charting routines of Microsoft Excel (Lee 2012). The results are listed in Table 2.
Figure 5 compares the oxalate content measurement with the oxalate content calculated by Eq. 13. The model was found to be a reasonably good fit to the experi-mental data ( ), and it was judged to be reliable for the prediction of oxalate formation.
Fig. 5. Comparable result between predicted and measured oxalate content
Table 2. Estimate of the Parameters in Eq. 13 (R2 = 0.903)
Prediction of Pulp Kappa Number
During OD, exposure of kraft pulp to alkali and oxygen at elevated temperature results in an oxidative depolymerization of lignin and the solubilization of oxidized lignin fragments. The rate of lignin removal varies as a function of process conditions, including reaction temperature, reaction time, oxygen pressure, and alkali charge. The resultant effect of those process conditions can be represented by a lignin measurement parameter such as the kappa number.
A relationship between the kappa number and the oxalate concentration was observed from experimental data, as shown in Fig. 4 and a mathematical model was generated to describe this behavior, as shown in Eq. 13. Therefore, the kappa number of wheat straw pulp, under different OD conditions, can be predicted by the model described in Eq. 13. Table 3 presents the predicted kappa numbers of OD pulps at 30 min and 60 min as 7.2 and 6.6, respectively, when the wheat straw is oxygen-delignified under the conditions of 2.5% NaOH and 100 oC with 0.7 MPa oxygen applied. These predicted values compare reasonably well to the corresponding pulp kappa numbers 7.6 and 6.4 observed in the experiment.
Table 3. Kappa Number Prediction for Oxygen Delignified Wheat Straw Pulp
Oxygen Delignification Process Control Strategy
In assessing the entire OD process, the inorganic load, as well as the delignifi-cation selectivity, should be considered. Figure 6 shows the relationship between the viscosity and kappa number of pulps.
Fig. 6. Relationship between viscosity and kappa number
Fig. 7. Constant kappa number (7.2) curves at different combination of reaction temperature, caustic charge, and oxygen pressure (60 min)
It can be seen that there was a transition region of pulp viscosity at the kappa number range from ~7.2 to 7.8. However, the pulp viscosity significantly decreased if the kappa number of delignified pulp beyond this region. Based on these results, to obtain better OD selectivity with less oxalate formation, the target kappa number for the delignification of wheat straw kraft pulp should not be below ~ 7.2.
For control purposes, process parameters should be adjusted to achieve this target kappa number. Among oxygen delignification conditions, the reaction time of 60 min and the medium pulp consistency of 8.0 to 15.0% are usually chosen for economic and operating consideration. For oxygen delignification reactions at 10% pulp consistency for 60 min, the constant kappa number (= 7.2) at four different oxygen pressure levels (0.30, 0.50, 0.70, and 0.90 MPa) were obtained from Eqs. 1 and 13 by changing the reaction temperature and caustic charge (Fig. 7).
Any point on the curves represents the combination of a set of process parameters to achieve the same target pulp kappa number (7.2) at 10% pulp consistency for a 60-min reaction. Therefore, the reaction temperature, caustic charge, and oxygen pressure can be adjusted to obtain appropriate oxygen delignification conditions for better OD selectivity and less oxalate formation.
CONCLUSIONS
- The effects of process variables on the oxalate formation were investigated during OD of wheat straw pulp. It was found that raising the reaction temperature and/or oxygen pressure could promote oxalate formation. The oxalate concentration increased linearly with the consumption of alkali but logarithmically with reduction of kappa number.
- Based on the relation of the oxalate concentration and the kappa number of wheat straw pulp, an empirical model of oxalate formation was proposed. A reasonably high correlation coefficient (
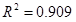 ) indicates that the model can be useful in predicting the oxalate formation from the kappa number. Therefore, the model can be used to optimize the OD process to reduce oxalate formation while retaining better preservation of the pulp viscosity.
) indicates that the model can be useful in predicting the oxalate formation from the kappa number. Therefore, the model can be used to optimize the OD process to reduce oxalate formation while retaining better preservation of the pulp viscosity.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (No. 31270638) and Major Science and Technology Projects of Fujian Province (No. 2008HZ0001), China. We also express sincere appreciation to Shandong Tralin Paper Co., Ltd. (Liaocheng, Shangdong Province, China) for providing wheat straw for this research.
REFERENCES CITED
Agarwal, S. B., Genco, J. M., Cole, B. J. W., and Miller, W. (1999). “Kinetics of oxygen delignification,” Journal of Pulp and Paper Science 25, 361-366.
Chai, X. S., Samp, J., Song, H. N., and Zhu, H. X. (2006). “Novel headspace gas chromatographic method for determination of oxalate in oxygen delignification liquor,” Journal of Chromatography A 1122, 209-214.
Cassland, P., Sjöde, A., Winestrand, S., Jönsson, L. J., and Nilvebrant, N. O. (2010). “Evaluation of oxalate decarboxylase and oxalate oxidase for industrial applications,” Applied Biochemistry and Biotechnology 161, 255-263.
DeMartini, N. A., and Verrill, C. L. (2005). “Evaporator fouling mitigation — Case studies,” 2005 TAPPI Engineering, Pulping and Environmental Conference, Philadelphia, PA, USA.
Elsander, A., Ek, M., and Gellerstedt, G. (2000). “Oxalic acid formation during ECF and TCF bleaching of kraft pulp,” Tappi Journal 83, 73-77.
Fu, L. M., Hu, H. C., Chai, X. S., and Luo, X. L. (2011). “An empirical model of methanol formation during oxygen delignification of wheat straw pulp,” China Pulp & Paper Industry 32, 13-16 (in Chinese).
Gendron, S., Bouchard, J., and Berry, R. (2002). “Optimal selection of operating conditions for an oxygen delignification tower,” Proceeding of TAPPI International Pulp Bleaching Conference, Portland, OR, USA, 121-137.
Ghosh, U. K. (2006). “Short sequence environment friendly bleaching of wheat straw pulp,” Journal of Scientific and Industrial Research 65, 68-71.
Gierer, J. (1997). “Formation and involvement of superoxide (O2·–/HO2·) and hydroxyl (OH·) radicals in TCF bleaching processes: A review,” Holzforschung 51(1), 34-46.
Gierer, J., Reitberger, T., Yang, E., and Yoon, B. (2001). “Formation and involvement of radicals in oxygen delignification studied by the autoxidation of lignin and carbohydrate model,” Journal of Wood Chemistry and Technology 21, 313-341.
Gullichsen, J., and Paulapuro, H. (1999). “Chemical pulping,” In: Papermaking Science and Technology, Book 6A, Gullichsen, J., and Fogelholm, C. J. (eds.), Fapet Oy, Helsinki, Finland, 144-145.
He, Z. B., Ni, Y. H., and Zhang, E. (2003). “Comparison of oxalate formation from spruce TMP: Conventional peroxide bleaching process versus the PM process,” Journal of Pulp and Paper Science 29(12), 391-394.
He, Z. B., Yang, Y., and Ni, Y. H. (2008). “Comparison of oxalate formation in peroxide bleaching of softwood and hardwood mechanical pulps,” Journal of Pulp and Paper Science 34, 153-160.
Hsu, C. L., and Hsieh, J. S. (1988). “Reaction kinetics in oxygen bleaching,” AIChE Journal 34(1), 116-122.
Iribarne, J., and Schroeder, L. R. (1997). “High pressure oxygen delignification of kraft pulps,” Tappi Journal 80, 241-250.
Kempf, A. W., and Dence, C. W. (1980). “The reactions of hardwood lignin model compounds with alkaline hydrogen peroxide,” In: Chemistry of Delignification with Oxygen, Ozone and Peroxides, Uni Publishers, Tokyo, 207-216.
Krasowski, J. A., and Marton, J. (1983). “The formation of oxalic acid during bleaching of kraft pulp,” Journal of Wood Chemistry and Technology 3, 445-458.
Lee, W. (2012). “Curve fitting in Microsoft Excel,” http://www.csupomona.edu/~seskandari/documents/Curve_Fitting_William_Lee.pdf (accessed December 20, 2012).
Li, H. L., Chai, X. S., and DeMartini, N. (2012). “Oxalate release and formation during alkaline pulping,” Journal of Wood Chemistry and Technology 32, 187-197.
Nilvebrant, N. O., and Reimann, A. (1996). “Xylan as a source for oxalic acid during ozone bleaching,” Proceedings of 4th European Workshop on Lignocellulosics and Pulp, Streza, Italy, 485-491.
Nilvebrant, N. O., Reimann, A., de Sousa, F., Cassland, P., Larsson, S., Hong, F., and Jönsson, L. J. (2002). “Enzymatic degradation of oxalic acid for prevention of scaling,” Progress in Biotechnology 21, 231-238.
Olm, L., and Teder A. (1981). “Extended delignification by combination of modified kraft pulping and oxygen bleaching,” Paperi ja Puu 63(4a), 315-326.
Perng, Y. S., and Oloman, C. W. (1994). “Kinetics of oxygen bleaching mediated by electrochemically generated ferricyanide,” Tappi Journal 77(7), 115-124.
Rovio, S., Kuitunen, S., Ohra-aho, T., Alakurtti, S., Kalliola, A., and Tamminen, T. (2011). “Lignin oxidation mechanisms under oxygen delignification conditions. Part 2. Advanced methods for the detailed characterization of lignin oxidation mechanisms,” Holzforschung 65, 575-585.
Schoon, N. H. (1982). “Interpretation of rate equations from kinetic studies of wood pulping and bleaching,” Svensk Papperstidning 85, 185-193.
Shevchenko, S. M., and Duggirala, P. Y. (2010). “Deposit management for the bleach plant,” IPPTA Journal 22, 135-140.
Sjöde, A., Jönsson, L. J., and Nilvebrant, N. O. (2005). “Oxalic acid in bleaching processes – formation and control,” Appita Annual Conference, 13th ISWFPC 2, 303-309.
Sjöde, A., Winestrand, S., Nilvebrant, N. O., and Jönsson, L. J. (2008). “Enzyme-based control of oxalic acid in the pulp and paper industry,” Enzyme and Microbial Technology 43, 78-83.
Tao, L., Genco, J. M., Cole, B. J. W., and Fort, R. C. (2011). “Selectivity of oxygen delignification for southern softwood kraft pulps with high lignin content,” TAPPI Journal 8, 29-38.
Tasman, J. E. and Berzins, V. (1959). “The permanganate consumption of pulp materials: IV. The kappa correction coefficient,” Pulp and Paper Canada 60, 231-235.
Ulmgren, P., and Rådeström, R. (2001). “Deposition of sodium oxalate in the black liquor evaporation,” International Chemical Recovery Conference, Montreal, Canada, 169-175.
van Heiningen, A., Krothapalli, D., Genco, J., and Justason, A. (2003). “A chemical reactor analysis of industrial oxygen delignification,” Pulp & Paper Canada 104, 331-336.
van Heiningen, A., and Ji, Y. (2012). “Southern pine oxygen delignified pulps produced in a Berty throughflow reactor: How to obtain the highest degree of delignification while maintaining pulp yield and quality,” TAPPI Journal 11(3), 9-18.
Verrill, C. L., and DeMartini, N. (2006). “Evaporator boilout guidelines,” Proceedings to 2006 TAPPI Engineering Pulping and Environmental Conference, Atlanta, GA.
Violette, S. M. (2003). “Oxygen delignification kinetics and selectivity improvement,” Ph.D. thesis, University of Maine, Orono, ME, USA, 12-14.
Yu, L., and Ni, Y. H. (2005). “Oxalate formation during peroxide bleaching of mechanical pulp,” Appita Journal 58, 138-142.
Yu, L., Rae, M., and Ni, Y. H. (2004). “Formation of oxalate from the Mg(OH)2-based peroxide bleaching of mechanical pulp,” Journal of Wood Chemistry and Technology 24, 341-355.
Yu, L., and Ni, Y. H. (2006). “Decreasing calcium oxalate scaling by partial substitution of Mg(OH)2 for NaOH in the peroxide bleaching of mechanical pulps,” TAPPI Journal 5, 9-12.
Yu, L., and Ni, Y. H. (2007). “Partition of soluble and precipitated oxalate and its impact on decreasing oxalate-related scaling during peroxide bleaching of mechanical pulp,” Pulp and Paper Canada 10, 39-43.
Zhang, J. X., Yu, L., Ni, Y. H., Zhou, Y., and Joliette, D. (2006). “Calcium oxalate related scaling in a BCTMP line,” Pulp Paper Canada 107, 52-55.
Article submitted: March 20, 2013; Peer review completed: April 29, 2013; Revised version received and accepted: October 9, 2013; Published: October 24, 2013.
