Abstract
This research evaluated a sequential process of thermal-alkali pretreatment, enzymatic hydrolysis, and anaerobic co-digestion applied on a mixture of corn straw (CS) and cattle manure (CM). The results showed that the optimal conditions of thermo-alkaline pretreatment were a Ca(OH)2 dosage of 1.5% and temperature of 120 °C. The optimal conditions of enzymatic hydrolysis in terms of cellulase loading, operational time, and protease loading were determined. Co-digestion with enzymes and thermal-alkali pretreatment achieved the highest methane yield of 0.41 m3 kg-1-VS from the liquid of hydrolysates. An maximum applicable organic loading rate (OLR) of 13.7 kg-SCOD m-3 d-1 was found with a soluble chemical oxygen demand (SCOD) removal of 96.4% in an expanded granular sludge blanket (EGSB) reactor. The optimization of conditions could lead to the industrial-scale treatment of organic solids with a high energy yield and efficiency.
Download PDF
Full Article
Thermal-alkali and Enzymes for Efficient Biomethane Production from Co-digestion of Corn straw and Cattle Manure
Ye Yuan,a,b,c,d Aiqin Bian,a,e Lulu Zhang,c Zhengliang Chen,c Fei Zhou,c Fan Ye,a Tianlu Jin,a Mei Pan,a,b Tianming Chen,a,b Jinlong Yan,a,b Aijie Wang,a,d Zhaoxia Li,a,b,e,* and Cheng Ding a,b,e,*
This research evaluated a sequential process of thermal-alkali pretreatment, enzymatic hydrolysis, and anaerobic co-digestion applied on a mixture of corn straw (CS) and cattle manure (CM). The results showed that the optimal conditions of thermo-alkaline pretreatment were a Ca(OH)2 dosage of 1.5% and temperature of 120 °C. The optimal conditions of enzymatic hydrolysis in terms of cellulase loading, operational time, and protease loading were determined. Co-digestion with enzymes and thermal-alkali pretreatment achieved the highest methane yield of 0.41 m3 kg-1-VS from the liquid of hydrolysates. An maximum applicable organic loading rate (OLR) of 13.7 kg-SCOD m-3 d-1 was found with a soluble chemical oxygen demand (SCOD) removal of 96.4% in an expanded granular sludge blanket (EGSB) reactor. The optimization of conditions could lead to the industrial-scale treatment of organic solids with a high energy yield and efficiency.
Keywords: Thermal-alkali pretreatment; Enzymatic hydrolysis; Anaerobic co-digestion; Methane production
Contact information: a: School of Environmental Science and Engineering, Yancheng Institute of Technology, Yancheng 224003, P. R. China; b: Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051, P. R. China; c: Jiangsu Keyida Environmental Science and Technology Co. LTD., Yancheng 224007, P. R. China; d: Key Laboratory of Environmental Biotechnology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, P. R. China; e: School of Environmental Science and Engineering, Jiangsu University, Zhenjiang 212013, P. R. China;
* Corresponding authors: lzxdzc@163.com; ycdingc@163.com
INTRODUCTION
Renewable energy has received worldwide attention because of the growing energy needs and increasing environmental pollution (Ding et al. 2012; Wang et al. 2015; Hassan et al. 2017; Yuan et al. 2019). Methane as a high-energy fuel (39829 kJ/m3) can be bio-generated from the anaerobic digestion (AD) of agricultural residues and livestock manure, such as lignocellulosic-rich corn straw and nutrient-rich cattle manure (Song and Zhang 2015; Wei et al. 2015; Yang et al. 2017). Agricultural residues are treated by various methods for production of energy and animal feed, but the utilization rate is less than 50% (Yuan et al. 2015; Tsapekos et al. 2017). Livestock manure is often used as a fertilizer for agricultural fields (Yuan et al. 2015; Haase et al. 2017; Tsapekos et al. 2017). However, the improper disposal of biowaste is a misuse of resources and source of pollution (Song et al. 2018). The AD process offers a potential way of converting biowaste into biomethane, which meets growing energy needs and reduces environmental concerns (Zhang et al. 2015; Yuan et al. 2019).
Anaerobic co-digestion (AcoD) of agricultural residues with livestock manure has attracted increasing attention because of its technological and economic benefits (Mehryar et al. 2017; Neshat et al. 2017). The AcoD process regulates nutrient balance, carbon to nitrogen ratio, and buffering ability, eventually improving the biomethane yield (Wei et al. 2015; Awais et al. 2018). Methane production from the AcoD of plant residues with animal manure is 0.5 to 3 times that from the AD of a single feedstock (Song and Zhang 2015). The intensive farms, which are usually surrounded by crop fields, can offer sufficient raw materials for the AcoD process (Wang et al. 2018). However, hydrolysis is a slow process in the AcoD of plant and livestock wastes. Lignin and crude protein are difficult for fermentative bacteria to utilize directly during the hydrolysis process, due to their complex and recalcitrant structures. Pretreatments, which can include mechanical breaking and physicochemical methods, have been investigated for promoting hydrolysis (Song and Zhang 2015; Zhang et al. 2016; Yang et al. 2017). Mechanical pretreatments such as grinding and extrusion are an efficient way to increase the surface area and decrease the crystallinity of lignocellulosic biomass (Kalamaras and Kotsopoulos 2014). Physicochemical pretreatment opens the chemical bonds between lignin and other macromolecular organic compounds (such as cellulose, hemicellulose, and protein) in raw materials (Krishania et al. 2013).
A combined process of enzymatic hydrolysis and liquid AD is a prospective way to obtain bioenergy from biowastes (Wang et al. 2015; Yuan et al. 2019). After physicochemical pretreatment, the remaining organic solids can be accessed by specific hydrolytic enzymes, producing a large amount of saccharides and other organics from cellulose and protein into liquid hydrolysates (Wang et al. 2015; Yuan et al. 2019). For example, Wang et al. (2015) introduced an integrated process of enzymatic hydrolysis and liquid AD for the methanization of brewers’ spent grain, achieving double the methane production compared with direct AD of raw material. Nkemka and Murto (2013) conducted AD of liquid hydrolysates from crop stalk after pre-hydrolysis in an upflow anaerobic sludge bed reactor, which is used for organic wastewater treatment. Obata et al. (2015) found that chemical-enzyme treatment showed a better performance on the hydrolysis of seaweed when compared with chemical only treatment. Jin et al. (2016) reported that the reducing sugar yield of catalpa sawdust was increased by 1185.7% under the optimal condition of thermal-Ca(OH)2 pretreatment and enzymatic hydrolysis. In summary, previous research has concentrated on applying enzymatic hydrolysis to a single feedstock, such as plant residues or animal manure, for bioenergy production (Cekmecelioglu and Uncu 2013; Odnell et al. 2016; Abada et al. 2018). However, the application of enzymatic hydrolysis to a mixed feedstock for biomethane production has not been reported.
The aim of this study was to evaluate a sequential process of thermal-alkali pretreatment, enzymatic hydrolysis, and anaerobic co-digestion applied on the mixture of corn straw (CS) and cattle manure (CM). First, the optimal conditions of thermal-alkali pretreatment and enzymatic hydrolysis were examined. The effects of thermal-alkali pretreatment and enzymatic hydrolysis on the methane potential (for characterizing a substrate’s influence on the anaerobic digestion process) were evaluated by a series of tests. The liquid hydrolysates with different organic loading rate (OLR) were continuously fed into an expanded granular sludge blanket (EGSB) reactor to examine the methane yield.
EXPERIMENTAL
Raw Materials
The CS was collected from a corn field located in Yancheng, Jiangsu province, China. The CS was oven-dried at 105 °C until it reached a constant weight and then chopped into approximately 2-mm-long pieces. The fresh CM was collected from a livestock farm near the corn field. The CM was stored in plastic bucket at -18 °C. The characterizations of raw CS and CM are shown in Table 1.
Table 1. Characterization of Raw CS and CM
Thermal-alkali Pretreatment
Thermal-Ca(OH)2 pretreatment was performed on the mixtures of CS and CM. In a previous study, the animal manure and straw residues were digested at a mass mixing ratio of 5:5, which was determined as the appropriate mixture for anaerobic co-digestion (Li et al. 2014). In this study, 10 kg mixtures of CS and CM (1:1) were placed in a stainless steel tank with a working volume of 30 L and stirred at a constant speed (n = 40 rpm) by speed anchor type mixer. The tank was equipped with a pH probe and heating system. In this process, a series of tests were carried out (Fig. 1). The temperature was kept at 60, 90, 120, and 150 °C, and the time maintained for 3 h. The additions of Ca(OH)2 content were 0.5, 1.0, 1.5, and 2.0% of the water of the mixtures. A control group without any pretreatment was established to compare the experimental results of the thermal-Ca(OH)2 pretreatment. After the thermal-Ca(OH)2pretreatment, the pretreated mixtures under optimum temperature and time conditions were stored and then used for enzymatic hydrolysis. The changes of total chemical oxygen demand (TCOD), soluble chemical oxygen demand (SCOD), solubilization, cellulose, hemicellulose, and protein content after the thermal-alkali pretreatment under different conditions are shown in Table 2.
Enzymatic Hydrolysis
Sufficient concentrations of applied alkali break down cellulose and crude protein crystallinity and hydrolyze the hemicellulose. Cellulose and protein are the main macromolecular organics in pretreated mixtures, and they are difficult to degrade via AD. Thus, two kinds of enzymes (Cellucast® and Alcalase® provided by Novozymes (China) Biotechnology Co., Ltd, Tianjin) were used for the enzymatic hydrolysis after thermal-alkali pretreatment.
Table 2. Influence of Different Thermo-alkali Pretreatment Conditions on Mixed Substances
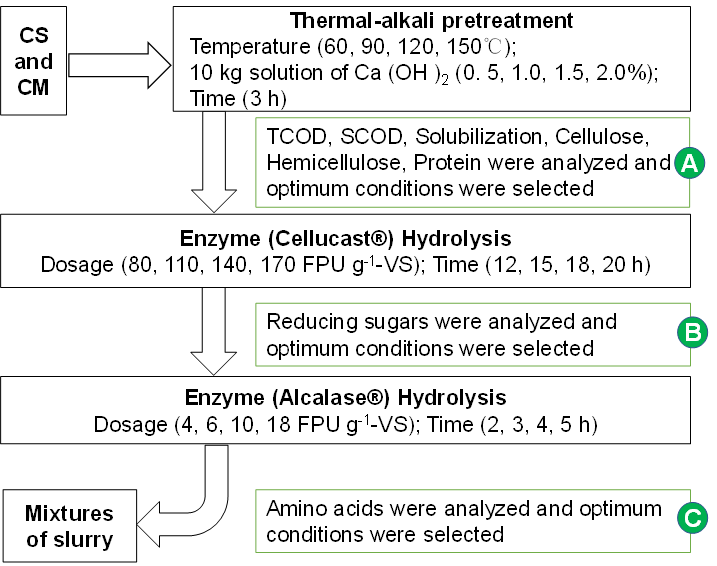
Fig. 1. Processing flow of corn straw (CS) and cattle manure (CM) mixtures under different treatment conditions
The working conditions of Cellucast® and Alcalase® were pH of 5.5 to 6.5, temperature of 50 °C to 60 °C and pH of 8.5 to 9.5, temperature of 55 °C to 80 °C, respectively. Enzymatic hydrolysis was conducted in the stainless steel tank with working volume of 25 L and continuous stirring (n = 40 rpm). The tank was equipped with a pH probe and heating system. Cellulase and protease hydrolysis were conducted under the following conditions (Fig. 1): enzyme loading of 80, 110, 140, 170 FPU g-1-VS and 4, 6, 10, 18 FPU g-1-VS, respectively, enzyme time of 12, 15, 18, 20 h and 2, 3, 4, 5 h, respectively. The pH was adjusted by adding HCl or NaOH. After the enzymatic hydrolysis, the liquid and solid fractions were separated using a multifilament filter cloth (Yongning model no: PP2400 Zhejiang, China). The samples for subsequent tests were taken after each processing step and stored at 4 °C (Fig. 2).
Anaerobic Digestion Tests
Batch tests of methane potential
The batch tests of methane potential were performed on the fractions of 1, 2, 3, 4, 4-1 and 4-2 from the process steps shown in Fig. 2. Each fraction was conducted in a glass bottle with a total volume of 2.0 L and working volume of 1.0 L. The inoculum used in the batch tests was obtained from a pilot-scale digester located in Yancheng, Jiangsu province, China. The TS of the inoculum was 6.9%, where 71.3% was the VS. The substrate to inoculum ratio was 1 to 2, in terms of VS. The six bottles were placed in a shaking water bath. The shaker maintained a mesophilic temperature of 35 ± 2 °C at a speed of 60 rpm. The tests were terminated when no biogas production was detected. All treatments were repeated three times.
Methane production in EGSB reactor
The liquid hydrolysate (4-1) for producing methane was examined in a lab-scale EGSB reactor under a continuous mode. The plexiglass-made EGSB reactor had a diameter of 60 mm, height of 120 cm, total volume of 3.4 L, and working volume of 2.0 L. The operational temperature was maintained at 35 ± 1 °C by an automatic thermostat (Shinko, model PCD-33A, Osaka, Japan). A gas-washing device installed at the column top was used to collect the gas. The anaerobic granular sludge used for inoculum was taken from a full-scale upflow anaerobic sludge blanket that treated food processing wastewater (Yancheng, Jiangsu province, China). The granular sludge was stored at 35 ± 2 °C until fully degassed. The EGSB reactor was initially inoculated with 1.5 L anaerobic granular sludge with biomass VS of 3.87 g L-1 (VS/TS = 0.93). A peristaltic pump (Longer, model BT100-2J, Baoding, China) introduced constant liquid hydrolysates into the reactor at the column bottom. The hydraulic retention times (HRT) was reduced from 16 d to 1 d, which corresponded to an increase in OLR of liquid hydrolysates from 2.18 kg SCOD m-3 d-1 to 35.21 kg SCOD m-3 d-1, as displayed in Table 3. The composition and volume of the gas and other biochemistry parameters such as NH4+-N, SCOD, and volatile fatty acids (VFAs) were measured.
Fig. 2. Schematic diagram and material flow for anaerobic digestion (AD) from pretreated corn straw (CS) and cattle manure (CM) mixtures
Analytical Methods
The pH was measured using a pH meter (Mettler-Toledo, model FE20, Shanghai, China). The TS, VS, TN, NH4+-N, hydrogen, and sulfur were analyzed according to the standard methods (APHA 2005). The TC content was determined by TOC analyzer (Elementar, model Liqui TOC II, Hanau, Germany). The TCOD and SCOD concentrations were determined as previously reported (Cho et al. 2013; Nkemka and Murto 2013). The chemical composition of the CS and CM was determined as described by Van Soest et al. (1991) using a raw fiber determination extraction system (Lai-Heng, model L-807, Beijing, China). The contents of cellulose, hemicellulose, and lignin were analyzed by fiber determination analyzer (Model CXC-06, Shanghai, China). The amount of reducing sugars and amino acids were measured by a HPLC system (Agilent Infinity 1260, Santa Clara, CA, USA). The methane contents in the biogas were analyzed using a gas chromatography (Agilent, model 6890, Santa Clara, CA, USA). The contents of the VFAs were determined by a gas chromatograph (Shimadzu, model GC-2010 Plus, Kyoto, Japan). Liquid samples were centrifuged at 10000 rpm for 10 min at room temperature and filtered through a 0.45 μm fiberglass filter for VFAs analysis.
The solubilization efficiency of the thermal-alkali pretreatment was calculated by Eq. 1,
Solubilization (%) = (SCODaf – SCODbe) / (TCODbe – SCODbe) × 100 (1)
where SCODaf was the SCOD after the thermal-alkali pretreatment, SCODbe is the SCOD before the thermal-alkali pretreatment, and TCODbe is the TCOD before the thermal-alkali pretreatment.
The SCOD removal was calculated by Eq. 2,
SCOD removal (%) = (SCODin – SCODef) / SCODin × 100 (2)
where SCODin and SCODef were the influent and effluent SCOD values, respectively, during the anaerobic digestion process.
RESULTS AND DISCUSSION
Optimization of Thermal-alkali Pretreatment
The main objective of the pretreatment was to break the complex structures of lignocellulosic materials, to solubilize lignocellulosics, and to enhance subsequent enzymatic hydrolysis. In this research, Ca(OH)2 was selected for thermal-alkali pretreatment due to its low cost and excellent performance. The results after thermal-alkali pretreatment are presented in Table 2. The initial TCOD and SCOD of the unpretreated raw mixtures were 20,300 mg L-1 and 4010 mg L-1, respectively. In all pretreatments to which mixtures were applied, the SCOD was increased noticeably from 4010 mg L-1 to 10080 mg L-1 (Table 2). The solubilization increased with increasing Ca(OH)2 dosage, but when the Ca(OH)2 dosage reached 1.5%, the solubilization did not significantly increase when further increasing the Ca(OH)2 dosage. Moreover, thermal-Ca(OH)2 pretreatment effectively reduced cellulose (from 3.94% to 35.86%), hemicellulose (from 16.55% to 56.26%), and lignin (from 3.59% to 27.93%). Hemicellulose was more easily solubilized than cellulose and lignin, possibly because the hemicellulose in the mixtures reacted more strongly with thermal-alkali pretreatment. The results are similar to previous research in which the reductions of cellulose, hemicellulose, and lignin of catalpa sawdust were 6.3%, 33.4%, and 5.0% after thermal-Ca(OH)2 pretreatment, respectively (Jin et al. 2016). Furthermore, the reductions of cellulose, hemicellulose, and lignin increased little when the dosage of Ca(OH)2 was increased from 1.5% to 2.0%, which were similar to the varying trends of solubilization.
Temperature is also an important factor when investigating thermal-alkali pretreatment. More SCOD was produced at higher temperature, and the reduction of cellulose, hemicellulose, and lignin increased with increasing temperature. The maximum content of SCOD (10082 mg L-1) and the maximum reductions of cellulose, hemicellulose, and lignin (35.86%, 56.26%, and 27.93%, respectively) were achieved at 150 °C. However, the content of SCOD and the reductions of cellulose, hemicellulose, and lignin changed slightly from 120 °C to 150 °C. Considering the above results and the overall cost of the process, the optimal parameters were 1.5% Ca(OH)2 and 120 °C.
Optimization of Enzymatic Hydrolysis
Hydrolysis is the rate-determining step during anaerobic digestion. Enzymatic hydrolysis is essential for the hydrolysis of complex organic matters into simple compounds. Cellulase and protease may be effective in enhancing hydrolysis. Figure 3 shows the effects of cellulase loading and hydrolysis time on the reducing sugar yield of the thermal-alkali pretreated mixtures with optimum conditions. The conditions chosen for the cellulase hydrolysis were based on reducing sugar yield. The reducing sugar yield changed remarkably with cellulase dosage and hydrolysis time. The highest reducing sugar yield (511.4 mg g-1) occurred at 20 h and 170 FPU g-1-VS. Jin et al. (2016) found that the maximum reducing sugar yield of 518.1 mg g-1 was achieved with 150 FPU g-1 dry biomass cellulase for 96 h, which was consistent with the results in this research. At 12 h and 15 h, the reducing sugar yield increased with an increase in cellulase loading, indicating the insufficiency of the enzymatic hydrolysis. When the hydrolysis time reached 18 h, the change of reducing sugar yield was weakened with increased cellulase loading. Additionally, under the higher cellulase loading (> 140 FPU g-1-VS), the reducing sugar yield of pretreated mixtures remained almost unchanged at 18 h and 20 h, suggesting that the accessible cellulose surface was saturated with hydrolytic enzymes at an enzyme loading of 140 FPU g-1-VS and the yield of reducing sugar nearly reached the maximum level after 18 h. The enzymatic hydrolysis time of 18 h and the cellulase loading of 140 FPU g-1-VS were the best conditions in view of the reducing sugar yield.
Figure 4 shows that the variation tendency of amino acid yield was similar with the reducing sugar yield of cellulase treated mixtures. The amino acid yield improved with the increased enzyme loading and hydrolysis time. Protease converts proteins to peptides and amino acids and improves the hydrolysis efficiency (Bjarnadóttir et al. 2018). Figure 4 clearly shows that more amino acid was generated with longer hydrolysis time for the same protease loading. The amino acid yield was unchanged after 4 h. When the hydrolysis time reached 5 h, the maximum amino acid yield (23.12 mg g-1) was achieved at 18 FPU g-1-VS. Moreover, the conversion line became flat from 10 FPU g-1-VS to 18 FPU g-1-VS after 4 h, indicating that the protease surface was saturated with hydrolytic enzymes when the enzyme loading reached 10 FPU g-1-VS. Based on the above results, the optimal conditions of 10 FPU g-1-VS and 4 h were selected for protease hydrolysis.
Fig. 3. Effect of cellulase loading and enzymatic hydrolysis time on reducing sugar yield
Fig. 4. Effect of protease loading and enzymatic hydrolysis time on amino acid yield
Batch Tests of Methane Potential
The methane yields of raw mixtures, thermal-alkali pretreated mixtures, and pretreated mixtures subsequently hydrolyzed by cellulase and protease are presented in Fig. 5. The methane yield from raw mixtures (process 1) was 0.22 m3 kg-1-VS. The methane production from the raw material after process 1 had a slow rate, as the raw mixtures contained slowly degrading lignocellulose. Thermal-alkali was used to pretreat a large group of feedstock, such as agricultural residuals and municipal solid waste, and the methane yield was increased by 3.2% to 230% (Zheng et al. 2014). In this research, through the thermal-alkali pretreatment (process 2), the methane yield increased by 31.82% to be 0.29 m3 kg-1-VS, which was similar to the previous research. Compared with thermal-alkali treatment, the cellulase hydrolysis (process 3) increased the methane yield by 13.79%. The crystalline structures of cellulose were broken down by the thermal-alkali treatment, and thereby the accessibility of hydrolytic enzymes to cellulose was improved. Therefore, methanogens could obtain the fermentable sugars from hydrolysates and produce more methane. Wang et al. (2015) found that protease slightly improves methane production by 1.47%. In contrast, in this research, the protease (process 4) showed an increase of 63.64% in the methane yield compared with process 1 and reached 0.36 m3 kg-1-VS. This difference was possibly because Wang et al. (2015) directly added amylase and protease to the substances, allowing some essential enzymes to be degraded by protease. In this research, the thermal-alkali pretreated mixtures were treated by cellulase followed by protease. The methane yield from the liquid fraction (process 4-1) was 0.41 m3 kg-1-VS, which was consistent with the expectation, because the liquid fraction contained quite a few hydrolysable sugars and soluble organic substances after filtration. The methane yield from the solid fraction (process 4-2) was 0.19 m3 kg-1-VS, which was lower than the methane production from the raw mixtures produced in process 1. The methane production of mixtures after process 4-2 was low, probably due to the slowly degrading lignin in solid fraction. Nkemka and Murto (2013) reported that the methane potential of the mixtures was higher than that of only wheat straw, indicating that co-digestion improves the methane production than the mono-digestion of feedstock due to better nutrient balance of the former one (Li et al. 2014). Nitrogen is an essential nutrient for microorganisms, livestock manure usually contains high TN, co-digestion of livestock manure and crop residues can supply a proper C/N ratio for microorganisms (Wei et al. 2015; Awais et al. 2018). Overall, the liquid process fraction (process 4-1) produced the highest methane.
Fig. 5. Process performance during anaerobic digestion of liquid of hydrolysate in EGSB reactor
Methane Production from Liquid of Hydrolysate in EGSB Reactor
An appropriate OLR could provide sufficient nutrients for methanogens to mineralize organic matters into methane and carbon dioxide. In this research, the maximum applicable OLR was explored to improve the degradation of organic matter. The experiment was composed of five stages according to different OLRs, as shown in Fig. 6 and Table 3. The methane production rate (MPR) was usually used as an indicator to evaluate the efficiency of biogas production. The MPR of the five stages had a similar increasing trend as the AD process progressed (Fig. 6). The MPR of the 4-1 fraction increased from 0.74 L Lreactor-1 d-1 to 4.79 L Lreactor-1 d-1 when OLR increased from 2.18 kg-SCOD m-3 d-1 to 13.66 kg-SCOD m-3 d-1, while the MPR under OLR of 35.21 kg-SCOD m-3 d-1 was increased from 4.98 L Lreactor-1 d-1 to 5.13 L Lreactor-1 d-1 first and then decreased to 4.42 L Lreactor-1 d-1. This phenomenon was consistent with the results obtained by Wang et al. (2015). Mu et al. (2017) found that methane yield increased with the increase of OLRs. In contrast, in this research, the methane yield decreased from 0.31 L g-1-SCOD to 0.23 L g-1-SCOD with the increase of OLRs. This result may be because the organic materials were washed out at short HRTs and high OLRs, and thus methanogens had less time to convert the hydrolysate into biogas. Table 3 shows that the methane content of biogas was approximately 67.92% with OLR increasing from 2.18 kg-SCOD m-3 d-1 to 13.66 kg-SCOD m-3 d-1, corresponding to the SCOD removal ranging from 93.52% to 96.41%.
An optimal NH4+-N concentration could provide a balanced nitrogen source for the growth of methanogens. Nkemka and Murto (2013) indicated that ammonia inhibition could be avoided by controlling the concentration of NH4+-N at less than 1 kg m-3. As indicated by Table 3, the concentration of NH4+-N increased from 0.28 kg m-3 to 1.01 kg m-3 when OLR increased from 2.18 kg-SCOD m-3 d-1 to 13.66 kg-SCOD m-3 d-1, which was beneficial for methanogens to produce more methane. In stage V, the concentration of NH4+-N was 2.78 kg m-3, resulting from the rapid increase of OLR, which caused an accumulation of ammonia and reduced the production of methane. The stability of anaerobic digestion was directly associated with the VFAs (Hassan et al. 2017). Neshat et al. (2017) indicated that the VFAs of 1.5 to 2.0 kg m-3 could inhibit anaerobic digestion. As shown in Table 3, the concentration of VFAs of effluent was below 0.20 kg m-3 when OLR increased from 2.18 kg-SCOD m-3 d-1 to 13.66 kg-SCOD m-3 d-1. Kalamaras and Kotsopoulos (2014) found that the VFAs in the co-digestion of wheat straw with cattle manure was far below the inhibitory concentration of VFAs, as co-digestion with mixtures could produce ammonia to neutralize VFAs. The rapid increase of OLR led to an increase in the VFAs concentration of effluent (Table 3). The highest VFAs concentration of effluent was achieved at the OLR of 35.21 kg-SCOD m-3 d-1, which was 0.24 kg m-3. The increase in VFAs concentration negatively affected the methane production (Fig. 6 and Table 3), indicating a close relationship between VFAs concentration and methane production. In this research, the maximum applicable OLR was 13.66 kg-SCOD m-3 d-1, which was a comfortable environment for biomass growth.
Fig. 6. Effect of different organic loading rate (OLR) on the methane production rate (MPR)
Table 3. Operating Conditions and Process Performance during Anaerobic Digestion of Liquid of Hydrolysate
CONCLUSIONS
- A sequential process was applied on the mixture of CS and CM. Thermal-alkali pretreatment and enzymatic hydrolysis helped to open chemical bonds in the cellulose, hemicellulose, and protein, which were further converted into small molecule organics (reducing sugar and amino acid) in the hydrolysates.
- The solubilization and reductions of cellulose, hemicellulose, and lignin increased with increasing Ca(OH)2 dosage and temperature. A high methane yield was achieved from liquid hydrolysates during the batch tests of methane potential of thermal-alkali pretreatment and enzymatic hydrolysis.
- The maximum applicable OLR (13.66 kg-SCOD m-3 d-1) provided a comfortable environment for biomass growth and improved the degradation of CS and CM hydrolysates in an EGSB reactor. This study provides an optimal control and selection of conditions to treat organic solids. The optimization of conditions could be a promising reference for the industrial-scale treatment of organic solids with a high energy yield and a high efficiency.
ACKNOWLEDGMENTS
The authors are grateful for the financial support from the Special Guidance Funds for Agricultural Science and Technology Innovation of Yancheng City (Grant No. yk2016030), the Joint Open Fund of Jiangsu Collaborative Innovation Center for Ecological Building Material and Environmental Protection Equipment and Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, the National Natural Science Foundation of China (NSFC, Grant No. 51608467), the Open Project of Key Laboratory of Environmental Biotechnology, CAS (Grant No. kf2016005), and the Open Project of State Key Laboratory of Urban Water Resource and Environment (Grant No. QA201716).
REFERENCES CITED
Abada, E. A., Masrahi, Y., Abboud, M. A., Alnashiri, H. M., and El-Gayar, K. E. (2018). “Bioethanol production with cellulase enzyme from Bacillus cereus isolated from sesame seed residue from the Jazan region,” BioResources 13(2), 3832-3845. DOI: 10.15376/biores.13.2.3832-3845
American Public Health Association (APHA) (2005). Standard Methods for the Examination of Water and Wastewater (21st Ed.), Washington, DC, USA.
Awais, M., Gulfraz, M., Asad, M. J., Kabir, F., Khan, K. S., and Naqvi, S. M. Z. A. (2018). “Mesophilic anaerobic co-digestion of cattle manure with Malus domestica and Dalbergia sissoo during biomethane potential assays,” BioResources 13(2), 3144-3156. DOI: 10.15376/biores.13.2.3144-3156
Bjarnadóttir, M., Aðalbjörnsson, B. V., Nilsson, A., Slizyte, R., Roleda, M. Y., Hreggviðsson, G. Ó., Friðjónsson, Ó. H., and Jónsdóttir, R. (2018). “Palmaria palmata as an alternative protein source: Enzymatic protein extraction, amino acid composition, and nitrogen-to-protein conversion factor,” J. Appl. Phycol. 30(3), 1-10. DOI: 10.1007/s10811-017-1351-8
Cekmecelioglu, D., and Uncu, O. N. (2013). “Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production,” Waste Manage.33, 735-739. DOI: 10.1016/j.wasman.2012.08.003
Cho, S., Park, S., Seon, J., Yu, J., and Lee, T. (2013). “Evaluation of thermal, ultrasonic and alkali pretreatments on mixed-microalgal biomass to enhance anaerobic methane production,” Bioresource Technol. 143(17), 330-336. DOI: 10.1016/j.biortech.2013.06.017
Ding, S., Liu, Y., Zeng, Y., Himmel, M. E., Baker, J. O., and Bayer, E. A. (2012). “How does plant cell wall nanoscale architecture correlate with enzymatic digestibility,” Science 338(6110), 1055-1060. DOI: 10.1126/science.1227491
Haase, M., Rösch, C., and Ulrici, O. (2017). “Feasibility study on the processing of surplus livestock manure into an organic fertilizer by thermal concentration – The case study of Les Plenesses in Wallonia,” J. Clean. Prod. 161, 896-907. DOI: 10.1016/j.jclepro.2017.05.207
Hassan, M., Ding, W., Umar, M., Hei, K., Bi, J., and Shi, Z. (2017). “Methane enhancement and asynchronism minimization through co-digestion of goose manure and NaOH solubilized corn stover with waste activated sludge,” Energy 118, 1256-1263. DOI: 10.1016/j.energy.2016.11.007
Jin, S., Zhang, G., Zhang, P., Fan, L., Fan, S., and Li, J. (2016). “Thermo-chemical pretreatment and enzymatic hydrolysis for enhancing saccharification of catalpa sawdust,” Bioresource Technol. 205, 34-39. DOI: 10.1016/j.biortech.2016.01.019
Kalamaras, S. D., and Kotsopoulos, T. A. (2014). “Anaerobic co-digestion of cattle manure and alternative crops for the substitution of maize in South Europe,” Bioresource Technol. 172, 68-75. DOI: 10.1016/j.biortech.2014.09.005
Krishania, M., Vijay, V. K., and Chandra, R. (2013). “Methane fermentation and kinetics of wheat straw pretreated substrates co-digested with cattle manure in batch assay,” Energy 57(8), 359-367. DOI: 10.1016/j.energy.2013.05.028
Li, J., Wei, L., Duan, Q., Hu, G., and Zhang, G. (2014). “Semi-continuous anaerobic co-digestion of dairy manure with three crop residues for biogas production,” Bioresource Technol. 156(2), 307-313. DOI: 10.1016/j.biortech.2014.01.064
Mehryar, E., Ding, W., Hemmat, A., Talha, Z., Hassan, M., Mamat, T., and Hei, K. (2017). “Anaerobic co-digestion of oil refinery wastewater with bagasse: Evaluating and modeling by neural network algorithms and mathematical equations,” BioResources 12(4), 7325-7340. DOI: 10.15376/biores.12.4.7325-7340
Mu, H., Zhao, C., Zhao, Y., Li, Y., Hua, D., Zhang, X., and Xu, H. (2017). “Enhanced methane production by semi-continuous mesophilic co-digestion of potato waste and cabbage waste: Performance and microbial characteristics analysis,” Bioresource Technol. 236, 68-76.
Neshat, S. A., Mohammadi, M., Najafpour, G. D., and Lahijani, P. (2017). “Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production,” Renew. Sust. Energ. Rev. 79, 308-322. DOI: 10.1016/j.rser.2017.05.137
Nkemka, V. N., and Murto, M. (2013). “Biogas production from wheat straw in batch and UASB reactors: The roles of pretreatment and seaweed hydrolysate as a co-substrate,” Bioresource Technol. 128(1), 164-172. DOI: 10.1016/j.biortech.2012.10.117
Obata, O., Akunna, J. C., and Walker, G. (2015). “Hydrolytic effects of acid and enzymatic pre-treatment on the anaerobic biodegradability of Ascophyllum nodosum and Laminaria digitata species of brown seaweed,” Biomass Bioenerg. 80, 140-146. DOI: 10.1016/j.biombioe.2015.05.001
Odnell, A., Recktenwald, M., Stensén, K., Jonsson, B. H., and Karlsson, M. (2016). “Activity, life time and effect of hydrolytic enzymes for enhanced biogas production from sludge anaerobic digestion,” Water Res. 103, 462-471. DOI: 10.1016/j.watres.2016.07.064
Song, C., Shan, S., Müller, K., Wu, S., Niazi, N. K., Xu, S., Shen, Y., and Rinklebe, J. (2018). “Characterization of pig manure-derived hydrochars for their potential application as fertilizer,” Environ. Sci. Pollut. R. 25, 25772-25779. DOI: 10.1007/s11356-017-0301-y
Song, Z., and Zhang, C. (2015). “Anaerobic codigestion of pretreated wheat straw with cattle manure and analysis of the microbial community,” Bioresource Technol. 186, 128-135. DOI: 10.1016/j.biortech.2015.03.028
Tsapekos, P., Kougias, P. G., Treu, L., Campanaro, S., and Angelidaki, I. (2017). “Process performance and comparative metagenomic analysis during co-digestion of manure and lignocellulosic biomass for biogas production,” Appl. Energ. 185, 126-135. DOI: 10.1016/j.apenergy.2016.10.081
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). “Carbohydrate methodology, metabolism, and nutritional implications in dairy cattle: Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition,” J. Dairy Sci. 74 (10), 3583-3597. DOI: 10.3168/jds.S0022-0302(91)78551-2
Wang, H., Tao, Y., Temudo, M., Schooneveld, M., Bijl, H., Ren, N., Wolf, M., Heine, C., Foerste, A., Pelenc, V., Kloek, J., Lier, J. B. V., and Kreuk, M. D. (2015). “An integrated approach for efficient biomethane production from solid bio-wastes in a compact system,” Biotechnol. Biofuels 8(1), 1-14. DOI: 10.1186/s13068-015-0237-8
Wang, X., Li, Z., Bai, X., Zhou, X., Cheng, S., Gao, R., and Sun J. (2018). “Study on improving anaerobic co-digestion of cow manure and corn straw by fruit and vegetable waste: methane production and microbial community in CSTR process,” Bioresource Technol. 249, 290-297. DOI: 10.1016/j.biortech.2017.10.038
Wei, Y., Li, X., Yu, L., Zou, D., and Yuan, H. (2015). “Mesophilic anaerobic co-digestion of cattle manure and corn stover with biological and chemical pretreatment,” Bioresource Technol. 198(1), 431-436. DOI: 10.1016/j.biortech.2015.09.035
Yang, Q., Wang, H., Larson, R. A., and Runge, T. (2017). “Comparative study of chemical pretreatments of dairy manure for enhanced biomethane production,” BioResources 12(4), 7363-7375. DOI: 10.15376/biores.12.4.7363-7375
Yuan, Y., Bian, A., Zhang, L., Chen, T., Pan, M., He, L., Wang, A., and Ding, C. (2019). “A combined process for efficient biomethane production from corn straw and cattle manure: Optimizing C/N Ratio of mixed hydrolysates,” BioResources 14(1), 1347-1363. DOI: 10.15376/biores.14.1.1347-1363
Yuan, H., Li, R., Zhang, Y., Li, X., Liu, C., Ying, M., Lin, M., and Yang, Z. (2015). “Anaerobic digestion of ammonia-pretreated corn stover,” Biosyst. Eng. 129, 142-148. DOI: 10.1016/j.biosystemseng.2014.09.010
Zhang, T., Mao, C., Zhai, N., Wang, X., and Yang, G. (2015). “Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk,” Waste Manage. 35(7), 119-126. DOI: 10.1016/j.wasman.2014.09.004
Zhang, C., Pei, H., Wang, S., Cui, Z., and Liu, P. (2016). “Enhanced enzymatic hydrolysis of poplar after combined dilute NaOH and fenton pretreatment,” BioResources 11(3), 7522-7536. DOI: 10.15376/biores.11.3.7522-7536
Zheng, Y., Zhao, J., Xu, F. Q., and Li, Y. (2014). “Pretreatment of lignocellulosic biomass for enhanced biogas production,” Prog. Energ. Combust. 2014, 42(1), 35-53. DOI: 10.1016/j.pecs.2014.01.001
Article submitted: January 30, 2019; Peer review completed: March 23, 2019; Revised version received: May 21, 2019; Accepted: May 22, 2019; Published: May 24, 2019.
DOI: 10.15376/biores.14.3.5422-5437
