Abstract
As an immerging lignocellulose pretreatment strategy, cellulose solvent-based pretreatment can break down inter- and intra-molecular hydrogen bonds and disrupt the rigid structure of cellulose. Two cellulose solvent pretreatments were examined and compared in this study: NaOH/urea and concentrated phosphoric acid. Pretreated corn stover substrates were characterized by optical microscopy, confocal laser scanning microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, and chemical analyses. It was found that both alkaline- and acid-based cellulose solvent pretreatments can disrupt cell wall structures and cause partial dissolution of the cell wall components. The results indicated that the alkaline-based cellulose solvent was more effective at removing lignin as compared with the phosphoric acid-based cellulose solvent. The initial enzymatic saccharification rate of corn stover pretreated by alkaline-based cellulose solvent was greatly enhanced; complete saccharification of the glucans was achieved within 24 h at an enzyme loading of 15 filter paper units (FPU)/g substrate. The enzymatic digestibility of corn stover pretreated by phosphoric acid was lower than that of the alkaline-based system; this was probably caused by the presence of a high concentration of lignin.
Download PDF
Full Article
Comparative Study of Alkali and Acidic Cellulose Solvent Pretreatment of Corn Stover for Fermentable Sugar Production
Qianqian Wang,a,b,* Wei Wei,a Xia Li,a Jianzhong Sun,a Jing He,b,c and Mingxiong He b,c
As an immerging lignocellulose pretreatment strategy, cellulose solvent-based pretreatment can break down inter- and intra-molecular hydrogen bonds and disrupt the rigid structure of cellulose. Two cellulose solvent pretreatments were examined and compared in this study: NaOH/urea and concentrated phosphoric acid. Pretreated corn stover substrates were characterized by optical microscopy, confocal laser scanning microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, and chemical analyses. It was found that both alkaline- and acid-based cellulose solvent pretreatments can disrupt cell wall structures and cause partial dissolution of the cell wall components. The results indicated that the alkaline-based cellulose solvent was more effective at removing lignin as compared with the phosphoric acid-based cellulose solvent. The initial enzymatic saccharification rate of corn stover pretreated by alkaline-based cellulose solvent was greatly enhanced; complete saccharification of the glucans was achieved within 24 h at an enzyme loading of 15 filter paper units (FPU)/g substrate. The enzymatic digestibility of corn stover pretreated by phosphoric acid was lower than that of the alkaline-based system; this was probably caused by the presence of a high concentration of lignin.
Keywords: Cellulose solvent pretreatment; Crystallinity; Lignin removal; Enzymatic hydrolysis
Contact information: a: Biofuels Institute, School of the Environment, Jiangsu University, Zhenjiang 212013, China; b: Key Laboratory of Development and Application of Rural Renewable Energy, Ministry of Agriculture, Chengdu 610041, China; c: Biomass Energy Technology Research Centre, Biogas Institute of Ministry of Agriculture, Chengdu 610041, China;
* Corresponding author: qianqian.wz@gmail.com; wqq@ujs.edu.cn
INTRODUCTION
Lignocellulose, the most abundant natural and renewable polymer in the world, is envisaged to provide a significant portion of fuels, chemicals, and materials (Ragauskas et al. 2006). Agricultural residues constitute one of the primary lignocellulose feedstocks in agriculture-based countries, like China. Six hundred fifty million tons of crop straws are produced each year in China (Chen et al. 2012). Most crop straws are left in the field and burnt on-site. Open field burning releases a large amount of greenhouse gas, contributes to air pollution, and underutilizes this bioresource in China (Li et al. 2007). Recently, the use of crop straws for bioenergy production is being encouraged because of the limited supplies of petroleum resources.
Cellulose solvent-based lignocellulose pretreatments have been developed and may offer unique advantages in lignocellulose pretreatment (Sathitsuksanoh et al. 2013). Cellulose solvent-based lignocellulose pretreatments can efficiently disrupt inter- and intra-molecular hydrogen bonds and van der Waals forces and alter the ultra physico-chemical structure of lignocellulose. This results in the disruption of cellulose crystalline structure, partial degradation of hemicellulose, and increased cellulase accessibility to cellulose. Recently, many derivative and non-derivative cellulose solvents, such as ionic liquids (ILs), concentrated phosphoric acid, NaOH/urea, N-methylmorpholine-N-oxide (NMMO), and N-dimethylacetamide (DMAc)/LiCl, have been applied as pretreatments for lignocelluloses (Kuo and Lee 2009; Sathitsuksanoh et al. 2013).
ILs have been widely studied as a potential strategy to pretreat lignocellulose materials (Tadesse and Luque 2011). Swelling and dissolution of cell wall components in ILs have been characterized (Sun et al. 2013; Ji et al. 2014; Zhang et al. 2014). It was reported that ILs initially liquefy the cellulose and then the hemicelluloses (Miyafuji et al. 2009). Cellulose solvent (concentrated phosphoric acid) and organic solvent-based lignocellulose fractionation (COSLIF) result in the disruption of highly ordered hydrogen bonds in the crystalline cellulose and the removal of some acid-insoluble lignin (Rollin et al. 2011). COSLIF works well on a wide range of feedstocks (Sathitsuksanoh et al. 2013). Cellulose dissolution in a NaOH/urea solution was developed for functional cellulose material such as fibers, membranes, aerogels, and hybrid composites (Zhou and Zhang 2000; Luo and Zhang 2013). Low-temperature NaOH/urea solution can disrupt the crystalline structure of cellulose and remove a large amount of lignin and hemicellulose because of its alkalinity. A NaOH/urea-based pretreatment was found to perform well on lignocellulose ( Zhao et al. 2008; Wang et al. 2014).
Significant enhancement in enzymatic saccharification of cotton (near 100% cellulose content) was achieved when it was pretreated by a cellulose solvent (Kuo and Lee 2009). Comparisons of the performances of alkaline- and acid-based cellulose solvent pretreatments using actual lignocellulose materials have not been reported to date. In the present study, pretreatments of corn stover with NaOH/urea solution and concentrated phosphoric acid were conducted. The effects of pretreatment on the chemical composition and the structure of the fiber cell walls were compared. Cellulose crystallinity was examined and the chemical changes were verified.
EXPERIMENTAL
Substrate, Enzyme, and Chemicals
Corn stover was provided by a local farmer. Leaves and pith tissues were manually removed; the remaining rind tissues were milled to pass through a 40-mesh screen. Cellic® CTec2 enzyme was obtained from Novozyme, North America. Sodium hydroxide, urea, phosphoric acid, sodium carbonate, sulfuric acid, and other reagent chemicals were purchased from a local laboratory chemical supplier company and used as received.
NaOH/Urea Pretreatment
Five grams of corn stover meal was mixed with 50 g of NaOH/urea solution (NaOH:urea:H2O of 7:12:81 (by weight)) in a flask using a shaker at room temperature (25 °C) for 3 h. The mixture was kept in a -20 °C refrigerator for 12 h. The resulting mixture was stirred at room temperature for 5 min and then thoroughly washed and filtered. The obtained substrate was denoted NU-CS.
Phosphoric Acid Pretreatment
Phosphoric acid pretreatment was conducted in a similar way to NaOH/urea pretreatment. In brief, 5 g of corn stover was mixed with 50 g of 85% phosphoric acid in a flask using a shaker for 3 h at room temperature. The mixture was then incubated at 50 °C while shaking for 1 h. The resulting mixture was then neutralized and washed. The obtained substrate was denoted PA-CS.
Enzymatic Hydrolysis of Corn Stover
Enzymatic hydrolysis of the treated substrate was conducted at a substrate consistency of 2% and an enzyme loading of 15 FPU/g substrate using 50 mM sodium acetic buffer (pH 4.8) with 0.1% (w/v) tetracycline. Hydrolysis experiments were carried out at with a shaker operating at 200 rpm and 50 °C (KYC 100B; Shanghai Fume Test Equipment Co., Ltd., China). Aliquots were taken periodically from the flask for sugar analysis.
Optical Microscopy (OM) and Confocal Laser Scanning Microscopy (CLSM) Analysis
Corn stover cross sections (30 µm) were obtained using a Leica CM 1900 cryostat (Leica Microsystems, Heidelberg, Germany) at -20 °C onto coverslips. Eighty microliters of an alkaline solution (NaOH:urea:H2O: 7:12:81 by weight) or an 85% phosphoric acid solution was placed on these microtome sections of corn stover and placed in a refrigerator or shaker under the conditions mentioned above.
Optical microscopy was conducted using a Keyence VHX-1000 digital microscope (Keyence Int. Trading Co. Ltd., Japan) equipped with a CCD camera. CLSM images were obtained using a Leica TCS SP5 confocal laser scanning microscope. Lignin autofluorescence was detected using a UV laser emitting at a wavelength of 405 nm and a krypton/argon laser emitting at a wavelength of 488 nm.
Component Analysis of Corn Stover
Chemical composition analysis of corn stover was determined according to NREL LAP protocols (Sluiter et al. 2011). Glucose and xylose were measured with a Shimadzu HPLC system (Japan) equipped with a BioRad Aminex HPX-87H sugar column (Hercules, California) and a refractive index detector (RID 10A). The column was operated at 50 °C using 50 mM sulfuric acid eluent as the mobile phase at a rate of 0.4 mL/min.
X-Ray Diffraction (XRD) Analysis
Freeze dried corn stover substrate was evenly distributed in the sample holder and then pressed down lightly to form a smooth surface. X-ray diffractograms of corn stover samples were analyzed by a D8 Advance diffractometer (Bruker, Germany) with a CuKa X-ray source (1.54 A˚) operated at 15 mA and 30 kV at a scanning speed of 5°/min, ranging from 5° to 40°. XRD spectra were used to investigate cellulose structural changes.
Fourier Transform Infrared (FTIR) Spectroscopy
2 mg freeze dried sample was finely ground with 200 mg of KBr in an agate mortar and pressed into a transparent pellet for measurement. The FTIR spectra were recorded on a Nicolet Nexus 470 FTIR spectrometer (Thermo Nicolet, USA). FTIR spectrum was recorded from 4000 to 400 cm-1 with 16 scans at a resolution of 4 cm-1 using the transmission mode.
RESULTS AND DISCUSSION
Optical microscopy images were made of the cross sections of corn stover at 500x magnification. Figure 1a shows the intact morphology of the untreated corn stover. In Fig. 1b, it is clear that the NaOH/urea pretreatment noticeably altered the cell wall structure of corn stover, which left the structure indistinguishable. In contrast, the cell wall structure was partially disrupted after the phosphoric acid pretreatment. The major cell wall structures were still preserved, as shown in Fig. 1c.
Confocal laser scanning microscopy (CLSM) was used to qualitatively determine the lignin distribution in corn stover substrate, as shown in Fig. 1d, 1e, and 1f. The untreated substrate exhibited the greatest autofluorescence, as shown in Fig. 1d. After the NaOH/urea pretreatment, the fluorescence intensity underwent an obvious decrease, which indicated that a considerable amount of delignification had occurred during pretreatment. The fluorescence of the corn stover substrate pretreated by phosphoric acid was still very strong in some regions, which suggested that this pretreatment had a limited impact on lignin removal.
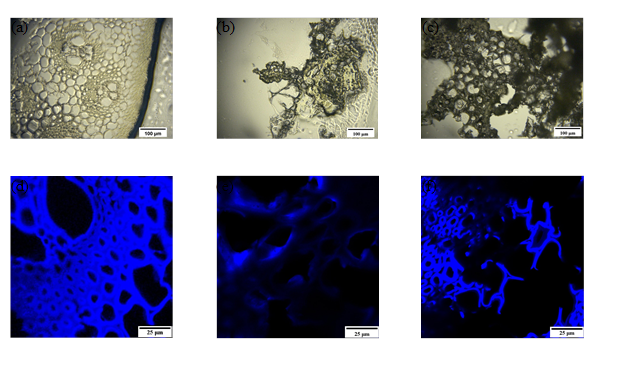
Fig. 1. Optical images (a, b, c) and CLSM images (d, e, f) after NaOH/urea and phosphoric acid pretreatment: (a, d), before pretreatment; (b, e), after NaOH/urea pretreatment for 1 h; (c, f) after phosphoric acid pretreatment for 1 h. Scale bar = 100 μm for (a, b, c); and = 25 μm for (d, e, f).
Previous studies indicate that both NaOH/urea and concentrated phosphoric acid solutions were suitable solvents for cellulose ( Zhang et al. 2006; Luo and Zhang 2013). Dissolved cellulose could be easily recovered by the addition of water. The chemical composition of corn stover before and after pretreatments is presented in Table 1. Corn stover used in this study contained 37.15% glucan, 15.16% hemicellulose, and 27.69% lignin. The recovered corn stover samples had appreciably reduced hemicellulose content when compared to the untreated substrate, which indicated that both NaOH/urea and phosphoric acid pretreatment effectively removed xylan. Both pretreatments left 6.37% vs. 6.21% residual xylan in pretreated substrates. It was found that both alkaline and acidic cellulose solvent pretreatments produced higher glucan content, ranging from 47.59% to 70.61%. It was also observed that the alkaline-based solvent (i.e., NaOH/urea) was more effective at lignin removal than the acid-based (i.e., 85% phosphoric acid) pretreatment. During the NaOH/urea pretreatment, the extent of lignin removal was approximately 90%, calculated from the total lignin values of the untreated corn stover and the NaOH/urea-treated substrate and the substrate yield (Table 1). In contrast, the phosphoric acid pretreatment reduced the lignin content in the substrate by 30% when compared to the untreated corn stover. The huge difference in delignification between the NaOH/urea and the 85% phosphoric acid-pretreated substrates was probably a result of lignin being more easily dissolved in alkaline than in acidic solution.
Table 1. Compositional Analysis of Untreated and Pretreated Corn Stover
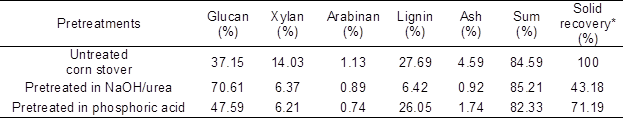
*: solid recovery is defined as the percentage of solids remaining following pretreatment divided by original oven-dry substrate weight.
It was hypothesized that cellulose crystallinity is an important factor affecting enzymatic hydrolysis. X-ray diffraction was employed to investigate the crystallinity of corn stover before and after cellulose solvent pretreatments. As shown in Fig. 2, untreated corn stover exhibited a typical cellulose I structure, which had diffraction angles near 22.0° 2θ for the (200) peak and 15.9° 2θ for an overlap of the (1-10) and (110) peaks.
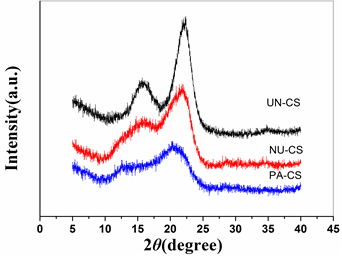
Fig. 2. XRD diffraction spectra for corn stover
The diffraction pattern of corn stover pretreated with NaOH/urea showed a broad peak near 21° 2θ, and an overlapped peak near 14° 2θ. The 21º 2θ peak is attributed to the (110) and (020) peaks of cellulose II at 20° and 22º 2θ, overlapped with the (200) reflection of cellulose I. The peak near 14° similarly arises from the (1-10) peaks of cellulose II at 12° 2q, overlapped with the (1-10) and (110) peaks of cellulose I (French 2014; Wang et al. 2015). This mixture of peaks was due to the partial conversion of cellulose I to cellulose II. This result suggests that both cellulose I and II structures coexisted in the corn stover after NaOH/urea pretreatment. In the case of phosphoric pretreatment, the changes involved a considerable weakening of peaks at 22.0° and 15.9°.
The appreciable changes in cellulose structure with the phosphoric acid pretreatment did not lead to an enhancement in enzymatic hydrolysis when compared with the NaOH/urea pretreatment. The relationship between cellulose structure changes and subsequent enzymatic hydrolysis is very complicated (Sathitsuksanoh et al. 2011). The reason for this low efficacy might be the high amount of lignin remaining, which could have caused non-productive cellulase binding.
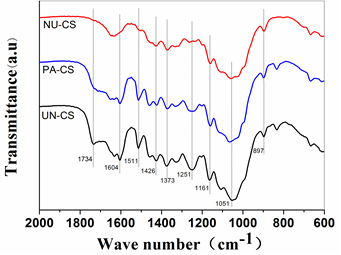
Fig. 3. FTIR spectra in the 2000 to 600 cm-1 region for corn stover samples
FTIR spectra of untreated and pretreated corn stover are shown in Fig. 3. The spectra of untreated and NaOH/urea-treated corn stover showed differences in band intensities at 1734 cm-1 (ester-linked acetyl, feruloyl and p-coumaroyl groups between hemicellulose and lignin), 1511 cm-1 (aromatic C=C stretching from lignin), and 1373 cm-1 (aliphatic C–H stretching in methyl and phenol OH) (Chen et al. 2010; da Costa Lopes et al. 2013). The weakening or disappearance of bands at 1734, 1511, and 1373 cm-1 for NaOH/urea-pretreated corn stover implies that considerable amounts of lignin were removed during pretreatment. In contrast, the differences between untreated and phosphoric acid-treated corn stover were minor at 1734, 1511, and 1373 cm-1, which indicated a minimum amount of lignin removal with concentrated phosphoric acid treatment. This observation is consistent with the chemical analysis results discussed earlier. The bands at 1426 cm-1 (CH2 scissoring motion), 1373 cm-1, 1161 cm-1 (C–O–C vibration in cellulose and hemicellulose), 1051 cm-1 (C–O stretching in cellulose and hemicellulose), and 897 cm-1 (characteristic of β-glycosidic C-H deformation with ring vibration) can be assigned to cellulose (Oh et al. 2005; Pandey and Pitman 2003; da Costa Lopes et al. 2013). The decrease in these band intensities suggests that highly crystallized cellulose structures were disrupted. Appreciable decreases in band intensities were also detected at 1373 cm-1, 1251 cm-1 (characteristic bands of hemicellulose), 1161 cm-1 (C–O–C vibration in cellulose and hemicellulose), and 1051 cm-1 (C–O stretching in cellulose and hemicellulose) after pretreatment; these changes are likely due to the removal of hemicelluloses ( Pandey and Pitman 2003; da Silva et al. 2013).
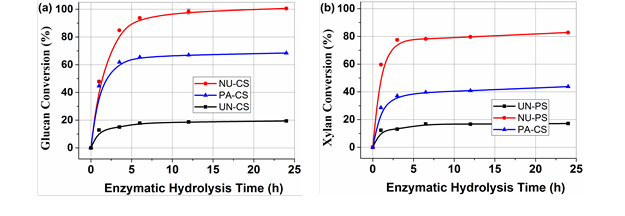
Fig. 4. Enzymatic hydrolysis profiles for UN-CS, NU-CS, and PA-CS at an enzyme loading of 15 FPU per gram substrate
Enzymatic hydrolysis was conducted on the various substrates to evaluate enzymatic digestibility. Figure 4 shows the time course of saccharification for untreated and pretreated corn stover substrates at an enzyme loading of 15 FPU/g substrate. The glucan conversion increased rapidly for 12 h and then plateaued. NaOH/urea-pretreated corn stover showed appreciable glucan digestibility. Complete glucan conversion was reached within 24 h, whereas conversion with the phosphoric acid-pretreated substrate only reached 65.5%. In the case of xylan conversion, similar trends were found. The conversion rates of xylan for the NaOH/urea- and phosphoric acid-pretreated substrates were 82.8% and 43.8%, respectively. One of the reasons for this could be that there was a considerable amount lignin left in the phosphoric acid-pretreated substrate, which caused non-productive enzymes adsorption, preventing the enzymes from accessing the carbohydrates.
CONCLUSIONS
- A comparative study of alkali and acidic cellulose solvent pretreatments of corn stover was successfully carried out for the production of fermentable sugars. XRD data indicated that cellulose solvents can destroy the rigid cellulose structure, resulting in substrates with a more easily digestible cellulose II structure.
- NaOH/urea pretreatment markedly decreased biomass recalcitrance by removing a majority of lignin and xylan from the lignocellulosic biomass. The cellulose-rich substrate achieved nearly complete enzymatic hydrolysis within 24 h with an enzyme loading of 15 FPU/g substrate.
- Phosphoric acid-based cellulose solvent pretreatment exhibited a relatively lower performance with respect to sugar recovery. This might be due to the large amount of lignin remaining in the substrate. The glucose recovery of the NaOH/urea pretreatment was 82.1%, as compared with 62.4% with the phosphoric acid pretreatment. A future study will be conducted to focus on the dissolution mechanism of cellulose and hemicellulose in the alkali and acidic cellulose solvents.
ACKNOWLEDGMENTS
The authors would like to acknowledge financial support from the National Natural Science Foundation of China (31300493), the China Postdoctoral Science Foundation (2015M581740), the Jiangsu Planned Projects for Postdoctoral Research Fund (1501072C), the Start-Up support (13JDG018) and the Young Scholars Program of Jiangsu University, the open funding from the Key Laboratory of Development and Application of Rural Renewable Energy, Ministry of Agriculture (2013007), and the project funding by PAPD.
REFERENCES CITED
Chen, H., Ferrari, C., Angiuli, M., Yao, J., Raspi, C., and Bramanti, E. (2010). “Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis,” Carbohyd. Polym. 82(3), 772-778. DOI: 10.1016/j.carbpol.2010.05.052
Chen, L., Zhao, L., Ren, C., and Wang, F. (2012). “The progress and prospects of rural biogas production in China,” Energy Policy 51, 58-63. DOI: 10.1016/j.enpol.2012.05.052
da Costa Lopes, A. M., João, K. G., Rubik, D. F., Bogel-Łukasik, E., Duarte, L. C., Andreaus, J., and Bogel-Łukasik, R. (2013). “Pre-treatment of lignocellulosic biomass using ionic liquids: Wheat straw fractionation,” Bioresource Technol. 142, 198-208. DOI: 10.1016/j.biortech.2013.05.032
da Silva, S. P. M., da Costa Lopes, A. M., Roseiro, L. B., and Bogel-Łukasik, R. (2013). “Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids,” RSC Advances 3(36), 16040-16050. DOI: 10.1039/c3ra43091j
French, A. D. (2014). “Idealized powder diffraction patterns for cellulose polymorphs,” Cellulose 21(2), 885-896. DOI: 10.1007/s10570-013-0030-4
Ji, Z., Ma, J., and Xu, F. (2014). “Multi-scale visualization of dynamic changes in poplar cell walls during alkali pretreatment,” Microsc. Microanal. 20(2), 566-576. DOI: 10.1017/s1431927614000063
Kuo, C.-H., and Lee, C.-K. (2009). “Enhancement of enzymatic saccharification of cellulose by cellulose dissolution pretreatments,” Carbohyd. Polym. 77(1), 41-46. DOI: 10.1016/j.carbpol.2008.12.003
Li, X., Wang, S., Duan, L., Hao, J., Li, C., Chen, Y., and Yang, L. (2007). “Particulate and trace gas emissions from open burning of wheat straw and corn stover in China,” Environ. Sci. Technol. 41(17), 6052-6058. DOI: 10.1021/es0705137
Luo, X., and Zhang, L. (2013). “New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system,” Food Res. Int. 52(1), 387-400. DOI: 10.1016/j.foodres.2010.05.016
Miyafuji, H., Miyata, K., Saka, S., Ueda, F., and Mori, M. (2009). “Reaction behavior of wood in an ionic liquid, 1-ethyl-3-methylimidazolium chloride,” J. Wood Sci. 55(3), 215-219. DOI: 10.1007/s10086-009-1020-x
Oh, S. Y., Yoo, D. I., Shin, Y., Kim, H. C., Kim, H. Y., Chung, Y. S., Park, W. H., and Youk, J. H. (2005). “Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of x-ray diffraction and FTIR spectroscopy,” Carbohyd. Res. 340(15), 2376-2391. DOI: 10.1016/j.carres.2005.08.007
Pandey, K. K., and Pitman, A. J. (2003). “FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi,” Int. Biodeter. Biodegr. 52(3), 151-160. DOI: 10.1016/s0964-8305(03)00052-0
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., Frederick, W. J., Jr., Hallett, J. P., Leak, D. J., Liotta, C. L., Mielenz, J. R., Murphy, R., Templer, R., and Tschaplinski, T. (2006). “The path forward for biofuels and biomaterials,” Science 311(5760), 484-489. DOI: 10.1126/science.1114736
Rollin, J. A., Zhu, Z., Sathitsuksanoh, N., and Zhang, Y. H. (2011). “Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia,” Biotechnol. Bioeng. 108(1), 22-30. DOI: 10.1002/bit.22919
Sathitsuksanoh, N., George, A., and Zhang, Y. H. P. (2013). “New lignocellulose pretreatments using cellulose solvents: A review,” J. Chem. Technol. Biot. 88(2), 169-180. DOI: 10.1002/jctb.3959
Sathitsuksanoh, N., Zhu, Z., Wi, S., and Zhang, Y. H. (2011). “Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass,” Biotechnol. Bioeng. 108(3), 521-529. DOI: 10.1002/bit.22964
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2011). “Determination of structural carbohydrates and lignin in biomass,” Laboratory analytical procedure NREL/TP-510-42618 National Renewable Energy Laboratory, Golden, Colorado, USA,
Sun, L., Li, C., Xue, Z., Simmons, B. A., and Singh, S. (2013). “Unveiling high-resolution, tissue specific dynamic changes in corn stover during ionic liquid pretreatment,” RSC Advances 3(6), 2017-2027. DOI: 10.1039/C2RA20706K
Tadesse, H., and Luque, R. (2011). “Advances on biomass pretreatment using ionic liquids: An overview,” Energy & Environmental Science 4(10), 3913-3929. DOI: 10.1039/c0ee00667j
Wang, Q., Wei, W., Kingori, G., and Sun, J. (2015). “Cell wall disruption in low temperature NaOH/urea solution and its potential application in lignocellulose pretreatment,” Cellulose 22(6), 3559-3568. DOI: 10.1007/s10570-015-0767-z
Wang, Q., Zhu, Q., Xu, J., and Sun, J. (2014). “Combined mechanical destruction and alkaline pretreatment of wheat straw for enhanced enzymatic saccharification,” BioResources 9(4), 6841-6850. DOI: 10.15376/biores.9.4.6841-6850
Zhang, X., Ma, J., Ji, Z., Yang, G.-H., Zhou, X., and Xu, F. (2014). “Using confocal raman microscopy to real-time monitor poplar cell wall swelling and dissolution during ionic liquid pretreatment,” Microsc. Res. Techniq. 77(8), 609-618. DOI: 10.1002/jemt.22379
Zhang, Y. H., Cui, J., Lynd, L. R., and Kuang, L. R. (2006). “A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure,” Biomacromolecules 7(2), 644-648. DOI: 10.1021/bm050799c
Zhao, Y. L., Wang, Y., Zhu, J. Y., Ragauskas, A., and Deng, Y. L. (2008). “Enhanced enzymatic hydrolysis of spruce by alkaline pretreatment at low temperature,” Biotechnol. Bioeng. 99(6), 1320-1328. DOI: 10.1002/bit.21712
Zhou, J., and Zhang, L. (2000). “Solubility of cellulose in NaOH/urea aqueous solution,” Polym. J. 32(10), 866-870. DOI: 10.1295/polymj.32.866
Article submitted: July 28, 2015; Peer review completed: November 1, 2015; Revised version received and accepted: November 5, 2015; Published: November 19, 2015.
DOI: 10.15376/biores.11.1.482-491
