Abstract
Birch veneers were coated with Ni-P films by a combined process of KBH4 activation and electroless plating. The plated veneers were further chemically corroded to obtain hydrophobic surfaces on wood. The effect of chemical corrosion on the contact angle of the veneers was investigated. The hydrophobic veneers were characterized by X-ray photo electron spectroscopy (XPS), scanning electron microscopy (SEM), and X-ray diffraction (XRD). The surface contact angle of birch veneer before and after it was plated with Ni-P alloy coating was 41º and 121º, respectively. The contact angle reached 136.7º when the nickel-coated veneers were corroded in CuSO4 aqueous solution for 30 min. XPS analysis showed that Cu0 cluster doped with little CuO formed on the corroded surface of Ni-P alloy film after chemical corrosion. SEM and XRD showed that rough copper clusters formed on the surface of the wood veneer and revealed the reason of the surface hydrophobicity. This study provides a new pathway for fabricating hydrophobic wood.
Download PDF
Full Article
Fabrication of Hydrophobic Surface on Wood Veneer via Electroless Nickel Plating Combined with Chemical Corrosion
Zhaojun Tang, Changhong Shi, Shu Wu, Zengfu Jiang, and Lijuan Wang *
Birch veneers were coated with Ni-P films by a combined process of KBH4 activation and electroless plating. The plated veneers were further chemically corroded to obtain hydrophobic surfaces on wood. The effect of chemical corrosion on the contact angle of the veneers was investigated. The hydrophobic veneers were characterized by X-ray photo electron spectroscopy (XPS), scanning electron microscopy (SEM), and X-ray diffraction (XRD). The surface contact angle of birch veneer before and after it was plated with Ni-P alloy coating was 41º and 121º, respectively. The contact angle reached 136.7º when the nickel-coated veneers were corroded in CuSO4 aqueous solution for 30 min. XPS analysis showed that Cu0 cluster doped with little CuO formed on the corroded surface of Ni-P alloy film after chemical corrosion. SEM and XRD showed that rough copper clusters formed on the surface of the wood veneer and revealed the reason of the surface hydrophobicity. This study provides a new pathway for fabricating hydrophobic wood.
Keywords: Birch veneer; Hydrophobicity; Electroless plating; Chemical corrosion; Contact angle
Contact information: Key Laboratory of Bio-based Material Science and Technology of Ministry of Education, Northeast Forestry University, 26 Hexing Road, Harbin 150040, China;
* Corresponding author: donglinwlj@163.com
INTRODUCTION
Wood is a renewable, natural composite material with many superior properties, including high strength-to-mass ratio, low thermal conductance, and a beautiful natural grain (Jiang et al. 2014). However, one of the key potential disadvantages of pristine wood is its hydrophilicity, which is due to its cellulose, hemicellulose, and lignin components (Devi et al. 2003). High hydrophilicity results in distortion and damage by fungi and termites, which limits its applications and decreases the service life of wood or wood-based products. To hinder water adsorption, hydrophobic surfaces are fabricated on wood via physical or chemical modifications such as acetylation and silylation (Mohammed-Ziegler et al. 2008), polymer coating (Magalhaes and de Souza 2002; Fu et al. 2012), nanocrystal coating (Li et al. 2010b; Sun et al. 2010), or other methods (Shi et al. 2014; Zheng et al. 2015).
Electroless plating is a method for depositing metals such as nickel and copper onto an insulating substrate through the catalyzed chemical reduction of solution-phase metal ions at the substrate surface (Fujii et al. 2014). Nickel, copper, and nickel-based alloy coatings have been deposited onto wood surfaces to prepare electromagnetic shielding materials (Wang et al. 2005, 2011; Li et al. 2010a; Wang and Liu 2011; Wang and Li 2013). The coatings are uniform and continuous, exhibiting high conductivity and electromagnetic shielding effectiveness. However, fabrication of hydrophobic wood surfaces via electroless plating has not been reported.
In this study, nickel films were plated on birch veneer via a simple process. The plated veneers were immersed in a copper ion solution for chemical corrosion, which resulted in a rough surface. The effects of corrosion time on the hydrophobicity were investigated. The surface changes were characterized by XPS, SEM, and XRD. This study provided a new pathway to improve dimensional stability for application in electro-conductive and electromagnetic shielding fields.
EXPERIMENTAL
Materials
Birch wood veneers with a thickness of 0.6 mm were purchased from a plywood factory. The veneers were polished by emery papers to remove fine fibers or dust on the surface and cut into squares of 50 mm × 50 mm. Only analytical grade chemicals were used. The composition of the electroless bath and the operation conditions are listed in Table 1. The pH of the bath was adjusted with ammonium hydroxide (NH4OH) solution.
Activation and plating procedure
Birch veneers were first dipped in a potassium borohydride (KBH4) solution containing 3 g/L sodium hydroxide (NaOH) for 10 min at room temperature. The veneers were air-dried for 1 min to allow KBH4 to diffuse to the inner pores. Next, veneers were placed in the plating bath at 70 °C. The plated specimens were rinsed in water for 1 h and dried at 100 °C for 1 h.
Table 1. Composition and Operation Condition of Electroless Nickel Plating

Corrosion procedure
The plated birch veneers were immersed into a copper sulfate (CuSO4) solution (0.25 M) for a set amount of time, washed with water to remove residues, and dried in an oven at 100 °C for 1 h. This procedure is illustrated in Fig. 1.
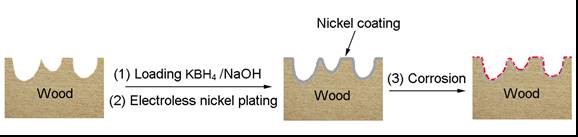
Fig. 1. Fabrication of hydrophobic surface on birch veneer
Characterization Methods
The chemical composition and state of coatings before and after chemical corrosion were analyzed by X-ray photoelectron spectroscopy (XPS, model PHI 5700 ESCA System, America Physical Electronic Company, USA) after etching by argon ion sputtering for 30 s.
The surface morphologies of the samples were determined by scanning electron microscopy (SEM, Quanta 200, Philips-FEI Co., The Netherlands). The phase structures of the birch veneers after various treatments were investigated via X-ray diffraction (XRD, D/max2200 diffractometer, Rigaku, Japan) using a Cu Kα radiation generator operated at 1200 W (40 kV × 30 mA).
Measurement of contact angle
The wettability of the samples was determined by the sessile drop method using a contact angle measurer (OCA20, Dataphysics Company, Germany). A 20-µL drop of distilled water was placed on the film surface. The angle between the drop and the film surface was measured.
RESULTS AND DISCUSSION
Effect of CuSO4·5H2O on Corrosion Time
Corrosion time is an important parameter. The plated birch veneers were corroded in CuSO4·5H2O solution to obtain roughness on the surface. As shown in Fig. 2, the surface contact angle of the nickel plated wood was 121° and the surface contact angle increased from 130.2 to 136.7° with an increase in corrosion time from 20 to 30 min. Then the contact angle decreased from 136.7 to 130.1° with further corrosion time. The maximum value occurred at 30 min.
The purpose of chemical corrosion is to obtain roughness by breaking the continuous nickel film. Suitable corrosion time results in a rough microstructure for the fabrication of the hydrophobic surface. In the time range from 20 min to 40 min, longer corrosion time erodes too much of the deposited nickel film, which leads to fabrication failure.
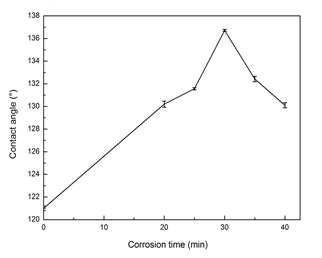
Fig. 2. The effects of corrosion time on hydrophobicity
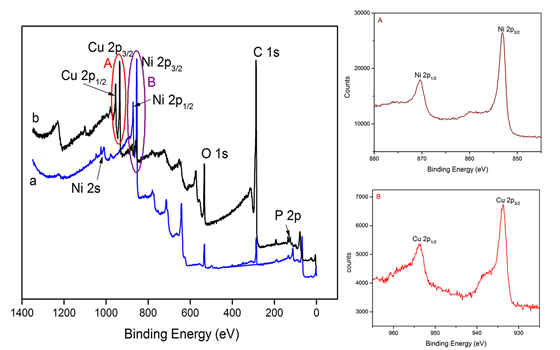
Fig. 3. XPS spectra of Ni-P alloy-coated birch veneer before (a) and after (b) corrosion in CuSO4 aqueous solution
XPS Analysis
XPS analysis was used to examine the chemical state of the coating. Representative XPS wide spectra of the plated veneer surface before and after chemical corrosion indicated the presence of Cu, P, O, and C (Fig. 3).
The peaks of Ni2P3/2 and Ni2P1/2 were stronger and thinner in the XPS high-resolution scan of Ni2p (Fig. 3A), showing that Ni0 is the main component of the coating. Therefore, the Ni-P alloy coating was successfully deposited on birch veneer by the following set of reactions:
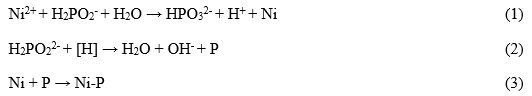
Because XPS only detects to a depth of several nanometers, the observed C and O came from the surface of the coating, suggesting that the surface was polluted by absorbed components in the plating solution.
After corrosion in Cu2+ solution, the XPS spectrum showed decreased Ni peaks, but Cu, O, and C increased noticeably. There was a shoulder peak at 935.88 eV in the XPS high-resolution scan of Cu2p (Fig. 3B), which indicated that Cu2+ was present. In addition, O and C came from the wood surface because the Ni-P coating under Cu clusters became rougher, and wood elements were detected at the thinner part after etching by argon ion sputtering for 30 s.
These observations further demonstrated that chemical corrosion caused the roughness on the surface.
SEM Observation
The nickel-plated birch veneer was immersed in CuSO4 aqueous solution to obtain rough copper clusters on the surface of the nickel coating. Copper clusters were deposited following the chemical reaction below:
![]()
Figure 4 shows the morphologies of nickel-coated wood veneer before and after corrosion in CuSO4 aqueous solution. A compact and continuous nickel coating was deposited on the wood surface, and the surface was entirely covered by the coating. After corrosion, the morphology changed. Some rough clusters built from rod-shaped particles formed on the surface. The increased roughness favors increased hydrophobicity on the surface.
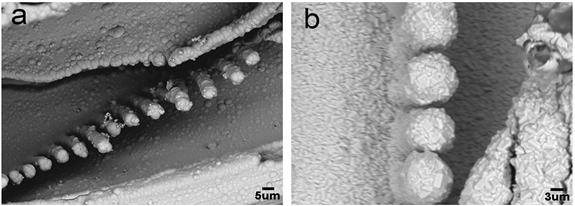
Fig. 4. SEM photographs of nickel-plated birch veneer (a) before and (b) after corrosion in CuSO4 aqueous solution
XRD Analysis
Figure 5 shows the XRD patterns of birch veneers after various processes. The peak at 2θ = 22.02° is the characteristic peak of cellulose in birch veneer.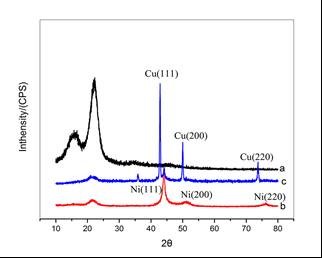
Fig. 5. XRD patterns of (a) the birch veneer, (b) the plated birch veneer, and (c) the corroded birch veneer
In the case of birch veneers coated with a nickel layer, the peaks at 2θ = 44.11°, 51.10°, and 76.41° (Kong et al. 2002) can be attributed to Ni (111), Ni (200), and Ni (220), respectively, which indicates the face-centered cubic phase of nickel. Moreover, the peak at 2θ = 22.02° became weaker, indicating that the birch veneer surface was entirely covered by the continuous nickel layer. After chemical corrosion, the new peaks at 2θ = 42.78°, 49.98°, and 73.81° were attributed to Cu (111), Cu (200), and Cu (220), which show the face-centered cubic phase of copper (Sun et al. 2012). Moreover, the characteristic peak of Ni (111) became weaker, demonstrating that the nickel layer was successfully corroded. Furthermore, the intensity of the peak at 2θ = 22.02° did not change; thus, the corrosion did not break the continuity of the nickel coating on wood surface. All XRD results were in agreement with the SEM photographs.
Comparison of Wettability
The wettability of pristine birch veneer and nickel-coated birch veneers before and after chemical corrosion is shown in Fig. 6. The surface of pristine wood was highly hydrophilic, with a contact angle of 40°. After nickel plating, the contact angle rose to 121°. The contact angle further increased to 136° after chemical corrosion. While water droplets spread on the surface of pristine wood, they adopted spherical shapes on treated surfaces.
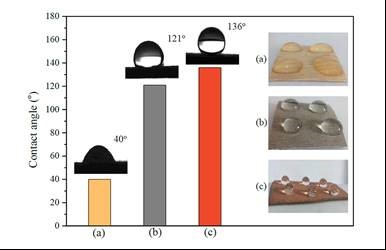
Fig. 6. The contact angle and water droplet photos of (a) pristine birch veneer and nickel-coated birch veneers (b) before and (c) after chemical corrosion
CONCLUSIONS
- Hydrophobic surfaces on birch wood were successfully obtained by electroless nickel plating followed with chemical corrosion.
- The corrosion time in Cu2+ solution was optimized to increase hydrophobicity on the plated surface. The contact angle increased to 136.7° after 30 min of corrosion.
- XPS, SEM, and XRD results show that the chemical corrosion produced rough copper clusters on the surface, which markedly improved surface hydrophobicity.
- This report demonstrates a facile and well-controlled method to fabricate hydrophobic surfaces on wood materials.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the National Innovation Experiment Program for University Students at Northeast Forestry University (NEFU) (201410225016).
REFERENCES CITED
Devi, R. R., Ali, I., and Maji, T. K. (2003). “Chemical modification of rubber wood with styrene in combination with a crosslinker: Effect on dimensional stability and strength property,” Bioresour. Technol. 88(3), 185-188. DOI: 10.1016/S0960-8524(03)00003-8
Fu, Y. C., Li, G., Yu, H. P., and Liu, Y. X. (2012). “Hydrophobic modification of wood via surface-initiated ARGET ATRP of MMA,” Appl. Surf. Sci. 258(7), 2529-2533. DOI: 10.1016/j.apsusc.2011.10.087
Fujii, S., Hamasaki, H., Takeoka, H., Tsuruoka, T., Akamatsu, K., and Nakamura, Y. (2014). “Electroless nickel plating on polymer particles,” J. Colloid Interf. Sci. 430, 47-55. DOI: 10.1016/j.jcjs.2014.05.041
Jiang, J. X., Li, J. Z., and Gao, Q. (2014). “Effect of flame retardant treatment on dimensional stability and thermal degradation of wood,” Constr. Build. Mater. 75, 74-81. DOI: 10.1016/j.conbuildmat.2014.10.037
Li, J., Wang, L. J., and Liu, H. B. (2010a). “A new process for preparing conducting wood veneers by electroless nickel plating,” Surf. Coat. Technol. 204(8), 1200-1205. DOI: 10.1016/j.surfcoat.2009.10.032
Li, J., Yu, H. P., Sun, Q.F., Liu, Y. X., Cui, Y. Z., and Lu, Y. (2010b). “Growth of TiO2 coating on wood surface using controlled hydrothermal method at low temperatures,” Appl. Surf. Sci. 256(16), 5046-5050. DOI: 10.1016/j.apsusc.2010.03.053
Magalhaes, W. L. E. and de Souza, M. F. (2002). “Solid softwood coated with plasma-polymer for water repellence,” Surf. Coat. Technol. 155(1), 11-15.
Mohammed-Ziegler, I., Tánczos, I., Hórvölgyi, Z., and Agoston, B. (2008). “Water-repellent acylated and silylated wood samples and their surface analytical characterization,” Colloids Surf. A 319(1-3), 204-212. DOI: 10.1016/j.colsurfa.2007.06.063
Shi, Y. L., Yang, W., Bai, J. J., Feng, X. J., and Wang, Y. S. (2014). “Fabrication of flower-like copper film with reversible superhydrophobicity–superhydrophilicity and anticorrosion properties,” Surf. Coat. Technol. 253, 148-153. DOI: 10.1016/j.surfcoat.2014.05.027
Sun, Q. F., Yu, H. P., Liu, Y. X., Li, J. A., Lu, Y., and Hunt, J. F. (2010). “Improvement of water resistance and dimensional stability of wood through titanium dioxide coating,” Holzforschung 64(6), 757-761. DOI: 10.1515/HF.2010.114
Sun, L.L., Li, J., and Wang, L. J. (2012). “Electromagnetic interference shielding material from electroless copper plating on birch veneer,” Wood Sci. Technol. 46(6), 1061-1071. DOI: 10.1007/s00226-012-0466-y
Wang, L. J., and Liu, H. B. (2011). “Electroless nickel plating on chitosan-modified wood veneer,” BioResources 6(2), 2035-2044. DOI: 10.15376/biores.6.2.2035-2044
Wang, L. J., and Li J. (2013). “Electromagnetic-shielding, wood-based material created using a novel electroless copper plating process,” BioResources 8(3), 3414-3425. DOI: 10.15376/biores.8.3.3414-3425
Wang, L. J., Li, J., and Liu, Y. X. (2005). “Surface characteristics of electroless nickel plated electromagnetic shielding wood veneer,” J. For. Res. 16(3), 233-236.
Wang, L. J., Li, J., and Liu, H. B. (2011). “A simple process for electroless plating nickel–phosphorus film on wood veneer,” Wood Sci. Technol. 45(1), 161-167. DOI: 10.1007/s00226-010-0303-0
Zheng, R. B., Tshabalala, M. A., Li, Q. Y., and Wang, H. Y. (2015). “Construction of hydrophobic wood surfaces by room temperature deposition of rutile (TiO2) nanostructures,” Appl. Surf. Sci. 328, 453-458. DOI: 10.1016/j.apsusc.2014.12.083
Article submitted: September 22, 2015; Peer review completed: November 16, 2015; Revised version received: November 23, 2105; Accepted: November 24, 2015; Published: December 3, 2015.
DOI: 10.15376/biores.11.1.1007-1014
