Abstract
The antioxidant activity, total polyphenol content (TPC), and total flavonoids content (TFC) of sea buckthorn juice was analyzed with electron paramagnetic resonance (EPR) and ultraviolet-visible (UV-VIS) in ethanol, methanol, and acetone extracts. The choice of a suitable solvent system is necessary so as not to skew the results. Undiluted juice of sea buckthorn berries is not suitable for the mentioned analyzes. Sea buckthorn juices were evaporated under vacuum until completely dry and then dissolved in 100% methanol, 96% ethanol, 70% ethanol, and 50% acetone. The 70% ethanol extract of sea buckthorn juice had an average of 1.3- and 1.6-times greater TPC and TFC values than other extracts, respectively. The 70% ethanol extract of juice contained 29 mg gallic acid equivalents (GAE)/g dw and 4 mg catechin equivalents (CE)/g dw. The results of the antioxidant activity of the extracts determined by EPR spectroscopy had an error rate ~ 2.5 times lower than the UV-VIS analysis. The highest antioxidant activity (123 mmol of Trolox equivalents/kg extract) was determined with EPR and ABTS radical in the 70% ethanol extract. This method correlated well with the TFC levels.
Download PDF
Full Article
Total Content of Polyphenols, Flavonoids, Rutin, and Antioxidant Activity of Sea Buckthorn Juice
František Kreps,a Blanka Tobolková,b Zuzana Ciesarová,b Marianna Potočňáková,a Lívia Janotková,a Svetlana Schubertová,a Aleš Ház,c Štefan Schmidt,a and Michal Jablonský c,*
The antioxidant activity, total polyphenol content (TPC), and total flavonoids content (TFC) of sea buckthorn juice was analyzed with electron paramagnetic resonance (EPR) and ultraviolet-visible (UV-VIS) in ethanol, methanol, and acetone extracts. The choice of a suitable solvent system is necessary so as not to skew the results. Undiluted juice of sea buckthorn berries is not suitable for the mentioned analyzes. Sea buckthorn juices were evaporated under vacuum until completely dry and then dissolved in 100% methanol, 96% ethanol, 70% ethanol, and 50% acetone. The 70% ethanol extract of sea buckthorn juice had an average of 1.3- and 1.6-times greater TPC and TFC values than other extracts, respectively. The 70% ethanol extract of juice contained 29 mg gallic acid equivalents (GAE)/g dw and 4 mg catechin equivalents (CE)/g dw. The results of the antioxidant activity of the extracts determined by EPR spectroscopy had an error rate ~ 2.5 times lower than the UV-VIS analysis. The highest antioxidant activity (123 mmol of Trolox equivalents/kg extract) was determined with EPR and ABTS radical in the 70% ethanol extract. This method correlated well with the TFC levels.
Keywords: Sea buckthorn; Folin-Ciocalte; Antioxidant; DPPH; ABTS; EPR; UV-VIS; Polyphenols; Flavonoids; Rutin
Contact information: a: Slovak Technical University, Department of Food Science and Technology; b: National Agricultural and Food Center, Food Research Institute, Priemyselná 5394/4, 821 08 Bratislava, Slovak Republic; c: Department of Wood, Pulp, and Paper, Radlinského 9, Bratislava, 812 37, Slovak Republic; *Corresponding author: michal.jablonsky@stuba.sk
GRAPHICAL ABSTRACT
INTRODUCTION
Sea buckthorn (Hippophae rhamnoides L.) is a drought-hardy, deciduous shrub that belongs to the family Elaeagnaceae. It is characterized by thorny branches, yellow flowers, and yellow to orange fruits, occurring only on female trees (Li and Beveridge 2003; Burčová et al. 2017). Sea buckthorn has been used in traditional folk medicine to treat various diseases since ancient times, and its effectiveness has been attributed to the presence of large amounts of bioactive substances, which it synthesizes in all parts of the plant (Liu et al. 2017). The berries from sea buckthorn are mainly used for the food industry. The two most common products derived from the sea buckthorn berries are juice from the fleshy tissue of the berries and oil produced from the seeds of the berries. The juice extracted from the berries is high in suspended solids and very high in vitamin C (1.4 to 30.0 g/kg), phytosterols (13.0 to 20.0 g/kg), and carotenoids (0.1 to 0.4 g/kg) (Ciesarová et al. 2020). The total concentration of flavonoids in sea buckthorn berries range from 1.7 to 8.6 g/kg fresh weight (Heinaaho and Julkunen-Tiitto 2011). In sea buckthorn, gallic acid in free form is dominant with variable concentrations in the leaves (79.0 mg/kg) and berries (16.9 mg/kg). Other phenolic acids, such as caffeic acid, p-coumaric acid, and ferulic acid of sea buckthorn were reported in lower amounts (Bittová et al. 2014; Ciesarová et al. 2020). Flavonoids, hydrolysable tannins, and phenolic acids are responsible for many of sea buckthorn’s bioactive and antioxidant properties (Chen et al. 2013; Ciesarová et al. 2020).
To determine the antioxidant activity of plant extracts, spectroscopic methods including electron paramagnetic resonance (EPR) and ultraviolet-visible (UV-Vis) spectroscopy are used. These methods analyze the ability of the extracts to scavenge free radicals such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS). The DPPH spectrophotometric method is one of the most widely applied methods and it is appreciated for its reliability (Sanna et al. 2012). For determining the capacities of antioxidants, the EPR spectroscopy method has been shown to be most reliable with all types of food and biomass matrices, since it is not dependent on the chemical composition of the extract (Sanna et al. 2012; Bartoszek and Polak 2016; Zang et al. 2017). This study compared these techniques and examined their correlation with the content of polyphenols, flavonoids, and rutin to best reflect the antioxidant activity of sea buckthorn berries. In this regard, the paper offers a new perspective on the analysis of the antioxidant potential of plant juices and polyphenol content. According to the present findings, results of the analysis depended mainly on the selection of extraction system before the analysis and only on the method of analysis and the spectrometer.
EXPERIMENTAL
Sea buckthorn (Hippophae rhamnoides L.) berries were obtained from Tvrdošovské zlato (Tvrdošovce, Slovak Republic). Sea buckthorn juice was obtained in the company Tvrdošovské zlato by pressing sea buckthorn berries of the cultivar Leikora. The lipid fraction was not centrifuged. In the production process, the juices were pasteurized at 82 °C in a circulation pasteurizer for 2 to 3 seconds and then immediately filled into plastic containers. The juice contained 102 g of total fat per kg of fresh berries.
Extraction and Sample Preparation
The extraction served as a pre-treatment of the sample, sea buckthorn juice, for further analysis. The extraction process is crucial for the determination of antioxidant capacity, total phenol, flavonoids, and rutin content. Sea buckthorn juice cannot be diluted only with water, because it would not extract a representative sample of antioxidant active substances and at the same time would not dissolve the reactants for the analysis of antioxidant potential. The antioxidant active compounds were extracted from the juice for 1 h using a shaker in the dark. This reduced the color of the sample and improved the reliability of the spectrophotometer analyses. The juice was evaporated overnight under vacuum at 45 °C. The dry residue with a ratio 1:15 was dissolved in 100% methanol, 96% and 70% aqueous ethanol, and 50% aqueous acetone (purity for analysis, Sigma-Aldrich, Slovakia). The extracts were filtered through Filtrak No. 390 filter paper (Munktell & Filtrak GmbH, Bärenstein, Germany).
Determination of Total Phenolic, Flavonoid, Rutin, and Tocopherol Content
According to Constantin et al. (2018), the total polyphenol content (TPC) was determined with Folin-Ciocalteu reagent expressed in gallic acid equivalents (GAE). The total content of flavonoids, expressed as catechin equivalents (CE), was determined by a colorimetric method using aluminum chloride and potassium acetate (Kalita et al. 2013). A Shimadzu UV-3600 (Kyoto, Japan) was used to conduct the UV-VIS-near-infrared analysis. The rutin content in the extracts was analyzed according to Sharma et al. (2005), and the tocopherol content was analyzed according to Burčová et al. (2017) via high-performance liquid chromatography-multiple wavelength detection (HPLC-MWD) with a 1260 Infinity II LC system (Agilent Technologies, Santa Clara, CA, USA).
Analysis of Antioxidant Capacity
The basis of the spectrophotometric method for determining the total antioxidant activity was the quenching of the cationic radical 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+), which was prepared as designed Re et al. (1999). The second radical was DPPH, whose solution in methanol was prepared according to Brand-Williams et al. (1995).
Calculation of Trolox Equivalent Antioxidant Capacity
According to the methodology of Polovka et al. (2010) and Tobolková et al. (2014), the radical-quenching activity of sea buckthorn juice extracts was determined. Results were expressed as the Trolox equivalent antioxidant capacity (TEAC) by EPR and UV-VIS spectrometers, calculated with following equation,
where is Trolox equivalent antioxidant capacity; is the initial concentration (or absorbance) of ABTS•+ or •DPPH solution; is the concentration (or absorbance) ABTS•+ or •DPPH solution after the addition into sample extract, determined in chosen time t (10.5 min); is the volume of •DPPH (ABTS•+) solution added to the system; is the stoichiometric response coefficient of ABTS • + or • DPPH with Trolox, which in both cases takes the value 𝜈 = 0,5; and is the dilution factor.
TEAC values were calculated for each sea buckthorn juice extract, for each of the reactions with ABTS or DPPH radical. TEAC values from electron paramagnetic resonance measurements were calculated for ABTS or DPPH values from the double integrals of their EPR spectra. TEAC values from UV/VIS were calculated from the absorbance of the respective solutions at 515 nm (DPPH) and 730 nm (ABTS).
Statistical analysis was carried out using Statgraphics Plus, software version 3.0 for Windows (Manugistic Inc., USA). The analysis was carried out three times with two from two batches of Sea buckthorn juice. The mean value of the measured data was determined with confidence interval (95 %), calculated with a single-variable analysis of data. The correlation factor between variables were obtained with correlation analysis.
RESULTS AND DISCUSSION
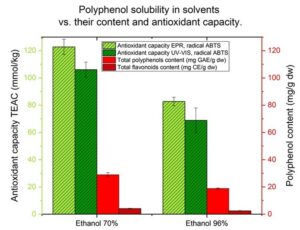
The total polyphenolic content was found to be directly dependent on the extractant and the water content of the solvent (Fig. 1). New findings are that the selection of a suitable solvent for extraction before analysis had more effect on the result than the choice of methods or spectrometer. According to Bartoszek and Polak (2016), Do et al. (2014), Staško et al. (2007), and Turkmen et al. (2006), various solvent combinations were selected and the water content was varied from 20 to 50%. According to mentioned authors, extract obtained from 20 to 50% water had the greatest antioxidant activity and polyphenol content. Such findings can be attributed to the solubility of polar polyphenols in a polar solvent. However, not all antioxidants are soluble only in a polar solvent. Tocopherols are easily dissolved in lipid, while rutin does not dissolve in water (Sun et al. 2007). The selection of a suitable solvent is therefore crucial for the determination of antioxidant capacity, total phenol, flavonoids, and rutin content. Improper solvent selection causes skewed results and undiluted juice of berries is not suitable for the mentioned analyzes. The present study examined which solvent system was more effective to extract polyphenols of sea buckthorn juice and evaluated the antioxidant activity. In 70% aqueous ethanol, the antioxidant activity was 28.97 mg GAE/g dw, which was the greatest content of total polyphenols in the obtained extract. In the 50% aqueous methanol extract, the antioxidant activity was 25.65 mg GAE/g dw. A similar trend can be observed in Fig. 1 regarding the flavonoids and rutin contents in the extracts. The 100% methanol solution was the second-best extract for flavonoids and rutin. In the 70% aqueous ethanol solution, the antioxidant activity was determined to be 4.13 mg CE/ g dw total flavonoids and 1.56 mg rutin/ g dw. According to Turkmen et al. (2006), extracts with the greatest total polyphenols were obtained in mate tea (132.5 mg GAE/g) and black tea (131.9 mg GAE/g) by using 50% dimethylformamide (DMF) and 50% aqueous acetone, respectively. Turkmen et al. (2007) found that all the 50% solvent extracts of black tea contained higher amounts of polyphenols, compared to their corresponding neat ones. The research in this study on sea buckthorn juice showed different results.
Fig. 1. Polyphenol content in the extracts obtained from sea buckthorn juice
The 70% aqueous ethanol solution was richer in polyphenol content than the 50% solvent extracts and their corresponding neat ones (Fig. 1). This trend can be explained by the fact that sea buckthorn juice is rich in polyphenols such as rutin and tocopherols. HPLC analysis confirmed that sea buckthorn juice contained 1.56 mg/g dw rutin and 1.01 mg/g tocopherols. Tocopherols are easily dissolved in lipid and do not dissolve in water. According to Sun et al. (2007), rutin is best dissolved in 70% aqueous ethanol. These findings match the results in Fig. 1. It can also be assumed that sea buckthorn juice is rich in phenolic compounds so it may possess more phenol groups or complex phenolic compound. These have higher molecular weights than the phenolic acids dissolved in the water extract. Do et al. (2014) had similar findings regarding the extract of Limnophila aromatica.
The extraction served as a pre-treatment of the sample before the analysis of the antioxidant capacity. This reduced the color of the sample and improved the analyzer with EPR spectrometers, but especially with UV-VIS. The results in Fig. 2 show that the highest antioxidant activity of extracts was determined with EPR. The EPR analysis also had an average of 2.5 times lower determination errors than the results obtained from the UV-VIS analysis. Other studies similarly found that EPR spectroscopy is the more adequate method for determining the TEAC values of fruit juices, drinks, and nectars (Sanna et al. 2012; Bartoszek and Polak 2016). The greatest antioxidant capacity, 122.8 mmol of Trolox equivalents/mg, was measured with EPR and ABTS radical in 70% aqueous ethanol extract of sea buckthorn juice. These results are in good correlation with publications that have examined antioxidant capacities of fruit juices, drinks, and nectars (Bartoszek and Polak 2016; Tobolková et al. 2017). As can be seen in Fig. 2, the extract with 50% aqueous methanol contained less antioxidant actives than 100% methanol, or 70% or 96% ethanol. This corresponded to the results from this study, illustrated by the total flavonoid content (TFC) and the rutin content in Fig. 1. Similarly, Staško et al. (2007) showed that in reaction media with high water content, aggregation phenomena of the DPPH molecules can occur, which reduces the availability of DPPH in the reaction with the antioxidants.
Fig. 2. Influence of different methods of analysis on the antioxidant capacity of sea buckthorn juice
Smaoui et al. (2019) compared the antioxidant activity of many extracts from pomegranate peel, and the highest antioxidant activity was found in a non-polar, hexane extract. This illustrated that each biomass is unique, and antioxidant active substances do not have to be just polar substances. This study confirmed that the content of polyphenols and antioxidant activity are related, and some methods of analysis have a stronger correlation than others (Fernández-Pachón et al. 2004; Kiselova et al. 2006; Paśko et al. 2009).
As shown in Table 1, the TFC had a strong positive relationship with the TEAC values determined using the cationic radical EPRABTS (r = 0.978) and UV-VISABTS (r = 0.967). Silva and Sirasa (2018) concluded that a strong correlation between vitamin C, TPC, and TFC with FRAP and DPPH in fruit showed their contribution to antioxidant capacity. The results determined by EPR with cationic radical ABTS (Fig. 2) showed the greatest antioxidant activity. Lissi et al. (1999) also confirmed that the results of antioxidant activity determined by ABTS radical have greater values than DPPH. Furthermore, this study found that the antioxidant capacity using a cationic radical correlated well with the determination of rutin. This may also be confirmed by the good correlation (r = 0.929) of rutin with the TFC (Table 1). In contrast, the correlation analysis revealed that the TPC is not specific enough to estimate the antioxidant activity of sea buckthorn juice. This is also related to the fact that the TPC expresses the total content of all reducing substances in the extract. The results in Table 1 showed that there was a strong positive relationship between the TEAC values measured using the cationic radical ABTS between EPR (EPRABTS) and UV-VIS spectroscopy (UV-VISABTS) at the level of r = 0.997. A similar trend was followed by a test using a radical DPPH, where the correlation between EPR spectroscopy (EPRDPPH) and UV-VIS spectroscopy (UV-VISDPPH) was at the level of r = 0.996.
CONCLUSIONS
- The new knowledge obtained in this work is that the selection of a suitable solvent for extraction is crucial before the determination of antioxidant capacity, total phenol, flavonoids, and rutin content. Only in the second place does it depend on the method used and the choice of spectrometer. The highest TPC levels (29.0 mg GAE/g dw), flavonoid (4.1 mg CE/g dw), and rutin (1.6 mg/g dw) as well as the highest antioxidant activity (123 mmol TEAC/kg) were determined in 70% aqueous ethanol extract of sea buckthorn juice.
- The determination of the TFC in the sea buckthorn juice extracts had a strong correlation (r = 0.978), and the antioxidant activity of sea buckthorn juice was determined by EPR with the cationic radical ABTS. Additionally, the determination of rutin provided a good estimate of the antioxidant activity of the extracts. In contrast, the TPC had a weaker correlation with the antioxidant activity of the extracts.
- Based on the statistical results, the antioxidant stability results determined by EPR can be considered to be more reliable than those determined by UV-VIS.
ACKNOWLEDGMENTS
This publication was supported by the Operational program Integrated Infrastructure within the project: Demand-driven research for the sustainable and innovative food, Drive4SIFood 313011V336, co-financed by the European Regional Development Fund. This publication was also supported by the Slovak Scientific Grant Agency VEGA based on contract no. VEGA 1/0012/19.
REFERENCES CITED
Bartoszek, M., and Polak, J. (2016). “A comparison of antioxidative capacities of fruit juices, drinks and nectars, as determined by EPR and UV–vis spectroscopies,” Spectrochim. Acta, Part A. 153, 546-549. DOI: 10.1016/j.saa.2015.09.022
Bittová, M., Krejzová, E., Roblová, V., Kubáň, P., and Kubáň, V. (2014). “Monitoring of HPLC profiles of selected polyphenolic compounds in sea buckthorn (Hippophaë rhamnoides L.) plant parts during annual growth cycle and estimation of their antioxidant potential,” Cent. Eur. J. Chem. 12(11), 1152-1161. DOI: 10.2478/s11532-014-0562-y
Brand-Williams, W., Cuvelier, M. E., and Berset, C. (1995). “Use of a free radical method to evaluate antioxidant activity,” LWT – Food Sci. Technol. 28(1), 25-30. DOI: 10.1016/S0023-6438(95)80008-5
Burčová, Z., Kreps, F., Schmidt, Š., Jablonský, M., Ház, A., Sládková, A., and Šurina, I. (2017). “Composition of fatty acids and tocopherols in peels, seeds and leaves of sea buckthorn,” Acta Chimica Slovaca 10(1), 29-34. DOI: 10.1515/acs-2017-0005
Chen, C., Xu, X.-M., Chen, Y., Yu, M.-Y., Wen, F.-Y. and Zhang, H. (2013). “Identification, quantification and antioxidant activity of acylated flavonol glycosides from sea buckthorn (Hippophae rhamnoides ssp. sinensis),” Food Chem. 141(3), 1573-1579. DOI: 10.1016/j.foodchem.2013.03.092
Ciesarová, Z., Murkovic, M., Cejpek, K., Kreps, F., Tobolková, B., Koplík, R., Belajová, E., Kukurová, K., Daško, Ľ., Panovská, Z., et al. (2020). “Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review,” Food Res. Int. 133, 109170. DOI: 10.1016/j.foodres.2020.109170
Constantin, O. E., Râpeanu, G., Kukurová, K., Turturică, M., Dubová, Z., Tobolková, B., Daško, L., Ciesarová, Z., and Croitoru, C. (2018). “Antioxidative capacity of and contaminant concentrations in processed plum products consumed in Romania,” J. Food Prot. 81(8), 1313-1320. DOI: 10.4315/0362-028X.JFP-18-066
Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., and Ju, Y.-H. (2014). “Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica,” J. Food Drug Anal. 22(3), 296-302. DOI: 10.1016/j.jfda.2013.11.001
Fernández-Pachón, M. S., Villaño, D., Garcı́a-Parrilla, M. C., and Troncoso, A. M. (2004). “Antioxidant activity of wines and relation with their polyphenolic composition,” Anal. Chim. Acta 513(1), 113-118. DOI: 10.1016/j.aca.2004.02.028
Heinaaho, M., and Julkunen-Tiitto, R. (2011). “Efficient extraction of flavonoids of sea buckthorn berries,” BioChem.: Indian J. 5(2), 83-87.
Kalita, P., Tapan, B. K., Pal, T. K., and Kalita, R. (2013). “Estimation of total flavonoids content (TFC) and anti oxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn,” J. Drug Delivery Ther. 3(4), 33-37. DOI: 10.22270/jddt.v3i4.546
Kiselova, Y., Ivanova, D., Chervenkov, T., Gerova, D., Galunska, B., and Yankova, T. (2006). “Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs, ” Phytother Res. 20(11), 961-965. DOI: 10.1002/ptr.1985
Li, T. S. C., and Beveridge, T. H. J. (2003). Sea buckthorn (Hippophae rhamnoides L.) Production and Utilization, National Research Council of Canada, Ottawa, Ontario.
Lissi, E. A., Modak, B., Torres, R., Escobar, J., and Urzua, A. (1999). “Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods,” Free Radic Res. 30(6), 471-477. DOI: 10.1080/10715769900300511
Liu, Y., Fan, G., Zhang, J., Zhang, Y., Li, J., Xiong, C., Zhang, Q., Li, X., and Lai, X. (2017). “Metabolic discrimination of sea buckthorn from different Hippophaë species by 1H NMR based metabolomics,” Sci. Rep. 7(1), 1585. DOI: 10.1038/s41598-017-01722-3
Paśko, P., Bartoń, H., Zagrodzki, P., Gorinstein, S., Fołta, M. and Zachwieja, Z. (2009). “Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth,” Food Chem. 115(3), 994-998. DOI: 10.1016/j.foodchem.2009.01.037
Polovka, M., Šťavíková, L., Hohnová, B., Karásek, P., and Roth, M. (2010). “Offline combination of pressurized fluid extraction and electron paramagnetic resonance spectroscopy for antioxidant activity of grape skin extracts assessment,” J. Chromatogr. A. 1217(51), 7990-8000. DOI: 10.1016/j.chroma.2010.08.003
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). “Antioxidant activity applying an improved ABTS radical cation decolorization assay,” Free Radic. Biol. Med. 26(9-10), 1231-1237. DOI: 10.1016/S0891-5849(98)00315-3
Sanna, D., Delogu, G., Mulas, M., Schirra, M., and Fadda, A. (2012). “Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV–Vis study,” Food Anal. Methods 5(4), 759-766. DOI:10.1007/s12161-011-9306-1
Sharma, V., Gulati, A., Ravindranath, S. D., and Kumar, V. (2005). “A simple and convenient method for analysis of tea biochemicals by reverse phase HPLC,” J. Food Compos. Anal. 18(6), 583-594. DOI: 10.1016/j.jfca.2004.02.015
Silva, K. D. R. R., and Sirasa, M. S. F. (2018). “Antioxidant properties of selected fruit cultivars grown in Sri Lanka,” Food Chem. 238, 203-208. DOI: 10.1016/j.foodchem.2016.08.102
Smaoui, S., Hlima, H. B., Mtibaa, A. C., Fourati, M., Sellem, I., Elhadef, K., Ennouri, K., and Mellouli, L. (2019). “Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products,” Meat Sci. 158, 107914. DOI: 10.1016/j.meatsci.2019.107914
Staško, A., Brezová, V., Biskupič, S., and Mišík, V. (2007). “The potential pitfalls of using 1,1-diphenyl-2-picrylhydrazyl to characterize antioxidants in mixed water solvents,” Free Radic. Res. 41(4), 379-390. DOI: 10.1080/10715760600930014
Sun, T., Powers, J. R., and Tang, J. (2007). “Effect of enzymatic macerate treatment on rutin content, antioxidant activity, yield, and physical properties of asparagus juice,” J. Food Sci. 72(4), S267-S271. DOI: 10.1111/j.1750-3841.2007.00345.x
Tobolková, B., Belajová, E., Benčičová, M., Jelemenská, V., Kukurová, K., and Ciesarová, Z. (2017). “Antioxidant properties of sea buckthorn and products with its content,” in: Symposium on New Directions of Food Production and Evaluation, Lísek, Czech Republic, pp. 22-24.
Tobolková, B., Polovka, M., Belajová, E., Koreňovská, M., and Suhaj, M. (2014). “Possibilities of organic and conventional wines differentiation on the basis of multivariate analysis of their characteristics (EPR, UV–Vis, HPLC and AAS study),” Eur. Food Res. Technol. 239(3), 441-451. DOI: 10.1007/s00217-014-2237-5
Turkmen, N., Sari, F., and Velioglu, Y. S. (2006). “Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods,” Food Chem. 99(4), 835-841. DOI: 10.1016/j.foodchem.2005.08.034
Turkmen, N., Velioglu, Y. S., Sari, F., and Polat, G. (2007). “Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea,” Molecules 12(3), 484-496. DOI: 10.3390/12030484
Zang, S., Tian, S., Jiang, J., Han, D., Yu, X., Wang, K., Li, D., Lu, D., Yu, A., and Zhang, Z. (2017). “Determination of antioxidant capacity of diverse fruits by electron spin resonance (ESR) and UV–vis spectrometries,” Food Chem. 221, 1221-1225. DOI: 10.1016/j.foodchem.2016.11.036
Article submitted: February 16, 2021; Peer review completed: April 26, 2021; Revised version received and accepted: April 29, 2021; Published: May 6, 2021.
DOI: 10.15376/biores.16.3.4743-4751
