Abstract
Download PDF
Full Article
Experimental Study on the Gasification Characteristics of Biomass with CO2/Air in an Entrained-flow Gasifier
Haimiao Yu,* Geng Chen, Yangyang Xu, and Dezhen Chen
This study explored the gasification characteristics of pine sawdust and rice straw with CO2/air in a bench-scale entrained-flow gasifier. The effects of various gasification parameters, i.e., CO2/C, temperature, and biomass type, on the syngas composition, gasification index, and tar yield were investigated. When compared to air gasification, the CO2/air agent for gasification improved the yield of CO, and it decreased the tar yield and the yield of CO2 produced from biomass. The cold gas efficiency (CGE) of pine sawdust reached 87.06% at the CO2/C equivalence ratio of 0.25, whereas that of rice straw reached 73.35% at the CO2/C equivalence ratio of 0.50. When compared with air gasification, the CO2/air gasification increased the CGE of pine sawdust and rice straw by 4.20% and 9.17%, respectively. However, excessive CO2 was unfavorable to the gasification process. As the temperature increased, the yields of CO and H2 increased, and the tar yield decreased, thus improving the syngas quality. This study indicated that the addition of the proper level of CO2 for gasification improved the overall gasification efficiency. Moreover, the improvement for rice straw (herbaceous plant) was more noteworthy than for pine sawdust (woody plant).
Keywords: Biomass; CO2; Mixed atmosphere; Tar; Gasification
Contact information: Institute of Thermal and Environmental Engineering, Tongji University, No.1239 Siping Road, Shanghai, P. R. China; *Corresponding author: hmyuzj@tongji.edu.cn
INTRODUCTION
In recent years, concerns about the increasing consumption of fossil fuels has promoted the development of unconventional energies (Prabowo et al. 2013). Biomass, as a clean and renewable resource, can partially replace traditional fossil fuel (Pereira et al. 2012). Biomass includes various plant materials, such as forest and agricultural residues (Basu 2010; Senapati and Behera 2012). In developing countries, especially in China, these residues are often thrown away or burned in situ and may lead to energy waste and serious environmental pollution. The process of biomass gasification can solve these problems. The selection of the gasification agent largely affects the result. Commonly used gasification agents include air, oxygen, steam, or a mixture of several gases. Although CO2 is not a popular gasification agent, pure CO2 or CO2-contained mixtures allow the secondary utilization of CO2 by converting CO2 into CO, thus increasing the gasification effect.
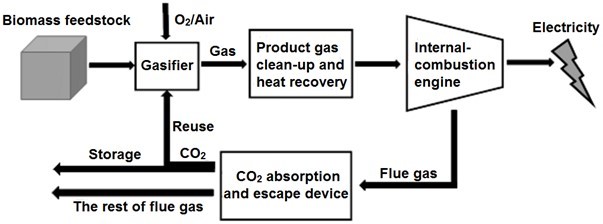
Carbon dioxide that is used in the industry usually comes from high-temperature calcined limestone (CaCO3) or the fermentation of alcohol; however, using CO2 from these processes in gasification is inconvenient and increases the economic burden. It is important to reasonably obtain CO2 and effectively combine it with gasification. In carbon capture and storage (CCS) technologies, CO2 in the flue gas is captured, evolved, liquefied, compressed, and transported to a deep underground storage area for long-term or permanent sequestration. Combined with CCS technology, the CO2 gasification power generation system can reuse CO2 as a gasifying agent, thus tending to reduce the net CO2 emissions (Fig. 1).
Fig. 1. Biomass gasification power generation and CO2-reuse system
Previous studies on CO2 gasification of biomass were mainly focused on the reaction kinetics (Yan et al. 2010; Xiao et al. 2012; Guizani et al. 2013; Cho et al. 2015; Zuo et al. 2015). Thilakavathi et al. (2010) calculated the reaction kinetic parameters of wheat straw char in a CO2 atmosphere using thermogravimetric analysis (TGA) and found that the char-CO2reaction activity increased when the temperature rose from 750 °C to 900 °C. Butterman and Castaldi (2007) found that the biomass gasification reaction characteristics changed in a mixture of CO2 and steam prepared according to different proportions, indicating that the addition of a small quantity of CO2 allowed the most significant improvement in the steam gasification reactivity. The above researchers found that CO2 enhanced the pore structure, particularly the micropores, of the residual carbon skeleton after drying and volatilization, thus efficiently gasifying the solid.
The characteristics of biomass gasification using CO2 are seldom studied. Mei et al. (2010) studied the gasification characteristics of seaweed powder in a small-scale entrained-flow gasifier and explored changes in the gasification characteristics under different O2/CO2 ratios. Under the O2/CO2 gasification conditions, the yield of CO2 decreased with increasing CO2/B ratios and a CO2/B ratio of 0.9 resulted in the highest yields of H2 and CO. These results indicated that the addition of CO2 increased the gasification effect to a certain degree and decreased the energy consumption. Pohorely et al. (2014) added different gases (CO2, H2O, and N2) in O2 gasification according to a certain proportion to explore gasification characteristics of oak wood chips in a fluidized bed at 850 °C under different mixed atmospheres. Compared to the other two conditions (O2/H2O and O2/N2), the O2/CO2 agent allowed the highest carbon conversion efficiency and cold gas efficiency as well as the lowest tar yield. Thus, the addition of CO2 improved the energy conversion and increased the syngas yield. Although experiments have confirmed that adding CO2 improves the gasification process, the trends of gas composition, gasification index, and tar yield in biomass gasification, under the gasification agent of CO2 (pure CO2 or the mixture of CO2 and other agents), are rarely studied when considering different reaction conditions.
This study explored the gasification characteristics of pine sawdust (woody plant) and rice straw (herbaceous plant) with a CO2/air atmosphere in a bench-scale entrained-flow gasifier. The effects of the CO2/C ratio (0 to 1.0), temperature (700 °C to 1100 °C), and the biomass resource type (pine sawdust or rice straw) on the biomass gasification characteristics were investigated. Moreover, this paper provides the proper CO2/C ratio for different biomass resources for practical applications.
EXPERIMENTAL
Materials
Two types of biomass resources were used in the experiment: pine sawdust and rice straw. The biomasses were pulverized and sieved into a particle size of less than 0.3 mm for gasification. The ultimate and proximate analysis results are listed in Table 1.
Table 1. Ultimate and Proximate Analysis of the Biomass Feedstock*
* values as reported on a delivered basis
Fig. 2. Schematic diagram of the gasification system
Experimental Devices and Procedures
Tests were performed in an entrained-flow gasifier that was designed based on the Badzioch-type reactor (Badzioch and Hawksley 1970) with a CO2/air atmosphere. As shown in Fig. 2, the gasification system consisted of the following components: an entrained-flow gasifier, a gas supply system, a temperature control system, a preheater, a biomass feeder, a tar collecting system, and other auxiliary devices. The height of the reaction tube is 600 mm, and its inner diameter is 48 mm. The reactor has eight globars to heat the tube.
During the experiments, biomass particles were cast into the reactor by the feeding gas (N2) at a rate of 4.0 g/min when the temperature reached the set value. At the same time, preheated gas (500 °C), consisting of CO2, N2, and O2, was introduced into the gasifier. The flow rate of O2 was calculated with the equivalence ratio (ER) of 0.25, and the ratio of N2/O2 was adjusted to 79:21 in order to simulate air. After cooling and purification, the producer gas was collected and analyzed with a gas chromatography (GC) analysis system (GC-9160, Shanghai Precision & Scientific Instrument; Shanghai, China), and the tar contained in the flue gas was collected according to the cold trapping method (Claes and Chen 1997). To avoid the tar condensation before being collected, especially heavy tar condensation, the temperature of the collection hopper was retained above 220 °C. Table 2 shows the operating conditions of the experiment.
The gas obtained from gasification was sampled and analyzed by gas chromatography to detect the concentrations of CO, H2, CO2, O2, N2, CH4, and some lighter hydrocarbons, such as C2H4 and C2H6. The yields of different gases were calculated with N2 as a tracer, according to the data obtained from gas chromatography (Qin et al. 2012).
Table 2. Experimental Conditions
Nomenclature and Calculations
The ratio of carbon dioxide to carbon (CO2/C) was defined as:
The carbon conversion efficiency (ηc) was defined as:
In this experiment, partial CO2 in the gasification agent was consumed in some reactions, such as the reaction (8) to be described later, while the remaining CO2 was discharged as gas production. At the same time, CO2 in the syngas had two sources: the gasification agent and gasification reactions. It was difficult to experimentally differentiate the two sources of CO2. Therefore, we introduced the concept of the relative yield of CO2 (G’CO2) in order to compare the amount of consumed CO2 with that of generated CO2. In this way, one can analyze the experimental results better,
The cold gas efficiency (CCE) was defined according to Ravikiran et al. (2012),
where Vsyngas is the total volume of collected gas (Nm3), Mfeedstock is the total mass of used biomass feedstock (kg), and LHVsyngas and LHVfeedstock are the lower heating value (LHV) of the syngas (kJ/Nm3) and the LVH of the biomass feedstock (kJ/kg), respectively.
The gasification tar yield (mg) per cubic meter of syngas was calculated as follows,
Tarp = Mtar / (Mb x t x Gp) (5)
where Tarp is the tar yield (mg/Nm3); Mtar is the total mass of collected tar (mg); Mb is the feeding rate (kg/min); t is the reaction time (min); Gp is the gas yield (Nm3/kg).
RESULTS AND DISCUSSION
The gasification process was essentially the thermal chemical reaction of a fuel at high temperature, mainly involving the following reactions (Butterman and Castaldi 2009):
Effect of CO2/C
The effects of different CO2/C ratios on the syngas compositions of pine sawdust and rice straw were investigated with an ER of 0.25 and reaction temperature of 1000 °C (Fig. 3). As the CO2/C ratio was increased, the yield of CO2 from pine sawdust and rice straw increased considerably, and the yield of CH4 decreased slowly. However, changes in the yield of CO and H2were notably different between the pine sawdust and rice straw. When the CO2/C ratio increased from 0 to 0.75, the CO yield of pine sawdust increased from 0.49 Nm³/kg to 0.61 Nm³/kg, respectively, while the H2 yield remained unchanged. When the CO2/C ratio was increased above 0.75, the yields of CO and H2 began to decline. The maximum CO yield of rice straw (0.37 Nm³/kg) was obtained at the CO2/C ratio of 0.50. The yields of CO and H2 gradually decreased as the ratio of CO2/C increased beyond 0.50.
Fig. 3. Effects of CO2/C on the gas yield of (a) pine sawdust and (b) rice straw at 1000 °C
The addition of CO2 effectively promoted the reaction (8) and reversible reaction (10), increasing the CO gas production yield. According to the results by Basu (2010), hydrogen was mainly derived from the reaction (9) and reaction (10), but the increase of CO2 and CO was not indicative of a conversion towards the right side of two reactions. However, the addition of CO2 weakened the interaction between H2 and the char matrix and increased the H2 fluidity (Pilon and Lavoie 2013). In addition, the specific surface area and pore volume of the char were increased to different degrees in CO2 atmosphere, meaning that the char gasification reaction was promoted more fully (Borrego et al. 2009; Rathnam et al. 2009; Guizani et al. 2013). After a certain amount of CO2 was added, the H2 yield was not much changed for similar reasons. According the results by to Zhou et al. (2009), when the biomass residence time in the entrained flow gasifier was reduced to a certain degree, the gasification reaction was not completed. If a large amount of CO2 is added, the residence time in the furnace is reduced and the gasification reaction is not completed, thus leading to a lower yield of CO and H2. In addition, the residence time required for the complete reaction of pine sawdust was different from that of rice straw due to the differences in the composition and structure of the two biomass materials. Therefore, the CO and H2 yields of pine sawdust and rice straw started to decline under different CO2/C ratios.
As shown in Fig. 4, the CO2 yield decreases with the increase in the CO2/C ratio, indicating that the addition of CO2 had an inhibiting effect on the reaction (7) rate according to the Le Chatelier’s principle. The inhibition effect was consistent with the observations by Ahmed and Gupta (2009). When the CO2/C ratio rose above 0.75, the CO2 yield from the biomass became a negative value. The negative value did not mean that no CO2 was generated during gasification. On the contrary, the negative value meant that the volume of CO2 generated from some reactions, such as the reaction (7) and (10), was less than the volume of CO2 consumed in the other reactions, such as the reaction (8).
Fig. 4. Effects of CO2/C on the relative yield of CO2 at 1000 °C
The effects of CO2/C on the gasification indexes are shown in Fig. 5. When the CO2/C ratio was 0, the lower heating value (LVH) of the syngas produced from pine sawdust and rice straw was 7505 kJ/Nm³ and 6952 kJ/Nm³, respectively. As the CO2/C ratio increased, the value of LHV decreased gradually, and the producer gas yield (PGY) increased. When the CO2/C ratio was 0.75, the PGY of pine sawdust remained at 2.5 Nm³/kg, which was 26.8% higher than that of air gasification. After the CO2/C ratio was increased above 0.25, the PGY of rice straw slowly increased by 8%, from 1.57 Nm³/kg to 1.70 Nm³/kg. The increase in PGY was mainly attributed to the addition of CO2 and the generation of CO.
Fig. 5. Effects of CO2/C on the gasification indexes at 1000 °C
Pine sawdust and rice straw differed in their carbon conversion efficiency and cold gas efficiency under CO2/C infusion. For pine sawdust, under the CO2/C ratio of 0.25, the carbon conversion efficiency reached the maximum value of 99.37% and the cold gas efficiency reached 87.06% with the relative increase of 4.20%. This was followed by a rapid decline in both parameters when the CO2 level was increased. Under the CO2/C ratio of 0.25, the carbon conversion efficiency of rice straw reached its maximum value of 99.62%, which was 7% higher than that of air gasification. Unlike pine sawdust, the CO2/C ratio of 0.50 was optimal for the cold gas efficiency of rice straw, resulting in a maximum value of 73.35%, which was 9.17% higher than that of pure air gasification. Changes in the carbon conversion efficiency can be interpreted as follows. When a small volume of CO2 was used as the gasifying agent, CO2promoted the breaking of benzene rings and the fracturing of hydroxyl, methyl, and methylene groups (Gao et al. 2013), thus increasing the carbon conversion efficiency. After a large amount of CO2 was added, it was not conducive to the reaction (7) toward the right. At the same time, the higher global gas flow rate led to the shorter particle residence time. The above two aspects caused the decrease in the carbon conversion efficiency.
The above results indicated that moderate CO2 addition allowed positive improvements in the cold gas efficiency, PGY, and carbon conversion efficiency. However, considering the inhibition of chemical balance, excessive CO2 limits the carbon conversion efficiency and is not conducive to improving cold gas efficiency.
Effect of the Reaction Temperature
The gasification temperature is an important parameter in the gasification process. Under the following conditions: CO2/C of 0.25 and ER of 0.25, the reaction temperature was increased from 700 °C to 1100 °C to study its effect on the gasification characteristics. The effect of temperatures on the syngas composition is shown in Fig. 6. As the temperature rose, the yields of CO and H2 of pine sawdust and rice straw were considerably higher. When the temperature was 1100 °C, the yields of H2 and CO of pine sawdust reached 0.46 Nm³/kg and 0.64 Nm³/kg, respectively, and the yields of H2 and CO of rice straw reached 0.35 Nm³/kg and 0.41 Nm³/kg, respectively.
Fig. 6. The effect of temperature on the syngas composition of (a) pine sawdust and (b) rice straw at the CO2/C of 0.25
During the gasification process, CO was mainly produced by the cleavage of the ether bonds at temperatures above 700 °C. The fracturing extent of the ether bond was enhanced by higher reaction temperatures. Reaction (8) was an endothermic reaction and its reaction activity would be strengthened with the temperature rise, thus substantially improving the CO yield. Moreover, the rising temperature was more conducive to the endothermic properties of the reaction (9), thus leading to the cleavage of hydrocarbons and production of more free radicals and H2. In this way, the H2 yield was dramatically increased.
The changes in the yield of CO2 with increasing temperature exhibited different tendencies for pine sawdust versus rice straw. As the temperature rose, the CO2 yield of pine sawdust gradually decreased, while the CO2 yield of rice straw slowly increased. These variations might be related to the component characteristics and ash content of the biomass. After the temperature rose above 900 °C, due to the endothermic reduction of reaction (8), the CO2 yields of both pine sawdust and rice straw decreased considerably.
Fig. 7. Effects of temperature on the gasification indexes at the CO2/C of 0.25
The effect of the reaction temperature on the gasification indexes are shown in Fig. 7. The rise in temperature notably improved the carbon conversion efficiency and cold gas efficiency of both pine sawdust and rice straw. When the temperature was increased from 700 °C to 1000 °C, the cold gas efficiencies of pine sawdust and rice straw, respectively, increased from 55.71% and 44.72% to 87.06% and 70.37%, while the carbon conversion efficiencies of pine sawdust and rice straw were increased to the higher level. In addition, the temperature increase also promoted the PGY and LHV of syngas. The PGY of pine sawdust increased by 24%, from 1.84 Nm³/kg to 2.28 Nm³/kg, and the PGY of rice straw increased by 34%, from 1.22 Nm³/kg to 1.63 Nm³/kg.
Analysis of the Tar Yield
The major biomass components include lignin, cellulose, and hemicellulose. Lignin yields more tar than the other two components (Yu et al. 2014). In our experiment, the lignin content (21.42%) in rice straw was less than that (31.75%) in pine sawdust. In addition, the high ash content in rice straw played a greater role in the catalytic cracking of tar. In this experiment, the tar yield of rice straw was less than that of pine sawdust no matter for air or a mixture of CO2 and air as a gasification agent.
The effects of CO2/C and temperature on tar yield are shown in Fig. 8. As the CO2/C ratio was increased, the tar yield of pine sawdust and rice straw gradually decreased, especially for the pine sawdust. The tar yield of pine sawdust under the CO2/C of 0.25 was 1.44 g/Nm³, which was 31% lower than that of air gasification (2.09 g/Nm³). The difference in the tar yield between CO2/air and air gasification can be interpreted as follows. The added CO2 favored reaction (12), in which the tar was cracked to generate CO and H2, as shown in Eq. (12):
Fig. 8. Effects of (a) CO2/C level and (b) the reaction temperature on the tar yield
The effect of temperature on the tar yield was more noteworthy. When the reaction temperature was above 800 °C, the tar yield of pine sawdust and rice straw declined sharply. The amount of tar that was produced from the pine sawdust decreased by 65%, from 7.96 g/Nm³ to 2.82 g/Nm³. The amount of tar that was produced from rice straw decreased by 75%, from 3.22 g/Nm³ to 0.81 g/Nm³. The effect of temperature on the tar yield may be interpreted as follows. As the temperature increases, the cracking reaction rate of the tar components increases, thus prompting tar reforming reactions and converting more primary tar into permanent gases and other small molecules.
CONCLUSIONS
- Adding CO2 drastically improved the CO yield. The addition of the appropriate volume of CO2 improved the cold gas efficiency, PGY, and carbon conversion efficiency. The tar yield declined with the addition of CO2, indicating that CO2 acts as a gasifying agent that inhibits tar generation to a certain degree.
- The effect of the reaction temperature on the gasification characteristics was noteworthy. A higher reaction temperature was conducive for increasing the production of H2 and CO, thus improving the cold gas efficiency, LHV, PGY, and carbon conversion efficiency, and decreasing the tar yield, which indicated that the syngas was high quality.
- This investigation revealed differences in the components and structures of pine sawdust versus rice straw, with respect to their CO2/air gasification characteristics. The PGY and tar yield of pine sawdust was higher than that of rice straw. The cold gas efficiency of pine sawdust was optimal at a CO2/C ratio of 0.25, whereas the cold gas efficiency of rice straw was optimal at a CO2/C ratio of 0.50.
- The proper addition volume of CO2 for the gasification process varies according to the type of biomass. Moreover, improvements in the gasification efficiency, by the addition of CO2 for herbaceous plants (rice straw), was more noteworthy than that for woody plants (pine sawdust).
ACKNOWLEDGMENTS
This research was supported by the China National 863 Plan project (Grant No. 2012AA063504).
REFERENCES CITED
Ahmed, I., and Gupta, A. K. (2009). “Characteristics of cardboard and paper gasification with CO2,” Appl. Energy 86(12), 2626-2634. DOI: 10.1016/j.apenergy.2009.04.002
Badzioch, S., and Hawksley, P. G. W. (1970). “Kinetics of thermal decomposition of pulverized coal particles,” Ind. Eng. Chem. Process Des. Dev. 9(4), 521-530. DOI: 10.1021/i260036a005
Basu, P. (2010). Biomass Gasification and Pyrolysis: Practical Design and Theory, Academic Press, London, UK.
Borrego, A. G., Garavaglia, L., and Kalkreuth, W. D. (2009). “Characteristics of high heating rate biomass chars prepared under N2 and CO2 atmospheres,” Int. J. Coal Geol. 77(3-4), 409-415. DOI: 10.1016/j.coal.2008.06.004
Butterman, H. C., and Castaldi, M. J. (2007). “Influence of CO2 injection on biomass gasification,” Ind. Eng. Chem. Res. 46(26), 8875-8886. DOI: 10.1021/ie071160n
Butterman, H. C., and Castaldi, M. J. (2009). “CO2 as a carbon neutral fuel source via enhanced biomass gasification,” Environ. Sci. Technol. 43(23), 9030-9037. DOI: 10.1021/es901509n
Cho, S. H., Kim, K. H., Jeon, Y. J., and Kwon, E. E. (2015). “Pyrolysis of microalgal biomass in carbon dioxide environment,” Bioresour. Technol.193, 185-191. DOI: 10.1016/j.biortech.2015.06.119
Claes, Y. Q., and Chen, G. (1997). “Use of amino phase absorbent of biomass tar sampling and separation,” Fuel 76(2), 137-142. DOI: 10.1016/S0016-2361(96)00199-8
Gao, S. P., Zhao, J. T., Wang, Z. Q., Wang, J. F., Fang, Y. T., and Huang, J. J. (2013). “Effect of CO2 on pyrolysis behaviors of lignite,” J. Fuel Chem. Technol. 41(3), 257-264. DOI: 10.1016/S1872-5813(13)60017-1
Guizani, C., Sanz, F. J. E., and Salvador, S. (2013). “The gasification reactivity of high-heating-rate chars in single and mixed atmospheres of H2O and CO2,” Fuel 108, 812-823. DOI: 10.1016/j.fuel.2013.02.027
Mei, Q. F., Zhou, J. S., and Chen, Q. (2010). “Experimental study on the effect of different gasification agents on seaweed powder gasification in an entrained flow gasifier,” Energy Engin.0(3):32-36. DOI:10.3969/j.issn.1004-3950.2010.03.007
Pereira, E. G, da Silva, J. N., de Oliveira, J. L., and Machado, C. S. (2012). “Sustainable energy: A review of gasification technologies,” Renew. Sust. Energ. Rev. 16(7), 4753-4762. DOI: 10.1016/j.rser.2012.04.023
Pilon, G., and Lavoi, J. M. (2013). “Pyrolysis of switchgrass (Panicum virgatum L.) at low temperatures within N2 and CO2 environments: Product yield study,” ACS Sustain. Chem. Eng. 1(1), 198-204. DOI: 10.1021/sc300098e
Pohorely, M., Jeremias, M., Svoboda, K., Kamenikova, P., Skoblia, S., and Beno, Z. (2014). “CO2 as moderator for biomass gasification,” Fuel 117(Part A), 198-205. DOI: 10.1016/j.fuel.2013.09.068
Prabowo, B., Umeki, K., Yan, M., Nakamura, M. R., Castaldi, M. J., and Yoshikawa, K. (2013). “CO2-steam mixture for direct and indirect gasification of rice straw in a downdraft gasifier: Laboratory-scale experiments and performance prediction,” Appl. Energy 113(6), 670-679. DOI:10.1016/j.apenergy.2013.08.022
Qin, K., Lin, W. G., Jensen, P. A., and Jensen, A. D. (2012). “High-temperature entrained flow gasification of biomass,” Fuel 93, 589-600. DOI: 10.1016/j.fuel.2011.10.063
Rathnam, R. K., Elliott, L. K., Wall, T. F., Liu, Y. H., and Moghtaderi, B. (2009). “Differences in reactivity of pulverised coal in air (O2/N2) and oxy-fuel (O2/CO2) conditions,” Fuel Process. Technol. 90(6), 797-802. DOI: 10.1016/j.fuproc.2009.02.009
Ravikiran, A., Renganathan, T., Pushpavanam, S., Voolapalli, R. K., and Cho, Y. S. (2012). “Generalized analysis of gasifier performance using equilibrium modelling,” Ind. Eng. Chem. Res. 51(4), 1601-1611. DOI: 10.1021/ie2006406
Senapati, P. K., and Behera, S. (2012). “Experimental investigation on an entrained flow type biomass gasification system using coconut coir dust as powdery biomass feedstock,” Bioresour. Technol.117, 99-106. DOI: 10.1016/j.biortech.2012.04.049
Thilakavathi, M., Nader, M., and Pulikesi, M. N. (2010). “Reaction kinetics and mass transfer studies of biomass char gasification with CO2,” Chem. Eng. Sci. 66(1), 36-41. DOI: 10.1016/j.ces.2010.09.033
Xiao, R. R., Chen, X. L., Wang, F. C., and Yu, G. S. (2012). “Research on kinetics characteristics of gasification on biomass semi-char with CO2,” Acta Energiae Solaris Sinica 33(2), 236-245. DOI: 10.3969/j.issn.0254-0096.2012.02.011
Yan, G. H., Xu, M., Li, X. X., Guan, H. B., Jian, J. G., Zhang, W. J., and Sun, R. F. (2010). “Gasification characteristics and kinetics of corncob char with CO2 by using isothermal thermogravimetry,” Trans. Chinese Soc. Agric. Eng. 26(11), 260-264. DOI: 10.3969/j.issn.1002-6819.2010.11.045
Yu, H. M., Zhang, Z., Li, Z. S., and Chen, D. Z. (2014). “Characteristics of tar formation 426 during cellulose, hemicellulose and lignin gasification,” Fuel 118, 250-256. DOI: 10.1016/j.fuel.2013.10.080
Zhou, J. S., Chen, Q., Zhao, H., Cao, X., Mei, Q., Luo, Z., and Cen, K. (2009). “Biomass-oxygen gasification in a high-temperature entrained-flow gasifier,” Biotechnol. Adv. 27(5), 606-611. DOI: 10.1016/j.biotechadv.2009.04.011
Zuo, H. B., Zhang, P. C., Zhang, J. L., Bi, X. T., Geng, W. W., and Wang, G. W. (2015). “Isothermal CO2 gasification reactivity and kinetic models of biomass char/anthracite char,” BioResources 10(3), 5242-5255. DOI: 10.15376/biores.10.3.5242-5255
Article submitted: January 19, 2016; Peer review completed: March 17, 2016; Revised version received and accepted: May 12, 2016; Published: May 24, 2016.
DOI: 10.15376/biores.11.3.6085-6096