Abstract
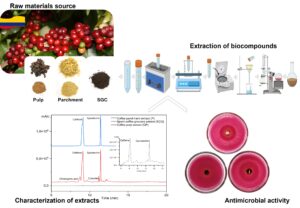
During coffee production, several types of waste such as pulp, mucilage, husk, parchment, coffee silver skin, and spent coffee grounds are generated. The amount of coffee waste and their environmental issues require effective valorization. Those wastes can be used as a source of bioactive compounds. In this work, solid-liquid extraction was used to obtain different solutions, and their phenolic contents, antioxidant capacities, fatty acid profiles, and antimicrobial activities were evaluated. Characterization of the waste materials showed that the highest yield (18.8%) was obtained for spent-coffee grounds. The highest total phenolic contents, caffeine and epicatechin, was observed for coffee pulp extract. Catechin was only observed for parchment. The lipid fraction in the coffee by-products extracts indicated that the spent coffee ground had a higher amount of total lipids, followed by the pulp, and finally the parchment. The most predominant fatty acids in all the extracts were palmitic, stearic, linoleic, oleic, arachidic, and behenic. However, parchment and coffee pulp extracts exhibited an inhibition halo against E. coli bacteria growth.
Download PDF
Full Article
Evaluation of Colombian Coffee Waste to Produce Antioxidant Extracts
Erika Arango-Agudelo,a Yazmín Rendón-Muñóz,a Edith Cadena-Chamorro,a,* Juan Felipe Santa,a,b and Robison Buitrago-Sierra.a,b
During coffee production, several types of waste such as pulp, mucilage, husk, parchment, coffee silver skin, and spent coffee grounds are generated. The amount of coffee waste and their environmental issues require effective valorization. Those wastes can be used as a source of bioactive compounds. In this work, solid-liquid extraction was used to obtain different solutions, and their phenolic contents, antioxidant capacities, fatty acid profiles, and antimicrobial activities were evaluated. Characterization of the waste materials showed that the highest yield (18.8%) was obtained for spent-coffee grounds. The highest total phenolic contents, caffeine and epicatechin, was observed for coffee pulp extract. Catechin was only observed for parchment. The lipid fraction in the coffee by-products extracts indicated that the spent coffee ground had a higher amount of total lipids, followed by the pulp, and finally the parchment. The most predominant fatty acids in all the extracts were palmitic, stearic, linoleic, oleic, arachidic, and behenic. However, parchment and coffee pulp extracts exhibited an inhibition halo against E. coli bacteria growth.
DOI: 10.15376/biores.18.3.5703-5723
Keywords: Coffee by-product wastes; Colombian coffee extracts; Antioxidant extracts; Antimicrobial activity
Contact information: a: Biofibers and Vegetables By-products Research Group, Universidad Nacional de Colombia, Medellín, Antioquia, C.P. 050034, Colombia; b: Instituto Tecnológico Metropolitano ITM, Facultad de Ingenierías, Medellín, Antioquia, C.P. 050013, Colombia;
* Corresponding author: emcadenac@unal.edu.co
GRAPHICAL ABSTRACT

INTRODUCTION
Coffee is a commodity produced by developing countries and consumed overwhelmingly all over the world. Its commercial importance has significantly grown during the last years, due to the consumption as beverage, being the second most commercialized product in the world, only preceded by petroleum (Murthy et al. 2012). There are three primary coffee growing regions – Central and South America, Africa and the Middle East, and Southeast Asia. However, coffee crops are widely grown throughout the tropical region and coffee is produced in at least 70 countries located along the equatorial zone. Colombia is among the globally largest coffee producers, preceded by Vietnam and Brazil (Ocampo and Álvarez 2017). These three countries together produce more than the whole three producing regions. Coffee crops are mainly produced by smallholder farmers and around 125 million people worldwide depend on coffee for their livelihoods.
Coffee beans go through a typical series of manual steps during production. During these steps, several residues are produced. The supplementary Fig. S1 (see Appendix) shows the coffee production process when the coffee by-products as husk, silverskin, spent coffee ground, pulp, and mucilage are obtained. The coffee cherry is composed of the bean (54%), silverskin (1.2%), parchment (6.1%), mucilage (6-8%), pulp (29%), and peel (12%) (Rodrigues da Silva et al. 2022). Of all the components, the bean is the only part of the coffee cherry used in the production of the coffee drink, and the other constituents are discarded. In most cases, the by-products are left in piles to rot. At the end of beverage production, spent coffee grounds (SCG) are also produced. Those wastes are produced at the end of the coffee supply chain (Hoseini et al. 2021). These diverse residues together are known as coffee by-products, and they are used as fertilizer in agricultural crops and animal consumption (dos Santos et al. 2021). These residues have also been used to produce biodiesel, used in the cosmetics industry (Kamil et al. 2019), and to produce filters (Ramos 2010).
Regarding dry matter, the coffee pulp (CP) has a similar composition to coffee parchment since these by-products are generated from the same part of a coffee cherry. However, the CP contains a significantly higher amount of moisture. CP has been used for the manufacturing of composite materials (Jaisan and Punbusayakul 2016). Coffee parchment is one of the least studied coffee by-products. The amount of CP by-products is around 20% of the residues from the coffee production (Benitez et al. 2019). Regarding the dry process, the parchment is extracted simultaneously with the pulp and the coffee peel, but in the wet process this by-product is obtained after the drying and hulling steps, and the parchment is separated from other by-products.
The biological activity of coffee is generated by its nutritional composition. Coffee has phenolic acids, alkaloids, flavonoids, xanthones, methylxanthines, and diterpenes (Wu et al. 2022). These bioactive compounds provide antioxidant, anti-inflammatory, antimicrobial, anti-cellulite, and emulsifying properties (Hall et al. 2022; Rodriguez da Silva et al. 2022). The CP contains an important value of macronutrients such as carbohydrates (35 to 85%), fiber (30.8%), proteins (5 to 11%), and minerals (3 to 11%) (Khochapong et al. 2021). In addition, CP has chlorogenic acid, tannins, cyanidins, caffeic acid, and ferulic acid, among others (Rodríguez et al. 2014). Those active compounds provide antimicrobial properties against the biological activity of bacteria such as Staphylococcus aureus, Enterococcus faecalis, Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli (Duangjai et al. 2016). The coffee parchment (P) is a material made of cellulose (40 to 49%), hemicellulose (25 to 32%), lignin (33 to 35%), and ash (0.5 to 1%) (Mirón et al. 2019). It also has traces of different biocompounds such as quercetin, tannins, caffeine, p-coumaric acid, synaptic acid, and mangiferin (Andrade et al. 2012) that provide antidiabetic, antiviral, and neuroprotective functions (Gemechu et al. 2020). In the case of SCG, there is a wide variety of compounds with physiological activity such as gallic, chlorogenic, vanillic, caffeic, ferulic, and synaptic acid (Abbasi-Parizad et al. 2021), and provide antioxidant, antiallergenic, and antimicrobial properties (Al-Dhabi et al. 2017).
The coffee bean also contains a wide variety of fatty acids. It is known that the amount of total lipids in the bean can be up to 17% in dry weight. The lipids have free diterpenes, steroid esters, free sterols, tocopherol, phospholipids, and triglycerides. The latter is the predominant group (Silva et al. 2020). The extraction of fatty material from the coffee bean and its residues have a prominent potential, since important concentrations of fatty acids such as lauric (C12:0), myristic (C14:0), palmitic (C16:0 ), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), linolenic (C18:3), arachidonic (C20:0), behenic (C22:0) , lignoceric (C24:0), and nervonic (C24:1) have been reported by other authors (Uddin et al. 2019; Coelho et al. 2020; Vidal et al. 2022).
Different authors have carried out studies on extracts obtained from coffee wastes. Benítez et al. (2019), obtained extracts from parchment and studied its phenolic content as an antioxidant product. Rebollo et al. (2019) analyzed the aqueous extract effect on the reduction of adipogenesis, due to the action of phenolic compounds in the decrease of lipid accumulation. Magoni et al. (2018) obtained an aqueous extract from coffee pulp rich in derivatives of quinic acid and procyanidins. The extract has high biological activity and affects the release of immunoregulatory cytokines. It also provides immune and inflammatory response of the organism. Ethanolic and methanolic extracts of spent coffee grounds have also been studied. Juan et al. (2020) found a variety of active compounds in extracts of hydroalcoholic mixtures of SCG. The main compound was chlorogenic acid, followed by gallic acid, vanillic acid, vanillin, 3-4-dihydroxycinnamic acid, hydroxybenzoic acid, caffeic acid, ferulic acid, p-coumaric acid, protocatechuic acid, and syringic acid. The variation in the content of polyphenols present in coffee by-products can be attributed to different factors, such as the variety of coffee beans, the extraction method, the solvents or binary mixtures used, and the solvent biomass ratio, among others.
From the literature review, the authors concluded that several researchers are trying to generate added-value products from coffee waste by using them as raw materials to develop novel products. Due to the chemical composition of coffee by-products, the aim of this work was to obtain and characterize extracts from different Colombian coffee by-products such as parchment, spent coffee ground, and pulp.
EXPERIMENTAL
Sourcing of Raw Materials
Coffee pulp (CP) was obtained from a farm located at Vereda La linda (coordinates 6.61839 – 75.83252) in the municipality of Jardín, Antioquia, Colombia. The pulp was obtained by using a mechanical depulping machine. After extraction, the pulp was collected and stored at 5 °C. The pulp was dried by convection drying at 40 °C for 30 h using air flowing at a velocity of 1 m s-1. The parchment (P) was obtained from green coffee in a collection plant from the same municipality. The green coffee beans were sun-dried in the field. The removal of the parchment was done using a mechanical dehuller machine. Spent Coffee Grounds (SCG) were obtained directly after brewing using a commercial coffee machine and, pouring water at 90 °C. The SCG was dried using the same conditions used for the pulp, but the drying time was done for 48 h. The particle size of Spent-Coffee grounds (SCG) was 250 µm. The particle size of Coffee Pulp (CP) and Parchment (P) was between 420 and 595 µm. The moisture content of CP, P, and SCG after drying was 9.641±0.048, 7.462±0.071 and, 2.835±0.079 (% dry weight, d.w.) respectively.
All solvents were reagent and HPLC-grade. They were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA).
Characterization of Raw Materials
The raw materials were analyzed using several techniques. The infrared analysis (FTIR) was performed on an infrared spectrometer (Advantage IR-Tracer 1000 Shimadzu, Kyoto, Japan). The pulp and parchment samples were analyzed using ATR. SCG were analyzed using DRIFT and samples were mixed in a KBr matrix using a 1:20 ratio. The tests were carried out using a wavelength between 4000 and 500 cm-1 with a resolution of 0.5 cm-1.
Thermogravimetry (TGA) analyses were performed simultaneously using a simultaneous TGA/DSC (SDT-Q600, TA Instruments, New Castle, Delaware, USA). The changes in mass of the samples were evaluated. For this purpose, the samples were heat treated from room temperature to 800 °C, using a heating ramp of 10 °C per min and a controlled nitrogen atmosphere. After 40 min at 800 °C, the atmosphere was changed to air and an isotherm at the same temperature was evaluated for 15 min.
Images of the samples were obtained by Field Emission Scanning Electron Microscopy FE/SEM (7100F, JEOL, Akishima, Tokio, Japan). The chemical composition was also evaluated by a coupled Energy Dispersive Spectrometry detector.
Lignocellulosic Characterization
The amount of lignocellulosic materials of the coffee waste (P, CP, and SCG) was evaluated by an acid-insoluble extraction of Klason lignin based on the TAPPI 222 om-02 standard (2011). Acid hydrolysis was carried out with sulfuric acid H2SO4 at 72% (w/w) at 30 °C. Later, the solution was taken at 110 °C for 1 h. The obtained hydrolyzate was filtered, and the remaining material was dried at 105 °C for 5 h. The remnant liquid was used to determine acid-soluble lignin. The sample was evaluated at 205 nm in a spectrophotometer (Genesys 10S UV-VIS, Thermo Scientific, Waltham, MA, USA).
Holocellulose was determined based on modified ASTM D1104-56 standard (1985). The extractives-free biomass was acid hydrolyzed with sodium chlorite and glacial acetic acid in a shaker water bath (LSB-015S, LabTech, Hopkinton, Massachusetts, USA) at 80 °C and 60 rpm for 1 h. After that, the hydrolyzed sample was filtered and dried at 105 °C for 5 h. Cellulose was extracted from the holocellulosic material.
Extraction of Biocompounds
Solid-liquid extraction was done using ethanol as extractive solvent (1:20 w:v). The solution was put in an ultrasonic bath using a frequency of 40 KHz. After that, the solution was stirred at 200 rpm at room temperature (25 °C) for 4 h. Finally, the solution was filtered using a 180 microns filter. The solution with the extract was concentrated by rotovaporation at 50 °C, 40 mbar at 80 rpm for 2 h. The extracts were stored in a freezer at -30 °C until its characterization (Dong et al. 2021). Supplementary material Fig. S2 shows a scheme of the extraction of biocompounds.
Characterization of Extracts
Total phenolic content (TPC)
The total phenolic content was evaluated using the modified Folin-Ciocalteu spectrophotometric method (Oktaviani et al. 2020). The samples were evaluated in triplicate and expressed as TPC in mg of gallic acid equivalent per 100 mg of dry weight of the sample
Antioxidant capacity
The antioxidant capacity was evaluated by two different methods. In both methods, the sample produces an inhibition of the free radicals DPPH (2,2-diphenyl-2-picrylhydrazyl radical scavenging) (Guija et al. 2015) and ABTS (2,20-Azinobis (3-ethylbenzithiazoline-6-sulfonic acid)) (Torres et al. 2020). Both spectrophotometric methods were analyzed in triplicate on a spectrophotometer (Genesys 10S UV-VIS, Thermo Scientific, Waltham, Massachusetts, USA). The results were reported as µmol of Trolox equivalent per gram of dried extract.
HPLC analysis
Samples were analyzed with High-Performance Liquid Chromatograph (HPLC) equipment (Prominence LC-20AT, Shimadzu, Kyoto, Japan) equipped with a diode array detector (PDA, SPD 20AT). The separation was performed on an Agilent Zorbax Eclipse Plus C-18 column (5 µ, 150 x 4.6 mm) and a Zorbax NH2 4-Pack pre-column (5 µ, 12.5 x 4.6 mm). Chlorogenic acid and caffeine were evaluated. Acidified water to 1% acetic acid (A) and acetonitrile to 1% acetic acid (B) were used as mobile phases, following a gradient of 10% B (0.2 min), 10 to 20% B (5.3 min), 20 to 25% B (2.7 min), 25 to 27% B (2 min), 27 to 30% B (2 min), 30 to 40% B (3 min), 40 to 60% B (2 min), 60 to 30% B (2 min), 30 to 0% B (1 min), and 0% B (2 min) at a flow of 0.9 mL/min for 22 minutes (Sualeh et al. 2020). The oven temperature was 30 °C, and 15 μL were injected for each sample. The reading was done at 280 nm. Two mobile phases were used for (-)-epicatechin and (+)-catechin, solvent A (1 % w. acetic acid) and solvent B (acetonitrile, 1% w. acetic acid), following a gradient of 0% B (5.3 min), 0 to 90% B (8.7 min), 90 to 70% B (3 min), 70 to 30% B (2 min), 30 to 0% B (2 min) and 0% B (4 min) at a flow rate of 1.3 mL/min for 25 minutes. The oven temperature was 40 °C, and 10 μL were injected for each sample. The concentration of flavonoids was determined by their absorbance at 280 nm.
Fatty acids
The extraction of fatty acids from the samples was carried out following the methodology described by Folch et al. (1957) with some variations. Specifically, dichloromethane was used instead of chloroform. The fatty acids in form of methyl ester or FAME’s (Fatty Acids Methyl Esters) were obtained following the methodology described by Villarreal et al. (2012). The FAME’s were extracted with 2 mL of hexane. The extracts were injected into a Gas Chromatography–Mass Spectrometrer (GC-MS) (QP2010, Shimadzu, Kyoto, Japan) using a DB-WAX column (30 mx 0.250 mm x 0.25 µm, Agilent J&W) and helium was selected as carrier gas at a rate of 1.21 mL/min and dichloromethane as solvent. A heating ramp was set up starting at 100 °C for 4 min. After that, the temperature increased up to 193 °C at a rate of 3 °C per min. After that, another increment was programmed up to 240 °C for 10 min at 1.5 °C/min, for a total time of 76 min. The injection volume was 1.0 µL with an injector temperature of 225 °C and a Split of 3:1. The equilibrium time of the system was 3 min and the data scan began after 4 min (Inhamuns et al. 2009). The qualitative identification of the fatty acids was carried out by comparing the retention times of the characteristic peaks generated by 37 standards of methyl esters contained in the FAME Mix Supelco 37 (Sigma, St. Louis, USA).
Evaluation of Antimicrobial Activity
The antibacterial activity of the coffee wastes extracts was determined by the Kirby-Bauer method (Shehata et al. 2021). This method was carried out following the procedure described below, using the bacterial strain Escherichia coli (ATCC 25922) as a Gram-negative bacterial model. The bacterial strain was grown in BHI (Brain Heart Infusion) growth broth for 24 hours in an incubator at 37 °C and 80% relative humidity. Then, in Petri dishes of 6 cm in diameter, 7 mL of selective agar for the bacteria was added and it was allowed to solidify. Subsequently, the bacteria were distributed on the surface of the agar. Finally, 10 µL of each of the extracts that were tested was placed in the center of the Petri dish.
RESULTS AND DISCUSSION
Extraction of Biocompounds
Figure 1 shows images of the pulp, parchment, and spent coffee grounds in the stereomicroscope (inserts in the SEM images) and the SEM. The micrographs of coffee pulp and parchment showed irregular edges, with sheet-like shape. SPG has a fibrillar morphology typical of lignocellulosic materials. The chemical composition measured using EDS analysis is shown in the same figure. Elements such as C, Ca, K, Cl and Mg were found in the sample. The amount of minerals in coffee wastes and its variability depend on the type of agronomic practices, soil type, altitude, and climatic conditions. The high carbon content is associated with the lignocellulosic materials. In pulp and parchment, the presence of minerals indicates that those residues can be used for soil fertilization. The EDS technique does not measure other light elements such as hydrogen. Minerals such as potassium, magnesium, and calcium have been found mainly in green coffee beans by other authors (Deshpande et al. 2019).
Coffee beans have a high amount of crude fiber composed of lignin, hemicellulose, cellulose, as well as poly-, oligo-, and monosaccharides (Massaya et al. 2019). Lignocellulosic contents, ash content, and chemical composition of coffee pulp and parchment were obtained (Table 1). In this work, the analysis was not performed on spent coffee grounds, but different authors (Franca et al. 2009; Zarrinbakhsh et al. 2016) have reported amounts of lignin between 17.6 and 26.0%, cellulose from 7 to 23%, hemicellulose from 19.4 up to 42%, and ash levels between 1.0 and 2.2%. Around 50% of SCG dry mass is composed of polysaccharides, the most relevant are cellulose and hemicellulose, which are comprised of galactose, arabinose, and mannose (McNutt and He 2019).
Table 1. Extractives, Lignin, Cellulose, Hemicellulose and Ashes of Coffee Pulp and Parchment
* Typical values from literature (Franca et al. 2009; Zarrinbakhsh et al. 2016)
Cellulose values of P and CP were similar to those obtained by Oliveira and Franca (2015) and Murthy et al. (2012). On the contrary, the hemicellulose contents (5.29 ± 1.65%) were lower than reported by Londoño et al. (2020) being around 28.7 % for coffee pulp. Lignin and ashes content were higher by 17.5% and 37.5% respectively. The difference could be related to the variety used during the analyses, geographic location and production parameters. The lignocellulosic material contained in those coffee by-products make them a potential source to be used to as fillers for polymer composites, fertilizers, compost, bioethanol, and also for the extractions of antioxidant compounds (Hejna et al. 2021).
Fig. 1. SEM-EDS analysis of the (a) coffee pulp, (b) parchment, and (c) spent-coffee grounds
Extracts Composition
The CP, SCG, and P extracts contained a total polyphenols content (TPC) of 12.628 ± 0.474, 5.737 ± 0.345, and 2.515 ± 0.545 mg GAE/g d.w., respectively. Bondam et al. (2022) reported similar results and found a higher TPC in CP ethanolic extracts compared to P and SCG. The TPC values were lower than those reported by different authors in P extracts from 2.84 mg GAE/g d.w. to 5 mg GAE/g d.w. (Aguilera et al. 2019; Mirón et al. 2019) and from 16 mg GAE/g d.w. to 31.03 mg GAE/g d.w. in SCG extracts (Hogan et al. 2010; Mussatto et al. 2011; Sette et al. 2020). However, the CP extracts presented a greater content of total polyphenols compared to those reported by Heeger et al. (2017) in an aqueous extraction. In that work, the authors measured polyphenol concentrations between 4.85 and 9.17 mg GAE/g d.w. The variation in the content of polyphenols present in coffee by-products can be attributed to different factors, such as the variety of coffee beans, the extraction method, the solvents or binary mixtures used, and the solvent biomass ratio, among others.
Polyphenols in coffee by-products can be classified into several class: flavonols, anthocyanins, hydroxycinanic acids, and flavan-3-ols (Bastian et al. 2006). These compounds are important in coffee and its by-products because of their antioxidant, anti-inflammatory, and antimicrobial effects, among others (Farah and de P. Lima 2019). The antioxidant capacity analyzed by the DPPH● radical method in the CP, P, and SCG ethanolic extracts is consistent with the content of total polyphenols, because of the direct relationship between functional compounds and their antioxidant effect (LIczbiński and Bukowska 2022). The analysis carried out by ABTS●⁺, showed that the SCG extract had a lower antioxidant capacity. This result may be related to a higher concentration of caffeine found in the parchment extract (Ramón et al. 2019). The polyphenols antioxidant capacity is attributed to the phenolic ring variations because of its arrangement of –OH groups (Khochapong et al. 2021).
The antioxidant capacity relative to ABTS●⁺ was greater than the values reported by the DPPH● method. This behavior may be based on the reaction mechanism of each radical. The ABTS●⁺ radical naturally has an affinity with hydrophilic and lipophilic compounds, while the DPPH● acts in an organic medium (Kuskoski et al. 2005). Caffeine is a central nervous system stimulant, and it is one of the most substances consumed in the world (Murthy and Madhava 2012). Is known to have a greater hydrophilic than lipophilic antioxidant power (Chu et al. 2012). This property reported in the literature agrees with the results for antioxidant capacity obtained in this study because there was a higher concentration of caffeine in all extracts.
The behavior related to caffeine content and antioxidant capacity of P and SCG seems contradictory. The authors do not have an experimental explanation for this behavior, but a plausible hypothesis is that, because of the total lipid content in the SCG, a rapid oxidation of Polyunsaturated Fatty Acids (PUFAs) is generated. This reaction can be slowed down with the presence of a hydrophilic antioxidant, such as caffeine. If caffeine was used in this reaction, its availability to react with the ABTS radical could have been reduced. Therefore, the results of DPPH could have increased by making the lipophilic antioxidants available, since they do not participate in the lipid oxidation reaction (Bahja et al. 2022).
The chromatographic profile of each extract evaluated by HPLC at a wavelength of 280 nm is shown in Supplementary material Fig. S3. The method was effective, generating a correct separation and resolution of the peaks. The peaks of interest were identified by their retention time and UV-vis spectrum in the extracts and standards used. The retention time of chlorogenic acid, caffeine, (+)-catechin, and (-)-epicatechin was 8.85 min, 9.33 min, 11.31 min, and 11.59 min, respectively. The amount of these flavonoids and phenolic acids present in the coffee by-products extracts are also shown (Table 2). In all extracts, caffeine was the most predominant compound. The highest concentration of caffeine (85.219 mg/g d.w.) was found in the CP extract, followed by P (24.478 mg/g d.w.), and ultimately the SCG extract (12.520 mg/g d.w.). The lower value in the SCG was expected since it has gone through a solubility caffeine process to obtain the beverage. The values detected in the different extracts are comparable with those reported by other authors (Heeger et al. 2017; Manasa et al. 2021), who used different extraction techniques such as Soxhlet and supercritical fluid technology.
Table 2. Coffee Waste Extracts Characterization
The chlorogenic acid found in the parchment extract (7.07 mg/100 g d.w.) was lower than the value reported by Iriondo et al. (2019), who used conventional methods of maceration and shaking with ethanol and water to obtain chlorogenic acid between 4.5 and 68.2 mg/100 g d.w. These variations can be attributed to the differences between each variety of coffee used in the different studies. The flavonoids evaluated were (+)-catechin, and (-)-epicatechin. The (+)-catechin content in parchment extracts was 19.4 mg/100 g of dry extract. That value is lower than the result obtained by Vallamkandu et al. (2021), who reported 24.43 mg/100 g from ethanolic extracts using compression systems. The (-)-epicatechin amount found in SCG extract (0.831 mg/g) was also lower compared to those reported by Abdullah et al. (2017) in extracts obtained by ultrasound (0.3 mg/g). However, regarding the parchment extract (1,155 mg/g), the (-)-epicatechin was comparable to those obtained by Andrade et al. (2012) using supercritical fluids extraction technology (1.4 to 2.1 mg/g). The amount of polyphenolic contained in SCG was affected by the brewing process. Since SCG are hydrophobic, it is possible to obtain a significant content of bioactive compounds in the coffee beverage. Polyphenolic compounds have antioxidant properties, and they are recognized for their preventive action against damage caused by oxidative stress.
The final contents of bioactive substances such as caffeine in coffee drinks depend on different parameters as temperature, time, process, pressure, coffee grounds variety, among others. In a traditional brewing process with water and a commercial coffee machine, around 7.908 and 0.139 g/L of caffeine content (Olechno et al. 2021) can be found. It is well known that caffeine increases its solubility with the temperature, is moderately soluble at 20 °C, and reaches its peak at 100 °C (Prankerd 2007). In this case, the SCG was collected after brewing commercial coffee in a coffee maker commonly used in Latin-America, called greca. The coffee maker consists of three chambers, one for the water, another for the ground coffee and finally, a chamber for coffee beverage. When the greca is heated up properly, pressure builds within the small lower water tank, forcing water through the ground coffee to produce rich, flavorful coffee. Therefore, some soluble materials such as caffeine are expected to be removed during the process.
Fig. 2. Chromatogram’s lipid profile of coffee wastes extracts
Table 3. Lipid Profile Extracts
The Fatty Acid Methyl Esters (FAME’s) found in the extracts (Table 3) and the chromatographic spectra (Fig. 2) are also shown. Each analyte was compared with the retention time of reference standards, and its mass spectrum was found in the NIST (2014) Database using GCMS solution software version 4.45. The lipid fraction in the coffee by-products extracts indicated the SCG had a higher amount of total lipids, followed by the pulp, and finally the parchment. The most predominant fatty acids in all the extracts were palmitic, stearic, linoleic, oleic, arachidic, and behenic. Only the pulp and parchment extracts had myristic, pentadecanoic, palmitoleic, and vaccenic acid. The SCG extract had the higher yield (18.9%) compared to P (5.77%) and CP (1.50%) extracts. These results can be attributed to a higher lipid fraction concentration in SCG extract. Quah et al. (2019) reported similar values by supercritical fluid and pressurized fluids technologies on SCG extracts. Linoleic (44.6 to 45.8%), palmitic (29.7 to 33.04%), oleic (8.43 to 8.89%), and stearic (7.69 to 8.60%) acids were found. The total saturated acids (SFA) found in the extracts were from 54.3 to 60.6%, polyunsaturated acids (PUFA) were between 19.2 and 38.2%, and monounsaturated acids (MUFA) were from 5.48 to 7.95%. The SFA were higher than those obtained by Uddin et al. (2019) (37.6%) in SCG oil for biodiesel. On the contrary, MUFA had lower levels (33.6%).
Supplementary material Fig. S4 shows the FTIR spectra for the three coffee residue extracts. The spectrum of coffee by-products extracts showed broad peaks at 3350 cm-1 and 3400 cm-1, and another at 3030 cm-1 for SCG extract. They can be attributed to the presence of carboxylic acid with dimer of OH. The O-H stretching absorbances could be related to the presence of hydroxyl compounds. The spectrum shows the coincidence of the bands generated by the vibrations of the functional groups of the fatty acids present in all the extracts. It is possible to observe the stretching of the carbonyl functional group (C=O) peaks in fatty acids to a length of 1732 cm-1, as well as the stretching and bending vibration at 2920 and 2850 cm-1 between the bonds of groups carboxyl (CH2) of the fatty acids alkyl chains (Sherazi et al. 2009; Alara and Abdurahman 2019).
Antibacterial Activity
Figure 3 shows the results of antibacterial tests. In Fig. 3(a) the positive control (ampicillin, 200 µg/L) and the blank made for the experiment with ethanol are shown. The ampicillin had a halo with a diameter of 12.05 ± 0.38 mm, indicating its antimicrobial activity. Figure 3 (b) shows the absence of inhibitory halo formation by SCG extract. On the other hand, P and CP extracts had a halo of 9.33 ± 0.58 mm and 7.67 ± 0.58 mm respectively. The antibacterial effect could be related to the presence of caffeine in the coffee pulp, as well as the chlorogenic acid in parchment extract.
According to different authors, caffeine has a major antioxidant effect among the bioactive compounds in coffee (Iriondo et al. 2016; Kieu et al. 2020). Phenolic acids, tannins, hydroxycinnamic acids, and malic acids have been reported as responsible of antimicrobial activity (Monente et al. 2015). The gram-negative bacteria cell membrane is composed of phospholipids, which makes it resistant to external attacks. Nevertheless, the phenolic compound on coffee by-products extracts may have caused a rupture of the membrane modifying its permeability due to a variety of hydrogen bonging interactions (Duangjai et al. 2016). This mechanism generates several membrane damages and can lead to the death of bacterial cells (Castaldo et al. 2020). Authors have reported the inhibitory effect of coffee by-products extracts on gram-positive and gram-negative bacteria such as as E. coli, S. aureus, P. aeruginosa (Monente et al. 2015), S. epidermis (Runti et al. 2015), S. mutans (Almeida et al. 2012), E. aerogenes, and P. mirabillis (Almeida et al. 2006).
Fig. 3. Antimicrobial activity of (a) positive control, (b) SCG, (c) CP, and (d) P extracts against the growth of E.coli
CONCLUSIONS
In this work, extracts from several coffee production by-products (pulp, parchment, and spent-coffee grounds) were obtained. The extracts were characterized to identify the physical-chemical properties of added-value products from coffee supply chain. The extracts were evaluated using antibacterial tests. From the results, the following conclusions were obtained:
- The characterization of raw materials used in this work, i.e., coffee pulp (CP), parchment (P), and spent coffee grounds (SCG), showed that the highest extraction yield (18.8%) was obtained for SPG.
- The highest total phenolic contents, caffeine, and epicatechin was observed for CP. Catechin was only observed for parchment (P).
- The lipid fraction in the coffee by-products extracts indicated the SCG had a higher amount of total lipids, followed by the pulp, and finally the parchment. The most predominant fatty acids in all the extracts were palmitic, stearic, linoleic, oleic, arachidic, and behenic. Only the pulp and parchment extracts had myristic, pentadecanoic, palmitoleic, and vaccenic acids.
- Spent-coffee grounds (SCG) showed a negative effect on the E. coli growth. On the other hand, P and CP extracts had a halo of 9.33 ± 0.58 mm and 7.67 ± 0.58 mm respectively. The antibacterial effect was related to the caffeine present in the coffee pulp, and chlorogenic acid in parchment extract.
ACKNOWLEDGMENTS
The authors are grateful to the Newton Fund for financial support through project valorisation of wastes from coffee supply chain in Colombia and UK to develop novel products. Support was provided by Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación “Francisco José de Caldas – MinCiencias Contrato 543 – 2020”. The authors would also like to acknowledge Instituto Tecnológico Metropolitano for financial support through young researchers and innovators program (Resolution No. 360 of 06 APR 2020 and Resolution No. 534 of 19 JUN 2020).
REFERENCES CITED
Abbasi-Parizad, P., de Nisi, P., Scaglia, B., Scarafoni, A., Pilu, S., and Adani, F. (2021). “Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties,” Food and Bioproducts Processing 127, 338-348. DOI: 10.1016/j.fbp.2021.03.015
Aguilera, Y., Rebollo, M., Cañas, S., Taladrid, D., and Martín, M. A. (2019). “Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis,” Food Funct 10(8), 4739-4750. DOI: 10.1039/c9fo00544g
Alara, O. R., and Adburahman, N. H. (2019). “GC–MS and FTIR analyses of oils from Hibiscus sabdariffa, Stigma maydis and Chromolaena odorata leaf obtained from Malaysia,” Chemical Data Collections, 20, Article no. 100200. DOI: 10.1016/j.cdc.2019.100200
Al-Dhabi, N. A., Ponmurugan, K., and Maran Jeganathan, P. (2017). “Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds,” Ultrasonics Sonochemistry 34, 206-213. DOI: 10.1016/j.ultsonch.2016.05.005
Almeida, A. A. P., Farah, A., Silva, D. A. M., Nunan, E. A., and Glória, M. B. A. (2006). “Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria,” Journal of Agricultural and Food Chemistry 54 (23), 8738-8743. DOI: 10.1021/jf0617317
Almeida, A. A. P., Naghetini, C. C., Santos, V. R., Antonio, A. G., Farah, A., and Glória, M. B. A. (2012). “Influence of natural coffee compounds, coffee extracts and increased levels of caffeine on the inhibition of Streptococcus mutans,” Food Research International 49(1), 459-461. DOI: 10.1016/j.foodres.2012.07.026
Andrade, K. S., Gonalvez, R. T., Maraschin, M., Ribeiro-Do-Valle, R. M., Martínez, J., and Ferreira, S. R. S. (2012). “Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition,” Talanta 88, 544-552. DOI: 10.1016/j.talanta.2011.11.031
ASTM D1104-56 (1985). “Method of test for holocellulose in wood,” ASTM International, West Conshohocken, PA, USA.
Bahja, J., Stewart, N., and Dymond, M. (2022). “Oxidative stress is inhibited by plant-based supplements: A quantitative lipidomic analysis of antioxidant activity and lipid compositional change,” Advances in Redox Research 6, 1-14. DOI: 10.1016/j.arres.2022.100054.
Benitez, V., Rebollo-Hernanz, M., Hernanz, S., Chantres, S., Aguilera, Y., and Martin-Cabrejas, M. A. (2019). “Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization,” Food Research International 122, 105-113. DOI: 10.1016/j.foodres.2019.04.002.
Bondam, A. F., Diolinda da Silveira, D., Pozzada dos Santos, J., and Hoffmann, J. F. (2022). “Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries,” Trends in Food Science and Technology 123, 172-186. DOI: 10.1016/j.tifs.2022.03.013.
Castaldo, L., Narváez, A., Izzo, L., Graziani, G., and Ritieni, A. (2020). “In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS,” Molecules 25(9), 2132. DOI: 10.3390/molecules25092132
Chu, Y. F., Chang, W. H., Black, R. J., Liu, J. R., Sompol, P., Chen, Y., Wei, H., Zhao, Q., and Cheng, I. H. (2012). “Crude caffeine reduces memory impairment and amyloid β1-42 levels in an Alzheimer’s mouse model,” Food Chemistry 135(3), 2095-2102. DOI: 10.1016/j.foodchem.2012.04.148
Coelho, J. P., Filipe, R. M., Paula Robalo, M., Boyadzhieva, S., Cholakov, G. S., and Stateva, R. P. (2020). “Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts,” Journal of Supercritical Fluids 161, Article no. 104825. DOI: 10.1016/j.supflu.2020.104825
Deshpande, S., Singh, S., Panneerselvam, A., and Rajeswari, V. D. (2019). “Nutrients in caffeinated beverages-an overview,” in: Caffeinated and Cocoa Based Beverages, Volume 8, The Science of Beverages, Elsevier, pp. 367-389.
Dong, W., Chen, Q., Wei, C., Hu, R., Long, Y., Zong, Y., and Chu, Z. (2021). “Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity,” Ultrasonics Sonochemistry 74, Article no. 105578. DOI: 10.1016/j.ultsonch.2021.105578
Duangjai, A., Suphrom, N., Wungrath, J., Ontawong, A., Nuengchamnong, N., and Yosboonruang, A. (2016). “Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts,” Integrative Medicine Research 5(4), 324-331. DOI: 10.1016/j.imr.2016.09.001.
Farah, A., and de P. Lima, J. (2019). “Consumption of chlorogenic acids through coffee and health implications,” Beverages 5(1). DOI: 10.3390/beverages5010011
Folch, J., Lees, M., and Sloane Stanley, G. H. (1957). “A simple method for the isolation and purification of total lipides from animal tissues,” J. Biol. Chem. 226(1), 497-509. DOI: 10.1016/s0021-9258(18)64849-5
Franca, A. S., Oliveira, L. S., and Ferreira, M. E. (2009). “Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds,” Desalination 249(1), 267-272. DOI: 10.1016/j.desal.2008.11.017
Gemechu, F. G. (2020). “Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation,” Trends in Food Science and Technology 104, 235-261. DOI: 10.1016/j.tifs.2020.08.005
Guija, E., Inocente, M., Ponce, J., and Zarzosa, E. (2015). “Evaluación de la técnica 2,2-difenil-1-picrilhidrazilo (DPPH) para determinar capacidad antioxidante,” Horizonte Médico 2(1), 0-4.
Hall, R. D., Trevisan, F., and de Vos, R. C. H. (2022). “Coffee berry and green bean chemistry – Opportunities for improving cup quality and crop circularity,” Food Research International 151, Article no. 110825. DOI: 10.1016/j.foodres.2021.110825
Heeger, A., Kosińska-Cagnazzo, A., Cantergiani, E., and Andlauer, W. (2017). “Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage,” Food Chemistry 221, 969-975. DOI: 10.1016/j.foodchem.2016.11.067
Hogan, S., Zhang, L., Li, J., Sun, S., Canning, C., and Zhou, K. (2010). “Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase,” Nutrition & Metabolism 7(1), 71. DOI: 10.1186/1743-7075-7-71
Hoseini, M., Cocco, S., Casucci, C., Cardelli, V., and Corti, G. (2021). “Coffee by-products derived resources. A review,” Biomass and Bioenergy 148, 1-10. DOI: 10.1016/j.biombioe.2021.106009
Inhamuns, A. J., Franco, M. R. B., and Batista, W. S. (2009). “Seasonal variations in total fatty acid composition of muscles and eye sockets of tucunaré (Cichla sp.) from the Brazilian Amazon area,” Food Chemistry 117(2), 272-275. DOI: 10.1016/j.foodchem.2009.03.113
Iriondo-DeHond, A., Aparicio García, N., Fernandez-Gomez, B., Guisantes-Batan, E., Velázquez Escobar, F., Blanch, G. P., San Andres, M. I., Sanchez-Fortun, S., and del Castillo, M. D. (2019). “Validation of coffee by-products as novel food ingredients,” Innovative Food Science and Emerging Technologies 51, 194-204. DOI: 10.1016/j.ifset.2018.06.010
Jaisan, C., and Punbusayakul, N. (2016). “Development of coffee pulp extract-incorporated chitosan film and its antimicrobial and antioxidant activities,” Asia-Pacific Journal of Science and Technology 21(2), 140-149. DOI: 10.14456/kkurj.2016.17
Juan, C., G. de Simone, G. Sagratini, G. Caprioli, J. Mañes, and A. Juan-García (2020). “Reducing the effect of beauvericin on neuroblastoma SH-SY5Y cell line by natural products,” Toxicon 188, 164–171. DOI: 10.1016/j.toxicon.2020.10.017
Kamil, M., Ramadan, K. M., Awad, O. I., Ibrahim, T. K., Inayat, A., and Ma, X. (2019). “Environmental impacts of biodiesel production from waste spent coffee grounds and its implementation in a compression ignition engine,” Science of the Total Environment 675, 13-30. DOI: 10.1016/j.scitotenv.2019.04.156
Khochapong, W., Ketnawa, S., Ogawa, Y., and Punbusayakul, N. (2021). “Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract,” Food Chemistry 348. DOI: 10.1016/j.foodchem.2021.129094
Kieu Tran, T. M., Kirkman, T., Nguyen, M., and van Vuong, Q. (2020). “Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora),” Heliyon 6(7), article e04498. DOI: 10.1016/j.heliyon.2020.e04498
Kuskoski, M., Asuero, A., Troncoso, A. N., Mancini Filho, J., and Fett, R. (2005). “Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos,” Ciencia y Tecnología de Alimentos 25(4), 726-732.
LIczbiński, P., and Bukowska, B. (2022). “Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations,” Industrial Crops and Products 175, 1-8. DOI: 10.1016/j.indcrop.2021.114265
Londoño-Hernandez, L., Ruiz, H. A., Cristina Ramírez, T., Ascacio, J. A., Rodríguez-Herrera, R., and Aguilar, C. N. (2020). “Fungal detoxification of coffee pulp by solid-state fermentation,” Biocatalysis and Agricultural Biotechnology 23, Article no. 101467. DOI: 10.1016/j.bcab.2019.101467
Magoni, C., Bruni, I., Guzzetti, L., Dell’Agli, M., Sangiovanni, E., Piazza, S., Regonesi, M. E., Maldini, M., Spezzano, R., Caruso, D., and Labra, M. (2018). “Valorizing coffee pulp by-products as anti-inflammatory ingredient of food supplements acting on IL-8 release,” Food Research International 112, 129-135. DOI: 10.1016/j.foodres.2018.06.026
Manasa, V., Padmanabhan, A., and Anu Appaiah, K. A. (2021). “Utilization of coffee pulp waste for rapid recovery of pectin and polyphenols for sustainable material recycle,” Waste Management 120, 762-771. DOI: 10.1016/j.wasman.2020.10.045
Marisol Ramos Rincon. n.d. “Estudio del proceso de biosorcion de colorantes sobre borra (cuncho) de café tesis de maestria,”
Massaya, J., Prates Pereira, A., Mills-Lamptey, B., Benjamin, J., and Chuck, C. J. (2019). “Conceptualization of a spent coffee grounds biorefinery: A review of existing valorisation approaches,” Food and Bioproducts Processing 118, 149-166. DOI: 10.1016/j.fbp.2019.08.010
McNutt, J., and He, Q. S. (2019). “Spent coffee grounds: A review on current utilization,” Journal of Industrial and Engineering Chemistry 71, 78-88. DOI: 10.1016/j.jiec.2018.11.054
Mirón-Mérida, V. A., Yáñez-Fernández, J., Montañez-Barragán, B., and Barragán Huerta, B. E. (2019). “Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films,” LWT 101, 167-174. DOI: 10.1016/j.lwt.2018.11.013
Monente, C., Bravo, J., Vitas, A. I., Arbillaga, L., de Peña, M. P., and Cid, C. (2015). “Coffee and spent coffee extracts protect against cell mutagens and inhibit growth of food-borne pathogen microorganisms,” Journal of Functional Foods 12, 365-374. DOI: 10.1016/j.jff.2014.12.006
Murthy, P. S., and Madhava Naidu, M. (2012). “Sustainable management of coffee industry by-products and value addition – A review,” Resources, Conservation and Recycling 66, 45-58. DOI: 10.1016/j.resconrec.2012.06.005
Mussatto, S. I., Ballesteros, L. F., Martins, S., and Teixeira, J. A. (2011). “Extraction of antioxidant phenolic compounds from spent coffee grounds,” Separation and Purification Technology 83(1), 173-179. DOI: 10.1016/j.seppur.2011.09.036
Ocampo-Lopez, O. L., and Álvarez-Herrera, L. M. (2017). “Tendencia de la producción y el consumo del café en Colombia,” Apuntes del Cenes 36(64), 139-165. DOI: 10.19053/01203053.v36.n64.2017.5419
Oktaviani, L., Astuti, D. I., Rosmiati, M., and Abduh, M. Y. (2020). “Fermentation of coffee pulp using indigenous lactic acid bacteria with simultaneous aeration to produce cascara with a high antioxidant activity,” Heliyon 6(7). DOI: 10.1016/j.heliyon.2020.e04462
Olechno, E., Púscion-Jakubik, A., Zujko, M. E., and Socha, K. (2021). “Influence of various factors on caffeine content in coffee brews,” Foods 10, Article no. 1208.
Oliveira, L. S., and Franca, A. S. (2015). “An overview of the potential uses for coffee husks,” in: Coffee in Health and Disease Prevention, Preedy, V. R. (ed), Elsevier, 283-291. DOI: 10.1016/B978-0-12-409517-5.00031-0.
Prankerd, R. J. (2007). “Critical compilation of pKa values for pharmaceutical substances,” Profiles of Drug Substances, Excipients and Related Methodology 33, 1-33.
Quah, R. V., Tan, Y. H., Mubarak, N. M., Khalid, M., Abdullah, E. C., and Nolasco-Hipolito, C. (2019). “An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts,” Journal of Environmental Chemical Engineering 7(4). DOI: 10.1016/j.jece.2019.103219.
Ramón-Gonçalves, M., Gómez-Mejía, E., Rosales-Conrado, N., León-González, M. E., and Madrid, Y. (2019). “Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses,” Waste Management 96, 15-24. DOI: 10.1016/j.wasman.2019.07.009
Rebollo-Hernanz, M., Zhang, Q., Aguilera, Y., Martín-Cabrejas, M. A., and Gonzalez-de Mejia, E. (2019). “Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways,” Food and Chemical Toxicology 132, Article no. 110672. DOI: 10.1016/j.fct.2019.110672
Rodrigues-da Silva, M., Sanchez-Bragagnolo, F., Lajarim-Carneiro, R., Carvalho-Pereira, I., Aquino-Ribeiro, J. A., Martins-Rodrigues, C., Jelley, R. E., Fedrizzi, B., and Soleo-Funari, C. (2022). “Metabolite characterization of fifteen by-products of the coffee production chain: From farm to factory,” Food Chemistry 369, Article no. 130753. DOI: 10.1016/j.foodchem.2021.130753
Rodríguez-Durán, L. v., Ramírez-Coronel, M. A., Aranda-Delgado, E., Nampoothiri, K. M., Favela-Torres, E., Aguilar, C. N., and Saucedo-Castañeda, G. (2014). “Soluble and bound hydroxycinnamates in coffee pulp (Coffea arabica) from seven cultivars at three ripening stages,” Journal of Agricultural and Food Chemistry 62(31), 7869-7876. DOI: 10.1021/jf5014956
Runti, G., Pacor, S., Colomban, S., Gennaro, R., Navarini, L., and Scocchi, M. (2015). “Arabica coffee extract shows antibacterial activity against Staphylococcus epidermidis and Enterococcus faecalis and low toxicity towards a human cell line,” LWT 62(1), 108-114. DOI: 10.1016/j.lwt.2014.12.039
Santos, É. M. dos, de Macedo, L. M., Tundisi, L. L., Ataide, J. A., Camargo, G. A., Alves, R. C., Oliveira, M. B. P. P., and Mazzola, P. G. (2021). “Coffee by-products in topical formulations: A review,” Trends in Food Science and Technology 111, 280-291. DOI: 10.1016/j.tifs.2021.02.064
Sette, P., Fernandez, A., Soria, J., Rodriguez, R., Salvatori, D., and G. Mazza, G. (2020). “Integral valorization of fruit waste from wine and cider industries,” Journal of Cleaner Production 242, Article no. 118486. DOI: 10.1016/j.jclepro.2019.118486
Shehata, M. G., Awad, T. S., Asker, D., el Sohaimy, S. A., Abd El- Aziz, N. M., and Youssef, M. M. (2021). “Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts,” Current Research in Food Science 4, 326-335. DOI: 10.1016/j.crfs.2021.05.001
Sherazi, S. T. H., Talpur, M. Y., Mahesar, S. A., Kandhro, A. A., and Arain, S. (2009). “Main fatty acid classes in vegetable oils by SB-ATR-Fourier transform infrared (FTIR) spectroscopy,” Talanta 80, 600-606. DOI: 10.1016/j.talanta.2009.07.030
Silva, A. C. R., da Silva, C. C., Garrett, R., and Rezende, C. M. (2020). “Comprehensive lipid analysis of green Arabica coffee beans by LC-HRMS/MS,” Food Research International 137. DOI: 10.1016/j.foodres.2020.109727
Sualeh, A., Tolessa, K., and Mohammed, A. (2020). “Biochemical composition of green and roasted coffee beans and their association with coffee quality from different districts of southwest Ethiopia,” Heliyon 6(12). DOI: 10.1016/j.heliyon.2020.e05812
TAPPI T222 om-11. (2011). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
Torres-Valenzuela, L. S., Ballesteros-Gómez, A., and Rubio, S. (2020). “Supramolecular solvent extraction of bioactives from coffee cherry pulp,” Journal of Food Engineering 278, Article no. 109933. DOI: 10.1016/j.jfoodeng.2020.109933
Uddin, M. N., Techato, K., Rasul, M. G., Hassan, N. M. S., and M. Mofijur, M. (2019). “Waste coffee oil: A promising source for biodiesel production,” Energy Procedia 160, 677-682. DOI: 10.1016/j.egypro.2019.02.221
Vidal, O. L., Barros Santos, M. C., Batista, A. P., Andrigo, F. F., Baréa, B., Lecomte, J., Figueroa-Espinoza, M. C., Gontard, N., Villeneuve, P., Guillard, V., Rezende, C. M., Bourlieu-Lacanal, C., and Larraz Ferreira, M. S. (2022). “Active packaging films containing antioxidant extracts from green coffee oil by-products to prevent lipid oxidation,” Journal of Food Engineering 312, Article no. 110744. DOI: 10.1016/j.jfoodeng.2021.110744
Villarreal-Peña, D., Baena-Clavijo, L. M., and Posada-Suárez, H. E. (2012). “Análisis de lípidos y ácidos grasos en café verde de líneas avanzadas de Coffea arabica cultivadas en colombia,” Revista Cenicafé 63(1), 19-40.
Wu, H., Gu, J., BK, A., Nawaz, M. A., Barrow, C. J., Dunshea, F. R., and Suleria, H. A. R. (2022). “Effect of processing on bioaccessibility and bioavailability – of bioactive compounds in coffee beans,” Food Bioscience 46, 1-13. DOI: 10.1016/j.fbio.2021.101373
Zarrinbakhsh, N., Wang, T., Rodriguez-Uribe, A., Misra, M., and Mohanty, A. K. (2016). “Characterization of wastes and coproducts from the coffee industry for composite material production,” BioResources 11(3), 7637-7653. DOI: 10.15376/biores.11.3.7637-7653
Article submitted: July 22, 2022; Peer review completed: March 18, 2023; Revised version received: April 24, 2023; Accepted: April 27, 2023; Published: July 10, 2023.
DOI: 10.15376/biores.18.3.5703-5723
SUPPLEMENTARY
Appendix
Fig. S1. Post-harvest coffee processing
Fig. S2. Scheme for the extraction of bioactive compounds from coffee wastes
Fig. S3. High-Performance Liquid Chromatography spectra of coffee wastes extracts
Fig. S4. Infrared analysis (FTIR) of coffee wastes extracts
