Abstract
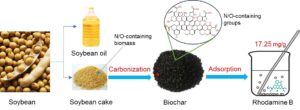
Nitrogen-doped biochar performs much better in dye adsorption due to its rich functional groups. Soybean cake, a by-product of soybean oil production, comprises rich contents of cellulose, lignin, and protein. Therein, simple direct carbonization was utilized to prepare self-nitrogen-doped biochar (SCB). The results showed that the N content of SCB was 6.81 wt%, and its specific surface area was 18.8 m2/g. X-ray photoelectron spectroscopic results confirmed that the surface of SCB was rich in pyridine-N, pyrrole-N, graphite-N, and oxidized-N functional groups. The adsorption capacity of SCB for Rhodamine B was 17.2 mg/g, which is higher compared with other unactivated biochars. The results of thermodynamic parameters indicate that Rhodamine B adsorption on SCB is an endothermic, entropy-increasing, and spontaneous process.
Download PDF
Full Article
Self-Nitrogen-Doped Biochar Derived from Soybean Cake for Rhodamine B Removal Prepared via Simple Carbonization
Xinyu Zhang,a,b Chao Yang,a Tingwei Zhang,a Jiaqi Guo,a Yusheng Gan,b Haibiao Wu,b Mohammad Rizwan Khan,c Huining Xiao,d and Junlong Song a,*
Nitrogen-doped biochar performs much better in dye adsorption due to its rich functional groups. Soybean cake, a by-product of soybean oil production, comprises rich contents of cellulose, lignin, and protein. Therein, simple direct carbonization was utilized to prepare self-nitrogen-doped biochar (SCB). The results showed that the N content of SCB was 6.81 wt%, and its specific surface area was 18.8 m2/g. X-ray photoelectron spectroscopic results confirmed that the surface of SCB was rich in pyridine-N, pyrrole-N, graphite-N, and oxidized-N functional groups. The adsorption capacity of SCB for Rhodamine B was 17.2 mg/g, which is higher compared with other unactivated biochars. The results of thermodynamic parameters indicate that Rhodamine B adsorption on SCB is an endothermic, entropy-increasing, and spontaneous process.
DOI: 10.15376/biores.18.4.7460-7473
Keywords: Soybean cake; Self-nitrogen-doped biochar; Rhodamine B; Adsorption; Thermodynamics
Contact information: a: Jiangsu Co-Innovation Center for Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing 210037, China; b: Hangzhou HUAWON New Material Technology Co., Ltd, Hangzhou 311305, China; c: Department of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia; d: Department of Chemical Engineering, University of New Brunswick, Fredericton, NB, E3B 5A3, Canada; *Corresponding author: junlong.song@njfu.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
Today, textile dyeing produces a large amount of dye wastewater. Since azo dyes are mutagenic, teratogenic, and carcinogenic (Chung 1983), they can cause severe damage to human health and the ecological environment if dye wastewater is not treated effectively or discharged directly (Al-Tohamy et al. 2022). Compared with membrane filtration and advanced oxidation processes, such as Fenton, electrocatalysis, ozone, and electrochemical oxidation (Albadarin et al. 2017; Boudissa et al. 2019; Crini and Lichtfouse 2019), adsorption is one of the most commonly used technologies to remove pollutants with small or medium molecular size in water since it is convenient, efficient, and inexpensive (Bushra et al. 2021). Among many adsorption materials, biochar is regarded as one of the most promising adsorbent materials because it has many excellent properties in respect of containing oxygen-containing functional groups, excellent stability, low cost, and easy availability (Goodman 2020).
Recently, the modification of biochar by nitrogen doping has attracted much interest, since the doped nitrogen on the surface of biochar enhances electron mobility and improves its hydrophobic properties (Kasera et al. 2022). As such, nitrogen-doped biochars perform much better in adsorbing heavy metals and organic chemicals (Yin et al. 2019). Nitrogen-doping in biochar is usually carried out in two ways: one is self-nitrogen-doped biochar, which uses the biomass that is rich in N element as raw material to prepare biochar; the other is using exogenous nitrogen additive or exogenous nitrogen (post-processing strategy) (Wan et al. 2020). The typical nitrogen additives used in the post-processing strategy include inorganic substances and organic matter (Guo et al. 2019; Lian et al. 2016). Compared with the post-processing strategy, the self-nitrogen-doping method provides an easier way to bind nitrogen atoms into the carbon lattice of biochar, which can maintain a well-structured and evenly distributed nitrogen (Duan et al. 2015). In addition, the self-nitrogen-doping method involves simpler operations and avoids potentially harmful or expensive chemical agents (Gao et al. 2016).
Soybean cake (SC), a by-product of soybean oil production, is mainly used to feed animals. Along with rich cellulose and lignin, which can serve as carbon sources, SC contains rich protein (more than 42%) (Yuan et al. 2010); therefore, it is an excellent potential raw material for self-nitrogen-doped biochar fabrication. For instance, Yin et al. (2019) used SC to prepare the self-nitrogen-doped biochar (SCB), and the adsorption capacity for Pb2+ reached 133.6 mg/g. However, the physicochemical properties of organic pollutants, such as molecular structure and molecular weight (Mw), may greatly affect the adsorption performance of activated carbon (Ahmed and Hameed 2018). Therefore, the adsorption of small organic molecules, e.g., dyes, by self-nitrogen-doped biochar still has excellent research prospects.
In this study, SC-derived self-nitrogen-doped biochars were prepared through direct carbonization at different temperatures (600, 700, and 800 ℃) and characterized. Then the adsorption performance of SCB for Rhodamine B (RhB) was evaluated, and the adsorption kinetics and thermodynamic analysis were assessed.
Experimental
Materials
Soybean cake was acquired from Chaiji grain station (Fuyang, China). Rhodamine B (RhB, Mw = 479.01 g/mol, λmax = 554 nm) was purchased from Aladdin Bio-Chem Technology Co., LTD (Shanghai, China). Ethanol (99.5 wt%) was supplied by Yasheng Chemical Co., LTD (Wuxi, China), HCl (36.7 wt%) and NaOH were provided by Sinopharm Chemical Reagent Co. Ltd. (China). All reagents were analytical grade and used as received.
Preparation of Adsorbents
To begin, SC powder was added into a ceramic ark and placed in a tubular furnace (Kejing, GSL-1100X-XX-S2). It was heated with a heating rate of 5 °C/min under 99.99% N2 atmosphere and then kept at different temperatures (600, 700, and 800 ℃) for 1 h. The pyrolytic residues were soaked in 2 mol/L HCl solution for several hours, then washed thoroughly using deionized water. Finally, the dry SCB adsorbents were obtained by drying the collected samples in an oven at 80 ℃ for 8 h.
The pre-experiment results showed that SCB prepared at 800 ℃ demonstrated the highest adsorption capacity for RhB; it was mainly due to the increase of pore volume and specific surface area caused by the removal of volatile organic compounds at a high temperature (Chen et al. 2008). The SCB used below was the sample prepared at 800 ℃ unless mentioned.
Characterizations
The details of the characterization methods are described in Appendix S1 (Supporting Information).
Adsorption Performance Test
Briefly, 100 mL of RhB solution was added into a 250 mL flask with an initial concentration of 1 to 10 mg/L. The pH of RhB solutions was adjusted to 2, 4, 6, 8, 10, and 12 with dropping HCl (0.1 mol/L) or NaOH (0.1 mol/L) solution. Approximately 200 mg SCB was added into each solution every time, and the adsorption time ranged from 1 to 720 min. During adsorption, all conical flasks were kept in an incubator shaker (ZWY-2101, Shanghai, China) at constant stirring (150 rpm). More information related to removal efficiency, adsorption capacity, adsorption kinetics, adsorption isotherm models, and thermodynamic analysis is addressed in detail in Appendix S2 & 3.
RESULTS AND DISCUSSION
Characterization of Adsorbent
Fig. 1. N2 adsorption-desorption isotherm and pore size distribution based on DFT model (a), SEM diagram (b), Raman spectra (c) of SCB, and XRD patterns of SC and SCB (d)
Fig. 2. XPS spectrum (a), C 1s (b), N 1s (c) for SCB and SCB/RhB, and FT-IR spectra of SCB and SCB/RhB (d)
The textural properties of SCB were characterized using a surface area analyzer (Quanta Chrome Nova 1200). Figure 1(a) shows the result for the N2 adsorption-desorption isotherm, which was a Ⅲ(b) type isotherm with H3 hysteresis loop, and the SBET was 18.79 m2/g obtained from the DFT model. According to the t-plot method, the external surface area was also 18.79 m2/g, while the micropore surface area was negligible. The pore size distribution (insert in Fig. 1(a)) shows that SCB has few micropores or mesopores. This was also confirmed by the SCB pore volume of only 0.021 cm3/g, and the dominant pore size was 5.39 nm. Figure 1(b) shows that SCB is depicted as some particles aggregates of different sizes with a relatively smooth and flat surface. The Raman spectrum of SCB is demonstrated in Fig. 1(c); two prominent diffraction peaks appear at 1337 and 1587 cm−1, corresponding to the “D-band” and “G-band” of carbon materials, respectively. The disordered degree of carbon materials can be measured by the ratio of ID/IG. A higher ID/IG value indicates a higher defect degree and lower graphitization degree. The calculated ID/IG of SCB was 0.97. The 2D peak (~2705 cm−1) is another characteristic peak of the graphite material, and the position and shape can be used to identify the stratification of carbon materials.
The XRD pattern of SC is displayed in Fig. 1(d). That pattern was similar to that of cellulose because cellulose contributes most to SC due to its crystalline structure. After carbonization, two distinct diffraction peaks at approximately 22.8° and 42.2° were present, corresponding to the (002) and (101) crystallographic planes of graphitic carbon (Xiao et al. 2020). Similar XRD diffraction patterns of biochar derived from different precursors were also reported in the literature (Ho et al. 2019; Sevilla et al. 2011). It was also noted that some sharp peaks appeared at 2θ = 28.3, 40.5, 47.8, 50.1, 58.6, 66.3, and 73.6°, which are due to the mineral potassium salts in the inorganic material (Pariyar et al. 2020). This indicates that the HCl solution cannot effectively remove impurities inside the biochar without activation. Thus, the remaining ash in SCB is high.
In addition, X-ray photoelectron spectroscopy (XPS) was employed to detect the chemistry changes on the adsorbent surface. Figure 2(a) shows that SCB is mainly composed of C, O, and N. Figure 2(b) shows several fitting peaks at 284.8, 285.8, 286.5, and 288.9 eV, which represent the C−C/C=C, C−O/C−N, C=O, and −COOH groups, respectively (Mena-Durán et al. 2018; Wu et al. 2020b). Figure 2(c) shows the N 1s spectrum, which can be deconvoluted into four peaks around 398.1 to 398.4 eV (pyridine-N), 399.1 to 400.5 eV (pyrrole-N and/or pyridine-N), 400.8 to 401.3 eV (graphite-N), and 402.8 eV (oxide-N) (Lian et al., 2016). Pyridines and graphite-N were the main components of N-doped biochar, and the content of oxide-N is less. In Fig. 2(d), the broad bands of each sample at approximately 3450 cm−1 are due to either the water absorbed by the biochar or the stretching vibration peaks of −OH contained on the biochar surface. The characteristic peak was detected at 1690 to1590 cm−1, which is attributed to the stretching vibrations of the −C=C/ and C=N bonds, while peaks near 1440 and 1025 cm−1 are attributed to C=C stretching/–C–H2 bending and symmetric C–O stretching or C–N stretching vibration, respectively (Chanajaree et al. 2021; Keiluweit et al. 2010). Oxygen-containing groups would be beneficial to electrostatic interaction and surface complexation between adsorbent and organic dyes. In addition, nitrogen content in SCB was 6.81 wt.% obtained by elemental analysis (Table S1), confirming it contains sufficient N content.
Adsorption Performance Experiments
Effect of pH on adsorption performance
Figure 3(a) shows the relationship between the initial pH of the adsorbent solution and the adsorption capacity of SCB. It can be seen that the adsorption capacity of SCB achieved its maximum at approximately pH = 6.0. When the pH of the solution is higher than 3.7 (the pKa of RhB), RhB molecules change from cationic to zwitterion due to the deprotonation of the carboxyl group in RhB. When SCB interacts with RhB molecules, the positive charge causes electrostatic repulsion under pH < 6.2; whereas when pH > 3.7, RhB molecules may form dimers, thus affecting dye molecules’ adsorption (Deng et al. 2019).
Fig. 3. Effects of pH (a) and adsorption time (b) on the adsorption capacity of SCB for RhB
Adsorption kinetic models fitting
Figure 3(b) shows the adsorption process of RhB on SCB over time. The adsorption process presents two stages (fast and slow adsorption). The fast adsorption rate in the early stage is due to the presence of a large number of adsorption sites on SCB. Subsequently, the adsorption rate decreases as adsorption progresses due to the decreasing number of sites on SCB surface and the decrease of adsorbate concentration in the solution (Wu et al. 2020). The results of the parameters are shown in Table 1. Regarding the fitting results, considering the values of R2 are very close to 1, these two models can fit the experimental adsorption data well, especially the pseudo-second-order model.
Table 1. Parameters of Kinetics Model for RhB Adsorption on SCB
Adsorption isothermal models fitting
Figure 4(a) shows that the adsorption capacity of SCB for RhB increased with initial concentration and temperature. On the one hand, an increase in initial adsorbent concentration will lead to an increase in adsorbent capacity, possibly due to the significant driving force for mass transfer at a higher initial dye concentration; on the other hand, as the adsorption temperature increases, the mobility of dye molecules increases, and the number of adsorbable active sites also increases (Salleh et al. 2011).
Fig. 4. Effect of temperature and concentration on the adsorption capacity (a), Function of lnKL and 1/T of the adsorption rate constant of RhB on SCB (b)
Table 2. Parameters of Isotherm Model for RhB Adsorption on SCB
Table 2 shows the parameters of the isothermal models obtained by non-linear fitting. According to the Langmuirian isotherm model fitting, the theoretical adsorption capacity of SCB for RhB at 25 and 55 °C was 17.25 and 21.16 mg/g, respectively. Compared with other adsorption materials reported in the literature, such as nosean composite (14.1 mg/g) (Ni et al. 2022), waste coconut shell biochar (12.41 mg/g) (Li et al. 2022), and Fe-N-biochar (12.4 mg/g) (Li et al. 2022), the adsorption capacity of SCB can be regarded as good enough.
The results of FT-IR and XPS show that the surface of SCB contains carboxyl, hydroxyl, and nitrogen-containing functional groups. Carboxyl and hydroxyl groups can form hydrogen bonds with hydroxyl groups on RhB molecules. In addition, the electrostatic adsorption between SCB and RhB cannot be ignored. Raman spectroscopy and XRD confirm that SCB has a graphite structure, RhB molecules are planar molecular structures, which can form π-π accumulation on the surface of biochar.
Ethanol desorption technology was used to evaluate the feasibility of reproducing SCB. The percentage of RhB desorption results is as high as 99%. Therefore, it can be considered that almost all adsorption points of SCB are recovered from the desorption process.
Adsorption thermodynamics
Figure 4(b) shows the fitting of adsorption thermodynamic parameters, and the fitting results are shown in Table 3. The ΔG° values of RhB adsorbed on SCB at multiple different temperatures were all negative, indicating that the adsorption was spontaneous in the system under a normal state (Fenti et al. 2019). Positive ΔH° values suggest that the adsorption of RhB on SCB is an endothermic process. Positive ΔS° values indicate that the adsorption of RhB on SCB leads to increased disorder at the biochar/adsorbate solution interface.
Table 3. Thermodynamic Parameters Related to Adsorption of RhB on SCB
CONCLUSIONS
- In this study, soybean cake-derived SCB was successfully prepared through simple direct carbonization. Its specific surface area reached 18.8 m2/g and its nitrogen content was 6.81 wt.%.
- According to Langmuir isotherm model fitting, the theoretical adsorption capacity of SCB for RhB reached 17.2 mg/g, which is relatively good for biochar prepared without any activation.
- The ΔG° values were all negative, indicating that the adsorption of RhB on SCB was spontaneous; the positive ΔH° values indicate that the adsorption was endothermic; the positive ΔS° values indicate that the adsorption of dyes on SCB resulted in increased disorder at the biochar/adsorbate solution interface.
ACKNOWLEDGMENTS
This project was financially supported by the National Natural Science Foundation of China (Nos. 32101468 and 31770623), Natural Science Foundation of Jiangsu Province (No. BK20210622), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJKY19-0904), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. M.R.K. acknowledged the Researchers Supporting Project (No. RSP2023R138), King Saud University, Riyadh, Saudi Arabia.
REFERENCES CITED
Ahmed, M. J., and Hameed, B. H. (2018). “Adsorption behavior of salicylic acid on biochar as derived from the thermal pyrolysis of barley straws,” J. Clean. Prod. 195, 1162-1169. DOI: 10.1016/j.jclepro.2018.05.257
Al-Tohamy, R., Ali, S. S., Li, F., Okasha, K. M., Mahmoud, Y. A. G., Elsamahy, T., and Sun, J. (2022). “A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety,” Ecotoxicol. Environ. Saf. 231, 113160. DOI:10.1016/j.ecoenv.2021.113160
Albadarin, A. B., Collins, M. N., Naushad, M., Shirazian, S., Walker, G., and Mangwandi, C. (2017). “Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue,” Chem. Eng. J. 307, 264-272. DOI: 10.1016/j.cej.2016.08.089
Boudissa, F., Mirilà, D., Arus, V.-A., Terkmani, T., Semaan, S., Proulx, M., and Azzouz, A. (2019). “Acid-treated clay catalysts for organic dye ozonation-Thorough mineralization through optimum catalyst basicity and hydrophilic character,” J. Hazard. Mater. 364, 356-366. DOI:10.1016/j.jhazmat.2018.09.070
Bushra, R., Mohamad, S., Alias, Y., Jin, Y., and Ahmad, M. (2021). “Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review,” Micropor. Mesopor. Mater. 319, 111040. DOI:10.1016/j.micromeso.2021.111040
Chanajaree, R., Sriuttha, M., Lee, V. S., and Wittayanarakul, K. (2021). “Thermodynamics and kinetics of cationic/anionic dyes adsorption on cross-linked chitosan,” J. Mol. Liq. 322, artcile 114507. DOI:10.1016/j.molliq.2020.114507
Chen, B., Zhou, D., and Zhu, L. (2008). “Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures,” Environ. Sci. Technol. 42(14), 5137-5143. DOI:10.1021/es8002684
Chung, K.-T. (1983). “The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes,” Mutation Research/Reviews in Genetic Toxicology 114(3), 269-281. DOI:10.1016/0165-1110(83)90035-0
Crini, G., and Lichtfouse, E. (2019). “Advantages and disadvantages of techniques used for wastewater treatment,” Environ. Chem. Lett. 17(1), 145-155. DOI:10.1007/s10311-018-0785-9
Deng, H., Mao, Z., Xu, H., Zhang, L., Zhong, Y., and Sui, X. (2019). “Synthesis of fibrous LaFeO3 perovskite oxide for adsorption of Rhodamine B,” Ecotoxicol. Environ. Saf. 168, 35-44. DOI:10.1016/j.ecoenv.2018.09.056
Duan, X., O’Donnell, K., Sun, H., Wang, Y., and Wang, S. (2015). “Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions,” Small 11(25), 3036-3044. DOI:10.1002/smll.201403715
Fenti, A., Iovino, P., and Salvestrini, S. (2019). “Some remarks on ‘A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption’ – Journal of Molecular Liquids 273 (2019) 425-434,” J. Mol. Liq. 276, 529-530. DOI:10.1016/j.molliq.2018.12.019
Gao, Y., Xu, S., Yue, Q., Ortaboy, S., Gao, B., and Sun, Y. (2016). “Synthesis and characterization of heteroatom-enriched biochar from keratin-based and algous-based wastes,” Adv. Powder Technol. 27(4), 1280-1286. DOI:10.1016/j.apt.2016.04.018
Goodman, B. A. (2020). “Utilization of waste straw and husks from rice production: A review,” J. Bioresour. Bioprod., 143-162. DOI: 10.1016/j.jobab.2020.07.001
Guo, S., Gao, Y., Wang, Y., Liu, Z., Wei, X., Peng, P., and Yang, Y. (2019). “Urea/ZnCl2 in situ hydrothermal carbonization of Camellia sinensis waste to prepare N-doped biochar for heavy metal removal,” Environ. Sci. Pollut. Res. Int. 26(29), 30365-30373. DOI:10.1007/s11356-019-06194-8
Ho, S.-H., Chen, Y.-d., Li, R., Zhang, C., Ge, Y., Cao, G., and Ren, N.-q. (2019). “N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation,” Water Res. 159, 77-86. DOI:10.1016/j.watres.2019.05.008
Kasera, N., Kolar, P., and Hall, S. G. (2022). “Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review,” Biochar 4(1), 1-30. DOI:10.1007/s42773-022-00145-2
Keiluweit, M., Nico, P. S., Johnson, M. G., and Kleber, M. (2010). “Dynamic molecular structure of plant biomass-derived black carbon (biochar),” Environ. Sci. Technol. 44(4), 1247-1253. DOI:10.1021/es9031419
Li, X., Shi, J., and Luo, X. (2022). “Enhanced adsorption of rhodamine B from water by Fe-N co-modified biochar: Preparation, performance, mechanism and reusability,” Bioresour. Technol. 343, 126103. DOI:10.1016/j.biortech.2021.126103
Lian, F., Cui, G., Liu, Z., Duo, L., Zhang, G., and Xing, B. (2016). “One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity,” Journal of Environmental Management 176, 61-68. DOI:10.1016/j.jenvman.2016.03.043
Mena-Durán, C. J., Alonso-Lemus, I. L., Quintana, P., Barbosa, R., Ordoñez, L. C., and Escobar, B. (2018). “Preparation of metal-free electrocatalysts from cassava residues for the oxygen reduction reaction: A sulfur functionalization approach,” Int. J. Hydrogen Energy 43(6), 3172-3179. DOI:10.1016/j.ijhydene.2017.12.139
Ni, X., Sun, X., Xu, Y., and Xu, D. (2022). “A green and facile synthesis of nosean composite from coal fly ash for optimizing Rhodamine B adsorption using response surface methodology,” J. Mol. Liq. 359, 119262. DOI:10.1016/j.molliq.2022.119262
Pariyar, P., Kumari, K., Jain, M. K., and Jadhao, P. S. (2020). “Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application,” Sci. Total Environ. 713, 136433. DOI: 10.1016/j.scitotenv.2019.136433
Salleh, M. A. M., Mahmoud, D. K., Karim, W. A. W. A., and Idris, A. (2011). “Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review,” Desalination 280(1-3), 1-13. DOI: 10.1016/j.desal.2011.07.019
Sevilla, M., Valle-Vigón, P., and Fuertes, A. B. (2011). “N-doped polypyrrole-based porous carbons for CO2 capture,” Adv. Funct. Mater. 21(14), 2781-2787. DOI:10.1002/adfm.201100291
Wan, Z., Sun, Y., Tsang, D. C., Khan, E., Yip, A. C., Ng, Y. H., and Ok, Y. S. (2020). “Customised fabrication of nitrogen-doped biochar for environmental and energy applications,” Chem. Eng. J. 126136. DOI:10.1016/j.cej.2020.126136
Wu, J., Yang, J., Huang, G., Xu, C., and Lin, B. (2020). “Hydrothermal carbonization synthesis of cassava slag biochar with excellent adsorption performance for Rhodamine B,” J. Clean. Prod. 251, 119717. DOI:10.1016/j.jclepro.2019.119717
Wu, J., Yang, J. W., Huang, G. H., Xu, C. H., and Lin, B. F. (2020). “Hydrothermal carbonization synthesis of cassava slag biochar with excellent adsorption performance for Rhodamine B,” J. Clean. Prod. 251. DOI: 10.1016/j.jclepro.2019.119717
Xiao, W., Garba, Z. N., Sun, S., Lawan, I., Wang, L., Lin, M., and Yuan, Z. (2020). “Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye,” J. Clean. Prod. 253, 119989. DOI: 10.1016/j.jclepro.2020.119989
Yin, W., Dai, D., Hou, J., Wang, S., Wu, X., and Wang, X. (2019). “Hierarchical porous biochar-based functional materials derived from biowaste for Pb(II) removal,” Appl. Surf. Sci. 465, 297-302. DOI:10.1016/j.apsusc.2018.09.010
Yuan, S., Zhou, Z.-j., Li, J., Chen, X.-l., and Wang, F.-c. (2010). “HCN and NH3 released from biomass and soybean cake under rapid pyrolysis,” Energy Fuels 24(11), 6166-6171. DOI:10.1021/ef100959g
Article submitted: July 10, 2023; Peer review completed: July 26, 2023; Revised version received and accepted: August 21, 2023; Published: September 15, 2023.
DOI: 10.15376/biores.18.4.7460-7473
