Abstract
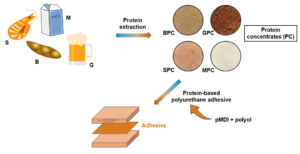
Wood structures generally rely on synthetic adhesives for their strength and versatility. However, environmental concerns linked to the chemical composition of these adhesives have stimulated the search for more environmentally friendly adhesives. Researchers have explored replacing petroleum-based constituents with natural raw materials such as lignins, tannins, and proteins. Of these alternatives, proteins, being biological macromolecules, are recognized for their capacity to enhance adhesion to wood substrates. This study considered the development of protein-based adhesives derived from diverse sources, including soybean meal, microbrewery spent grains, shrimp shells, and skim milk powder. These raw materials were subjected to mild alkaline conditions to yield protein concentrates. The resulting adhesives were formulated at various protein content levels: 5%, 10%, 15%, and 20%. The study’s findings showed that the incorporation of proteins into the polyurethane adhesive system not only can preserve but also augment adhesive performance. This enhancement encompasses deeper penetration into wood substrates and an overall improvement in mechanical strength. These results underscore the promise of proteins as a sustainable alternative to petroleum-based polyols in adhesive formulations.
Download PDF
Full Article
Upcycling of Protein Concentrates from Industrial Byproducts into Polyurethane Wood Adhesives
Alex Mary,a Pierre Blanchet,a Simon Pepin,a Julien Chamberland,b and Véronic Landry a,c,*
Wood structures generally rely on synthetic adhesives for their strength and versatility. However, environmental concerns linked to the chemical composition of these adhesives have stimulated the search for more environmentally friendly adhesives. Researchers have explored replacing petroleum-based constituents with natural raw materials such as lignins, tannins, and proteins. Of these alternatives, proteins, being biological macromolecules, are recognized for their capacity to enhance adhesion to wood substrates. This study considered the development of protein-based adhesives derived from diverse sources, including soybean meal, microbrewery spent grains, shrimp shells, and skim milk powder. These raw materials were subjected to mild alkaline conditions to yield protein concentrates. The resulting adhesives were formulated at various protein content levels: 5%, 10%, 15%, and 20%. The study’s findings showed that the incorporation of proteins into the polyurethane adhesive system not only can preserve but also augment adhesive performance. This enhancement encompasses deeper penetration into wood substrates and an overall improvement in mechanical strength. These results underscore the promise of proteins as a sustainable alternative to petroleum-based polyols in adhesive formulations.
DOI: 10.15376/biores.19.1.1165-1189
Keywords: Wood; Engineered wood products; Mass timber; Polyurethane adhesive; Industrial byproducts; Proteins
Contact information: a: Department of Wood and Forest Sciences, NSERC Industrial Research Chair on Eco-Responsible Wood Construction (CIRCERB), Université Laval, Québec, QC G1V 0A6, Canada; b: Department of Food Sciences, STELA Dairy Research Center, Institute of Nutrition and Functional Foods (INAF), Université Laval, Québec, QC, G1V 0A6, Canada; c: Department of Wood and Forest Sciences, NSERC Canlak Industrial Research Chair in interior Wood-Products finishes, Université Laval, Québec, QC G1V 0A6, Canada; *Corresponding author: veronic.landry@sbf.ulaval.ca
GRAPHICAL ABSTRACT
INTRODUCTION
In construction, wood is frequently manufactured into engineered wood products such as glulam or even cross-laminated timber (CLT) to enhance its characteristics and allow long-span or high-rise buildings. However, many of these products rely on non-bio-based adhesives, which can tarnish wood materials’ otherwise positive environmental profile (Chen et al. 2019). Among the synthetic resins commonly used in this industry today are phenol-formaldehyde or polyurethane (Arias et al. 2022). Addressing the growing societal interest in eco-friendly adhesives calls for intensified research into testing alternative, less harmful, renewable, and bio-based elements for prospective adhesive formulations. Polyurethane adhesives (PU), known for their effectiveness and formaldehyde-free nature, are widely utilized in this industry. They can be either one-component or two-component adhesives. Two-component polyurethanes are composed of at least one isocyanate prepolymer and a polyol. While these adhesives exhibit strong bonding capabilities and compatibility with wood, most are derived from petrochemical sources (Pizzi and Mittal 2005; Kumar and Pizzi 2019).
Several studies have been carried out to increase the biobased content of these adhesives. The main biobased adhesives studied to date are tannin-, lignin-, and protein-based (Pizzi and Mittal 2005; Vnučec et al. 2017). Tannins and lignins are plant-based polyphenols used in adhesive formulations to substitute phenol in phenol-formaldehyde resins (Pizzi 2016). This partial substitution reduces the consumption of petrochemical phenol. The results have shown that the addition of tannins results in improved water and moisture resistance (Bisanda et al. 2003). As far as lignin is concerned, the reactive sites are less available than in phenol, resulting in a lower reactivity of phenol towards formaldehyde (Pizzi 2006). Furthermore, this substitution of phenol by lignin revealed a reduction in the water resistance of the final resin (Solt et al. 2019). Another approach that has been extensively studied in the literature is the use of proteins in the development of adhesives. Proteins are biological macromolecules known to improve the adhesion of the adhesive to the wood substrate (Yang et al. 2006; Yan et al. 2023). The isocyanates will then be able to react not only with the hydroxyl groups of the polyol, but also with the amino groups of the amino acids of the proteins. The most studied proteins are soy, cotton, and milk proteins. Although soy proteins increase the durability of adhesives, this comes at the cost of increased viscosity and decreased water resistance (Huang and Li 2008; Vnučec et al. 2017). These adhesives can be replaced by protein-based adhesives derived from cotton, which offer better water resistance (Cheng et al. 2013). Milk protein-based adhesives can create strong bonds with wood, but their long-term resistance in humid environments must be improved (Detlefsen 1989; Vick and Rowell 1990). To minimize environmental impact, it is preferable to aim for local raw materials derived from non-recyclable or excess industrial byproducts (Chalapud et al. 2020; Badouard et al. 2021).
This study revolves around incorporating diverse industrial byproducts into a polyurethane adhesive system. The focus lies on exploring the potential of specific raw materials – soybean meal, microbrewery spent grains, shrimp shells, and skim milk powder – as viable protein sources within adhesive development. Soybean meals are mainly composed of soy protein. Soy, recognized for its renewability and widespread availability, contributes substantially to agricultural yields in countries such as the United States, Brazil, Argentina, and China (Huang and Li 2008; Eslah et al. 2016). Originating from grain crops, microbrewery spent grains are typically disregarded or minimally used as livestock feed. Shrimp shells accumulation raises environmental concerns and disposal complexities. These shells can be ground into flour and used as a natural fertilizer. Skim milk powder is derived from the dehydration of skim milk. The increase in demand, and therefore production, of butter and cream in Canada has resulted in a more important accumulation of skim milk, a product whose demand has remained stable over time. Although this excess skim milk can be used as powder for animal feed, the supply remains greater than the demand, resulting in a surplus of skim milk powder in Canada. Protein concentrates have been obtained from these raw materials.
This research aims to assess the suitability of these protein concentrates as replacements for petroleum-based polyols. Initially, the study involves characterizing the proteins to gain insights into their structure and chemical composition, which are essential for understanding their behavior upon incorporation. Subsequently, protein concentrates are introduced at varying content levels (5%, 10%, 15%, and 20%) to investigate the influence of proteins on the kinetic and mechanical properties of adhesives.
EXPERIMENTAL
Materials
The raw materials used as protein sources are non-recyclable or excess industrial co-products from local resources. Soybean meal (B) in pellet form was obtained from Sollio Agriculture (Lévis, Canada). Microbrewery spent grains (G) were from the microbrewery Le Corsaire (Lévis, Canada), and had been used to produce a Pilsner beer type made of 88% barley, 8% oats, and 4% wheat. Shrimp shells flour (S) was provided by Les Pêcheries Marinard ltée (Gaspé, Canada). Skim milk powder (M) was provided by Agropur (Longueuil, Canada). Polymeric methylene diphenyl diisocyanate (pMDI) (mass equivalent amine=32.5 wt.%, viscosity=129 mPa.s at 20 °C) and a polypropylene oxide-based triol (Multranol 8175) (acid value=350-390 mg KOH/g sample, molecular weight=450 Da, viscosity=232-412 mPa.s at 25 °C) from Covestro (Pittsburgh, USA) were provided by EMCO-Inortech (Terrebonne, Canada). All chemicals were used as received.
Proteins Extraction
Protein concentrates were prepared by alkaline extraction for B, G, and S. The extraction details are presented in Table 1. After 60 min of extraction, the solutions were centrifuged at 2,650 × g for 10 min at 20 °C, and the supernatants were collected (Celus et al. 2009). Samples were then washed with 2 mL of distilled water to be centrifuged again, and the filtrates were collected. Proteins in the filtrates were precipitated by acidification to pH 4.0 using 2.0 M citric acid and then placed at 4 °C for 3 h. The obtained protein precipitates were then centrifuged at 4,250 × g for 10 min at 4 °C. The supernatants were disposed of, and the precipitates were washed with 2 mL of 0.1 M NaOH or 0.5 M NaOH for S solutions and centrifuged. The protein precipitates were finally freeze-dried to recover the samples in powder form and remove traces of water. A distinct method, already studied in the literature, was used for producing M protein concentrates (Husnaeni et al. 2019). Specifically, acetic acid was added to the mixture when its temperature had reached 40 °C, leading to precipitation. Afterward, the mixture was filtered, and the collected product precipitate was subjected to a temperature of 40 °C in an oven until it was completely dry. The B, G, S, and M protein concentrates, all in powder form, are respectively noted as BPC, GPC, SPC, and MPC.
Table 1. Solution, Ratio, and Temperature of Extraction for Soybean Meal, Microbrewery Spent Grains, Shrimp Shells, and Skim Milk Powder
Protein Concentrates Characterization
Composition
The composition of the protein concentrates was determined to identify the various elements that might react during adhesive formulation. Before their transformation into protein concentrates, the raw materials predominantly comprised proteins, fats, ashes, and carbohydrates. Subsequent analyses were conducted to quantify the percentage composition of these constituents within the protein concentrates.
The nitrogen percentage must be determined to ascertain the samples’ protein content. The analysis was performed using the carbon, nitrogen, and sulfur (CNS) in Plant Tissue method, from LECO Corporation, at a temperature of 1,350 °C on a TruMAC CNS (LECO Corporation, Midland, Canada). Once the nitrogen content was obtained, the protein content could be determined using a conversion factor specific to each raw material (Table 2). The analyses were performed in triplicate.
Table 2. Conversion Factors from Nitrogen Content to Protein Content of Raw Materials
The Soxhlet method served as the benchmark technique for ascertaining fat content in dehydrated solid food products. In each sample set, approximately 3 g of protein concentrate was subjected to extraction, and the results were extrapolated to the complete mass of the sets. The extractions were carried out employing a Soxhlet apparatus employing 100 mL of ethyl ether for 2 h and 30 min. Following the ethyl ether evaporation, the extracts were placed in an oven at 103 °C overnight before weighing. The analysis were performed in duplicate
To ascertain the ash content in the samples, around 2 g of each protein concentrate was loaded into a porcelain crucible and subjected to an oven at 600 °C for 6 h. After cooling, the crucible, now containing the ash residue, was weighed after cooling in a desiccator to determine the ash content. This analysis was conducted in triplicate.
According to the nutrition labeling regulations of the US Food and Drug Administration 21 CFR 101.9 (c)(6)(i)–(iv), the value of carbohydrate content of foods was calculated as presented by Eq. 1 (Anon 2016). Moisture results were determined by dynamic vapour sorption as described below.
(1)
Molecular weight and protein identification
The molecular weight of the proteins contained in the protein concentrates was determined, as this value can have an impact on adhesive properties such as viscosity. Protein digestion and mass spectrometry analyses were performed by the Proteomics Platform of CHU Research Center (Quebec, Canada). The analysis, as detailed by Mary et al. (2023), employs gel electrophoresis and includes a series of steps: sample solubilization, gel migration, protein digestion, mass spectrometry, and database searching (Mary et al. 2023). Concerning database searching, Mascot generic format peak list files were created using Proteome Discoverer 2.3 software (Thermo Fisher Scientific, San Jose, USA). Mascot generic format sample files were then analyzed using Mascot (Matrix Science, London, UK; version 2.5.1). Mascot was set up to search a contaminant database, and depending on the species, the following databases from Uniprot were used: Glycine max (UP000008827, 74,863 entries) for BPC samples, Hordeum vulgare (UP000011116, 35,907 entries) for GPC samples, Penaeus (all proteins under taxon id 133894, 29,183 entries) for SPC samples, and Bos Taurus (UP000009136, 37,880 entries) for MPC samples, assuming the digestion enzyme trypsin and with a fragment ion mass tolerance of 0.60 Da and a parent ion tolerance of 10.0 ppm. Carbamidomethylation of cysteine was specified in Mascot as a fixed modification. Deamidation of asparagine and glutamine and oxidation of methionine were specified as variable modifications.
Amino acid profiles
Amino acids fall into three categories: hydrophilic, hydrophobic, and amphipathic (Vnučec et al. 2017). The nature of amino acids can influence the moisture resistance of protein concentrates and adhesives. Amino acid analysis was conducted by Merinov (Gaspé, Canada). Total amino acids, excluding tryptophan due to its susceptibility to acid hydrolysis, were determined using the Official Methods of Analysis of AOAC International, 17th Edition, as detailed in Method 45.4.04. The determination of hydrolysis times and the quantification of tryptophan were conducted following the procedures described by Albin et al. (2000) and Sanchez-Machado et al. (2008), respectively.
Dynamic vapor sorption (DVS)
Sorption analyses assess the water absorption capacity of the protein concentrate, which can influence the water resistance of adhesives. Sorption data were collected on a water vapor sorption analyzer at a constant temperature of 25 °C. The instrument used was the DVS Adventure water vapor sorption analyzer (Surface Measurement Systems, Allentown, USA). The samples were dried for at least 24 h at 40 °C before being analyzed. This initial drying was followed by 4 h at 0% relative humidity and 25 °C. The sample was then exposed to 2 cycles of humidity variations for MPC samples, according to the Moisture Stability of Powdered Milk Formulations method from the DVS Application Note 11 (Surface Measurement Systems, Allentown, USA), and one cycle for the other protein samples. A cycle was characterized by a series of relative humidity steps from 0% to 95% and then to the same series in reverse order. The steps were 10% humidity between 0 and 90% relative humidity, then 5% up to 95% relative humidity.
X-Ray diffraction (XRD)
XRD is a nondestructive technique widely used to determine the structure of materials. The X-ray diffractograms of BPC, GPC, SPC, and MPC were recorded by using AERIS powder X-ray diffraction (Malvern PANalytical, Malvern, United Kingdom) with a scanning range of 5 to 70° and a 0.01° step.
Thermal stability
Thermal analyses were conducted on the protein concentrates to establish the temperature range suitable for their application. The thermal stability of protein concentrates was determined using thermogravimetric analysis (TGA). TGA was performed on a TGA/DSC 3+ (Mettler Toledo, Columbia, USA). Protein concentrate samples of 4 to 10 mg were placed in a 70 uL reusable sapphire crucible. The samples were heated from 25 to 700 °C with a 20 °C/min heating rate under nitrogen flow. The temperature at which the protein begins to degrade was considered as the starting temperature for the second stage of weight loss, the first being attributed to moisture loss (Ricci et al. 2018). The analyses were performed in triplicate.
Preparation of Polyurethane Adhesives
Polyurethane adhesive formulations were prepared with a ratio of isocyanate to hydroxyl functions (NCO/OH) of 1.13, to ensure a complete reaction between the polyol and the isocyanate (Meier-Westhues 2019). The incorporation of proteins was done by substituting part of the hydroxyl groups of the polyol with the amine groups of the proteins. Proteins were incorporated into the polyol and dispersed at 1000 rpm for 3 min with a Dispermat LC30 Dissolver (VMA-Getzmann, Reichshof, Germany) with a 45 mm flat turbine. The substitution of polyol with protein concentrates was performed at 5%, 10%, 15%, and 20%. The percentages were determined considering the nitrogen content of the protein concentrates, the hydroxyl content of the polyol and on the consideration that one NH function is equivalent to one OH function. The petrochemical reference, formulated with the same chemicals as the protein-based adhesives, is represented by the formulation containing 0% protein. Polyurethane adhesives composed of BPC, GPC, SPC, and MPC are respectively named PU-BPC, PU-GPC, PU-SPC, and PU-MPC.
Adhesives Characterization
Moisture uptake
Moisture uptake analysis evaluates how well the adhesive can withstand exposure to moisture. The adhesive samples, cured at ambient humidity and temperature, were ground into powder and were dried at 50 °C until their weight remained constant. After that, 1 g of the samples were placed in a chamber with a constant temperature of 22 ± 1 °C and 90 ± 1 % relative humidity. The weight of the adhesive samples was measured until the weight remained constant. Finally, the moisture uptake of the samples was calculated using the following equation,
(2)
where mwet is the weight (g) of the adhesive after moisture uptake, and mdry is the weight (g) of the dried sample. The analyses were performed in triplicate.
Time-dependant viscosity
Viscosity is an important physical parameter that affects the behavior of the adhesive. Proper viscosity gives the adhesive good flowability, facilitates handling to achieve high bond strength of the bonded product, and allows penetration into the first cells of the wood, which is necessary for mechanical anchoring (Luo et al. 2016). Viscosity measurements were conducted at a temperature of room temperature using a Bohlin Visco88 viscometer (Malvern Instruments Limited, Worcestershire, UK) by taking a viscosity measurement every 2 min. This particular viscometer adopts a concentric cylindrical design comprising a rotating inner cylinder and a stationary outer cylinder. The inner cylinder has a diameter of 25 mm, while the outer cylinder has a diameter of 27.55 mm. The analyses were carried out at a rotational speed of 6.39 rad·s-1. The torque developed on the inner cylinder by the sample is directly correlated with the viscosity of the sample and should be in a range of 0.5 to 9.5 mN.m to ensure accurate measurement. Approximately 15 mL of the sample was introduced into the viscometer cylinder.
Effective penetration
Adhesive penetration analysis is essential to assess the adhesive’s ability to penetrate the wood surface and pores, ensuring the creation of a strong, durable bond. Two-layer glued laminated wood panels were produced for each adhesive and then cut into 1 cm3 cubes. A HistoCore AUTOCUT automatic rotary microtome (Leica Biosystems, Buffalo Grove, USA) was used to cut 40 µm-thick cross-sections. Cross-sections were placed on glass slides with deionized water, covered with a glass coverslip, and sealed with nail varnish to prevent water evaporation. The penetration of adhesives into the wood was observed using a VHX-7000 digital microscope at ×700 magnification (Keyence Co. Ltd., Osaka, Japan). Full ring lighting was used to allow dark field observation. The quantitative examination of adhesive penetration into the wood substrate was conducted through the measurement of effective penetration depth (EP) (Bastani et al. 2016; Qin et al. 2016; Sernek et al. 1999). EP represents the total area of adhesive detected within the interphase region, divided by the width of the bondline. The EP was calculated as follows,
(3)
where EP is the effective penetration depth (µm), Ai the area of adhesive object i (µm²), and X0 the width of the maximum rectangle defining measurement area (µm). Three measurement zones were studied for each adhesive. The measurement parameters outlined in Eq. 3 are visually depicted in Fig. 1. Subsequently, ImageJ software was employed to quantify and measure these parameters.
Fig. 1. Measurement parameters in experimental image
Gel time
The gel time, which defines the adhesive’s handling and curing duration, refers to the time needed for the adhesive to attain a specific level of gelation or stiffness after the components are mixed (Desai et al. 2003). The analyses were performed using a Gel time meter 22A (Sunshine, Philadelphia, USA). Samples were poured into a test tube, which was immersed in an oil bath maintained at 100 °C. The samples were heated until they polymerized, and the time was recorded in seconds. The analyses were performed in triplicate.
Kinetics and conversion
Kinetic analyses serve to investigate whether protein concentrates influence the polymerization process of adhesives. The polymerization kinetics of the adhesive systems were studied by real-time FTIR (RT-FTIR). The instrument used was the INVENIO® R (Bruker Optics Inc., Billerica, USA). Spectra were recorded at room temperature in the range 450 to 4000 cm-1 for 215 min, corresponding to 400 measurements of 32 scans with a resolution of 4 cm-1. A baseline correction was performed for all absorption peaks. Details of the expected FTIR bands are shown in Table 3 (Maji and Bhowmick 2009). The analyses were performed in duplicate.
The urethane formation can be monitored by the disappearance of the isocyanate’s NCO vibration band at 2260 cm-1 and the appearance of the urethane’s C=O vibration band at 1730 cm-1. The isocyanate conversion can be used as the degree of curing reaction as follows (Eq. 4), assuming that there is no side reaction (Maji and Bhowmick 2009).
(4)
where is the 2260 cm-1 peak intensity at the initial time,
is the 2260 cm-1 peak intensity of absorbance at a specified time during the curing,
is the 1730 cm-1 peak intensity at the initial time, and
is the 1730 cm-1 peak intensity of absorbance at a specified time during the curing.
Table 3. Principal Peak Assignments in the FTIR Spectra of the Isocyanate, Polyol, and Cured Polyurethane
Block shear strength
Block shear tests were used to determine the shear strength of adhesives used to bond wood. Wood cutting, bonding, and testing were conducted according to the ASTM D905:2008 test method. Black spruce (Picea mariana, Mill.) wood specimens were cut into rectangular panels of 32 × 65 × 20 mm3, and 3.75 g of adhesive were applied to the wood within 24 h after cutting. The two pieces, one with adhesive and one without, were placed together for the adhesives to be cured at room temperature with a pressure of 150 psi exerted onto the contact area for 24 h. After that, the glued elements were cut according to the ASTM D905:2008 method. Wood specimens were conditioned at 20 °C with a relative humidity of 65% for seven days. Block-shear strength tests were performed on the Alliance RT/50 (Frank Bacon Machinery Sales Co., Warren, USA). The load was applied with continuous movement of the moving head at a 5 mm/min rate until failure. The analyses were performed on ten samples per adhesive. The shear results were analyzed using a least significant difference (LSD) test.
RESULTS AND DISCUSSION
The following sections present the results of the protein concentrate and adhesive analyses to evaluate their suitability as adhesive resin components.
Protein Concentrates Characterization
Nitrogen content
Figure 2 presents the protein content before and after the extraction. The increased protein content observed in the post-extraction samples, in contrast to the pre-extraction counterparts, indicates the viability of the employed extraction protocol.
Fig. 2. Protein content in soybean meal (B), microbrewery spent grains (M), shrimp shells (S), and skim powder milk (M) before and after extraction
Fig. 3. Composition of soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC), and skim powder milk protein concentrate (MPC)
Specifically, the extraction process led to a protein content enhancement of 40.8%, 37.1%, 44.1%, and 44.3% for B, G, S, and M, respectively. Among the samples, M exhibited the most substantial protein content, with S ranking second, followed by B and G. Consequently, to attain the targeted substitution rate when formulating adhesives, a comparatively larger quantity of protein concentrate is necessitated for G, in contrast to the amount required for M.
The components present in the different protein concentrates are presented in Fig. 3. It is necessary to know the composition of these different raw materials because they can impact the polymerization reaction. For example, the OH groups present in the carbohydrates, such as starch, cellulose, chitin, and lactose, can theoretically react with isocyanates, which must be taken into account in the analysis of the different results.
Molecular weight and protein identification
Protein separation by polyacrylamide gel electrophoresis is used to determine the molecular weight of the different proteins present in the samples. (Fig. 4). Liquid chromatography coupled with mass spectrometry ensured the identification and quantification of the various proteins present in raw materials. A molecular weight below 80 kDa is not a barrier to the adhesive formulation because, as the literature has shown, protein-based adhesives up to 100 kDa can be obtained while presenting satisfactory results in terms of adhesion (Jenkins et al. 2013). In fact, having a lower molecular weight helps to promote protein incorporation into the adhesive and minimize the final viscosity of the system.
Fig. 4. Molecular weight of proteins contained in soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC) and skim powder milk protein concentrate (MPC)
The predominant bands within the protein concentrates and their associated proteins are detailed in Table 4. Although some faintly labeled bands were not explicitly identified due to their lower abundance compared to the major proteins specified in Table 4, they are likely related to protein fragments. Table 5 displays the amino acid composition of the protein concentrates according to their properties determined by Vnučec et al., indicating that SPC exhibited slightly higher hydrophilicity than the other concentrates (Vnučec et al. 2017). Subsequent analyses will aim to assess whether these differences in amino acid composition have any effect on the water resistance of both the protein concentrates and the adhesives derived from them.
Table 4. Proteins in Different Concentrates
Note: soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC), and skim powder milk protein concentrate (MPC)
Table 5. Total Amino Acids Content
Note: soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC), and skim powder milk protein concentrate (MPC)
Dynamic Vapour Sorption Analysis
To evaluate the hygroscopicity of proteins under different relative humidity conditions, DVS analyses were performed (Zhao et al. 2021). Two isotherms are presented in Fig. 5: first, the adsorption isotherm, which represents the wetting of the dry sample, and second, the desorption isotherm, which represents the drying of the water-saturated sample. The observed increase in mass is explained by the presence of peptides and potential hydrogen bonding sites in the protein samples (Zhao et al. 2021).
Peptides are polymers of amino acids that make up proteins. When the relative humidity increases, the polar groups of the peptides bind to water molecules, increasing the sample’s mass. The variation in mass among samples can be attributed to the structural dissimilarities among various amino acids. Considering the amino acid compositions detailed in Table 5, it was anticipated that SPC would display higher moisture sensitivity due to its elevated content of hydrophilic amino acids compared to other protein concentrates. The findings depicted in Fig. 5 align with these expectations. Furthermore, as shown in Fig. 3, SPC contained a lower fat content, known for its hydrophobic nature, compared to BPC and GPC. This disparity could also contribute to the observed differences, in addition to the distinct hydrophilic nature of the various protein concentrates.
Fig. 5. Vapour sorption isotherms of soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC) and skim powder milk protein concentrate (MPC)
X-Ray diffraction
The physical states of the various protein concentrates were investigated by XRD (Fig. 6). The relatively smooth curves obtained indicate that the protein concentrates do not contain crystalline matter. These results are consistent with the literature, which has shown that proteins derived from these raw materials are also amorphous (Wang et al. 2017; Zhou et al. 2021; Zeng et al. 2023). The XRD peaks at 9.8 and 19.8° correspond to the α-helix and β-leaf structures of the proteins (Wang et al. 2017; Zhou et al. 2021; Zeng et al. 2023). The peaks observed at 12.5° and 38.4°, as well as the shoulder observed on the peak at 19.8°, may be linked to the residual lactose within MPC, a connection supported by the carbohydrate composition indicated in Fig. 3 (Haque and Roos 2005).
Fig. 6. X-ray diffraction curves of soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC) and skim powder milk protein concentrate (MPC)
Fig. 7. a) Thermogravimetry (TG) and b) differential thermogravimetry (DTG) analysis of soybean meal protein concentrate (BPC), microbrewery spent grains protein concentrate (GPC), shrimp shells protein concentrate (SPC) and skim powder milk protein concentrate (MPC)
Thermo gravimetric analysis
TGA experiments were performed to study the thermal stability of different protein concentrates, which had been placed in an oven for 24 h at 50 °C. The samples showed several peaks of mass loss following thermal events (Fig. 7). The first mass losses between 25 and 200 °C are attributed to the dehydration of the protein concentrates and protein denaturation (Boussetta et al. 2022). For BPC samples, gases, such as CO2, NO2, and NH3, are emitted from the decomposition of the peptide backbone at 323 °C (Wang et al. 2020; Wang et al. 2017). A similar phenomenon occurred at 327 °C for GPC samples, and the gases emitted were CO, CO2, CH4, and C2H4 (Borsato et al. 2019). For the SPC samples, the mass loss at 306 °C can be attributed to the decomposition of proteins and chitin, which leads to emissions of CH4, CO2, CO, and NH3 (Zhang et al. 2019; Liu et al. 2021). The amino acid chains of casein are cleaved during thermal degradation, before cross-linking and dehydration (Lee et al. 2020). This phenomenon produces CO2, CO, and NH3 emissions from the MPC samples at 333 ± 3 °C (Mocanu et al. 2012; Lee et al. 2020). Ultimately, the higher decomposition temperatures of protein concentrates in comparison to the formulation and usage temperatures of adhesives of this study, i.e., room temperature, eliminate the risk of protein decomposition during adhesive formulation.
Adhesives Characterization
Moisture uptake
The moisture uptake tests aimed to determine whether the water sensitivity observed in protein concentrates, as depicted in Fig. 5, is transmitted to adhesives once these proteins are integrated (Fig. 8). Initially, it is consistent that the introduction of protein concentrates caused a minor reduction in moisture uptake values. This decrease is attributed to the substitution of OH groups, which can form hydrogen bonds with environmental moisture and consequently increase mass, with NH groups found in amino acids, which can have a more hydrophobic nature. The consistent increase for PU-SPC and the upturn to 20% observed for PU-BPC can be explained by the fact that PU-SPC contains amino acids with higher hydrophilicity, as shown in Table 5, followed closely by PU-BPC. The differences in results remain relatively marginal, considering the scale used. Thus, it can be inferred that the presence of proteins does not notably impact the moisture resistance of the adhesives.
Fig. 8. Moisture uptake value of polyurethane adhesives at different protein contents of soybean meal protein concentrate (PU-BPC), microbrewery spent grains protein concentrate (PU-GPC), shrimp shells protein concentrate (PU-SPC) and skim powder milk protein concentrate (PU-MPC)
Time-dependent viscosity
Viscosity measurements were conducted at room temperature on all the adhesives (Fig. 9). These results illustrate that the inclusion of proteins led to an increase in viscosity, consistent with established findings in the literature, where the addition of fillers to polymer systems commonly results in heightened viscosity (Markovičová 2021; Schulze et al. 2003). As anticipated, GPC and SPC adhesives exhibited higher viscosity at shorter times. For PU-SPC adhesives, the increased viscosity can be attributed to the higher molecular weight of the concentrate compared to GPC and MPC. Regarding GPC, the variation in viscosity may be connected to its lower protein content, necessitating a larger quantity to achieve the desired protein substitution rate. Conversely, PU-MPC adhesives, with the higher protein content of MPC, require a reduced amount of product for the desired protein substitution rate. Consequently, a longer time is needed for viscosity to increase in these systems. For the PU-BPC, higher viscosity was expected due to β-conglycinin proteins within this concentrate having greater molecular weights than the other samples.
Fig. 9. Viscosity analysis of polyurethane adhesives at different protein contents of soybean meal protein concentrate (PU-BPC), microbrewery spent grains protein concentrate (PU-GPC), shrimp shells protein concentrate (PU-SPC), and skim powder milk protein concentrate (PU-MPC)
Gel time
The gel times of the various adhesives are depicted in Fig. 10. PU-BPC and PU-GPC adhesives, regardless of their protein content, exhibited equivalent or even shorter gel times than the reference adhesive, accounting for standard deviations. Conversely, PU-SPC and PU-MPC adhesives displayed similar behavior, with gel times remaining below the reference up to 15% protein content but subsequently exceeding the reference at 20% protein content. This observation aligns with the reactivity of amine groups incorporated in the protein-based adhesives, which tend to accelerate the polymerization reaction compared to the reference’s hydroxyl groups (Afagh and Yudin 2010). The increase to 20% for PU-SPC can be attributed to the minerals present in SPC. SPC exhibited a higher ash content, as depicted in Fig. 3, signifying a heightened concentration of salts compared to other protein concentrates. Elevated salinity imparts greater flexibility to polymer chains and facilitates agglomeration, thereby reducing gelation time. Consequently, with an increase in protein content, the growing influence of salt content leads to the prolonged gel time observed in PU-SPC adhesives (Li et al. 2022). It is also worth noting that during the production of protein concentrates, B, G, and S are precipitated at a pH of 4 after hydrolysis. In contrast, skimmed milk powder is precipitated using acetic acid, resulting in a lower pH of around 3. Literature indicates that a low pH can slow down urea formation and urethane formulation, implying a longer gel time (Maillard et al. 2021). Therefore, introducing greater protein concentrate at a lower pH might lead to longer gel times, particularly in the case of PU-MPC adhesives.
Fig. 10. Gel time of polyurethane adhesives (PU) at different protein contents of soybean meal protein concentrate (PU-BPC), microbrewery spent grains protein concentrate (PU-GPC), shrimp shells protein concentrate (PU-SPC) and skim powder milk protein concentrate (PU-MPC)
Effective penetration
Effective penetration analyses were conducted to investigate the impact of protein incorporation on adhesive penetration in wood (Fig. 11). Both sides of the bonded element, upper and lower, were studied to determine penetration. During the penetration process; three main factors interact: adhesive properties, wood properties, and processing parameters (Kamke and Lee 2007). As wood and gluing/pressing conditions remain consistent across all adhesives, these parameters were not considered when comparing different adhesives. However, adhesive properties such as viscosity can influence the penetration of various adhesives into the wood substrate (Kamke and Lee 2007). The results indicate a positive relationship between effective penetration and protein content, regardless of the protein concentrate used. This observed trend can be attributed to the varying viscosities of adhesives at different protein contents. Notably, it has been observed that adhesive penetration correlates positively with adhesive viscosity. While penetration increases were similar across adhesives containing protein concentrates from soybean meal, shrimp shells, and skim milk powder, a noticeable difference was observed for adhesives containing protein concentrate from microbrewery spent grains, particularly at a protein content of 15% and 20%. This deviation can also be explained by viscosity. As shown in Fig. 9, the viscosities of PU-GPC adhesives at 15% and 20% protein content increased more rapidly than those of the other adhesives in this study, leading to an increase in effective penetration. In instances of low-viscosity adhesives, the lack of penetration may contribute to adhesive flow through the radial bands of parenchyma (Vnučec et al. 2015, 2016). This is substantiated by the consistent observation that the penetrated adhesive was exclusively on the lower side of the sample (Fig. 12 a). Figure 12 underwent contrast enhancement to improve the visibility of adhesive-filled cells. This indicates that low-viscosity adhesives tend to flow into the lower side of the samples during the pressing process, thereby diminishing the quantity of adhesive that successfully penetrates the wood (Vnučec et al. 2015, 2016). Increased penetration could benefit the mechanical properties of adhesives because improved penetration typically leads to stronger bonding, potentially boosting the mechanical strength of bonded components.
Fig. 11. Effective penetration of polyurethane adhesives at different protein contents of soybean meal protein concentrate (PU-BPC), microbrewery spent grains protein concentrate (PU-GPC), shrimp shells protein concentrate (PU-SPC) and skim powder milk protein concentrate (PU-MPC)
Fig. 12. Optical micrographs of wood samples bonded with a) polyurethane adhesive at 5% microbrewery spent grains protein concentrate content and b) polyurethane adhesive at 20% microbrewery spent grains protein concentrate content. Glueline is marked with GL and penetrated adhesive is marked with PA
Kinetic and conversion of adhesives
FTIR spectra of adhesives at different percentages of protein incorporation were similar in absorption bands. RT-FTIR spectra are shown in Fig. 13. The OH and NH stretching bands were at 3335 cm-1, the -CH stretching vibration band at 2920 cm-1, the NCO stretching vibration band at 2260 cm-1, the -C=O- stretching vibration band of urethane at 1730 cm-1, and the -NH stretching band at 1620 cm-1.
Fig. 13. Fourier-transform infrared spectroscopy spectra of polyurethane adhesive with protein over time
Fig. 14. Isocyanate achieved degree of conversion vs. time of a) microbrewery spent grains protein concentrate (PU-GPC), b) polyurethane adhesives with different protein concentrates at 10% protein content
Urethane formation can be monitored by the disappearance of the isocyanate’s NCO vibration band at 2260 cm-1 and the appearance of the urethane’s C=O vibration band at 1730 cm-1 (Fig. 14a). Observations revealed that the reaction rate tended to decrease as protein content increased, while the conversion rate remained unaffected. Polyurethane adhesives made with the other protein concentrates of this study behaved similarly. These results are consistent with the viscosity and gel time data shown in Figs. 9 and 10, respectively, as higher viscosity and longer gel times gave slower polymerization reactions. However, initial expectations suggested that adhesives with 10% protein content (Fig. 14b), such as PU-MPC, might exhibit faster reactions due to their lower viscosity compared to higher protein-content adhesives. In traditional polymerization reactions, lower viscosity typically facilitates reactant mobility, promoting more rapid reactions. The unexpected results suggest the involvement of other factors, hypothetically linked to the other components of the protein concentrates and to the unique characteristics of proteins and their interactions within the adhesive system, influencing reaction dynamics. Haut du formulaireHaut du formulaire
Block shear strength
Block shear analysis aimed to determine the comparative failure point of adhesives used for wood bonding when subjected to shear tests. Adhesives containing protein concentrates were compared with the petrochemical reference, which is represented by the adhesive with a 0% incorporation rate. In this analysis, the adhesive bond exhibited a notable characteristic – no fibers were observed on the surface of the fractured samples. This absence of wood fibers indicates a 100% failure in the adhesive joint for all the adhesives studied. It implies that the bond strength of the adhesive was weaker than the cohesive strength of the adherent material, resulting in bond failure. The comparative shear strength of adhesives with increasing protein content is presented in Fig. 15. The figure also includes values obtained from standard error analysis, quantifying sampling error, as well as the results of statistical analysis. These results suggest that incorporating proteins into adhesives can enhance the maximum load capacity that wood structures can support before reaching the adhesive breaking point. This aligns with the effective penetration results in Fig. 11, where improved penetration typically leads to stronger bonding, potentially boosting the mechanical strength of bonded components (Johnson and Kamke 1992). However, it is important to note that effective penetration, while an important factor, is not the sole determinant of these outcomes. Further insight into the mechanical strength of PU-BPC adhesives at 20% protein content can be gained by examining the condition of the adhesive joint. Microscopic analysis revealed voids within the adhesive joint, particularly in areas where the adhesive deeply penetrated into the wood. These voids may provide an explanation for the reduced mechanical strength observed in this adhesive during mechanical testing.
Fig. 15. Shear strength of polyurethane adhesives at different protein contents of soybean meal protein concentrate (PU-BPC), microbrewery spent grains protein concentrate (PU-GPC), shrimp shells protein concentrate (PU-SPC) and skim powder milk protein concentrate (PU-MPC). Letters represent LSD test groups.
Statistical analysis revealed a significantly superior performance for the PU-SPC adhesive with 20% protein content compared to the other adhesives investigated in this study. Additionally, it is noteworthy that adhesives within group b, while demonstrating a performance inferior to PU-SPC with 20% protein content, encompass adhesives made with diverse protein types, excluding those derived from milk. Improvements are therefore needed in adhesives containing MPC. Finally, all adhesives not belonging to group g exhibited a statistically significant improvement over the petrochemical reference PU employed as a benchmark in this study. However, it is essential to highlight that further enhancements are needed to ensure that the failure point occurs within the wood substrate rather than within the adhesive. This is crucial for attaining the desired market performance standards.
CONCLUSIONS
- The introduction of proteins into polyurethane adhesives resulted in several notable improvements. The increase in protein content increased, or stabilized, adhesive gel time, promoted better penetration into wood, and impacted positively the mechanical properties of the adhesives.
- Importantly, it was observed that the polymerization process of protein-containing adhesives achieved degrees of conversion similar to petrochemical references.
- Protein-based adhesives are both formaldehyde-free and increase the biobased content of adhesives while utilizing industrial byproducts. These findings emphasize the potential of incorporating protein-based materials into polyurethane adhesives, paving the way for the production of renewable based structural adhesives. These adhesives may be feasible from an industrial point of view, due to the few steps required to obtain the protein concentrates from the raw materials and the non-completeness of the adhesive formulation, while at the same time making it possible to manage industrial waste. Future research endeavors should focus on optimization strategies and further enhancing biobased content.
ACKNOWLEDGMENTS
The authors are grateful to Marie Soula, Solène Péllerin, Aurélien Hermann, Jérémy Winninger, and Sacha Tremblay for their valuable assistance. Thanks to Yves Bédard, Diane Gagnon, Martin Boudreault, Daniel Bourgault, Luc Germain, Benoit St-Pierre, and Jean Ouellet for their help with the various analyses. The authors would also like to thank Richard Nadeau (Sollio Agriculture), Audrey Cote (Sollio Agriculture), Serge Lacasse (Les Pêcheries Marinard), the Renewable materials research center, and the NSERC industrial chair on eco-responsible wood construction (CIRCERB) and its industrial partners.
Funding
The authors are grateful to Natural Sciences and Engineering Research Council of Canada for the financial support through its IRC, and CRD programs (IRCPJ 461745- 18 and RDCPJ 524504-18) as well as the industrial partners of the NSERC industrial chair on eco-responsible wood construction (CIRCERB). The authors are also grateful to the Ministère de l’Économie et de l’Innovation du Québec through the PSO-I-009 project.
Author Contributions
Conceptualization, A.M., P.B., S.P., and V.L.; methodology, A.M., P.B., S.P., J.C., and V.L; software, A.M.; validation, A.M.; formal analysis, A.M.; investigation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M., P.B., S.P., J.C., and V.L.; supervision, P.B., and V.L.; project administration, P.B., and V.L.; funding acquisition, P.B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that there is no conflict of interest. The funders had no role in the design of the study; the collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
REFERENCES CITED
Afagh, N. A., and Yudin, A. K. (2010). “Chemoselectivity and the curious reactivity preferences of functional groups,” Angewandte Chemie – International Edition 49(2), 262-310. DOI: 10.1002/anie.200901317
Albin, D. M., Wubben, J. E., and Gabert, V. M. (2000). “Effect of hydrolysis time on the determination of amino acids in samples of soybean products with ion-exchange chromatography or precolumn derivatization with phenyl isothiocyanate,” Journal of Agricultural and Food Chemistry 48(5), 1684-1691. DOI: 10.1021/jf990599q
Anon. (2016). Code of Federal Regulations, Title 21, Part 101.9 – Food Nutrition Labeling of Food. U.S. Government Printing Office, Washington, DC.
AOAC International. (2000). AOAC Official Method 997.09:1997 Nitrogen in Beer, Wort, and Brewing Grains Protein (Total) by Calculation. Combustion Method.
Arias, A., Entrena-Barbero, E., Feijoo, G., and Moreira, M. T. (2022). “Sustainable non-isocyanate polyurethanes bio-adhesives for engineered wood panels are revealed as promising candidates to move from formaldehyde-based alternatives,” Journal of Environmental Chemical Engineering 10(1), article 107053. DOI: 10.1016/j.jece.2021.107053
Badouard, C., Bogard, F., Bliard, C., Lachi, M., Abbes, B., and Polidori, G. (2021). “Development and characterization of viticulture by-products for building applications,” Construction and Building Materials 302, article 124142. DOI: 10.1016/J.CONBUILDMAT.2021.124142
Bastani, A., Adamopoulos, S., Koddenberg, T., and Militz, H. (2016). “Study of adhesive bondlines in modified wood with fluorescence microscopy and X-ray micro-computed tomography,” International Journal of Adhesion and Adhesives 68, 351-358. DOI: 10.1016/j.ijadhadh.2016.04.006
Bisanda, E. T. N., Ogola, W. O., and Tesha, J. v. (2003). “Characterisation of tannin resin blends for particle board applications,” Cement and Concrete Composites 25(6), 593-598. DOI: 10.1016/S0958-9465(02)00072-0
Borsato, V. M., Jorge, L. M. M., Mathias, A. L., and Jorge, R. M. M. (2019). “Thermodynamic properties of barley hydration process and its thermostability,” Journal of Food Process Engineering 42, article e12964. DOI: 10.1111/jfpe.12964
Boussetta, A., Benhamou, A. A., Ihammi, A., Ablouh, E. H., Barba, F. J., Boussetta, N., Grimi, N., and Moubarik, A. (2022). “Shrimp waste protein for bio-composite manufacturing: Formulation of protein-cornstarch-mimosa-tannin wood adhesives,” Industrial Crops and Products 187, article 115323. DOI: 10.1016/j.indcrop.2022.115323
Boyd, C. E. (2018). “Protein conversion efficiency in aquaculture,” in: Global Aquaculture Advocate.
Celus, I., Brijs, K., and Delcour, J. A. (2009). “Fractionation and characterization of brewers’ spent grain protein hydrolysates,” Journal of Agricultural and Food Chemistry 57(12), 5563-5570. DOI: 10.1021/jf900626j
Chalapud, M. C., Herdt, M., Nicolao, E. S., Ruseckaite, R. A., Ciannamea, E. M., and Stefani, P. M. (2020). “Biobased particleboards based on rice husk and soy proteins: Effect of the impregnation with tung oil on the physical and mechanical behavior,” Construction and Building Materials 230, article 116996. DOI: 10.1016/J.CONBUILDMAT.2019.116996
Chen, C. X., Pierobon, F., and Ganguly, I. (2019). “Life cycle assessment (LCA) of cross-laminated timber (CLT) produced in Western Washington: The role of logistics and wood species mix,” Sustainability (Switzerland) 11(5), article 11051278. DOI: 10.3390/su11051278
Cheng, H. N., Dowd, M. K., and He, Z. (2013). “Investigation of modified cottonseed protein adhesives for wood composites,” Industrial Crops and Products 46, 399-403. DOI: 10.1016/j.indcrop.2013.02.021
Desai, S. D., Patel, J. V., and Sinha, V. K. (2003). “Polyurethane adhesive system from biomaterial-based polyol for bonding wood,” International Journal of Adhesion and Adhesives 23(5), 393-399. DOI: 10.1016/S0143-7496(03)00070-8
Detlefsen, W. D. (1989). Adhesive from Renewable Ressources, Chapter 31: Blood and Casein Adhesives for Bonding Wood, American Chemical Society, pp. 445-452. https://pubs.acs.org/sharingguidelines
Eslah, F., Jonoobi, M., Faezipour, M., Afsharpour, M., and Enayati, A. A. (2016). “Preparation and development of a chemically modified bio-adhesive derived from soybean flour protein,” International Journal of Adhesion and Adhesives 71, 48-54. DOI: 10.1016/j.ijadhadh.2016.08.011
Haque, M. K., and Roos, Y. H. (2005). “Crystallization and X-ray diffraction of spray-dried and freeze-dried amorphous lactose,” Carbohydrate Research 340(2), 293-301. DOI: 10.1016/j.carres.2004.11.026
Huang, J., and Li, K. (2008). “A new soy flour-based adhesive for making interior type II plywood,” JAOCS, Journal of the American Oil Chemists’ Society 85(1), 63-70. DOI: 10.1007/s11746-007-1162-1
Husnaeni, Maruddin, F., Malaka, R., and Prahesti, K. I. (2019). “Study on the use of various concentration of acetic acid and different precipitation duration on casein characteristics,” IOP Conference Series: Earth and Environmental Science 343(1). DOI: 10.1088/1755-1315/343/1/012035
Jenkins, C. L., Meredith, H. J., and Wilker, J. J. (2013). “Molecular weight effects upon the adhesive bonding of a mussel mimetic polymer,” ACS Applied Materials and Interfaces 5(11), 5091-5096. DOI: 10.1021/am4009538
Johnson, S. E., and Kamke, F. A. (1992). “Quantitative analysis of gross adhesive penetration in wood using fluorescence microscopy,” The Journal of Adhesion 40(1), 47-61. DOI: 10.1080/00218469208030470
Kamke, F. A., and Lee, J. N. (2007). “Adhesive penetration in wood – A review,” Wood and Fiber Science 39(2), 205-220.
Kumar, R. N., and Pizzi, A. (2019). Adhesives for Wood and Lignocellulosic Materials, John Wiley & Sons, Scrivener Publishing LLC.
Lee, H., Kim, N. K., Jung, D., and Bhattacharyya, D. (2020). “Flammability characteristics and mechanical properties of casein based polymeric composites,” Polymers 12(9), article 2078. DOI: 10.3390/POLYM12092078
Li, R., Ge, H.-J., Xiong, Y., Guo, Z.-Q., Deng, L.-Q., Xu, H.-E., Lin, Y., Wang, A.-L., Li, Y., and Wang, X.-G. (2022). “Factors influencing gelation time of high temperature crosslinked polymer gel and its mathematical function,” Springer Series in Geomechanics and Geoengineering 2274-2282. DOI: 10.1007/978-981-19-2149-0_210
Liu, J., Yang, X., Liu, H., Jia, X., and Bao, Y. (2021). “Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: Co-pyrolysis characteristics and its adsorption capability,” Chemosphere 282, article 131116. DOI: 10.1016/j.chemosphere.2021.131116
Luo, J., Luo, J., Li, X., Gao, Q., and Li, J. (2016). “Effects of polyisocyanate on properties and pot life of epoxy resin cross-linked soybean meal-based bioadhesive,” Journal of Applied Polymer Science 133(17), article 43362. DOI: 10.1002/app.43362
Maillard, D., Osso, E., Faye, A., Li, H., Ton-That, M. T., and Stoeffler, K. (2021). “Influence of lignin’s pH on polyurethane flexible foam formation and how to control it,” Journal of Applied Polymer Science 138(18), article 50319. DOI: 10.1002/app.50319
Maji, P. K., and Bhowmick, A. K. (2009). “Influence of number of functional groups of hyperbranched polyol on cure kinetics and physical properties of polyurethanes,” Journal of Polymer Science, Part A: Polymer Chemistry 47(3), 731-745. DOI: 10.1002/pola.23185
Markovičová, L. (2021). “The effect of filler content on the viscosity of polymer composites,” Conference Quality Production Improvement–CQPI, Vol. 3, No. 1, pp. 293-298. DOI: 10.2478/cqpi-2021-0028
Mary, A., Blanchet, P., and Landry, V. (2023). “Polyurethane wood adhesives from microbrewery spent grains,” in: Bio-Based Building Materials, S. Amziane, I. Merta, and J. Page (eds.), ICBBM 2023. RILEM Bookseries, Springer, Vol. 45, pp. 14-28. DOI: 10.1007/978-3-031-33465-8_2
Maubois, J. L., and Lorient, D. (2016). “Dairy proteins and soy proteins in infant foods nitrogen-to-protein conversion factors,” Dairy Science and Technology 96(1), 15-25. DOI: 10.1007/s13594-015-0271-0
Meier-Westhues, U. (2019). “Polyurethanes: coatings, adhesives and sealants,” in: European Coatings, Vincentz Network, Hannover, Germany.
Mocanu, A. M., Moldoveanu, C., Odochian, L., Paius, C. M., Apostolescu, N., and Neculau, R. (2012). “Study on the thermal behavior of casein under nitrogen and air atmosphere by means of the TG-FTIR technique,” Thermochimica Acta 546, 120-126. DOI: 10.1016/j.tca.2012.07.031
Pizzi, A. (2006). “Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues,” Journal of Adhesion Science and Technology 20(8), 829-846. DOI: 10.1163/156856106777638635
Pizzi, A. (2016). “Wood products and green chemistry,” in: Annals of Forest Science, Springer-Verlag France, Vol. 73, Issue 1, pp. 185-203. DOI: 10.1007/s13595-014-0448-3
Pizzi, A., and Mittal, K. L. (2005). Handbook of Adhesive Technology, Third Edition, CRC Press, pp. 573-599.
Qin, L., Lin, L., and Fu, F. (2016). “Microstructural and micromechanical characterization of modified urea-formaldehyde resin penetration into wood,” BioResources 11(1), 182-194.
Ricci, L., Umiltà, E., Righetti, M. C., Messina, T., Zurlini, C., Montanari, A., Bronco, S., and Bertoldo, M. (2018). “On the thermal behavior of protein isolated from different legumes investigated by DSC and TGA,” Journal of the Science of Food and Agriculture 98(14), 5368-5377. DOI: 10.1002/jsfa.9078
Sánchez-Machado, D. I., Chavira-Willys, B., and López-Cervantes, J. (2008). “High-performance liquid chromatography with fluorescence detection for quantitation of tryptophan and tyrosine in a shrimp waste protein concentrate,” Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 863(1), 88-93. DOI: 10.1016/j.jchromb.2008.01.011
Schulze, K. A., Zaman, A. A., and Söderholm, K. J. M. (2003). “Effect of filler fraction on strength, viscosity and porosity of experimental compomer materials,” Journal of Dentistry 31(6), 373-382. DOI: 10.1016/S0300-5712(03)00091-5
Sernek, M., Resnik, J., and Kamke, F. A. (1999). “Penetration of liquid urea-formaldehyde adhesive into beech wood,” Wood and Fiber Science 1, 41-48.
Solt, P., van Herwijnen, H. W. G., and Konnerth, J. (2019). “Thermoplastic and moisture-dependent behavior of lignin phenol formaldehyde resins,” Journal of Applied Polymer Science 136(40), article 48011. DOI: 10.1002/app.48011
Vick, C. B., and Rowell, R. M. (1990). “Adhesive bonding of acetylated wood,” International Journal of Adhesion and Adhesives 10, 4.
Vnučec, D., Goršek, A., Kutnar, A., and Mikuljan, M. (2015). “Thermal modification of soy proteins in the vacuum chamber and wood adhesion,” Wood Science and Technology 49(2), 225-239. DOI: 10.1007/s00226-014-0685-5
Vnučec, D., Kutnar, A., and Goršek, A. (2017). “Soy-based adhesives for wood-bonding–a review,” Journal of Adhesion Science and Technology 31(8), 910-931. DOI: 10.1080/01694243.2016.1237278
Vnučec, D., Mikuljan, M., Kutnar, A., Šernek, M., and Goršek, A. (2016). “Influence of process parameters on the bonding performance of wood adhesive based on thermally modified soy proteins,” European Journal of Wood and Wood Products 74(4), 553-561. DOI: 10.1007/s00107-016-1018-1
Wang, Y., Liu, C., Lai, J., Lu, C., Wu, X., Cai, Y., Gu, L., Yang, L., Zhang, G., and Shi, G. (2020). “Soy protein and halloysite nanotubes-assisted preparation of environmentally friendly intumescent flame retardant for poly(butylene succinate),” Polymer Testing 81, article 106174. DOI: 10.1016/j.polymertesting.2019.106174
Wang, Z., Kang, H., Zhang, W., Zhang, S., and Li, J. (2017). “Improvement of interfacial interactions using natural polyphenol-inspired tannic acid-coated nanoclay enhancement of soy protein isolate biofilms,” Applied Surface Science 401, 271-282. DOI: 10.1016/j.apsusc.2017.01.015
Wang, Z., Zhao, S., Song, R., Zhang, W., Zhang, S., and Li, J. (2017). “The synergy between natural polyphenol-inspired catechol moieties and plant protein-derived bio-adhesive enhances the wet bonding strength,” Scientific Reports 7(1). DOI: 10.1038/s41598-017-10007-8
Yan, Q., Ji, W., Feng, J., Shen, Y., Shan, S., Xia, C., and Zhang, S. (2023). “Development of soybean meal based adhesives with excellent wet-resistance and prepressing bonding strength via disulfide bond reshuffling strategy,” Construction and Building Materials 377, article 131040. DOI: 10.1016/J.CONBUILDMAT.2023.131040
Yang, I., Kuo, M., Myers, D. J., and Pu, A. (2006). “Comparison of protein-based adhesive resins for wood composites,” Journal of Wood Science 52(6), 503-508. DOI: 10.1007/s10086-006-0804-5
Zeng, G., Zhu, F., Aladejana, J. T., Zhou, Y., Li, K., Luo, J., Li, X., Dong, Y., Wang, K., and Li, J. (2023). “Barley – A yet un-tapped feedstock for improved vegetable protein-based wood adhesives,” Journal of Materials Chemistry A 11(21), 11310-11325. DOI: 10.1039/d3ta00619k
Zhang, P., Hu, H., Tang, H., Yang, Y., Liu, H., Lu, Q., Li, X., Worasuwannarak, N., and Yao, H. (2019). “In-depth experimental study of pyrolysis characteristics of raw and cooking treated shrimp shell samples,” Renewable Energy 139, 730-738. DOI: 10.1016/j.renene.2019.02.119
Zhao, Y., Lin, S., Yang, R., Chen, D., and Sun, N. (2021). “Proton dynamics of water diffusion in shrimp hydrolysates flour and effects of moisture absorption on its properties,” Foods 10(5), article 1137. DOI: 10.3390/foods10051137
Zhou, W., Zhang, Y., Li, R., Peng, S., Ruan, R., Li, J., and Liu, W. (2021). “Fabrication of caseinate stabilized thymol nanosuspensions via the pH-driven method: Enhancement in water solubility of thymol,” Foods 10(5), article 10051074. DOI: 10.3390/foods10051074
Article submitted: November 14, 2023; Peer review completed: November 27, 2023; Revisions received: December 10, 2023; Revisions accepted: December 13, 2023; Published: January 2, 2024.
DOI: 10.15376/biores.19.1.1165-1189
