Abstract
Empirical studies on the effects of urban forests on the health of humans and other animals are needed to rationalize the construction of urban forests for healthcare. The effects of urban forests (coniferous, broad-leaved, and mixed coniferous and broad-leaved) were studied relative to the physical and mental health of mice and the major environmental factors influencing them. Compared with the control group, the total movement distance of the mice that stayed in the coniferous forest, mixed coniferous and broad-leaved forest, and broad-leaved forest increased 9.7% to 18.1%, the central grid movement distance increased 7.2% to 23.9%, central grid dwell time increased 10.7% to 16.3%, and the number of entries increased 5.2% to 18.6%, indicating that the mental state and the exploration and cognitive abilities of mice were significantly improved in the three urban forests. The broad-leaved forest had greater positive effects than the other two forest types. This can be attributed to a decrease in temperature and the increases in humidity and concentrations of oxygen and negative ions in the air in the broad-leaved forest. The research results provide a theoretical basis for building urban forests that are beneficial to the health of urban residents.
Download PDF
Full Article
Broad-leaved Forest’s Impact on Spontaneous Activities of Mice and their Mental State
Donglin Wang,a,† Qian Wang,b,†,* Weining Du,b Yuebin Wang,b Jianhua Shu,b Luyao Ma,b Xiaolei Cao,b Lili Han,b,* and Yuerong Wang b,*
Empirical studies on the effects of urban forests on the health of humans and other animals are needed to rationalize the construction of urban forests for healthcare. The effects of urban forests (coniferous, broad-leaved, and mixed coniferous and broad-leaved) were studied relative to the physical and mental health of mice and the major environmental factors influencing them. Compared with the control group, the total movement distance of the mice that stayed in the coniferous forest, mixed coniferous and broad-leaved forest, and broad-leaved forest increased 9.7% to 18.1%, the central grid movement distance increased 7.2% to 23.9%, central grid dwell time increased 10.7% to 16.3%, and the number of entries increased 5.2% to 18.6%, indicating that the mental state and the exploration and cognitive abilities of mice were significantly improved in the three urban forests. The broad-leaved forest had greater positive effects than the other two forest types. This can be attributed to a decrease in temperature and the increases in humidity and concentrations of oxygen and negative ions in the air in the broad-leaved forest. The research results provide a theoretical basis for building urban forests that are beneficial to the health of urban residents.
DOI: 10.15376/biores.19.1.1465-1478
Keywords: Broad-leaved forest; Mice; Spontaneous activities; Forest environmental factors; Open-field test
Contact information: a: Institute of Ecological Conservation and Restoration, Chinese Academy of Forestry, Beijing 100091, China; b: Beijing Academy of Landscape and Greening Science, Beijing 100102, China; *Corresponding author: wangqian-200@163.com;13701251147@139.com; 467309192@qq.com
GRAPHICAL ABSTRACT
INTRODUCTION
Environmental pollution is becoming increasingly prominent in urban areas, including the heat island effect, noise pollution, automobile exhaust emissions, and other problems. Consequently, urban residents are paying more attention to the impact of the environment on their own health. As an urban ecosystem with an important regulatory role, the urban forest is also an air conditioner and cleaner for the city. It can improve the urban environment through various functions such as cooling and humidification, carbon sequestration and oxygen release, and air purification. An increasing number of urban residents are walking in urban forests to experience nature and relieve stress. At present, a large number of studies have provided evidence that the urban forest environment is beneficial to health (Barthod and Fournier 2019; Carnicer et al. 2021; Miao et al. 2022). For example, the forest environment can help people reduce stress and restore their attention. People perform various leisure activities in the forest environment, including walking, running, playing; these activities can increase the activity and the number of Natural Killer (NK) cells and the cellular levels of anti-cancer proteins (Wen et al. 2019; Chae et al. 2021; Pei et al. 2022). Studies have shown that compared with the urban environment, the human body’s blood pressure, heart rate, skin conductivity, and muscle tension are reduced in the forest environment; negative emotions, such as depression and pessimism, are significantly diminished, mental stress is relieved, and attention fatigue is relieved (Li et al. 2010; Li et al. 2022). In addition, the forest environment can also prevent and treat chronic diseases, such as obesity, high blood pressure, and diabetes to a certain extent. Workouts boost metabolism (Antonelli et al. 2022). However, there are minimal studies on the impact of different types of urban forest environments, such as coniferous forests (CF), broad-leaved forests (BF), and mixed coniferous and broad-leaved forests (MF), on human health or the health of animals.
Due to the influence of their growth environment, personality, social factors, etc., humans are complex social beings with changing emotions (Doimo et al. 2020). Therefore, human-based experiments are difficult to conduct. In contrast, the open-field test is a simple, effective, and widely used animal behavior analysis method in animal psychology. Because it is more controllable than human experiments, it is more objective in studying the impact of forest environment on human health (Dai et al. 2018). This method can track and evaluate the adaptability, excitability, tension, exploration, and memory of animals in response to novel environments through indicators such as the total movement distance, the movement distance and the residence time in the central grid, the number of crossings, the body weight, the number of fecal particles, and other behaviors (Yang et al. 2015). In 2015, the authors studied a series of spontaneous behaviors of mice after “forest bathing” in the single forest environments such as Phyllostachys edulis forests and Fokienia hodginsii forests. Mice showed the exploration and cognitive abilities, indicating that using mice to simulate human behavior is scientific. In the study presented here, the authors analyzed the changes in spontaneous behavior of mice in coniferous, broad-leaved, and mixed coniferous and broad-leaved forests and measured a number of the environmental quality parameters to provide a basis for assessing the “health bathing”/human recuperation suitability of urban forests (Qian et al. 2015).
EXPERIMENTAL
Study Area
The research site was the Beijing Olympic Forest Park in the northern section of the central axis of Beijing (40°00’N, 116°22’E). It is the green legacy of the 2008 Beijing Olympic Games, covering an area of about 680 hectares. Its design (“Axial Nature”) aims to balance large-scale development with the urban ecological buffer zone and the biodiversity creation area. Around 90% of the park area is covered by vegetation, and a large part (70%) of it is woodland dominated by tree species such as Pinus tabuliformis, Ginkgo biloba L., Sophora japonica L., Platycladus orientalis, and Fraxinus chinensis. Forsythia suspensa, Lonicera japonica, Syringa oblata, and other shrubs are planted in the woodland, forming a variety of plant community patterns such as arbor, shrubbery or arbor, shrubbery, and grass. Lawns and flower beds are also common in the park, especially in the important spaces, such as in the vicinity of seed plants (e.g., dandelions and plantains). Most plant species in the park are commonly cultivated garden plants (Xiao et al. 2019).
Fig. 1. (a) Map Of China and Beijing City (red area), with the blue area representing the Chaoyang District of Beijing (116°23′ E, 40°01′ N); (b) Aerial image of Beijing Olympic Forest Park (red solid line), with the red dots indicating monitoring sites
Plant Composition of the Research Plot
The study site was selected from the three types of typical recreation forests adjacent to the south part of Olympic Forest Park (Fig. 1). The broad-leaved forest (BF) is composed of Platycladus orientalis, Sophora japonica L., and Ginkgo biloba L.; the mixed coniferous and broad-leaved forest (MF) is composed of Pinus tabuliformis and Populus L.; and the coniferous forest (CF) contains Pinus tabulaeformis and Platycladus orientalis. A survey of the plant community composition was conducted, and the plant name, height, age, breast-high diameter, density, and other descriptors were recorded. The types of measurements undertaken in the sampled areas are shown in Table 1.
Table 1. Plant Community Composition of the Recreational Forests in Olympic Forest Park
Research Methods
Animal Open-Field Test (OFT)– Experimental animals
The experimental animals in this study were Kunming mice provided by Beijing Wu’s Experimental Animal Center. They were 5 weeks old, weighing 18 to 22 g, and half male and half female; there were 80 mice in total. After receiving them, they were raised in the laboratory for one week. Five mice of the same sex were kept in a cage (30 cm × 18 cm × 16 cm), and sufficient water and feed were supplied during this period.
All of the procedures performed in this study followed the ethical standards of the Welfare Ethics Committee of Beijing Langke Biotechnology Co., Ltd., Beijing, China.
Field test simulation box
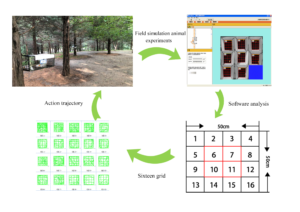
Mice were placed in a cuboid iron cage of 28 cm × 15 cm × 17 cm and placed in a forest environment with an S-shaped iron hook (Fig. 2). A hard board was fixed at the bottom of the iron cage, which was convenient for collecting and recording the number of fecal particles of mice (FPN).
Fig. 2. Mice And open field laboratory exposed to forest environment
Fig. 3. Sixteen house grid diagram of open field experiment on mice
Exposure of mice to the forest environments
Experiments were performed after mice had spent 3 days acclimatizing to the laboratory environment. The mice were weighed before and after the experiment each day from 8:30 a.m. to 15:30 p.m. There were four groups (broad-leaved forest, coniferous forest, mixed coniferous and broad-leaved forest, and control). In each group, 10 males were housed in two cages and 10 females were housed in the other two cages and marked on their tails. The mice cages were suspended in three forest habitats at a height of 1.3 to 1.5 m. In addition, two pairs of cages were suspended (1.3 to 1.5 m above the ground) over a small concrete-paved square as a control (CK) group.
Cages were left hanging from 9:00 to 15:00, and mice did not drink water or eat anything during this period. The FPN produced by the mice in the forest groups and the CK group was recorded at 15:00. Then, the cages were carefully brought to the laboratory (the distance between the laboratory and the experimental sites was approximately 500 m), trying to avoid the impact of transportation on the mice. They were then left for 20 to 30 min until they calmed down, and were weighed at 15:30. During the experiment, each mouse was placed in the center of the open field experiment box and was allowed to move freely in it for 5 min.
To avoid the influence of mice urine and feces on the results of the experiment, the open-field test boxes were wiped with a clean towel dipped in 75% v/v alcohol after each experiment. This experiment was repeated for 6 consecutive days (August 12 to 17, 2020). Based on the usual mice behavior, the first 3 days were regarded as the exploration period, and the last 3 days were regarded as their adaptation period.
Observations of spontaneous behavior
A high-definition camera (Panasonic w3-BP332) was used to observe the activity of each mouse. The camera was fixed on a tripod (60 cm high) and placed near the open field test box. The bottom of the chamber was divided by a software system into 16 squares (Fig. 3). Open fields 6, 7, 10, and 11 represented the central area, and the movement behavior of the mice in the central area can reflect their exploration activities and emotional states.
Monitoring of forest environmental factors— Observation methods
To clarify the impact of the forest environment on mice, a range of environmental factors were simultaneously monitored (air temperature, relative humidity, wind velocity, light intensity, particulate matter, negative ion concentration, and air oxygenation) in the three types of forest and control every 2 h from 1:00 to 23:00. Each sample was taken for 20 min, and the monitored altitude was approximately 1.3 to 1.5 m, which was the height at which the mice cages were positioned. In each forest, six observation points were set up at more than 50 m away from the edge of the forest and under the canopy of trees of the same size. The distance between any two points was at least 50 m, and the six replicate measurements were separated by 5 m. To compare the effect of the forest environments, the environmental factors of the tested area were observed simultaneously.
Measuring instruments
The microclimate of the forest environment was measured by a NK5920 portable weather meter (Kestrel 3500 pocket weather meter, Nielsen-Kellerman Co., Boothwyn, PA, USA). The resolution was 0.1% RH (relative humidity). The wind speed measurement range was 0.40 to 40 m·s-1, and the resolution was 0.1 m·s-1. The concentration of air particulate matter was measured using a Dustmate monitor (Turnkey, London, UK). A KEC-990 air ion tester (NKE, Tokyo, Japan) was used for measuring concentration of negative ions. Oxygen concentration in the air was determined using a domestic PTM400-O2 portable oxygen analyzer (Ozone analyzer manufacturer, Beijing, China).
Data Analysis
The significance test was performed using the SPSS 19.0 (IBM, Armonk, NY, USA). The differences in spontaneous activity of mice in different experimental groups were detected by one-way ANOVA (analysis of variance). For all tests, the p value of < 0.05 was considered statistically significant. The charts were drawn using Excel 2010 (Microsoft Corp., Redmond, WA, USA).
RESULTS AND DISCUSSION
Different Forest Environments’ Effects on the Spontaneous Behavior of Mice
Changes in the total movement distance and the movement distance in the central grid
Compared to the control group, the total exercise distance after the three forests were longer in the exploration than the adaptation stages, indicating that with the duration of the test (6 days), mice gradually adapted to the environment, and the amount of exercise decreased. The daily average exercise distance was highest in the BF and lowest in the CK group. The exercise distance in the BF group was 9.6%, 11.3%, and 14.9% higher than in the CF, MF, and CK groups, respectively. The ANOVA showed that the total exercise distance between the BF and the CK groups on the 2nd, 3rd, and 6th day of the experiment was significantly different (P = 0.021, P = 0.005, and P = 0.032, respectively).
The active area of the central lattice reflects the exploration and cognitive abilities of mice. The BF group had the highest (and the CK group the lowest) daily average value for the central grid movements (Fig. 4). The central grid movements in the BF group were 8.1%, 7.1%, and 19.3% higher than in the CF, MF, and CK groups, respectively. The ANOVA showed significant differences between the BF and the CK groups on the 2nd and 3rd day of the experiment (P = 0.017 and P = 0.003, respectively), between the CF and the CK groups on the 2nd (P = 0.032) and 3rd day (P = 0.004), and between the MF and the CK groups on the 2nd (P = 0.035) and 3rd day (P = 0.006), indicating that the mice had increased excitability after spending time in the three forest environments (especially BF).
The OFT is an experimental method assessing mice behavior and is used commonly in medicine. Open-field test analysis can track and evaluate animal behaviors, such as adaptability, excitability, tension, exploration, and memory, as influenced by novel environments (Oroszi et al. 2022). In this study, animal simulation experiments were used to estimate the effects of three urban forest environments on the human body from the perspective of animal behavior and psychology (Perreault et al. 2010). Analysis showed that the mice that stayed in the three types of urban forest environment, especially BF, increased their levels of exercise and improved their exploration and cognitive abilities significantly, and also increased appetite and relieved tension effectively (Fig. 4). There may be three main reasons for these findings. Firstly, the forests are lush in summer (especially BF), and the forest environment has an obvious cooling and humidifying effect, which can relieve the tension of mice and increase their appetite (Jin et al. 2021). Secondly, all three types of urban forest environments tested here retained PM 2.5 particles at least to some extent and thus showed the capacity to purify the air, which is conducive to improving the stability and agility of animals’ psychological activities (Kwak et al. 2019; Tan et al. 2022). It is helpful for the animal’s brain activities and the enhancement of its exploration capacity (Hu et al. 2022). Thirdly, the physiological activity of plants in summer is strong, and can release a large amount of negative ions, which can eliminate fatigue and improve the immunity of animals (Tyagi and Malik 2012; Luo et al. 2020).
Fig. 4. The pattern of changes In total distance and the central grid movement distance covered: BF: Broad-Leaved Forest; MF: Mixed Coniferous And Broad-Leaved Forest; CF: Coniferous Forest; CK: Control (concrete-paved area)
Staying time and number of crossings in the central grid
The central area residence time and crossing times of mice living in recreation forests increased first and then decreased in the exploration stage (Fig. 4). Compared with the CK group, there were significant differences on the 2nd (P = 0.004) and 3rd day (P = 0.003) in the CF group, on the 2nd, 3rd, and 6th day in the BF group (P = 0.015, P = 0.002, and P = 0.043, respectively), and on days 3, 4, and 6 (P = 0.022, P = 0.018, and P = 0.035, respectively) in the MF group. The daily mean values of the central lattice residence time were 15.6%, 16.3%, and 10.7% higher in the CF, BF, and MF groups, respectively, than the CK group.
The daily mean value of the crossing times was highest in the BF group and lowest in the CF group (Fig. 5). The CF, BF, and MF groups had the mean crossing time values, respectively, 5.1%, 18.6%, and 5.9% higher than the CK group. The CF and BF groups differed significantly from the CK group on the 2nd (P = 0.048 and P = 0.008, respectively) and 3rd day (P = 0.007 and P = 0.003, respectively), whereas the MF group was significantly different from the CK group on day 3 (P = 0.009). Overall, the mice showed increased exploratory abilities after staying in any of the three forest environments.
Fig. 5. Dynamics of animal central grid movement time and crossing times
Body weight and fecal particle count
The changes in body weight reflect the appetite of mice. The body weight of the mice in each forest group increased with the increase in the number of days of treatment, with the exception of decreased weights of the CK group on the 3rd day and the CF group on the 4th day (Fig. 6). Both the CF (P = 0.04) and the BF (P = 0.03) groups differed significantly in body weight on the 6th day compared with the 1st day. Therefore, after the stay in the forest, the emotional state of mice improved significantly, resulting in an increase in appetite.
The fecal particle number can reflect the state of tension (anxiety) in mice. More fecal particles indicate higher levels of stress, and fewer particles indicate higher levels of relaxation (Guo et al. 2012). Figure 6 shows that the number of fecal particles of mice in each field decreased with the prolonged duration of the treatment, with the BF group having the fastest decrease, followed by the MF group, and the CK group had the slowest decrease. The numbers of fecal particles in the CF, BF, and MF groups were, respectively, 7.3%, 10.6%, and 7.6% higher than in the CK group. This showed that the mice were significantly less anxious and more relaxed when they were exposed to the forest environment.
Fig. 6. Changes in mice body weight and fecal particle numbers
Comparative analysis of environmental factors
Compared with CK, the air temperature was lower in the three forest environments (by 32.1% in BF, 19.1% in CF, and 30.3% in MF) (Fig. 7). The relative humidity was lower in CK compared with BF (by 15.5%), CF (by 10.2%), and MF (by 14.2%). Compared with CK, the average wind speed was lower by 3.13-fold in BF, 1.45-fold in CF, and 1.32-fold in MF. Hence, the BF environment was noticeably cooler, more humid, and had reduced wind speed compared with the other environments.
Fig. 7. Diurnal variation in microclimate indicators in the four plots. Columns show relative humidity and wind velocity, and the lines show air temperature and light intensity.
Regarding the influence of environmental factors on spontaneous activities, mice are not adaptable to the high temperature and high humidity environments. When the ambient temperature is above 30 ℃, mice growth is retarded, and recovery sleep and spontaneous slow wave sleep are inhibited. The optimal environmental temperature for mice is 23 to 25 °C and relative humidity is 55 to 60% (Cureau et al. 2022). Weak light can increase the behavioral and instinctive activities of mice; in contrast, mice exposed to too strong light are lethargic, have slow growth, and lose weight. This is consistent with the present research conclusion. Due to the larger canopy density, suitable temperature and humidity, and stronger shading in BF than in MF and CF (Fig. 7), the amount of exercise of mice that stayed in BF was greater than for those that stayed in the other two forests (Gu et al. 2019).
Environmental noise is also closely related to the behavior of rodents. For example, some scholars have found that the mice exposed to noise have decreased vitality, slow weight gain, poor behavioral learning function and neuromuscular activity, and altered tolerance. Most notably, there are few reports on the effects of wind velocity on the behavior of mice. Wind can affect the activities of mice directly by influencing the skin sensation, or indirectly by changing the environmental temperature. Therefore, the effects of environmental factors on experimental animals are complex, and the synergistic effect of multiple factors is one of the important topics the authors will study in the future.
During the experiment, the concentration of PM 2.5 particles in CK was 31%, 28%, and 37% higher than in BF, MF, and CF, respectively (Fig. 8). The concentration of PM 1.0 was higher in CK 23%, 19%, and 29% than in BF, MF, and CF, respectively. It showed that the three forest types exhibited the effect of dust holding.
Guo et al. (2012) found that the PM 2.5 particles could cause a dose-dependent decrease in the enzyme activity of alveolar macrophages in mice, potentially inducing alveolar oxidation and an imbalance between the oxidative stress and antioxidant systems in mice, damaging the lung tissue, and causing an inflammatory response (Geng et al. 2005). The current study found that the three forest environments all had good dust retention effects (Fig. 8), significantly reducing the concentration of small-sized particles, which was associated with promoting the exercise activity, increasing appetite, and enhancing the cognitive and exploration abilities of mice.
On average, the concentration of negative ions in the air in CK was 45%, 39%, and 31% lower than in BF, CF, and MF, respectively. The concentration of oxygen in the air in CK was 0.04%, 0.03%, and 0.02% lower than in BF, MF, and CF (Fig. 9).
Increased concentration of negative ions in the air can improve learning and memory in mice (Ekkel and De 2017), which is consistent with the present study. The concentration of negative ions in BF and CF was relatively high (Fig. 9), and the weight of mice that stayed in these forests increased significantly over time, and the residence time and crossing times in the central grid were significantly higher than those of the control group (Figs. 5 and 6). It may be that the behavior and emotional expression of mice were related to their brain activity, with the NIC gene reducing the level of serotonin in the mice brain, which was involved in the regulation of pain, sleep and body temperature (Hershey et al. 2018). Some physiological and psychological investigations showed that exposure to negative ions in the open environment can improve performance efficiency and mental status, increase blood oxygen content, and reduce heart rate (Cureau et al. 2022). These studies demonstrate that increased concentrations of negative ions and oxygen in the air are beneficial to the health of both animals and humans, with improvements in spontaneous behavior of mice after the stay in the three types of recreational forests. In contrast, Hedge and Collis (1987) and Wen et al. (2019a) found no beneficial effects of increased concentrations of negative ions and oxygen in the air on mood and performance of mice. This indicated that there may be a certain concentration threshold for the positive effects of negative ions and oxygen (Hedge and Collis 1987; Wen et al. 2019b). Therefore, the role of air concentrations of negative ions and oxygen in forests on promoting psychophysiological health of mice remains a topic for more systematic investigation.
Previous researchers have quantitatively analyzed the visual characteristics and psychological relationship of different plant communities in broad-leaved pure forests, coniferous pure forests, and mixed coniferous and broad-leaved forests by using brainwave changes as an intrinsic scientific indicator of human emotional changes. Research has found that the visual and olfactory characteristics of broad-leaved forests and coniferous forests have similar effects on human relaxation and stress relief, although the impact of broad-leaved forests on human health is more positive. This previous finding was consistent with the conclusion of the present study, indicating that the forest environment, especially the broad-leaved forest environment, has a positive promoting effect on the health of both mice and humans (Ning and Meiling 2017)
Fig. 8. Diurnal variation in the concentrations of various particle sizes in the air in the monitored locations. Columns show TSP particle concentration and PM 1.0 particle concentration, and the lines show PM10 particle concentration and PM 2.5 particle concentration
Fig. 9. Changes in the concentrations of oxygen and oxygen anions in the air. Columns show air oxygenation while the lines show negative ion concentration.
CONCLUSIONS
- After the mice stayed in the three types of urban forest habitats, their excitability and the exploration, cognition, and learning, and memory abilities were all significantly improved. In addition, their appetite increased significantly, and the number of fecal particles gradually decreased. Their stress and anxiety were significantly reduced, and their emotional state was significantly improved.
- The broadleaf forest (BF) had the best effect, followed by the mixed broadleaf and coniferous forest (MF), and the third was the coniferous forest (CF).
- The effect of forest environment on the spontaneous behavior of mice was the result of the interactions among various factors, with high negative ion concentration and air oxygenation having positive, and high air temperature, relative humidity, wind velocity, and particulate matter, and negative effects on mice.
- In the future, it is necessary to study more forest environments to provide a research basis for the selection of suitable plant compositions for the development of “forest bathing”/human recuperation.
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by National Natural Science Foundation of China (32201621), Beijing Natural Science Foundation Municipality (8192018), Beijing Key Laboratory of Ecological Function Evaluation and Regulation Technology of Garden Green Space (STZD202303), and Beijing Key Laboratory of Ecological Function Evaluation and Regulation Technology of Garden Green Space (STQN202202).
REFERENCES CITED
Antonelli, M., Donelli, D., Carlone, L., Maggini, V., Firenzuoli, F., and Bedeschi, E. (2022). “Effects of forest bathing (shinrin-yoku) on individual well-being: An umbrella review,” International Journal of Environmental Health Research 32(8), 1842-1867. DOI: 10.1080/09603123.2021.1919293
Barthod, C., and Fournier, P. (2019). “Forests and health: Discourse and practises from the 18st to the 21st century,” Journal 13(5), 15-23. DOI:10.3917/spub.190.0015
Carnicer, J., Vives‐Ingla, M., Blanquer, L., Méndez‐Camps, X., Rosell, C., Sabaté, S., Gutiérrez, E., Sauras, T., Peñuelas, J., and Barbeta, A. (2021). “Forest resilience to global warming is strongly modulated by local‐scale topographic, microclimatic and biotic conditions,” Journal of Ecology 109(9), 3322-3339. DOI: 10.1111/1365-2745.13752
Chae, Y., Lee, S., Jo, Y., Kang, S., Park, S., and Kang, H. (2021). “The effects of forest therapy on immune function,” International Journal of Environmental Research and Public Health 18(16), Article Number 8440. DOI: 10.3390/ijerph18168440
Cureau, R. J., Pigliautile, I., Kousis, I., and Pisello, A. L. (2022). “Multi-domain human-oriented approach to evaluate human comfort in outdoor environments,” International Journal of Biometeorology 66, 2033-2045. DOI: 10.1007/s00484-022-02338-7
Dai, Z., Wu, S., and Cheng, H. (2018). “Effect of vegetation on summer microclimate in suburban forest park: A case study of Fuzhou National Forest Park,” Journal of Chinese Urban Forestry 16, 1-5. DOI: 10.3969/j.issn.1673-923X.2018.01.001
Doimo, I., Masiero, M., and Gatto, P. (2020). “Forest and wellbeing: Bridging medical and forest research for effective forest-based initiatives,” Forests 11(8), article 791. DOI: 10.3390/f11080791
Ekkel, E. D., and De, V. S. (2017). “Nearby green space and human health: Evaluating accessibility metrics,” Landscape and Urban Planning 157, 214-220. DOI: 10.1016/j.landurbplan.2016.06.008
Geng, H., Meng, Z., and Zhang, Q. (2005). “Effects of blowing sand fine particles on plasma membrane permeability and fluidity, and intracellular calcium levels of rat alveolar macrophages,” Toxicology Letters 157, 129-137. DOI: 10.1016/j.toxlet.2005.01.010
Gu, L., Wang, C., Wang, Y., Wang, X., Sun, Z., Wang, Q., and Sun, R. (2019). “Patterns of temporal variation of microclimate and extent of human comfort in the recreation forests in Huishan National Forest Park,” Scientia Silvae Sinicae 55, 150-159. DOI: 10.3969/j.issn.1001-7488.2019.06.019
Guo, X., Yan, Q. Q., and Zhao, X. B. (2012). “Experimental study on the toxicity of atmospheric PM2.5 in different regions to alveolar macrophages in rats,” Journal of Environment and Health 29, 5-8. DOI: 10.16241/j.cnki.1001-5914.2012.01.002
Hedge, A., and Collis, M. D. (1987). “Do negative air ions affect human mood and performance?,” Annals of Occupational Hygiene 31, 285-290. DOI: 10.1093/annhyg/31.3.285
Hershey, J. D., Gifford, J. J., Zizza, L. J., Pavlenko, D. A., Wagner, G. C., and Miller, S. (2018). “Effects of various cleaning agents on the performance of mice in behavioral assays of anxiety,” Journal of the American Association for Laboratory Animal Science 57, 335-339. DOI: 10.30802/AALAS-JAALAS-17-000161
Hu, Z., Gu, Y., Ye, M., Ma, Y., Wang, Y., Pan, S., Huang, C., and Lu, X. (2022). “Innate immune stimulation prevents chronic stress-induced depressive and anxiogenic-like behaviors in female mice,” International Immunopharmacology 111, article ID 109126. DOI: 10.1016/j.intimp.2022.109126
Jin, E. J., Yoon, J. H., Bae, E. J., Jeong, B. R., Yong, S. H., and Choi, M. S. (2021). “Particulate matter removal ability of ten evergreen trees planted in Korea urban greening,” Forests 12(4), article 438. DOI: 10.3390/f12040438
Kwak, M. J., Lee, J., Kim, H., Park, S., Lim, Y., Kim, J. E., Baek, S. G., Seo, S. M., Kim, K. N., and Woo, S. Y. (2019). “The removal efficiencies of several temperate tree species at adsorbing airborne particulate matter in urban forests and roadsides,” Forests 10(11), article 960. DOI: 10.3390/f10110960
Li, Q. (2010). “Effect of forest bathing trips on human immune function,” Environmental Health and Preventive Medicine 15, 9-17. DOI: 10.1007/s12199-008-0068-3
Li, Q. (2022). “Effects of forest environment (Shinrin-yoku/Forest bathing) on health promotion and disease prevention—the Establishment of “Forest Medicine,” Environmental Health and Preventive Med. 27, 43-43. DOI: 10.1265/ehpm.22-00160
Luo, L., Sun, W., Han, Y., Zhang, W., Liu, C., and Yin, S. (2020). “Importance evaluation based on random forest algorithms: Insights into the relationship between negative air ions variability and environmental factors in urban green spaces,” Atmosphere 11(07), article 706. DOI: 10.3390/atmos11070706
Miao, C., Cui, A., Xiong, Z., Hu, Y., Chen, W., and He, X. (2022). “Vertical evaluation of air quality improvement by urban forest using unmanned aerial vehicles,” Frontiers in Ecology and Evolution 10, article ID 1045937. DOI: 10.3389/fevo.2022.1045937
Ning, K., and Meiling, X. (2017). “Visual characteristics of different plant community types and their impact on human psychology,” Journal of Northwest Forestry University 32(1), 315-320. DOI: 10.3969/j.issn.1001-7461.
Oroszi, T., Geerts, E., Boer, S. F., Schoemaker, R. G., Zee, E. A., and Nyakas, C. (2022). “Whole body vibration improves spatial memory, anxiety-like behavior, and motor performance in aged male and female rats,” Frontiers in Aging Neuroscience 13, article ID 801828. DOI: 10.3389/fnagi.2021.801828
Pei, Y. L., Yin, X., and Jing, J. C. (2022). “Research progress on the effects of forest recuperation on physical and mental health,” Southeast National Defense Medicine, 24(4), 414-417. DOI: 10.3969/j.issn.1672-271X.
Perreault, M., Will, S., Panza, D., Gareski, T., Harding, K., Kubasiak, D., Jalenak, M., Gartrell, K., Wang, S., and Bollag, G. (2010). “Modulation of nutrient sensing nuclear hormone receptors promotes weight loss through appetite suppression in mice,” Diabetes, Obesity and Metabolism 12(3), 234-245. DOI: 10.1111/j.1463-1326.2009.01157.x
Qian, W., Cheng, W., and Yan, Y. W. (2015). “Effects of forest bathing in a Phyllostachys edulis forest on the spontaneous behavior of mice,” Scientia Silvae Sinicae 51, 78-86. DOI: 10.11707/j.1001-7488.20150509
Tan, X. Y., Liu, L., and Wu, D. Y. (2022). “Relationship between leaf dust retention capacity and leaf microstructure of six common tree species for campus greening,” International Journal of Phytoremediation 24, 1213-1221. DOI: 10.1080/15226514.2021.2024135
Tyagi, A. K., and Malik, A. (2012). “Bactericidal action of lemon grass oil vapors and negative air ions,” Innovative Food Science & Emerging Technologies 13, 169-177. DOI: 10.1016/j.ifset.2011.09.007
Wen, J. D., Cheng, W., Nan, C. P., Chang, Z., Lin, G., Sha, S. J., Ze, Z. H., and Xin, H. X. (2019a). “Urban forests increase spontaneous activity and improve emotional state of white mice,” Urban Forestry & Urban Greening 46, 247-255. DOI: 10.1016/j.ufug.2019.126449
Wen, J. D., Cheng, W., Nai, C. P., Chang, Z., Liu, G., Shan, S. J., Ze, Z. H., and Xin, H. X. (2019b). “Spatiotemporal ozone level variation in urban forests in Shenzhen, China,” Forests 10, 247-254. DOI: 10.3390/f10030247
Xiao, P. L., Shu, X. F., Pei, Y. H., and Li, D. (2019). “Temporal variations of spontaneous plants colonizing in different types of planted vegetation-a case of Beijing Olympic Forest Park,” Urban Forestry & Urban Greening 46, 125-134. DOI: 10.1016/j.ufug.2019.126459
Yang, J., Wang, H., Xie, B., Shi, H., and Wang, Y. (2015). “Accumulation of particulate matter on leaves of nine urban greening plant species with different micromorphological structures in Beijing,” Research of Environmental Sciences 28, 384-392. DOI: 10.13198/j.issn.1001-6929.2015.03.08
Article submitted: October 7, 2023; Peer review completed: November 26, 2023; Revised version received: December 24, 2023; Accepted: January 1, 2024; Published: January 11, 2024.
DOI: 10.15376/biores.19.1.1465-1478
