Abstract
A multistage process was employed to obtain value-added products from brewer’s spent grain (BSG). This paper is focused on the production and characterisation of cellulose nano-fibres (CNF) as one of the products obtained during the complete process. In the first stage, protein-rich liquor was separated via the alkaline (NaOH) treatment of dried BSG and stored for further utilisation. In the second stage, bleaching treatments were conducted to separate cellulose, which was later converted to CNF by high-pressure homogenisation. The lignocellulosic product from each step was analysed for its chemical composition by means of alkaline hydrolysis combined with the HPEAC method. The thermal properties were measured using thermogravimetric analysis (TGA). The morphology was studied using scanning electron microscopy (SEM) and atomic force microscopy (AFM). X-ray diffraction (XRD) was done to observe changes in crystallinity. The nano-cellulose produced can be regarded as a value-added material from the bio-refinery of BSG along with numerous already-reported products.
Download PDF
Full Article
Utilising Brewer’s Spent Grain as a Source of Cellulose Nanofibres Following Separation of Protein-based Biomass
Pawan Kumar Mishra,a,* Tomas Gregor,b and Rupert Wimmer a
A multistage process was employed to obtain value-added products from brewer’s spent grain (BSG). This paper is focused on the production and characterisation of cellulose nano-fibres (CNF) as one of the products obtained during the complete process. In the first stage, protein-rich liquor was separated via the alkaline (NaOH) treatment of dried BSG and stored for further utilisation. In the second stage, bleaching treatments were conducted to separate cellulose, which was later converted to CNF by high-pressure homogenisation. The lignocellulosic product from each step was analysed for its chemical composition by means of alkaline hydrolysis combined with the HPEAC method. The thermal properties were measured using thermogravimetric analysis (TGA). The morphology was studied using scanning electron microscopy (SEM) and atomic force microscopy (AFM). X-ray diffraction (XRD) was done to observe changes in crystallinity. The nano-cellulose produced can be regarded as a value-added material from the bio-refinery of BSG along with numerous already-reported products.
Keywords: Nanocellulose; Cellulose nano-fibres; Brewer‘s spent grain; Bio-refinery
Contact information: a: Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00 Brno, Czech Republic; b: Department of Food Technology, Mendel University in Brno; *Corresponding author: xmishra@mendelu.cz
INTRODUCTION
The brewing industry generates spent grains, hops, and yeasts from the fermentation process as its major wastes and byproducts. Spent grains constitute 85% of the total waste generated by a given brewery, with a composition of cellulose (16 to 25%), protein (15 to 24%), lignin (11 to 27%), and other components (Mussatto et al. 2006). It has also been widely used as an ingredient for human and animal food products (Ozturk et al. 2002), energy production (Ezeonu and Okaka 1996), charcoal manufacture (Okamoto et al. 2002), brick manufacture (Okamoto et al. 2002), paper production (Ishiwaki et al. 2000), and adsorbent production (Low et al. 2000). The bio-refining of BSG and techno-economic analysis is reported by Mussatto et al. (2006).
Nano-cellulose has been reported to be prepared from a wide range of sources, including raw cotton linter (Morais et al. 2013), sisal fibres (Morán et al. 2008), sugarcane bagasse (Mandal and Chakrabarty 2011), oil palm empty-fruit-bunch (Fahma et al. 2010), rice husk, (Ludueña et al. 2011), wood (Abe et al. 2007), bamboo (Abe and Yano 2009), cotton (Morais et al. 2013), soy hulls (Alemdar and Sain 2008), hemp (Wang et al. 2007), branch-barks of mulberry (Li et al. 2009), pineapple leaf fibres (Cherian et al. 2010), pea hull fibre (Chen et al. 2009), coconut husk fibres (Rosa et al. 2010), banana rachis (Zuluaga et al. 2009) and sugar beet (Dufresne et al. 1997; Dinand et al. 1999). In a very recent study (Berglund et al. 2016), production of CNF from BSG has been reported using an ultragrinding method with a main focus on energy consumption and feasibility of the process. However, in our research, the aim was to prepare cellulose nanofibres without destroying the protein content of the material, which can be later utilised for other various purposes. To achieve this, the protein-based material was separated using alkaline dissolution as the first step without affecting other contents significantly. Lignocellulosic biomass obtained in first step of alkaline treatment was bleached to obtain cellulose fibres. Cellulose fibres thus obtained were processed in a high pressure homogeniser (HPH) to obtain cellulose nanofibres. The advantages of HPH include continuous operation and easy scale up (Spence et al. 2011); problems include a high number of passes required and clogging of homogenizer due to larger fiber (Oksman et al. 2014). This postulated a method for complete utilisation of BSG including reclaimed lignin (Mishra and Wimmer 2016) and denaturated protein (reported as wood adhesive (Hettiarachchy et al. 1995)) along with CNF as products. Additionally, our work provides information and comparison on thermal and crystalline properties of products obtained at various stages of the proposed processing method.
EXPERIMENTAL
Materials
The BSG used in this research was obtained from a microbrewery of the Department of Food Science, Mendel University in Brno, Czech Republic. The material was washed with distilled water as soon as it was obtained until a neutral pH was reached, after which it was stored at -5 °C until further utilisation. The stored material was then dried at 50 ± 5 °C for 24 h to attain 10% moisture content (untreated material). The dried material was further stored in a hermetic container at -5 °C to prevent any microbial growth. The reagents used in chemical treatment were: sodium hydroxide (NaOH), sodium chlorite (NaClO2), and sodium sulphite (Na2SO4). All chemicals were purchased from Sigma Aldrich (Czech Republic) and used without any further purification.
Methods
The alkali treatment, first milder (for shorter time) to dissolve the protein based biomass followed by bleaching process and second alkali treatment (for complete removal of lignin), was carried out to obtain cellulose. This resulted in reclaimed lignin from bleaching liquor and protein based biomass as potentially usable products from the same process. Mainly the lignocellulosic biomass and cellulose were characterized for morphology, thermal properties, and CNF for obtained diameter.
Treatment of BSG
Dried BSG (P0) was extracted with 0.1 M NaOH at 60 °C for 60 min (17% w/v) with continuous stirring at 30 rpm. Centrifugation was done at 2000 rpm for 10 min at 4 °C to separate the protein extract from lignocellulosic part. Protein-rich supernatant was separated, and the lignocellulosic residue was washed with distilled water and used for further experiment. Lignocellulosic residue (P1) obtained in previous step was boiled with 0.7% (w/v) sodium chlorite (1:50 g/mL), for 2 h at pH 4. This residue was further treated with 5% (w/v) sodium bisulphite (1:50 g/mL) at room temperature for 1 h, washed with distilled water, and dried. This was followed by treatment with 17.5% NaOH (1:50 g/mL) at room temperature for 8 h, washed, and dried again (P2). A scheme of the treatments is shown in Fig. 1.
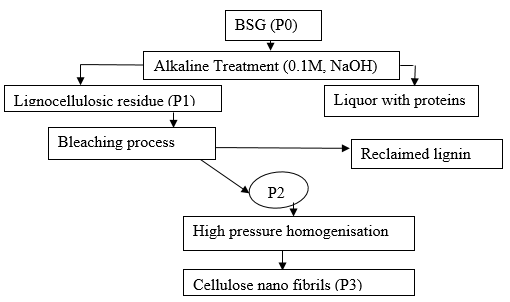
Fig. 1. Scheme of different steps of mechano-chemical treatment of BSG
Nano-cellulose production
The cellulose-based product (P2) obtained in the previous steps was collected, and a 1.5% dispersion was prepared in water. This dispersion was processed using an IKA-T10 (Ultra-Turrax supplied IKA, Germany) to disperse the fibres. The resultant dispersion was homogenised in a high-pressure homogeniser (APV 1000 from SPX Corporation, USA) at 700 to 800 atm for 20 cycles.
Chemical composition
The chemical composition of BSG was analysed at every stage (P0, P1, and P2) according to the following method. Approximately 200 mg of each sample was pre-hydrolysed with 2 mL of 72% H2SO4 at 30 °C for 1 h, followed by hydrolysis in an autoclave using 56 mL of ultra-pure water at 120 °C for 30 min. For the borate complex, anion exchange chromatography HPAEC-Borate analysis (Willför et al. 2009; Sinner et al. 1975), the wood sugars were separated on a 5.6 mm bore column 115 mm in length (omnifit) that was filled with a strong anion exchange resin (MCL gel CA08F (Mitsubishi chemicals, Japan) at 60 °C). The mobile phase (0.7 mL/min) was composed of A (0.3 potassium borate buffer with pH 9.2) and B (0.9 M potassium borate buffer with pH 9.5). After the sample injection, separation was started with 90%A and 10%B. A linear gradient was run within 35 min to 10% A and 90% B. The data acquisition was stopped after 50 min. The sugar quantification was performed after the column derivatisation with cu-bichincininate (0.35 mL/min) at 105 °C in a 30 m crocheted Teflon coil with a 0.3 mm inner diameter and detection at 560 nm (Sinner et al. 1975; Sinner and Puls 1978). Data were processed with Dionex Chromeleon software (Thermo Scientific, USA).
Thermo-gravimetric analysis (TG)
Thermogravimetric analysis was performed to compare the degradation characteristics of the raw BSG, bleached cellulose fibres, and cellulose fibres. The thermal stability of reinforcing fibres is very important for their greater application in reinforced-biocomposite materials because typical processing temperature for many thermoplastics exceeds 200 °C (Tadmor and Gogos 1979). The thermal stability of the different samples was determined by TGA measurements performed using a Netzch thermo-gravimetric analyser (TG 209 F1, Netzch, Germany). The amount of sample used for each measurement was 5 to 7 mg. All measurements were performed under a nitrogen atmosphere with a gas flow of 10 mL/min while the material was heated from room temperature to 800 °C at a heating rate of 10 °C/min.
Scanning electron microscopy
SEM images were taken using a Hitachi TM3030 tabletop model (Hitachi, Japan) to observe the surface morphology of BSG based CNF. The SEM images were used to assess the effect of chemical treatment on untreated, alkali-treated, and bleached fibres. Samples were observed at an accelerating voltage of 15 kV. No sputtering was required. Samples were placed on sampling pads procured from Christine-Gropl company, Tulln, Austria.
X-ray diffractometry (XRD)Ia-Ib
Cellulose crystallinity is a key factor for determining the mechanical and thermal properties of cellulose nanofibres. XRD studies on P0, P1, and P2 stages of cellulose fibres were conducted to investigate the crystalline behaviour of the fibres. XRD was performed using a PANalytical Empyrean diffractometer system (PANalytical, Netherlands) using copper Kα-radiation. Scans were collected from 10 to 40° with a scan step size of 0.026° and a time per step of 96.39 s. The Segal crystallinity index (CI (%)) was calculated using the following equation,
![]() (1)
(1)
where Ia is the total intensity of the (2 0 0) peak for cellulose representing amorphous and crystalline both regions at 22° 2θ and Ia is the amorphous region intensity at 18° 2θ (Segal et al. 1959).
Atomic force microscopy (AFM):
AFM imaging was done using a Bruker (Bruker Corporation) Dimension Icon. Measurements were performed in tapping mode with an OTESPA (Bruker Corporation) cantilever (rectangular shape, resonance frequency 300 (range 200 to 400), spring constant 42 (range 12 to 103), length 160 µm (range 140 to 180 µm), and width 50 µm (range 48 to 52 µm)). The scanning frequency varied between 0.5 and 1 Hz. The sample, as a suspension, was diluted to a dry content of 0.001%. A drop of the obtained suspension was placed on mica and placed in an oven to be dried at 103 °C for a few minutes.
RESULTS AND DISCUSSION
Chemical Composition of BSG at Different Steps of Processing
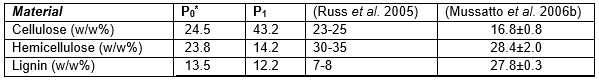
Materials from stages P0, P1, and P2 were analysed for their chemical compositions, as shown in Table 1. The composition of the original material included cellulose (24.5%), hemicellulose (9.8%), and lignin (15.8%). The cellulose and lignin percentages agreed closely with 25.4% and 11.8%, respectively (Kanauchi et al. 2001). The quantity of cellulose and lignin was in agreement with the reported composition of BSG, that is, 16% to 25% cellulose and 11% to 27% lignin (Mussatto et al. 2006b). The composition of BSG was found to be dependent on the type of malting barley, the process and conditions of malting, sample preparation, and method of chemical characterisation. Upon chemical treatment, the cellulose content increased as expected. Bleaching almost removed the lignin and hemicellulose. Chemical composition of P2 was not determined assuming it to be entirely cellulose which was later confirmed by characteristic data obtained by other methods of analysis (TG, DSC and XRD).
Table 1. Chemical Composition of BSG Utilised for Nanofibre Production
Morphological Evaluations
The visual morphology of the P0, P1, and P2 stages are shown in Fig. 1. The color changed from brownish to slightly brownish-orange after the first treatment due to removal of proteins and some part of lignin. The bleached material (P2) was clearly different and white in color, which was due to complete removal of non-cellulosic material like lignin and hemicellulose. White color is an indication of cellulose. The chemical treatment also induced some morphological changes. After the first treatment, the surface became much rougher, mainly because of some materials like waxes, pectin, some sugars, and proteins.
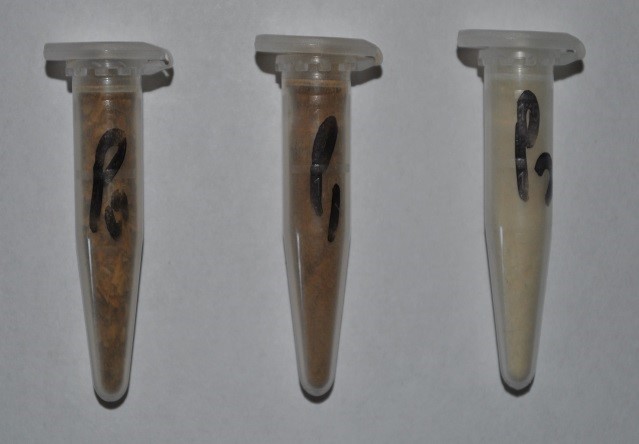
Fig. 2. Change in color of BSG with processing steps (P0, P1, and P2, left to right)
The fibres could easily be recognised in the optical microscope coupled to the AFM, which allowed a quick selection of the area to scan. Individual fibres were selected for diameter assessment. As seen in Fig. 3, the presence of nanofibrils is obvious. The height profiles shown in Fig. 3 and corresponding to the positions marked with a 3 in Fig. 4 revealed the fibre diameters to be about 5 nm. A statistical analysis of 20 nano-fibrils depicted in Fig. 4 indicated a mean diameter of 4.58 nm, the median value being 4.25 nm. Some fibrils had a diameter smaller than 3 nm, which corresponded to elementary fibrils.
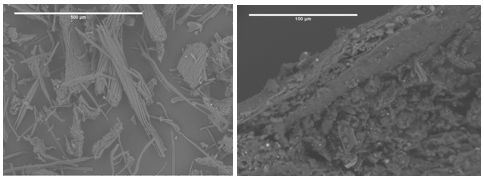
Fig. 3. Cellulose fibres visible in bundles (P2, left) and raw BSG (P0, right)
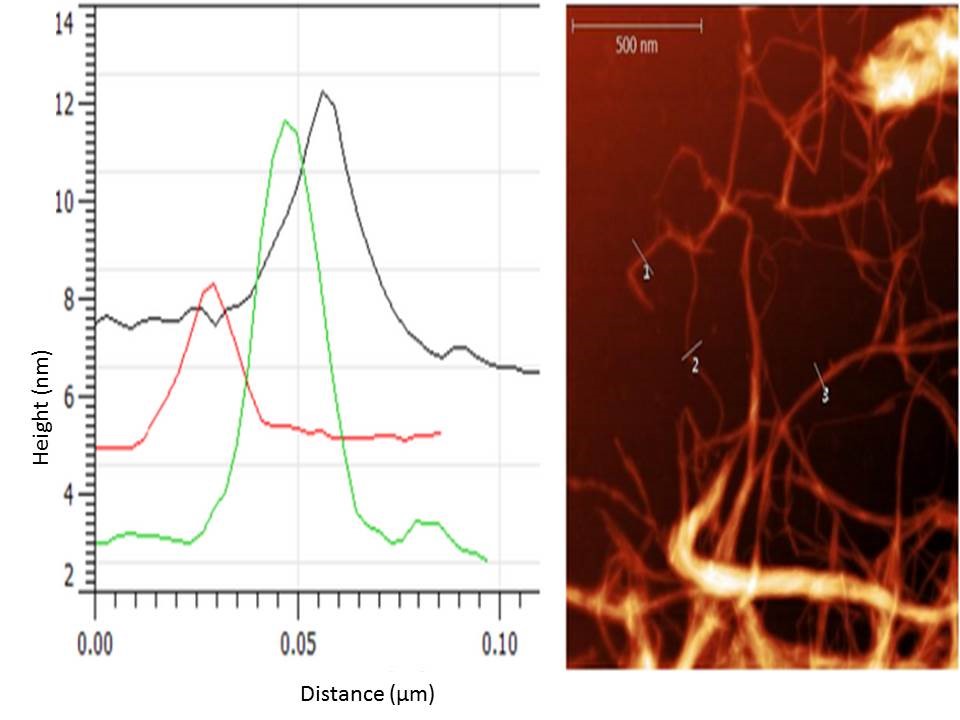
Fig. 4. BSG-nanocellulose picture obtained with the AFM and example of extracted height profiles
Thermal stability
The initial weight losses starting at ∼25 °C for P0, P1, and P2 could be attributed to the evaporation of loosely bound moisture on the surfaces of the materials. The chemisorbed water was found to be given off near 100° C for almost all the three materials. The degradation of P0 began at approximately 200 °C, and the rate of degradation reached two different peaks, at 300 and 340 °C. The early degradation could be explained by the presence of proteins and sugars in P0, which was followed by P1 and P2 because of the presence of hemicelluloses and lignin in P1 and the removal of the same during the bleaching process.
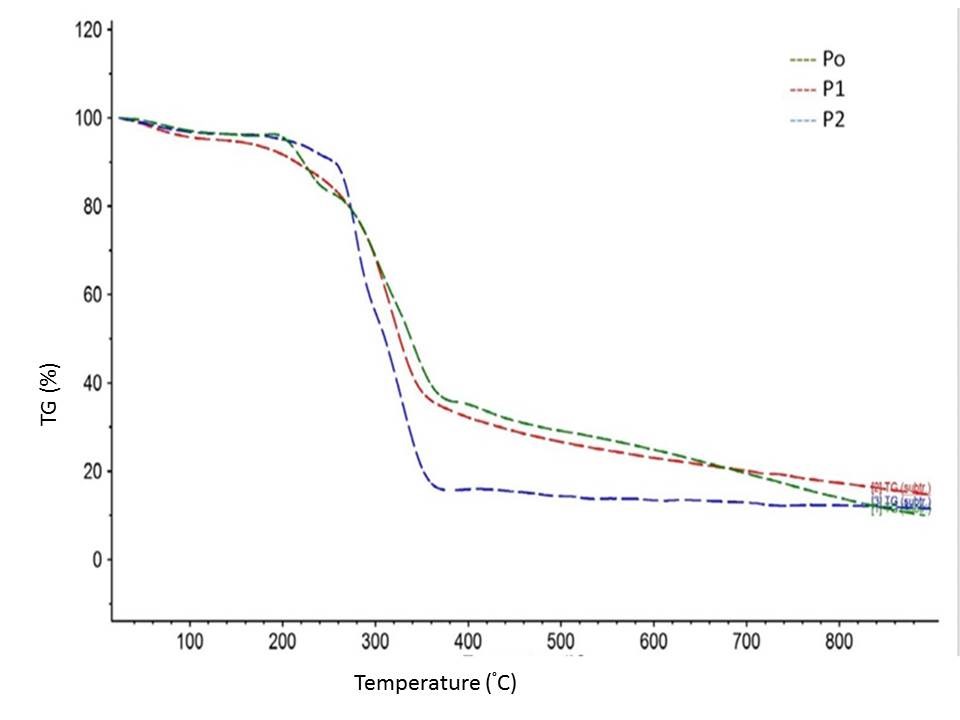
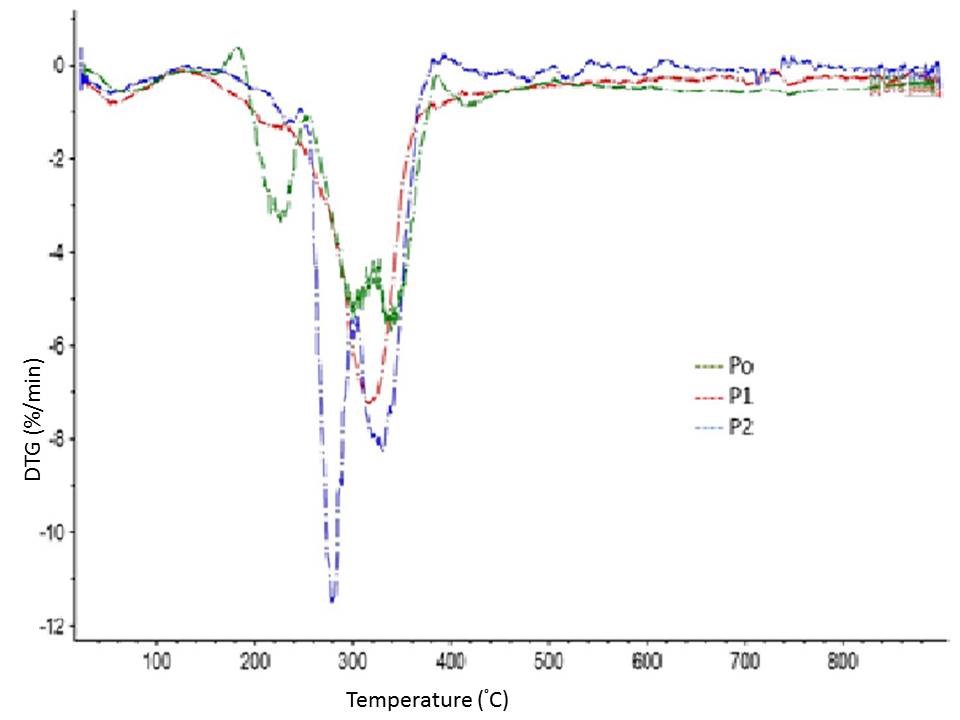
Fig. 5. TG and DTG curves of BSG at P0, P1, and P2 stages of processing
In the range of 400 to 640 °C, the residual weight decreased with the number of processing steps, which might have been due to char-forming substances (sugars, proteins, lignin, and hemicelluloses) and their subsequent removal during the different processing steps. The role of HPH in further improvement of thermal stability of bleached fibres has already been widely reported (Li et al. 2014).
In the XRD (Fig. 6), P2 showed a clear doublet at and asymmetric peak around 21.27 and 19.8, with a small shoulder around 12.05 degrees. In the raw BSG, cellulose is mixed with hemicellulose, lignin, and protein. As these components are removed with progress in processing steps, the crystallinity is expected to increase, as can be seen from the XRD curve (sharpening of curve) and increasing values (17.4% (P0), 18.1% (P1), and 48.8% (P2)) of crystallinity index. This marked the presence of cellulose II (Cheng et al. 2011). The conversion of cellulose I to cellulose II could have happened because of alkaline treatment, but the change was visible only after the bleaching process (P2 stage) (Gassan and Bledzki 1999; Klemm et al. 2005). Improvement in crystallinity of bleached fibres following the HPH treatment has been already reported (Li et al. 2014).
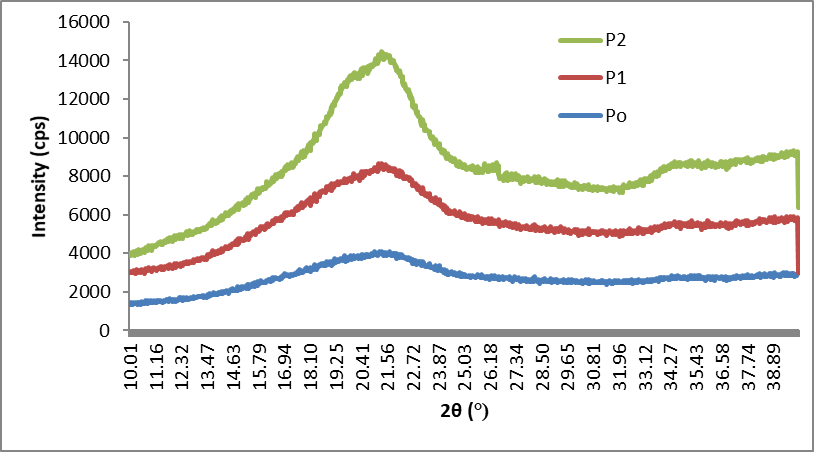
Fig. 6. XRD curves of P0, P1, and P2
CONCLUSIONS
- Brewer’s spent grain (BSG) can be used as a potential source of CNF, especially in countries like the Czech Republic (with significant size of brewing industry).
- Wastage can be minimised by potential utilisation of protein-based biomass (degenerated protein based adhesive reported elsewhere and reclaimed lignin).
- Reported work can be a useful part of multistep process for producing value-added products from BSG.
ACKNOWLEDGEMENT
This work was funded by the Internal Grant Agency of the Faculty of Forestry and Wood Technology, Mendel University in Brno, Grant number LDF_VP_2015003.
REFERENCES CITED
Abe, K., and Yano, H. (2009). “Comparison of the characteristics of cellulose microfibril aggregates isolated from fiber and parenchyma cells of Moso bamboo (Phyllostachys pubescens),” Cellulose 17(2), 271-277. DOI: 10.1007/s10570-009-9382-1
Abe, K., Iwamoto, S., and Yano, H. (2007). “Obtaining cellulose nanofibers with a uniform width of 15 nm from wood,” Biomacromolecules 8, 3276-3278. DOI:10.1021/bm700624p
Alemdar, A., and Sain, M. (2008). “Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls,” Bioresour. Technol. 99, 1664-1671. DOI:10.1016/j.biortech.2007.04.029
Berglund, L., Noël, M., Aitomäki, Y., Öman, T., and Oksman, K. (2016). “Production potential of cellulose nanofibers from industrial residues: Efficiency and nanofiber characteristics,” Ind. Crops Prod. 92, 84-92. DOI:10.1016/j.indcrop.2016.08.003
Chen, Y., Liu, C., Chang, P. R., Cao, X., and Anderson, D. P. (2009). “Bionanocomposites based on pea starch and cellulose nanowhiskers hydrolyzed from pea hull fibre: Effect of hydrolysis time,” Carbohydr. Polym. 76, 607-615.
Cheng, G., Varanasi, P., Li, C., Liu, H., Melnichenko, Y. B., Simmons, B. A., Kent, M. S., and Singh, S. (2011). “Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis,” Biomacromolecules 12, 933-941. DOI:10.1021/bm101240z
Cherian, B. M., Leão, A. L., de Souza, S. F., Thomas, S., Pothan, L. A., and Kottaisamy, M. (2010). “Isolation of nanocellulose from pineapple leaf fibres by steam explosion,” Carbohydr. Polym. 81, 720-725. doi:10.1016/j.carbpol.2010.03.046
Dinand, E., Chanzy, H., and Vignon, R. M. (1999). “Suspensions of cellulose microfibrils from sugar beet pulp,” Food Hydrocoll. 13, 275-283. DOI:10.1016/S0268-005X(98)00084-8
Dufresne, A., Cavaillé, J.-Y., and Vignon, M. R. (1997). “Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils,” J. Appl. Polym. Sci. 64, 1185-1194. DOI:10.1002/(SICI)1097-4628(19970509)64:6<1185::AID-APP19>3.0.CO;2-V
Gassan, J., and Bledzki, A. K. (1999). “Alkali treatment of jute fibers: Relationship between structure and mechanical properties,” J. Appl. Polym. Sci. 71, 623-629. DOI:10.1002/(SICI)1097-4628(19990124)71:4<623::AID-APP14>3.0.CO;2-K
Hettiarachchy, N. S., Kalapathy, U., and Myers, D. J. (1995). “Alkali-modified soy protein with improved adhesive and hydrophobic properties,” J. Am. Oil Chem. Soc. 72, 1461-1464. DOI:10.1007/BF02577838
Klemm, D., Heublein, B., Fink, H.-P., and Bohn, A. (2005). “Cellulose: Fascinating biopolymer and sustainable raw material,” Angew. Chem. Int. Ed. 44, 3358-3393. DOI:10.1002/anie.200460587
Li, M., Wang, L., Li, D., Cheng, Y.-L., and Adhikari, B. (2014). “Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp,” Carbohydr. Polym. 102, 136-143. DOI:10.1016/j.carbpol.2013.11.021
Li, R., Fei, J., Cai, Y., Li, Y., Feng, J., and Yao, J. (2009). “Cellulose whiskers extracted from mulberry: A novel biomass production,” Carbohydr. Polym. 76, 94-99. DOI:10.1016/j.carbpol.2008.09.034
Mishra, P. K., and Wimmer, R. (2016). “Aerosol assisted self-assembly as a route to synthesize solid and hollow spherical lignin colloids and its utilization in layer by layer deposition,” Ultrason. Sonochem. DOI:10.1016/j.ultsonch.2016.09.001
Morais, J. P. S., Rosa, M. de F., de Souza Filho, M. de sá M., Nascimento, L.D., do Nascimento, D. M., and Cassales, A. R. (2013). “Extraction and characterization of nanocellulose structures from raw cotton linter,” Carbohydr. Polym. 91, 229-235. DOI:10.1016/j.carbpol.2012.08.010
Mussatto, S. I., Dragone, G., Rocha, G. J. M., and Roberto, I. C. (2006). “Optimum operating conditions for brewer’s spent grain soda pulping,” Carbohydr. Polym. 64, 22-28. DOI:10.1016/j.carbpol.2005.10.033
Oksman, K., Mathew, A. P., Bismarck, A., Rojas, O., and Sain, M. (2014). Handbook of Green Materials: Processing Technologies, Properties and Applications (in 4 volumes), World Scientific.
Rosa, M. F., Medeiros, E. S., Malmonge, J. A., Gregorski, K. S., Wood, D. F., Mattoso, L. H. C., Glenn, G., Orts, W. J., and Imam, S. H. (2010). “Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior,” Carbohydr. Polym. 81, 83-92. DOI:10.1016/j.carbpol.2010.01.059
Russ, W., Mörtel, H., and Meyer-Pittroff, R. (2005). “Application of spent grains to increase porosity in bricks,” Constr. Build. Mater. 19, 117-126. DOI:10.1016/j.conbuildmat.2004.05.014
Segal, L., Creely, J. J., Martin, A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,” Text. Res. J. 29, 786-794. DOI:10.1177/004051755902901003
Spence, K. L., Venditti, R. A., Rojas, O. J., Habibi, Y., and Pawlak, J. J. (2011). “A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods,” Cellulose 18, 1097-1111. DOI:10.1007/s10570-011-9533-z
Wang, B., Sain, M., and Oksman, K. (2007). “Study of structural morphology of hemp fiber from the micro to the nanoscale,” Appl. Compos. Mater. 14, 89-103. DOI:10.1007/s10443-006-9032-9
Willför, S., Pranovich, A., Tamminen, T., Puls, J., Laine, C., Suurnäkki, A., Saake, B., Uotila, K., Simolin, H., Hemming, J., and Holmbom, B. (2009). “Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides–A comparison between different hydrolysis and subsequent chromatographic analytical techniques,” Ind. Crops Prod. 29, 571-580. DOI:10.1016/j.indcrop.2008.11.003
Zuluaga, R., Putaux, J. L., Cruz, J., Vélez, J., Mondragon, I., and Gañán, P. (2009). “Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features,” Carbohydr. Polym. 76, 51-59. DOI:10.1016/j.carbpol.2008.09.024
Article submitted: June 2, 2016; Peer review completed: October 22, 2016; Revised version received and accepted: November 3, 2016; Published: November 8, 2016.
DOI: 10.15376/biores.12.1.107-116
