Abstract
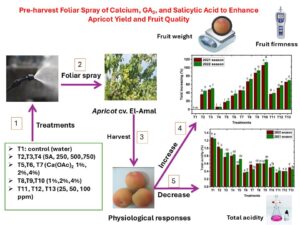
The impact of different pre-harvest foliar sprays was assessed relative to the yield and quality of apricot fruits (cv. El-Amal) under field conditions. Apricot trees were sprayed with various solutions, including salicylic acid (SA at 250 and 750 ppm), calcium acetate [Ca(OAc)₂ at 2% and 4%], calcium chloride (CaCl₂ at 2% and 4%), and gibberellic acid (GA₃ at 25 and 100 ppm) at the pit hardening growth stage before harvest. All foliar spray treatments positively affected fruit yield per tree compared to untreated plants. The most effective treatments were CaCl₂ at 4% and GA₃ at 100 ppm, followed by SA at 750 ppm and Ca(OAc)₂ at 4%. All treatments significantly increased fruit weight compared to the control group. GA₃ also significantly improved fruit firmness, outperforming all other treatments. Additionally, CaCl₂ at 2% and SA at 250 ppm resulted in higher firmness. SA at 750 ppm exhibited higher total soluble solid (TSS) content. While the foliar spray treatment without any solution resulted in the lowest fruit acidity, SA at 250 ppm had the highest acidity. In conclusion, pre-harvest foliar application of GA₃ (100 ppm), CaCl₂ (2%), and Ca(OAc)₂ (4%) can effectively enhance fruit yield and improve quality of apricots.
Download PDF
Full Article
Pre-harvest Foliar Spray of Calcium, GA3, and Salicylic Acid to Enhance Apricot Yield and Fruit Quality
Adel M. Al-Saif ,a,* Hosny F. Abdel-Aziz,b,* Abd El-wahed N. Abd El-wahed,b Sobhy M. Khalifa,b Ibrahim A. Elnaggar
,b Sania S El-Shershaby,d Mohammed H. Farouk,c Samah A. Abulmeaty,d Eman M. Hammad,d and Ashraf E. Hamdy
,b,*
The impact of different pre-harvest foliar sprays was assessed relative to the yield and quality of apricot fruits (cv. El-Amal) under field conditions. Apricot trees were sprayed with various solutions, including salicylic acid (SA at 250 and 750 ppm), calcium acetate [Ca(OAc)₂ at 2% and 4%], calcium chloride (CaCl₂ at 2% and 4%), and gibberellic acid (GA₃ at 25 and 100 ppm) at the pit hardening growth stage before harvest. All foliar spray treatments positively affected fruit yield per tree compared to untreated plants. The most effective treatments were CaCl₂ at 4% and GA₃ at 100 ppm, followed by SA at 750 ppm and Ca(OAc)₂ at 4%. All treatments significantly increased fruit weight compared to the control group. GA₃ also significantly improved fruit firmness, outperforming all other treatments. Additionally, CaCl₂ at 2% and SA at 250 ppm resulted in higher firmness. SA at 750 ppm exhibited higher total soluble solid (TSS) content. While the foliar spray treatment without any solution resulted in the lowest fruit acidity, SA at 250 ppm had the highest acidity. In conclusion, pre-harvest foliar application of GA₃ (100 ppm), CaCl₂ (2%), and Ca(OAc)₂ (4%) can effectively enhance fruit yield and improve quality of apricots.
DOI: 10.15376/biores.20.4.10504-10520
Keywords: Prunus armeniaca L.; Growth regulators; Yield; Vitamin C
Contact information: a: Department of Plant Production, College of Food and Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia; b: Department of Horticulture, Faculty of Agriculture, Al-Azhar University, Cairo, 11884, Egypt; c: Key Laboratory of Product Quality and Security, Ministry of Education, Jilin Agricultural University, Changchun, PR China; d: Department of Food Science and Technology, Faculty of Agriculture (for Girls), Al-Azhar University, 11884 Cairo, Egypt;
*Corresponding authors: adelsaif@ksu.edu.sa; ashrafezat@azhar.edu.eg
Graphical Abstract

INTRODUCTION
A climacteric fruit crop of significant economic importance on a global scale, apricots (Prunus armeniaca L.) are valued for their unique flavor, nutritional content, and adaptability in both fresh and processed forms (Bartolini and Andreini 2014). A major goal for apricot growers is to produce large quantities of fruit that can be sold. Fruit set, development, and quality characteristics like size, firmness, and postharvest shelf life can all be substantially impacted by several preharvest variables, such as environmental stressors, nutritional deficiencies, and hormonal imbalances (Ruiz et al. 2013). Effective pre-harvest techniques are therefore continuously needed to maximize apricot production (Crisosto and Kader 1999). For the best tree growth and fruit development, traditional orchard management techniques including fertilization and irrigation are crucial. However, to further increase yield and fruit quality, recent studies have increasingly concentrated on the potential of exogenous applications of plant growth regulators (PGRs) and other bioactive compounds. Salicylic acid (SA), calcium (Ca), calcium acetate [Ca(OAc)₂] and gibberellic acid (GA3) and have demonstrated potential as efficient pre-harvest treatments.
Salicylic acid (SA), a phenolic compound acting as a signaling molecule in plants, plays a critical role in regulating physiological processes, including stress tolerance, fruit ripening, and senescence (Hayat et al. 2010; Abd El-Naby, 2019; Asrey et al. 2023). Pre-harvest SA applications have been reported to enhance fruit firmness, total soluble solids (TSS), and antioxidant activity in various fruit crops (Abdel Wahab 2015; Shafiq et al. 2017). Acetylsalicylic acid-treated loquat fruit exhibited higher firmness retention at the initial harvesting period, and less changes in soluble solids content and titratable acidity values, thereby maintaining the sweet/acid equilibrium (Hadjipieri et al. 2021). Similarly, A pre-harvest spray of 2 mmol salicylic acid, applied two weeks before fruit harvest, yielded the best results for enhancing peach physical and chemical properties (Ismail et al. 2014; Salyari et al. 2022).
Calcium (Ca) is an essential nutrient involved in cell wall stabilization, membrane integrity, and enzyme activity (White and Broadley 2003). Pre-harvest calcium applications improve fruit firmness, delay softening, and extend shelf life by strengthening cell walls and limiting enzymatic degradation (Conway et al. 2002; Fallahi et al. 2010). Calcium chloride (CaCl₂) has been widely used to enhance these effects due to its high solubility and efficacy in increasing fruit firmness and reducing postharvest decay. Pre-harvest foliar applications of CaCl₂ (0.5 and 2%) at 72 and 74 days after full bloom improved the fruit quality of cvs. ‘Shahroudi’, ‘Dajie’, and ‘Canino’ apricots by increasing fruit size, weight, and firmness. These effects were most pronounced when CaCl₂ was applied at fruit set and approximately 10 days before harvest (Cui et al. 2020; Ennab et al. 2020; Moradinezhad and Dorostkar 2021; Okba et al. 2021; Liang et al. 2023).
Gibberellic acid (GA₃) regulates fruit development by promoting cell division and elongation, enhancing fruit size and weight, and delaying ripening (Agusti 2004; Davies 2010). In apricots, GA₃ has been shown to improve fruit color, size, and shelf life. Apricots cv. NS-4 treated with 200 ppm gibberellic acid (GA₃) at the early stage of sepal fall showed increased flesh firmness compared to untreated fruits (Khajehyar et al. 2014; Milović et al. 2022). Similarly, foliar application of GA₃ significantly enhanced the yield of apricot trees cv. ‘Zaghinia’. GA₃ at 75 mg L⁻¹ produced the highest fruit yield, weight, and volume, as well as total soluble solids and carotene content, while 25 mg L⁻¹ resulted in the lowest fruit acidity (Al-Janabi 2024). Preharvest foliar application of GA₃ at 0.005% produced the highest fruit yield, weight, and size in apricot cv. New Castle (Thakur et al. 2025).
Despite extensive research on the role of salicylic acid, calcium acetate, calcium chloride, and gibberellic acid in postharvest quality of various stone fruits, limited information is available on their comparative effects and optimal concentrations for enhancing fruit yield and quality in apricot trees, particularly the ‘El-Amal’ cultivar grown under Egyptian conditions. This lack of detailed understanding represents a significant gap in optimizing pre-harvest spray programs for this cultivar. Therefore, the present work aimed to fill this gap by evaluating the physiological and biochemical responses of ‘El-Amal’ apricot trees to different pre-harvest foliar applications. The novelty of this study lies in its integrated comparison of multiple bio-regulators and calcium sources applied at different concentrations and growth stages, providing the first comprehensive assessment of their combined influence on yield, fruit physical traits, and quality parameters in this locally important apricot cultivar.
EXPERIMENTAL
Experimental Site and Plant Material
The study was implemented across two continuous agricultural seasons, 2021 and 2022. The experimental site was a private orchard located in Wadi Elmollak, Ismailia Governorate, Egypt (30°35’N, 32°14’E, altitude). The annual rainfall and annual temperatures for the two studied seasons are given in Table 1. The region is characterized by a Mediterranean climate with an average annual temperature of 21.3 °C and an average annual rainfall of 26 mm. The plant material used in this study consisted of ten-year-old apricot trees (Prunus armeniaca L.), belonging to the “El-Amal” cultivar and grafted onto Balady rootstock. These trees were cultivated in sandy soil with a spacing of 5 × 5 m, equivalent to a planting density of 168 trees per feddan. Irrigation was applied via a drip system comprising two lateral lines per row, each tree receiving water through eight adjustable emitters with a flow rate of 8 L/h. The soil in the experimental area was characterized as sandy, with a composition of 94.72% sand.
Table 1. Climate Data for Wadi Elmollak, Ismailia Governorate, Egypt
Experimental Design and Treatments
The experimental treatments were arranged in blocks to minimize variability, and each treatment was applied to all blocks, ensuring full replication. The experiment consisted of 13 treatments, each treatment was replicated three times, and each replicate consisting of two trees. The treatments involved foliar applications of SA, Ca(OAc)2, CaCl2 and GA3 at varying concentrations. The specific treatments are shown in Table 2.
Table 2. Pre-harvest Foliar Sprays of ‘El-Amal’ Apricot Cultivar
The preparations of each chemical used in the pre-harvest foliar spray were as follows: Salicylic Acid (SA): To prepare the SA solution, the required amount of SA powder was dissolved in a small volume of ethanol and then diluted with distilled water to reach the final concentrations of 250, 500, and 750 ppm for T2, T3, and T4, respectively according to (Zahid et al. 2023). Calcium Acetate [Ca(OAc)₂]: The appropriate amount of Ca(OAc)₂ was dissolved directly in distilled water to prepare 1%, 2%, and 4% solutions for T5, T6, and T7, respectively. This preparation method is widely accepted in agricultural research for foliar applications. Calcium Chloride (CaCl₂): CaCl₂ powder was dissolved in distilled water to achieve 1%, 2%, and 4% solutions for T8, T9, and T10, respectively according to (Mazumder et al. 2021). Gibberellic Acid (GA₃): GA₃ powder was first dissolved in a small volume of 95% ethanol and then diluted with distilled water to obtain final concentrations of 25, 50, and 100 ppm for T11, T12, and T13, respectively. Control (T1): Distilled water only, serving as a baseline for comparison.
Foliar sprays were applied once at the pit hardening phenological stage, which occurred in the first week of May during both seasons. To enhance the efficacy of the foliar applications, polysorbate 20 (Tween 20) was added to each solution at a concentration of 0.1% (v/v) as a surfactant. Each tree was sprayed until it ran off using a hand sprayer with a capacity of 5 L/tree.
Yield Measurements
Fruit yield was determined at harvest, which took place on May 27th when fruits reached a yellowish-green color and a TSS content of 11%. Yield was recorded as the total weight of fruit harvested per tree (kg/tree), and total yield was calculated and expressed as tons per feddan. The percentage increase in yield for each treatment was calculated using the following Eq. 1:
Yield increase (%) = [(Yield treatment – Yield control) / Yield control] × 100 (1)
Fruit Physical Characteristics
At harvest, a random sample of 20 fruits was collected from each replicate (a total of 80 fruits per treatment) and transported to the laboratory of the Department of Horticulture, Faculty of Agriculture, Al-Azhar University, Cairo, for physical and chemical analyses. The following physical characteristics were measured:
• Fruit weight (g): Measured using an electronic balance.
• Fruit volume (cm3): Determined by water displacement.
• Fruit length and height (cm): Measured using a digital caliper.
• Fruit diameter (cm): Measured using a digital caliper.
• Pulp weight (g): Measured after separating the pulp from the seed.
• Seed weight (g): Measured after drying the seeds.
Fruit Chemical Characteristics
The following chemical characteristics of the fruit samples were determined.
Titratable acidity
Titratable acidity (%) was measured in 10 mL of fruit juice by titration with 0.1 N NaOH until the endpoint was reached, using phenolphthalein as an indicator. The acidity was expressed as a percentage of citric acid using the formula,
(2)
where is the volume of NaOH used (mL), is the normality of NaOH, and is the weight of fruit juice sample (g) the method described in A.O.A.C. (2000).
Total soluble solids
The TSS (%) was measured in 10 mL of fruit juice filtrate using a digital refractometer at room temperature, according to the method described in A.O.A.C. (2000).
Ascorbic acid (vitamin C)
Ascorbic acid content was determined in fruit juice (mg/100 mL) by direct titration with 2,6-dichlorophenolindophenol. The results were expressed as mg ascorbic acid per 100 g of fresh fruit using the following formula,
(3)
where V is the volume of 2,6-dichlorophenolindophenol solution used (mL), C is the concentration of DCPIP (mg/mL), W is the weight of fruit sample (g), according to the method described in A.O.A.C. (2000).
Statistical Analysis
The data collected for all parameters were subjected to statistical analysis using analysis of variance (ANOVA). Mean comparisons were performed using the Least Significant Difference (L.S.D.) test at a significant level of P ≤ 0.05. Statistical analyses were conducted using the Co-STAT software (version 4) according to (Stern 2023).
RESULTS AND DISCUSSION
Effect of Pre-Harvest Treatments on Apricot Tree Yield
The results of this study clearly demonstrated that pre-harvest foliar applications of GA3, SA, Ca(OAc)2, and CaCl2 significantly enhanced fruit yield per tree of the ‘El-Amal’ apricot cultivar compared to the untreated control. This finding underscores the potential of these treatments to improve apricot productivity. Particularly, a trend of increasing yield with higher concentrations of the applied substances was observed. The most effective treatment for enhancing yield was CaCl2 at 4%, followed by 2% and 1% levels, and GA3 at 100 ppm. In contrast, the control trees consistently exhibited the lowest yield across both seasons. These results align with recent studies demonstrating the positive effects of GA₃ on yield in various stone fruit crops. González-Villagra et al. (2024) reported a 9% increase in fruit yield following SA application in Prunus avium L. Similarly, Attia et al. (2022) observed enhanced fruit weight and size in apricots treated with SA and Ca(OAc)₂. Mazumder et al. (2021) found that preharvest foliar application of CaCl₂ increased fruit weight and yield. Similarly, Shahid et al. (2020) found that foliar application of calcium chloride significantly improved peach fruit quality and yield. The most favorable results were generally observed with a 2% solution applied at the pit hardening stage. The primary effects of our calcium treatments are a direct consequence of the physiological and biochemical activities expected of a divalent alkaline earth metal ion. The Ca²⁺ ion acts both structurally, by cross-linking cell wall components, and functionally, as a central node in cellular signaling networks. The formation of insoluble calcium salts is a competing process that can limit bioavailability, and thus the efficacy of a treatment is dependent on the applied form and its ability to deliver soluble Ca²⁺ to key cellular sites Xu et al. (2022).
The increase in yield due to GA₃ treatment can be attributed to its role in stimulating cell division and enlargement, resulting in an enhancement of fruit weight. Furthermore, GA₃ can create a strong sink effect. This is a physiological consequence of GA₃-induced growth, driven by hormonal signaling that alters gene expression and metabolic activity, rather than a property deducible from its atomic composition in fruit cells, attracting water and nutrients and further contributing to yield improvement Gupta et al. (2022).
Fruit Weight and Fruit Volume
The results illustrated in Fig. 2 (a and b) demonstrate that various pre-harvest applications significantly influenced both the weight and volume of the fruits, as also reflected in Fig. 1 (a and b). Among the treatments, gibberellic acid (GA₃) at 100 ppm yielded the heaviest fruits, followed in effectiveness by salicylic acid (SA) at 750 ppm and calcium acetate [Ca(OAc)₂] at a 4% concentration. All treatment groups, except for the untreated control, exhibited notable enhancements in fruit weight, with the control producing the lightest fruits. In a similar trend, fruit volume was markedly increased by both GA₃ at 100 ppm and [Ca(OAc)₂] at 4%, while the control again recorded the lowest values. The observed improvements in fruit weight and volume following GA₃ application are consistent with the findings of recent studies. Elmenofy et al. (2021) reported that GA₃ treatments led to increased fruit size and weight in apricot trees. The growth-promoting effect of GA₃ is likely due to its stimulation of cell elongation and expansion, as well as its role in enhancing cell wall elasticity and promoting starch breakdown into sugars, leading to decreased cellular water potential. The beneficial effect of SA observed in this study corresponds with findings by González-Villagra et al. (2023), who reported improved fruit weight and quality in apricot following SA application. This enhancement may be attributed to SA’s role in boosting physiological productivity, particularly through the stimulation of photosynthetic pigments and activity. Likewise, the improvement in fruit weight associated with calcium treatment is well-supported by prior studies. Research by Wu et al. (2025) indicated that calcium chloride treatments led to greater fruit weight in plums. Furthermore, Correia et al. (2020) found that foliar applications of GA₃, SA, and calcium compounds collectively improved physiological responses and yield in sweet cherry trees. These results suggest that the application of GA₃, SA, and calcium compounds can effectively enhance apricot fruit yield and quality. The combined use of these treatments may offer synergistic benefits, leading to improved fruit size, weight, and overall quality characteristics. Research by Wood et al. (2024) indicated that calcium chloride treatments led to greater fruit weight in apples.
Fruit Length and Fruit Diameter
Results in Figs. 2c and 2d showed that foliar treatments of GA3, particularly at 100 and 50 ppm, significantly increased fruit length and diameter. Ca(OAc)2 at 4% and CaCl2 at 1% also showed positive effects on these parameters. However, no significant differences were observed between other treatments, and the control group exhibited the lowest values for both fruit length and diameter. The primary hormonal effect of GA₃ is the promotion of growth. This is achieved through a well-defined signaling pathway where GA₃ binds to its GID1 receptor, leading to the degradation of DELLA repressor proteins. The removal of DELLAs unleashes the transcription of genes encoding cell wall-loosening enzymes including expansions and xyloglucan endotransglucosylase/hydrolases. This process increases cell wall extensibility, facilitating water uptake and cell elongation, which directly explains the observed increase in fruit dimensions (Gupta et al. 2022). Furthermore, GA₃ can promote cell division in the pericarp during early fruit development, contributing to a larger final fruit size. The significant increase in fruit size (both in length and diameter) following Gibberellic acid (GA₃) treatments aligns with established physiological mechanisms and is strongly supported by recent research on various stone fruits. This growth promotion is primarily attributed to GA₃’s fundamental role in stimulating cell elongation and expansion. Richard (2006) explained that GA₃ promotes growth by increasing cell wall plasticity and facilitating the conversion of starch into sugars, which osmotically enhances water uptake and drives cell elongation. A study on the ‘September Sun’ peach cultivar demonstrated that foliar applications of GA₃ increased fruit diameter, weight and improved marketable yield (Silva et al. 2021). Similarly, a study on Japanese plums found that GA₃ application was crucial for achieving optimal fruit size and quality by directly influencing the cell division and expansion phases post-pollination, thus increasing mesocarp cell volume (Reyes et al. 2022). Furthermore, the efficacy of GA₃ is influenced by application timing, as evidenced in sweet cherries, where specific phenological stages are more responsive to treatment, maximizing cell division and ultimately final fruit size (Zhang et al. 2023).
Fruit Firmness
Results in (Fig. 3a) showed that GA3 treatments at 100, 50, and 25 ppm led to marked improvements in fruit firmness, surpassing the effects of all other applications. CaCl2 at 2% and SA at 250 ppm also resulted in higher firmness. In contrast, SA at 750 and 500 ppm led to lower fruit firmness. The observed increase in fruit firmness following Gibberellic acid (GA₃) application is a well-documented phenomenon, consistent with foundational studies on plums and strongly supported by recent research. While early work by Hassan et al. (2010) established this effect, contemporary findings by Khan et al. (2024) confirm that GA₃ application positively regulates fruit firmness in peaches. The physiological mechanism underpinning this effect is attributed to GA₃’s role in modulating cell wall-degrading enzymes. Specifically, GA₃ is understood to suppress the activity of polygalacturonase and pectin methylesterase, which are key enzymes responsible for the breakdown of pectin and the subsequent softening of the fruit cell wall, a concept initially suggested by Webster et al. (2006). This enzymatic inhibition helps maintain structural integrity during fruit development and postharvest life.
The positive impact of calcium treatments on fruit firmness remains a cornerstone of postharvest science. The foundational work of Samara et al. (2008), demonstrating that CaCl₂ treatment maintained maximum firmness in peaches, has been corroborated by recent findings on plum. Wu et al. (2025) reported that pre-harvest calcium chloride sprays significantly reduced softening and improved the storage quality of ‘Fengtangli’ plum by reinforcing the cell wall matrix. The efficacy of calcium stems from its role in forming calcium pectate cross-bridges within the middle lamella. This enhances cell-to-cell adhesion and acts as a structural barrier against the enzymatic degradation that leads to fruit softening. The hormonal action of GA₃ directly interferes with the ripening program. Ripening is coordinated by a shift in hormone balance, particularly a rise in ethylene and a decline in auxins and gibberellins. By applying GA₃, a “youthful” hormonal status was exogenously maintained. This inhibits the expression and activity of key cell wall-degrading enzymes such as polygalacturonase and pectin methylesterase, thus preserving pectin structure and maintaining firmness (Milović et al. 2022). This delay in the ripening cascade also explains the observed modulation in TSS and acidity, as the metabolic conversion of starch to sugars and the respiration of organic acids are postponed.
Seed Weight
The data presented in Fig. 3b indicates that fruits treated with Ca(OAc)₂ exhibited significantly greater seed weight compared to those treated with salicylic acid (SA) at 250 ppm and gibberellic acid (GA₃) at 25 ppm. No statistically significant difference in seed weight was observed between the Ca(OAc)₂ treatment and the untreated control group. The superior seed weight associated with calcium acetate treatment can be attributed to calcium’s role in cell division and membrane stability. Calcium is a crucial secondary messenger involved in numerous signal transduction pathways that govern plant growth and development (He et al. 2023). During seed development, adequate calcium is essential for mitotic activity and the formation of new cells in the developing embryo and endosperm (Konrad et al. 2021). By providing a readily available calcium source, the Ca(OAc)₂ treatment likely supported sustained cellular proliferation and structural integrity within the seed, leading to greater final mass. In contrast, the primary modes of action for SA and GA₃ are less directly tied to seed mass accumulation. Salicylic acid is predominantly involved in stress response and defense signaling, which may divert energy resources away from reproductive growth (Rai et al. 2022). While gibberellic acid is critical for fruit set and cell expansion within the mesocarp, its impact on seed development can be variable and species-specific; in some cases, it may even promote parthenocarpic fruit development where seeds are absent or underdeveloped (Gomez et al. 2023). The lack of a significant difference between the calcium treatment and the control suggests that the inherent calcium levels in the control plants were sufficient to meet the basic requirements for seed development but were not optimal. The Ca(OAc)₂ application provided a supplemental boost, while the SA and GA₃ treatments, at the concentrations applied, did not enhance this particular trait and may have indirectly limited the resource allocation to seed fill.
Effect of Pre-harvest Treatments on Apricot Fruit Chemical Properties
Total Soluble Solids
As shown in Fig. 4a, the foliar application of the control and SA at 750 ppm led to higher TSS levels compared to the other treatments. In contrast, no statistically significant differences were observed among the remaining treatments. The lowest TSS values were recorded with SA at 250 ppm, followed by Ca(OAc)₂ at 2%. The present findings align with the established phenomenon that GA₃ can delay ripening, as documented by Elshazly et al. (2013) in peaches, where GA₃ treatments resulted in a decline in TSS. This reduction is likely attributable to a GA₃-induced postponement of the ripening process, which delays the comprehensive hydrolysis of starch and other polysaccharides into simple sugars that constitute TSS (Khan et al. 2024). In contrast, the potential of organic acids to enhance TSS, as observed by El Badawy (2013) for ascorbic and citric acids, finds support in recent research. Foliar applications of such compounds are understood to enhance photosynthetic efficiency and chlorophyll stability, thereby increasing the total photoassimilate pool available for translocation and accumulation in the fruit as sugars (Zahid et al. 2023).
The interaction between ripening delay and TSS accumulation is further clarified by considering the role of calcium. The fluctuations in TSS following applications of GA₃ and calcium salts, as historically reported by Kirmani et al. (2013), can be explained by calcium’s function in preserving membrane and cell wall integrity. As demonstrated in plums, calcium treatments significantly reduce water loss and slow down the degradation of cell wall components (Wu et al. 2025).
Titratable Acidity
The data revealed that the untreated control group exhibited the lowest level of fruit titratable acidity (%), whereas the highest acidity was observed in fruits treated with SA at a concentration of 250 ppm (Fig. 4b). No statistically significant differences were detected among the other treatment groups. This finding is consistent with the long-established principle that calcium treatments can mitigate this decline, a phenomenon initially documented by Drake and Spayd (1983) in apple, where CaCl₂ application led to increased titratable acidity. Calcium plays a dual role in preserving acidity by forming complexes with pectin in the middle lamella. Calcium enhances cellular structure integrity, reduces membrane permeability, and thereby limits the inter-mixing of substrates and enzymes necessary for acid degradation (Mazumder et al. 2021). Moreover, calcium ions are known co-factors for key enzymes involved in the regulation of respiration, and their sufficiency can help maintain a slower, more stable metabolic rate, thus delaying the consumption of organic acids (Wu et al. 2025). Consequently, the application of calcium salts effectively slows the postharvest senescence process, directly contributing to the retention of organic acids and a higher measurable titratable acidity, which is a critical component of fruit flavor and sensory quality.
TSS/Acidity Ratio
According to the results, the highest total soluble solids to acidity (TSS/acidity) ratio was recorded in fruits treated with GA₃ at 25 ppm, indicating a more advanced ripening stage or enhanced sugar accumulation. In contrast, the lowest ratio was found in the untreated control and the SA treatment at 250 ppm (Fig. 4c). The differences among the remaining treatments were not statistically significant.
The hormonal effect of GA₃ on fruit biochemistry is largely indirect, stemming from its role as a ripening retardant. The slower metabolic rate in GA₃-treated fruits results in a delayed accumulation of soluble solids and a slower degradation of titratable acids. This is consistent with the role of GA₃ in maintaining a pre-climacteric physiological state, thereby extending the period of acid retention and slowing sugar concentration (Elshazly et al. 2013; Khan et al. 2024).
CONCLUSIONS
- The findings of this study demonstrated that pre-harvest foliar applications of calcium (as CaCl2 and Ca(OAc)2), gibberellic acid (GA3), and salicylic acid (SA) can effectively improve fruit yield and fruit quality of the El-Amal apricot cultivar.
- GA3 at 100 ppm, CaCl2 at 2%, and Ca(OAc)2 at 4% were the most effective treatments for increasing yield and enhancing fruit quality.
- The results provide valuable insights for optimizing apricot production practices and enhancing the marketability of ‘El-Amal’ apricots. Specifically, the foliar application of gibberellic acid (GA₃) at 50 ppm is recommended to significantly increase fruit size and firmness, key drivers of consumer preference and market value. Furthermore, the application of calcium acetate (Ca(OAc)₂) offers a dual benefit: it further enhances fruit firmness for improved postharvest handling. By defining the optimal concentrations and expected outcomes for these phytoregulators, this study provides a clear, science-based protocol for growers to improve both the yield and quality of ‘El-Amal’ apricots, thereby increasing profitability.
ACKNOWLEDGMENTS
The authors extend their appreciation to Ongoing Research Funding Program (ORF-2025-334), King Saud University, Riyadh, Saudi Arabia.
REFERENCES CITED
A. O. A. C. (2000). “Official methods of analysis,” Association of Official Analytical Chemists, 17th Ed, Arlington, VA, USA.
Abd El-Naby, S. K. A. (2019). “Physiological responses of ‘Ceglsa’ olive seedlings to foliar spray with salicylic acid and putrescine under salinity stress conditions,” Middle East Journal of Agriculture Research 8(1), 278-291.
Abdel Wahab, W. M. (2015). “Effect of pre-harvest salicylic acid spray on yield and fruit quality of Canino apricot,” Alexandria Journal of Agricultural Research 60(1), 11-21.
Agusti, M. (2004). “Gibberellins and fruit set in citrus,” Plant Growth Regulation 42(1), 61-69.
Al-Janabi, A. M. I. (2024). “Impact of gibberellic acid and boron trioxide nanoparticles foliar spraying on growth, yield and fruit quality of ‘Zaghinia’ apricot trees,” Anbar Journal of Agricultural Sciences 22(2).
Asrey, R., Sharma, S., Barman, K., Prajapati, U., Negi, N., and Meena, N. K. (2023). “Biological and postharvest interventions to manage the ethylene in fruit: A review,” Sustainable Food Technology 1(6), 803-826. DOI: 10.1039/d3fb00037k
Bartolini, S., and Andreini, L. (2014). “Influence of rootstock on fruit entity, quality and antioxidant properties of fresh apricots (cv. ‘Pisana’),” New Zealand Journal of Crop and Horticultural Science 42(4), 265-274. DOI: 10.1080/01140671.2014.894919
Conway, W. S., Sams, C. E., and Miller, A. R. (2002). “Effects of calcium on plant disease and fruit quality,” HortScience 37(3), 510–520. DOI: 10.5958/2249-5258.2016.00048.8
Correia, S., Pinto, P., Monteiro, A., Ribeiro, J. M., Batista, J. L., Rodrigues, A. M., and Lidon, M. A. (2020). “Foliar sprays of gibberellic acid, salicylic acid and calcium improve the physiological performance and yield of sweet cherry trees,” Agronomy 10(9), article 1247. DOI:10.3390/agronomy10091247
Crisosto, C. H., and Kader, A. A. (1999). Apricot: Recommendations for Maintaining Postharvest Quality, University of California, Agriculture and Natural Resources.
Cui, K., Shu, C., Zhao, H., Fan, X., Cao, J., & Jiang, W. (2020). Preharvest chitosan oligochitosan and salicylic acid treatments enhance phenol metabolism and maintain the postharvest quality of apricots (Prunus armeniaca L.). Scientia Horticulturae 267, article 109334.
Davies, P. J. (2010). Plant Hormones: Biosynthesis, Signal Transduction, Action, Springer Science and Business Media. DOI: 10.1007/978-1-4020-2686-7
Drake, S. R., and Spayed, S. M. (1983). “Effects of calcium chloride treatment on the firmness and storage life of Stark Delicious apples,” Journal of Food Science 48(2), 403-405. DOI:10.1111/j.1365-2621.1983.tb10752.x
El‑Badawy, H. E. M. (2013). “Effect of some antioxidants and micronutrients on growth, leaf mineral content, yield and fruit quality of Canino apricot trees,” Journal of Applied Sciences Research 9(2), 1228-1237.
Elmenofy, H. M., Mazrou, Y. S. A., Elmenofy, H. M., and Salama, A. (2021). “Yield, fruit quality, and storability of ‘Canino’ apricot in response to aminoethoxyvinyl-glycine, salicylic acid, and chitosan,” Horticulturae 7(12), article 531. DOI: 10.3390/horticulturae7120531
Elshazly, S. M., Meligy, M. M., Abdal, A. M., and El-Sayed, A. A. (2013). “Effect of pre-harvest spray with gibberellic acid and urea on yield and fruit quality of Canino peach trees,” J. Hortic. Sci. Ornamental Plants 5(1), 66-71. DOI: 10.4236/ajps.2016.77098
Ennab, H. A. E. F., Abd El-Aziz, M. H., and Soliman, M. A. (2020). “Pre-harvest treatments on Canino apricot trees to improve yield, fruit quality at harvest and during storage,” Journal of Plant Production 11(12), 1633-1640.
Fallahi, E., Nejatzadeh, M. A., and Fallahi, M. (2010). “A review of the influence of preharvest calcium and magnesium applications on postharvest quality of pome and stone fruits,” Stewart Postharvest Review 6(3), 1-18. DOI:10.2212/spr.2010.3.2
Gomez, M. L., Tognetti, J. A., and Botto, J. F. (2023). “Interplay between gibberellins and other hormones in the regulation of fruit set and seed development,” Journal of Plant Growth Regulation 42(5), 3120-3135. DOI: 10.1007/s00344-022-10778-z
González-Villagra, J., Bravo, L. A., Reyes-Díaz, M., Cohen, J. D., Ribera-Fonseca, A., López-Olivari, R., Jorquera-Fontena, E., and Tighe-Neira, R. (2023). “Pre-harvest salicylic acid application affects fruit quality and yield under deficit irrigation in Aristotelia chilensis (Mol.) Plants,” Plants 12(18), article 3279. DOI: 10.3390/plants12183279
González-Villagra, J., Chicahual, C., Jorquera-Fontena, E., Falquetto-Gomes, P., Nunes-Nesi, A., and Reyes-Díaz, M. (2024). “Salicylic acid improves yield, fruit quality, and post-harvest storage in sweet cherry (Prunus avium L.) cv. Lapins subjected to late-deficit irrigation,” Horticulturae 10(7), 707. DOI: 10.3390/horticulturae10070707
Gupta, A., Singh, S., and Pandey, A. (2022). “Gibberellic acid signaling: A key regulator of plant growth and development,” Plant Physiology and Biochemistry 170, 273-283. DOI: 10.1016/j.plaphy.2021.12.013
Hadjipieri, M., Georgiadou, E. C., Drogoudi, P., Fotopoulos, V., and Manganaris, G. A. (2021). “The efficacy of acetylsalicylic acid, spermidine and calcium preharvest foliar spray applications on yield efficiency, incidence of physiological disorders and shelf-life performance of loquat fruit,” Scientia Horticulturae 289, article 110439. DOI: 10.1016/j.scienta.2021.110439
Hayat, Q., Hayat, S., Irfan, M., and Ahmad, A. (2010). “Effect of exogenous salicylic acid under changing environment: A review,” Crop Research 40(1–3), 1-38. DOI:10.1016/j.envexpbot.2009.08.005
He, L., Li, Y., and Liu, X. (2023). “Calcium signaling in plant development and stress adaptation,” Plant Cell Reports 42(8), 1287-1304. DOI:10.1007/s00299-023-03030-9
Ismail, E. A., Hussien, S. M., and Abou Fatma, I. G. (2014). “Studies on improving fruit yield and quality of peach cv. ‘Early Swelling’,” Egyptian Journal of Horticulture 41(1), 83-95. DOI: 10.21608/ejoh.2014.1355
Khajehyar, R., Rahemi, M., and Fallahi, E. (2015). “The impact of various rates and dates of gibberellic acid applications on fruit set in apricot,” International Journal of Fruit Science 15(3), 324–338. DOI: 10.1080/15538362.2015.1016381
Khan, M. J., Sakhi, S., Azam, N., Ahmad, Z., Hadayat, N., Ahmad, B., and Gaafar, A. R. Z. (2024). “Effect of chitosan and gibberellic acid on fruit yield and production of peach (Prunus persica L.),” Polish Journal of Environmental Studies 33(1), 721-726. DOI: 10.15244/pjoes/171982
Kirmani, S., Wani, G. M., Wani, M. S., and Malik, A. R. (2013). “Effect of preharvest application of calcium chloride (CaCl₂), gibberellic acid (GA₃) and naphthalene acetic acid (NAA) on storage of plum (Prunus salicina L.) cv. Santa Rosa under ambient storage conditions,” African Journal of Agricultural Research 8(9), 812-818. DOI: 10.5897/AJAR12.1708
Konrad, K. R., Wudick, M. M., and Feijó, J. A. (2021). “Calcium regulation of tip growth: New genes for old mechanisms,” Current Opinion in Plant Biology 64, article 102101. DOI: 10.1016/j.pbi.2021.102101
Liang, C., Cui, X., Sun, C., Ye, S., Huang, N., Chen, R., and Li, X. (2023). “Synergistic and antagonistic effects of preharvest salicylic acid and postharvest 1-methylcyclo-propene treatments on the storage quality of apricot,” Food Chemistry 405, article 134764. DOI: 10.1016/j.foodchem.2022.134764
Mazumder, M. N. N., Misran, A., Ding, P., Wahab, P. E. M., and Mohamad, A. (2021). “Preharvest foliar spray of calcium chloride on growth, yield, quality, and shelf life extension of different lowland tomato varieties in Malaysia,” Horticulturae 7(11), article 466. DOI: 10.3390/horticulturae7110466
Milović, M., Kevrešan, Ž., Mastilović, J., Kovač, R., Kalajdžić, J., Magazin, N., Bajić, A., Milić, B., Barać, G., and Keserović, Z. (2022). “Could an early treatment with GA and BA impact prolonged cold storage and shelf life of apricot?,” Horticulturae 8(12), article 1220. DOI: 10.3390/horticulturae8121220
Moradinezhad, F., and Dorostkar, M. (2021). “Pre-harvest foliar application of calcium chloride and potassium nitrate influences growth and quality of apricot (Prunus armeniaca L.) fruit cv. ‘Shahroudi’,” J. Soil Sci. Plant Nutr. 21, 1642-1652. DOI: 10.1007/s42729-021-00468-2
Okba, S. K., Mazrou, Y., Elmenofy, H. M., Ezzat, A., and Salama, A. M. (2021).
“New insights of potassium sources impacts as foliar application on ‘Canino’ apricot fruit yield, fruit anatomy, quality and storability,” Plants 10(6), article 1163. DOI: 10.3390/plants10061163
Rai, K. K., Pandey, N., and Rai, S. P. (2022). “Salicylic acid and nitric oxide-mediated signaling in plants under stress tolerance,” Physiologia Plantarum 174(2), article e13639. DOI: 10.1111/ppl.13639
Reyes, M., Crawford, S., and Alvaro, J. E. (2022). “Timing of gibberellic acid application modulates fruit development and quality in ‘Angeleno’ plum,” Scientia Horticulturae 295, article 110881. DOI: 10.1016/j.scienta.2021.110881
Richard, M. (2006). How to Grow Big Peaches, Dept. of Horticulture, Virginia Tech, Blacksburg, VA, USA. Available at: www.rce.rutgers.edu, 8 pages, August.
Ruiz, D., Egea, J., García-Brunton, E., Marsal, J., and Dicenta, F. (2013). Apricot (Prunus armeniaca L.): Breeding, Production and Physiology, CABI Publishing.
Salyari, R., Seifi, E., Varasteh, F., and Alizadeh, M. (2022). “Effects of pre-harvest salicylic acid treatment on the post-harvest quality of peach cultivar ‘Robin’,” Journal of Chemical Health Risks 12(3), 355-362. DOI: 10.22034/jchr.2021.1875114.1041
Samara, N. R., Mansour, A. M., Touky, M. N., and Tarabih, M. E. (2008). “Pre and postharvest treatments on peach fruits grown under desert conditions,” J. Agric. Sci. Mansoura Univ. 31(12), 7835-7846. DOI: 10.21608/jpp.2006.236422
Shafiq, M., Khan, M. R., Ahmad, M., Hameed, A., Ashraf, M. Y., and Waseem, M. (2017). “Salicylic acid-induced changes in growth, yield, and fruit quality of tomato (Lycopersicon esculentum L.) under water deficit conditions,” Front. Plant Sci. 8, article 1944. DOI: 10.3389/fpls.2017.01944
Shahid, M. O., Muhmood, A., Ihtisham, M., Rahman, M. U., Amjad, N., Sajid, M., Riaz, K., and Ali, A. (2020). “Fruit yield and quality of ‘Florida King’ peaches subjected to foliar calcium chloride sprays at different growth stages,” Acta Scientiarum Polonorum Hortorum Cultus 19(1), 131-139. DOI: 10.24326/asphc.2020.1.12
Silva, R. R., Damiano, C., and Gianfra, L. (2021). “Effects of gibberellic acid on fruit quality, yield, and phytochemical properties of ‘September Sun’ peach,” Journal of Plant Growth Regulation 40(4), 1452-1462. DOI: 10.1007/s00344-021-10374-7
Stern, R. D. (2023). Statistical Methodology, Statistical Services Centre, University of Reading.
Thakur, N., Singh, G., Sharma, D. P., and Sharma, U. (2025). “Fruiting and physicochemical–biochemical characteristics of apricot (Prunus armeniaca) cultivar ‘New Castle’ as influenced by foliar application of antioxidants and phytoregula-tors,” Agricultural Research 14(1), 121-129. DOI: 10.1007/s40003-024-00747-1
Webster, A. D., Spencer, J. E., Dover, C., and Atkinson, C. J. (2006). “The influence of sprays of gibberellic acid (GA₃) and aminoethoxyvinylglycine (AVG) on fruit abscission, fruit ripening and quality of two sweet cherry cultivars,” Acta Horticulturae 727, 467-472. DOI: 10.17660/ActaHortic.2006.727.57
White, P. J., and Broadley, C. L. (2003). “Calcium in plants,” Ann. Bot. 92(4), 487-511. DOI: 10.1093/aob/mcg164
Wood, R. M., de Freitas, S. T., Argenta, L. C., and Neuwald, D. A. (2024). “The influence of pre‑harvest calcium application on the concentration and distribution of ascorbic acid and mineral content in apple cultivars at harvest and during storage,” Postharvest Biology and Technology 214, article 112979. DOI: 10.1016/j.postharvbio.2024.112979
Wu, Y., Dong, X., Zhu, Y., and Chen, H. (2025). “Influence of pre-harvest sprays of calcium chloride on fruit storage quality and softening of ‘fengtangli’ (Prunus salicina L.) plum,” CyTA – Journal of Food 23(1), article 2446834. DOI: 10.1080/19476337.2025.2446834
Xu, T., Niu, J., and Jiang, Z. (2022). “Sensing mechanisms: Calcium signaling mediated abiotic stress in plants,” Frontiers in Plant Science 13, article 925863. DOI: 10.3389/fpls.2022.925863
Zahid, A., Razzaq, A. A., Munawar, M., Ramzan, M., Almutairi, B. O., and Almutairi, M. H. (2023). “Foliar spray of salicylic acid and ascorbic acid ameliorates the biochemical compounds in hybrid chillies,” Journal of King Saud University – Science 35(5), article 102660. DOI: 10.1016/j.jksus.2023.102660
Zhang, L., Wang, P., and Johnson, K. (2023). “Optimizing gibberellin application timing to enhance fruit size and firmness in ‘Bing’ sweet cherry,” HortScience 58(2), 215-222. DOI: 10.21273/HORTSCI16988-22
Article submitted: July 26, 2025; Peer review completed: October 5, 2025; Revised version received and accepted: October 7, 2025; Published: October 21, 2025.
DOI: 10.15376/biores.20.4.10504-10520
Erratum: November 9, 2025; The following has been changed in the paper, but results are not influenced by these changes: Figure captions (Figs 1-4), T9: CaCl2 2%
