Abstract
The freshness and safety of fruits and vegetables is important in our daily life. Paper products are often used for shipping, wrapping, and decoration in the retail for fruits and vegetables. When these paper products are modified with active substances, they can offer additional functions other than just packaging. Thus, introducing 1-methylcyclopropene (1-MCP) into paper products can impart a preservation function for fruits and vegetables. 1-MCP is an excellent and eco-friendly inhibitor of ethylene that can effectively retard the ripening of fruits and vegetables. This article reviews the ripening process induced by ethylene, the inhibition mechanism of 1-MCP, and the existing technologies and products for 1-MCP utilization. Novel active paper packaging products via the use of encapsulated 1-MCP complexes may have a great potential for commercialization. Such packaging containing 1-MCP active paper could be effective in prolonging the shelf-life and improving the quality of the product during the storage, shipping process, and retail market, and can be attractive economically, socially, and environmentally.
Download PDF
Full Article
1-Methylcyclopropene (MCP)-Containing Cellulose Paper Packaging for Fresh Fruit and Vegetable Preservation: A Review
Zhijun Hu,a,c Chunxia Tang,b,c Zhibin He,c Jiang Lin,a and Yonghao Ni c,*
The freshness and safety of fruits and vegetables is important in our daily life. Paper products are often used for shipping, wrapping, and decoration in the retail for fruits and vegetables. When these paper products are modified with active substances, they can offer additional functions other than just packaging. Thus, introducing 1-methylcyclopropene (1-MCP) into paper products can impart a preservation function for fruits and vegetables. 1-MCP is an excellent and eco-friendly inhibitor of ethylene that can effectively retard the ripening of fruits and vegetables. This article reviews the ripening process induced by ethylene, the inhibition mechanism of 1-MCP, and the existing technologies and products for 1-MCP utilization. Novel active paper packaging products via the use of encapsulated 1-MCP complexes may have a great potential for commercialization. Such packaging containing 1-MCP active paper could be effective in prolonging the shelf-life and improving the quality of the product during the storage, shipping process, and retail market, and can be attractive economically, socially, and environmentally.
Keywords: Preservation; Active paper packaging; Fruit preservation; Ethylene; 1-MCP; 1-MCP containing paper
Contact information: a: Zhejiang Province Collaborative Innovation Center of Agricultural Biological Resources Biochemical Manufacturing, Zhejiang University of Science and Technology, Hangzhou, 310023, China; b: College of Chemical and Biological Engineering, Changsha University of Science and Technology, Changsha 410004, China; c: Limerick Pulp and Paper Centre, Department of Chemical Engineering, University of New Brunswick, Fredericton, New Brunswick E3B 5A3, Canada;
* Corresponding author: yonghao@unb.ca
INTRODUCTION
Harvested fruits and vegetables are highly perishable agricultural commodities. The total loss can be massive as a consequence of poor post-harvest handling and the subsequent transport processes (Sharma et al. 2009). Related losses could turn into billions of dollars worldwide (Bapat et al. 2010). Moreover, the decay of fresh fruits and vegetables will produce pathogenic microorganisms that pose a hazard to human health (Beuchat 1996).
For a long time, the preservation of harvested fruits and vegetables largely has relied on synthetic chemical fungicides and low temperature storage (Eckert and Ogawa 1988). Synthetic chemicals can end up in the food chain and be directly available to consumers; this is potentially harmful to human health. Chemical food preservatives are responsible for occasional allergic reactions in sensitive individuals; thus, interest in antimicrobial compounds found in nature and the demand from consumers have recently increased (Corbo et al. 2010).
Increasing public demands for the improved safety and quality of fruits and vegetables in the fresh market call for novel technologies without the use of synthetic chemicals, leading to the development of some physical methods, such as ultraviolet light (UV-C) (Rameshkumar et al. 2012), ionizing-irradiation (Mostafavi et al. 2013), and biological control methods (Bautista-Baños et al. 2006), as well as active packaging (AP) (McMillin et al. 1999), and Modified Atmosphere Packing (MAP) (Gonzalez-Aguilar et al. 2003). Among these methods, AP has significant advantages.
By definition, active packaging is an intelligent or smart system that involves interactions between the package or package components and food or internal gas atmosphere. Such systems have potential to meet consumer demands for high quality, fresh-like, and safe products. Important active packaging systems currently known to date include oxygen scavengers, carbon dioxide emitters, moisture absorbers, ethylene absorbers/inhibitors, ethanol emitters, flavor releasing systems, and antimicrobial containing layers (Labuza and Breene 1989; Ozdemir and Floros 2004), of which, ethylene absorbers/inhibitors have received much attention. Ethylene (C2H4) acts as a plant hormone that has physiological effects on fresh fruit and vegetables. It accelerates respiration, leading to maturity and senescence and the softening and ripening of fruits/vegetables (Rooney 1995). Furthermore, ethylene accumulation can cause the yellowing of green vegetables and may be responsible for a number of specific postharvest disorders in fresh fruits and vegetables (Vermeiren et al. 1999). Hence, the removal/inhibition of ethylene gas in the package headspace is an effective method for the preservation of fruits and vegetables.
There are a number of methods that inhibit ethylene for the preservation of fruits and vegetables. 1-Methylcyclopropene (1-MCP) is an innovative AP agent for this purpose. 1-MCP is a gas at room temperature and pressure. It has been continually attracting research/commercial interest because of its effective, environmental, and non-toxic inhibition of ethylene perception at extremely low concentrations (Sisler 2006). The product was approved by the United States Environmental Protection Agency (EPA) in 1999 for ornamentals and is sold under the trade name EthylBloc (Blankenship and Dole 2003).
Paper packaging products are popular in our daily life, because they are cost- effective; in addition, paper products are green in view of their natural sourcing and easy recyclability (Liu et al. 2012; Zaman et al. 2012). Cellulose paper can also be chemically modified through the functional groups, such as the hydroxyl groups in the glucose units to expand their applications. Paper products are currently used for shipping, packaging, and wrapping/decoration for fruits/vegetables in the retail market, as shown in Fig. 1. Some of these paper products may be paraffined to avoid the high humidity environment, which is critical for their applications. Unlike plastics, such as polyethylene (PE) and polystyrene products, packaging made from paper provides a good airflow and prevents products from drying out. As noted by Tutus et al. (2016), paper-based products are suitable for packaging of various goods. Low- cost production and high- strength packaging papers are of practical importance.

Fig. 1. Cellulosic paper products for fruit/vegetable packaging
Paper packages can offer further enhancements for fruit preservation if the paper has been modified with antimicrobial agents or other functional active substances. Elamin (2015) investigated the effect of using potassium permanganate absorbed in paper on the quality and shelf-life of banana and found a delay in the fruit ripening and an extension of the shelf-life of banana fruits (Elamin 2015). Arfa et al. (2007) designed an antimicrobial paper based on soy protein isolates or modified starch coating, including carvacrol and cinnamaldehyde, and the results showed that the coated papers showed antimicrobial properties against the bacterium E. coli and the mold Botrytis cinerea (Ben Arfa et al. 2007). Surface applications of active ingredients to impart antimicrobial property to paper products have been a popular subject in the recent literature (Ziaee et al. 2014; Liu et al. 2015; Liu et al. 2016).
Guillaume et al. (2010) revealed that wheat gluten-coated paper was very effective to improve the shelf-life of mushrooms (Guillaume et al. 2010). Anca et al. (2016) investigated the microbiological and chemical characteristics of white bread during storage in paper-packages modified with Ag/TiO2-SiO2, which showed that the shelf life of bread can be extended by two additional days as compared with the control (unmodified paper-package) (Peter et al. 2016).
However, growing concern over the use of chemical fungicides on horticultural products, may limit the use of the above-mentioned chemicals, because of their potential hazards on human health and the emergence of pathogen resistance to fungicides.
CONCEPT OF 1-MCP CONTAINING PAPER PRODUCTS FOR FRUIT PRESERVATION
Paper packaging containing 1-MCP would serve well for the purpose of fruit preservation. Neoh et al. (2010) studied the dissociation of a crystalline complex of α-cyclodextrin (α-CD) and 1-MCP in response to the stepwise rising of relative humidity (RH) at 50 °C. The increase in relative humidity generally triggered the complex dissociation (Neoh et al. 2010). The typical real time dissociation profile of a 1-MCP complex subjected to stepwise increases in RH is shown in Fig. 2. The relative humidity corresponded to the dissociation of a crystalline complex of α-CD and 1-MCP owing to the collapse of the crystalline structure.
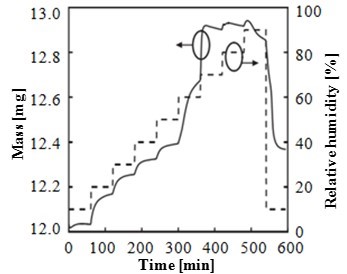
Fig. 2. Moisture induced release of 1- MCP from 1-MCP/α-CD complex, as a function of time and humidity (Neoh et al. 2010)
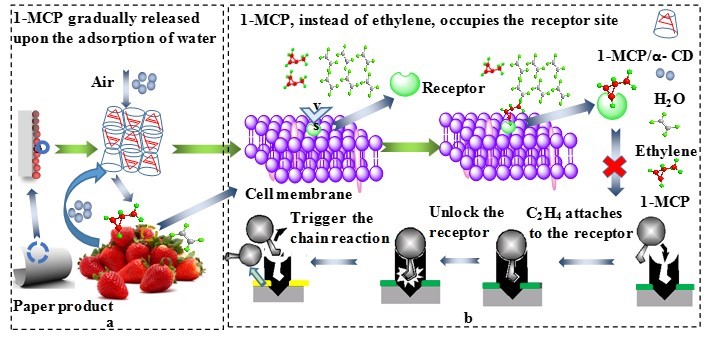
Fig. 3. Schematic of 1-MCP-containing paper packaging for fruit/vegetable preservation;
(a) 1-MCP will be gradually released upon the adsorption of moisture from air or fruit respiration process, and (b) 1-MCP, instead of ethylene, occupies the receptor site, thus blocking the ethylene-induced fruit ripening process
One method for utilizing 1-MCP for fruit preservation is to load the 1-MCP-containing complex particles onto the paper. The working mechanism is that when using the encapsulated 1-MCP paper products during transportation, and retail market, 1-MCP will be gradually released upon the adsorption of water that comes from the respiration of the fruits and/or air in the surroundings. The released 1-MCP will out-compete ethylene in binding to the receptor sites, thus inhibiting the formation of ethylene receptor complexes. In this way, the ethylene-induced fruit ripening process is blocked. Figure 3 illustrates the schematic of the overall concept.
The cost of such products would be competitive because cellulosic paper is rather cost-effective. These paper products would inherit the intrinsic properties of cellulosic paper, such as flexibility, biocompatibility, lightweight, and recyclability. The products can be made using conventional surface treatment processes, such as a coating that the inclusion complex particles adhere to on the paper surface.
In addition to the green/environmental advantages of using paper products for such applications, the proposed product concept of paper/1-MCP encapsulation composites may have a number of advantages. It would be convenient to bring the 1-MCP containing complex particles onto the surface of the paper using a conventional surface treatment process. It would be easy to adjust the amount of the 1-MCP by changing the amount of the coating applied to the paper substrate. Also, the sustaining and gradual release of 1-MCP would enhance the utilization efficiency of 1-MCP encapsulation (Sisler and Serek 1997; Feng et al. 2000; DeEll et al. 2002).
1-MCP CHEMISTRY
Mechanism of Ripening by Ethylene
Ethylene is a natural plant hormone that plays a vital role in the initiation of ripening, and is physiologically active at trace amounts (0.1 ppm) (Watada 1986). Excessive amounts of ethylene will lead to spoilage, reducing the shelf-life, flavor, texture, nutrition, and appearance of fruits (Saltveit 1999). First, ethylene binds the ethylene acceptor, and the precursor methionine (Met) forms. Then the Met is further converted to S-adenosyl-methionine (S-AdoMet) that is mediated by S-AdoMetsynthetase (SAMS). The S-AdoMet is further catalyzed to 1-aminocyclopropane-1-carboxylate (ACC) via ACC synthetase (ACS). Finally, the ACC oxidase (ACO) converts the ACC to ethylene (Sato and Theologis 1989; Hamilton et al. 1991; Kende 1993). Simultaneously, a cascade of events involve the enzymes according to different roles as follows: pectinases to break down the cell walls and soften the fruit, amylases to convert the carbohydrates into simple sugars, and hydrolases to degrade the chlorophyll content of the fruit resulting in color change (Nath et al. 2014). Figure 4 shows the ethylene induced biological process, leading to the regeneration of ethylene and the ripening of fruits. In a sense, when ethylene binds to the receptor, it is like a lock turning and a door opening. The binding of ethylene with the receptor “unlocks” the receptor and leads to a biological process in the plant tissue (Blankenship 2001). A series of events then take place, such as the fruit begins to soften and turn yellow (Giovannoni 2007).
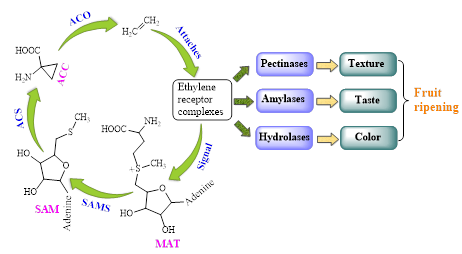
Fig. 4. Ethylene induced biological process leading to the regeneration of ethylene and ripening of fruits (Kende 1993; Saltveit 1999; Nath et al. 2014)
In light of the ethylene-induced ripening process, there have been commercial strategies that minimize ethylene production and/or its presence during the transportation and storage of horticultural products by temperature and atmosphere control (Daly and Kourelis 2001). Sisler and Blankenship made an exciting discovery that 1-methylcyclopropene (1-MCP) is an effective inhibitor of ethylene (Sisler and Blankenship 1996), and then the fruit/vegetable preservation science and technology entered a new era. So far, 1-MCP-related research has been focused on the fundamental processes that are involved in the inhibition of ripening, and the development of 1-MCP-based technology for the preservation of fruits and vegetables after harvest (Blankenship and Dole 2003).
Preservation Mechanism of 1-MCP
The action of 1-MCP is mediated through the inhibition of an ethylene-induced respiration process by interacting with the receptor and competing against the ethylene for binding in the receptor sites. 1-MCP binds the ethylene receptor and inhibits the formation of ethylene receptor complexes (Sisler and Serek 1997). Therefore, 1-MCP blocks the ethylene-induced signal transduction by binding to an ACC synthase enzyme and stopping the conversion of S-AdoMet to ACC, the immediate precursor in the ethylene biosynthesis pathway. 1-MCP also influences ethylene biosynthesis by exerting a feedback to lower the expression of the ACS and ACO enzymes (Hofman et al. 2001; Kluge and Jacomino 2002). 1-MCP acts as a ‘key’ that goes into the ‘lock’, but it is unable to turn on the ‘lock.’ When the 1-MCP ‘key’ is in the ‘lock’ it is not possible for the ethylene ‘key’ to go inside the ‘lock’ (Merisko-Liversidge and Liversidge 2011). Figure 5 shows a model of how the 1-MCP wins the competition against ethylene in binding the ethylene receptor, because the affinity of 1-MCP for the receptor is approximately 10 times greater than that of ethylene. Compared with ethylene, 1-MCP is active at much lower concentrations. In contrast, new ethylene receptors may be formed, and the cells regain the ethylene-induced ripening process (Northup 2013).
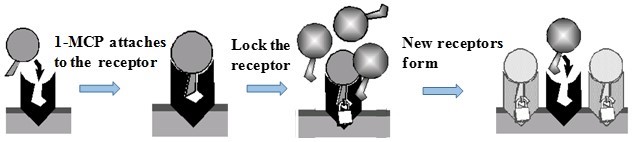
Fig. 5. When 1-MCP binds the ethylene receptor, it remains locked to the receptor preventing the binding of ethylene and the chemical reaction does not occur (Merisko-Liversidge and Liversidge 2011)
In this way, 1-MCP dramatically inhibits the ripening of fruit, thus extending the shelf-life and quality of plant products. Moreover, it is reported that the respiration rates of most products treated with 1-MCP decreased or were delayed, especially for climacteric fruits, the respiration rates of which are so high due to the high ethylene formation (Golding et al. 1999).
Characterization and Encapsulation of 1-MCP
1-MCP is a gaseous product at room temperature. Due to its unsaturated nature, it is chemically unstable and prone to self-polymerization. Furthermore, as a low molecular weight hydrocarbon, it presents an explosive hazard when being compressed (Sisler et al. 1996).
To solve these problems, various substrates have been studied to encapsulate 1-MCP, such as cyclodextrin (CD) (Trotta et al. 2011), cucurbit uril (Buschmann et al. 2005), molecular sieves, and modified starch (Chang et al. 2008) and sawdust (Sisler and Blankenship 1994). Among them, α-CD has been considered as the favorable substrate, because 1-MCP can be stably present as a single molecule in the cavities of α-CD, and the formed 1-MCP/α-CD complex products have been commercialized.
The encapsulated 1-MCP/α-CD complexes greatly reduce the explosion potential, due to decreased vapor pressure, and also they prevent 1-MCP from its self-polymerization, both of which can ease its handling. Neoh et al. (2007) investigated the molecular encapsulation of gaseous1-MCP into aqueous α-CD in a closed, agitated vessel with a flat gas-liquid interface. The molecular encapsulation of gaseous 1-MCP by α-CD is a simultaneous two-step process, which involves the dissolution of gaseous 1-MCP and the encapsulation of the dissolved 1-MCP into α-CD. Changes in the X-ray diffraction pattern suggested that the crystal lattice structure of α-CD was altered upon the inclusion of 1-MCP (Neoh et al. 2007).
Trotta et al. (2011) reported the complexing abilities of CD nanosponges to 1-MCP. They synthesized the CD-based carbonate nanosponges from native β-CD and active carbonyl compounds, and the 1-MCP included in β-CD nanosponges showed superior anti-ethylenic performances in long-lasting cut flowers in comparison with α-CD complex products, such as SmartFresh, due to the increased inner cavity (Seglie et al. 2011; Seglie et al. 2012).
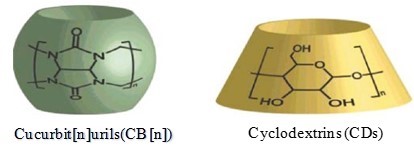
Fig. 6. Structures and schematic representations of CB and CD (Zhang et al. 2011)
Zhang et al. (2011) reported a study of encapsulating 1-MCP by Cucurbit[6]uril, a barrel-like macrocyclic molecule, which is prepared from the cheap starting materials glycoluril and formalin, and has the similar cavity size to α-CD (Fig. 6), as the absorbent to encapsulate 1-MCP. The effects of the encapsulation conditions on the formation of the inclusion complex, the amount of 1-MCP encapsulated by CB[6], encapsulation temperature and time, and the concentration of CB[6] were also investigated. Furthermore, the release of 1-MCP from the complex was made possible in different solutions, such as sodium bicarbonate, benzoic acid, and distilled water.
Other encapsulation methods were reported in the patent literature, i.e., CN100364394C (Chang et al. 2008) and CN101416658B (Feng 2009). The patent CN100364394C disclosed a microcapsule agent for fresh fruits, vegetables, and flowers. The core material of 1-MCP at a 0.5% to 3.5%, 20% to 30% wall shell 3A molecular sieve, 60% to 65% starch, 1% to 2% ammonium chloride and 3% to 18% sodium bicarbonate. CN101416658 B disclosed another 1-MCP containing product, with the following composition: 1-MCP of 0.15% to 4.8%, with the modified stabilizer, which is made of the following raw materials in parts by weight of the preparation: 20 to 100 parts of modified starch; β-CD or a derivative of 0 to 80 parts; α-CD or a derivative of 0 to 40 parts.
1-MCP Related Products and Applications
1-MCP was approved by the Environmental Protection Agency (EPA) in 1999 for use on ornamentals, and was marketed as EthylBloc by Floralife, Inc. (Walterboro, SC). AgroFresh, Inc. subsequently developed 1-MCP under the trade name SmartFresh. The synthesis method of 1-MCP/α-CD complex was then patented (Daly and Kourelis 2001). This product is based on the release of gaseous 1-MCP from a 1-MCP/α-CD complex in either powder or tablet form due to the moisture/water or base solution.
There are a number of SmartFresh commercial products including ProTabs, SmartTabs, and InBox (AgroFresh Corp.). ProTabs is a kind of tablet, which is designed for a closed environment and is applicable to a wide range of space and storage. SmartTabs can be used in any prepared existing storage space, for example, a cold storage facility for a small orchard, or a small batch harvest fruit. InBox is made as a convenient sachet format that can be used directly in the container.
The encapsulated 1-MCP technology and its products have been available in many countries for a wide variety of crops and produce, including such fruits as apple, apricot, avocado, kiwifruit, mango, melon, nectarine, papaya, peach, pear, pepper, persimmon, pineapple, plantain, plum, squash, tomatoes, and other products, such as tulip bulbs (Hofman et al. 2001; Paliyath et al. 2009). When applied to fruit preservation, 1-MCP dramatically inhibits the ripening and softening process. For example, 1-MCP-treated ‘Qingnai’ plum showed reduced ethylene formation and respiration rate and delayed softening (Luo et al. 2009). Upon being treated with 1 μL/L of 1-MCP for 10 h at 20 °C, kiwifruit (Actinidiadeliciosa cv. Hayward), showed a reduced respiration rate, ethylene formation, delayed softening, and color change, compared to the control, after storing at 2 °C for 10 d (Mao et al. 2007).
In most studies (Antunes et al. 2010; Acuna et al. 2011; Amornputti et al. 2014), 1-MCP has been applied at temperatures ranging from 20 °C to 25 °C. The effective concentrations of 1-MCP vary widely, depending on the products to be treated, for instance, the minimum concentration required was 2.5 nL/L on carnation, while for apples it was 1 μL/L. The treatment duration ranged from 12 h to 24 h. The importance of the time from harvest to the 1-MCP treatment varies with the crop species. Generally, the more perishable the crop, the more quickly the 1-MCP treatment after harvest should be applied.
There are many factors determining the 1-MCP treatment efficiency, i.e., pre-harvest factors, cultivar, maturity, and postharvest practices. For leafy or non-fruit vegetables, the 1-MCP technology may only be effective under strong conditions, such as high temperatures and exogenous ethylene exposure. Finally, the economics and benefits of the 1-MCP-based technology should be evaluated on a case-by-case basis, especially, in relation to quality as perceived by the consumer (Marsh and Bugusu 2007).
However, there are some disadvantages associated with the existing SmartFresh products. For example, the ProTabs need a closed environment for a 6 h to 12 h fumigation, which is a complicated operation and not convenient for large-scale storage (AgroFresh Corp.). Even after 1-MCP fumigation in the warehouse, fruits may recover their ethylene level, thus starting the ethylene induced maturation process again (Cameron and Reid 2001).
Innovations in technologies for the use of 1-MCP are still desirable. One option is to follow the so-called controlled release packaging (CRP) concept, which is being used widely for the delivery of active compounds, such as antimicrobials and antioxidants. The CRP concept is designed to achieve a slow release (timed delivery) of active compounds over longer periods of time (Lee et al. 2006).
CRP BASED ON PAPER PRODUCT CONTAINING 1-MCP
As noted above, the controlled release packaging (CRP) is an emerging technology by which active compounds, such as antimicrobials or antioxidants are incorporated into the package and then gradually released in a controlled manner. This technology is considered to have great potential in applications in various fields, including food packaging and medicine packaging (Bezemer et al. 2000; Piercey et al. 2012; Rooney 1995). For example, Loṕez et al. (2012) prepared low molecular weight antioxidant tocopherol by using PPG-PEG-PPG films that could be used for food packaging (Lópezet al. 2012). LaCoste et al. (2005) developed a concept of functional packaging films for the controlled release of active compounds, such as antimicrobials, antioxidants, and flavour compounds to extend the shelf-life of food (LaCoste et al. 2005). This concept can be extended to the 1-MCP technology in the wrapping/packaging of products for fruits/ vegetables, so that 1-MCP can be gradually released during the shipping/transportation, and storage/retail phases.
The gradual release of MCP during the fruit transportation/shipping process would be able to slow down the formation of ethylene. Therefore, a new 1-MCP containing paper package, with a sustained 1-MCP release, has great commercial potential for fruit preservation, such as strawberry, waxberry, mango, etc. The controlled release packaging that contains 1-MCP can be designed as follows.
The 1-MCP containing complex particles are used to prepare the paper/1-MCP encapsulation composites. The key elements of the paper/1-MCP encapsulation composites design can include a layer of encapsulated complex particles mixed with adhesives, and cellulosic paper as the supporting substrate.
Figure 7 shows a cross-sectional view of the design of the paper/encapsulated1-MCP composites. The general concept was to use the paper as a recyclable/biodegradable substrate that could support the1-MCP containing complex particles, which would then meet the requirements of controlled release packaging for preservation end-use applications.
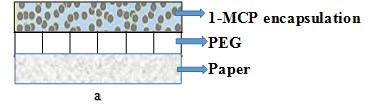
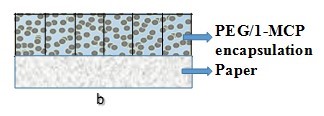
Fig. 7. Schematic representation of typical layered paper/encapsulated 1-MCP composites
The paper serves as the supporting substrate for the encapsulated 1-MCP complex particles. Two different methods of preparation are used: (a) the binder, such as polyethylene glycol (PEG), is applied to the paper surface, and then a thin layer of the encapsulated complex particles is sprayed/coated on the top; and (b) the binders and the encapsulated 1-MCP complex particles are mixed, and the mixture is then applied onto the paper surface via coating. The paper product can absorb the water from the air or the fruit respiration process, which then triggers the gradual release of 1-MCP. The products can ensure that a gradual release of 1-MCP takes place during the shipping. By this means, the retail market achieves the extension of the shelf life of the fruits or vegetables.
CONCLUDING REMARKS
The freshness quality and long shelf life are important factors for fruits and vegetables during the storage, shipping process, and retail market. Ethylene (C2H4) is a plant hormone, and it triggers the physiological respiration process, leading to the maturity and senescence, the softening and rotting of the fruit/vegetable. 1-MCP is a strong inhibitor of C2H4, thus, blocking the ethylene-induced respiration process.
Active packaging is an evolving technology in extending the shelf life of many food products, which continually calls for further developments and improvements. A novel active paper packaging with the use of encapsulated 1-MCP complexes is an interesting concept that may have a great potential for commercialization. Such paper products can effectively decrease the formation of ethylene of the packed fruits (vegetables) by the gradual release of 1-MCP. Hence it can prolong the shelf life and improve the quality of the product during the storage, shipping process, and retail market. These paper products are advantageous over synthetic products, for example, paper is environmental friendly and easy to be recycled. It is convenient to bring the 1-MCP containing complex particles onto the surface of the paper using a conventional surface treatment process, and the amount of 1-MCP can be adjusted by changing the amount of the coating applied to the paper substrate
Future research will be needed in the development of optimized surface application technologies to bring 1-MCP into paper products, likely after the paper is produced. In addition to the cyclodextrin, other 1-MCP absorbents/ complexing agents, such as calixarene, can be of practical interest.
ACKNOWLEDGMENTS
The authors are grateful for the support of the China Natural Science Foundation under Grant No. 51376162, and the Zhejiang Province Collaborative Innovation Center of Agricultural Biological Resources Biochemical Manufacturing Foundation under Grant No. 2016KF0019.
REFERENCES CITED
Acuna, M. G. V., Biasi, W. V., Mitcham, E. J., and Holcroft, D. (2011). “Fruit temperature and ethylene modulate 1-MCP response in ‘Bartlett’pears,” Postharvest Biol. Technol. 60(1), 17-23. DOI: 10.1016/j.postharvbio.2010.11.005
AgroFresh Corporation (2015). (http://www.agrofresh.com/smartfresh-technology), Accessed 16 October 2015.
Amornputti, S., Ketsa, S., and Van Doorn, W. G. (2014). “Effect of 1-methylcyclopropene (1-MCP) on storage life of durian fruit,” Postharvest Biol. Technol. 97, 111-114. DOI: 10.1016/j.postharvbio.2014.06.011
Antunes, M. D., Dandlen, S., Cavaco, A. M., and Miguel, G. (2010). “Effects of postharvest application of 1-MCP and postcutting dip treatment on the quality and nutritional properties of fresh-cut kiwifruit,” J. Agr. Food Chem. 58(10), 6173-6181. DOI: 10.1021/jf904540m
Bapat, V. A., Trivedi, P. K., Ghosh, A., Sane, V. A., Ganapathi, T. R., and Nath, P. (2010). “Ripening of fleshy fruit: Molecular insight and the role of ethylene,” Biotechnol. Adv. 28(1), 94-107. DOI: 10.1016/j.biotechadv.2009.10.002
Bautista-Baños, S., Hernandez-Lauzardo, A. N., Velazquez-Del Valle, M. G., Hernández-López, M., Barka, E. A., Bosquez-Molina, E., and Wilson, C. (2006). “Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities,” Crop Prot. 25(2), 108-118. DOI: 10.1016/j.cropro.2005.03.010
Ben Arfa, A., Preziosi-Belloy, L., Chalier, P., and Gontard, N. (2007). “Antimicrobial paper based on a soy protein isolate or modified starch coating including carvacrol and cinnamaldehyde,” J. Agr. Food Chem. 55(6), 2155-2162. DOI: 10.1021/jf0626009
Beuchat, L. R. (1996). “Pathogenic microorganisms associated with fresh produce,” J. Food Protect. 59(2), 204-216.
Bezemer, J., Radersma, R., Grijpma, D., Dijkstra, P., Feijen, J., and Van Blitterswijk, C. (2000). “Zero-order release of lysozyme from poly (ethylene glycol)/poly (butylene terephthalate) matrices,” J. Control. Release 64(1), 179-192. DOI: 10.1016/S0168-3659(99)00127-3
Blankenship, S. (2001). “Ethylene effects and the benefits of 1-MCP,” Perishables Handling Quarterly 11(108), 2-4.
Blankenship, S. M., and Dole, J. M. (2003). “1-Methylcyclopropene: A review,” Postharvest Biol. Technol. 28(1), 1-25. DOI: 10.1016/S0925-5214(02)00246-6
Buschmann, H.-J., Mutihac, L., Mutihac, R.-C., and Schollmeyer, E. (2005). “Complexation behavior of cucurbit [6] uril with short polypeptides,” Thermochim. Acta 430(1), 79-82. DOI: 10.1016/j.tca.2005.01.002
Cameron, A. C., and Reid, M. S. (2001). “1-MCP blocks ethylene-induced petal abscission of Pelargonium peltatum but the effect is transient,” Postharvest Biol. Technol. 22(2), 169-177. DOI: 10.1016/S0925-5214(00)00189-7
Chang, W. T. H., Chen, H. Y., and Wang, Y. C. (2008). “Microcapsuled fruit, vegetable, and flower antistaling agent and preparation thereof,” U. S. Patent No. 8461086-B2
Corbo, M. R., Speranza, B., Campaniello, D., Dˊamato, D., and Sinigaglia, M. (2010). “Fresh-cut fruits preservation: Current status and emerging technologies,” in: Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology,Formatex Research Center, Badajoz. 1143-1154. https://www.researchgate.net/publication/236552242
Daly, J., and Kourelis, B. (2001). “Synthesis methods, complexes, and delivery methods for the safe and convenient storage, transport, and application of compounds for inhibiting the ethylene response in plants,” U. S. Patent No. 6017849-A.
DeEll, J. R., Murr, D. P., Porteous, M. D., and Rupasinghe, H. V. (2002). “Influence of temperature and duration of 1-methylcyclopropene (1-MCP) treatment on apple quality,” Postharvest Biol. Technol. 24(3), 349-353. DOI: 10.1016/S0925-5214(01)00136-3
Eckert, J. W., and Ogawa, J. M. (1988). “The chemical control of postharvest diseases: Deciduous fruits, berries, vegetables, and root/tuber crops,” Annu. Rev. Phytopathol. 26(1), 433-469. DOI: 10.1146/annurev.py.26.090188.002245
Kende, H. (1993). “Ethylene biosynthesis,” Annual Review of Plant Biology 44(1), 283-307.
Elamin, M. A. (2015). “Effect of polyethylene film lining and potassium permanganate on quality and shelf-life of banana fruits,” Ph. D. Disscertation, University of Khartoum, Khartoum, Sudan. DOI: 10.5897/AJAR11.1203
Feng, X., Apelbaum, A., Sisler, E. C., and Goren, R. (2000). “Control of ethylene responses in avocado fruit with 1-methylcyclopropene,” Postharvest Biol. Technol. 20(2), 143-150. DOI: 10.1016/S0925-5214(00)00126-5
Feng, J. G. (2009). “1- MCP/modified stabilizer coating material, production method, and use thereof,” C. N. Patent No. 101416658-A.
Giovannoni, J. J. (2007). “Fruit ripening mutants yield insights into ripening control,” Curr. Opin. Plant Biol. 10(3), 283-289. DOI 10.1016/j.pbi.2007.04.008
Golding, J., Shearer, D., McGlasson, W., and Wyllie, S. (1999). “Relationships between respiration, ethylene, and aroma production in ripening banana,” J. Agr. Food Chem. 47(4), 1646-1651. DOI: 10.1021/jf980906c
Gonzalez-Aguilar, G. A., Buta, J. G., and Wang, C. Y. (2003). “Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain postharvest quality of papaya ‘Sunrise’ ,” Postharvest Biology and Technology 28(3), 361-370. DOI: 10.1016/S0925-5214(02)00200-4
Grichko, V., Serek, M., Watkins, C., and Yang, S. (2006). “Father of 1-MCP,” Biotechnol. Adv. 24(4), 355-356. DOI: 10.1016/j.biotechadv.2006.01.001
Guillaume, C., Schwab, I., Gastaldi, E., and Gontard, N. (2010). “Biobased packaging for improving preservation of fresh common mushrooms (Agaricus bisporus L.),” Innov. Food Sci. Emerg. 11(4), 690-696. DOI: 10.1016/j.ifset.2010.05.007
Hamilton, A., Bouzayen, M., and Grierson, D. (1991). “Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast,” P. Natl. Acad. Sci. USA 88(16), 7434-7437. DOI: 10.1073/pnas.88.16.7434
Hofman, P., Jobin-Decor, M., Meiburg, G., Macnish, A., and Joyce, D. (2001). “Ripening and quality responses of avocado, custard apple, mango, and papaya fruit to 1-methylcyclopropene,” Anim. Prod. Sci. 41(4), 567-572. DOI: 10.1071/EA00152
Kende, H. (1993). “Ethylene biosynthesis,” Annu. Rev. Plant Biol. 44(1), 283-307. DOI: 10.1146/annurev.pp.44.060193.001435
Kluge, R. A., and Jacomino, A. P. (2002). “Shelf life of peaches treated with 1-methylcyclopropene,” Sci. Agr. 59(1), 69-72. DOI: 10.1590/S0103-90162002000100010
Labuza, T., and Breene, W. (1989). “Applications of “active packaging” for improvement of shelf-life and nutritional quality of fresh and extended shelf-life foods 1,” J. Food Process. Pres. 13(1), 1-69. DOI: 10.1111/j.1745-4549.1989.tb00090.x
LaCoste, A., Schaich, K. M., Zumbrunnen, D., and Yam, K. L. (2005). “Advancing controlled release packaging through smart blending,” Packag. Technol. Sci. 18(2), 77-87. DOI: 10.1002/pts.675
Lee, Y. S., Beaudry, R., Kim, J. N., and Harte, B. R. (2006). “Development of a 1‐methylcyclopropene (1‐MCP) sachetrelease system,” J. Food Sci. 71(1), 1-6. DOI: 10.1111/j.1365-2621.2006.tb12380.x
Liu, H., Chen, Y., Zhang, H., Yuan, Z., Zou, X., Zhou, Y., and Ni, Y. (2012). “Increasing the use of high-yield pulp in coated high-quality wood-free papers: From laboratory demonstration to mill trials,” Ind. Eng. Chem. Res. 51(11), 4240-4246. DOI: 10.1021/ie2029514
Liu, K., Liang, H., Nasrallah, J., Chen, L., Huang, L., and Ni, Y. (2016). “Preparation of the CNC/Ag/beeswax composites for enhancing antibacterial and water resistance properties of paper,” Carbohydrate Polymers 142, 183-188. DOI : 10.1016/j.carbpol.2016.01.044
Liu, K., Chen, L., Huang, L., Ni, Y., and Sun, B. (2015). “Enhancing antibacterium and strength of cellulosic paper by coating triclosan-loaded nanofibrillated cellulose (NFC),” Carbohydrate Polymers 117, 996-1001. DOI: 10.1016/j.carbpol.2014.10.014
Liu, M., Pirrello, J., Chervin, C., Roustan, J. P., and Bouzayen, M. (2015). “Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation,” Plant Physiology 169(4), 2380-2390. DOI: 10.1104/pp.15.01361
Luo, Z., Xie, J., Xu, T., and Zhang, L. (2009). “Delay ripening of ‘Qingnai’ plum (Prunus salicina L.) with 1-methylcyclopropene,” Plant Sci. 177(6), 705-709. DOI: 10.1016/j.plantsci.2009.08.013
Mao, L., Wang, G., and Que, F. (2007). “Application of 1-methylcyclopropene prior to cutting reduces wound responses and maintains quality in cut kiwifruit,” J. Food Eng. 78(1), 361-365. DOI: 10.1016/j.jfoodeng.2005.10.004
Marsh, K., and Bugusu, B. (2007). “Food packaging- roles, materials, and environmental issues,” J. Food Sci. 72(3), 39-55. DOI: 10.1111/j.1750-3841.2007.00301.x
McMillin, K., Huang, N., Ho, C., and Smith, B. (1999). “Quality and shelf-life of meat in case-ready modified atmosphere packaging,” in: Quality Attributes of Muscle Foods, Springer, New York. DOI: 10.1007/s13197-012-0682-3
Merisko-Liversidge, E., and Liversidge, G. G. (2011). “Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology,” Adv. Drug Deliv. Rev. 63(6), 427-440. DOI: 10.1016/j.addr.2010.12.007
Mostafavi, H. A., Mirmajlessi, S. M., Fathollahi, H., Shahbazi, S., and Mirjalili, S. M. (2013). “Integrated effect of gamma radiation and biocontrol agent on quality parameters of apple fruit: An innovative commercial preservation method,” Radiat. Phys. Chem. 91, 193-199. DOI: 10.1016/j.radphyschem.2013.02.020
Neoh, T. L., Koecher, K., Reineccius, G., Furuta, T., and Yoshii, H. (2010). “Dissociation characteristic of the inclusion complex of cyclomaltohexaose (α-cyclodextrin) with 1-methylcyclopropene in response to stepwise rising relative humidity,” Carbohydr. Res. 345(14), 2085-2089. DOI: 10.1016/j.carres.2010.07.006
Neoh, T. L., Yamauchi, K., Yoshii, H., and Furuta, T. (2007). “Kinetics of molecular encapsulation of 1-methylcyclopropene into α-cyclodextrin,” J. Agr. Food Chem. 55(26), 11020-11026. DOI: 10.1021/jf072357t
Northup, J. (2013). “Biochar as a replacement for perlite in greenhouse soilless substrates,” Iowa State Univeristy, Ames, Iowa.
Ozdemir, M., and Floros, J. D. (2004). “Active food packaging technologies,” Crit. Rev. Food Sci. 44(3), 185-193. DOI: 10.1080/10408690490441578
Peter, A., Mihaly-Cozmuta, L., Mihaly-Cozmuta, A., Nicula, C., Ziemkowska, W., Basiak, D., Danciu, V., Vulpoi, A., Baia, L., Falup, A., et al. (2016). “Changes in the microbiological and chemical characteristics of white bread during storage in paper packages modified with Ag/TiO2–SiO2, Ag/N–TiO2, or Au/TiO2,” Food Chem. 197, 790-798. DOI: 10.1016/j.foodchem.2015.11.048
Piercey, M., Mazzanti, G., Budge, S., Delaquis, P., Paulson, A., and Hansen, L. T. (2012). “Antimicrobial activity of cyclodextrin entrapped allyl isothiocyanate in a model system and packaged fresh-cut onions,” Food Microbiol. 30(1), 213-218. DOI: 10.1016/j.fm.2011.10.015
Rameshkumar, A., Sivasudha, T., Jeyadevi, R., Ananth, D. A., and Pradeepha, G. (2012). “Effect of environmental factors [air and UV-C irradiation] on some fresh fruit juices,” Eur. Food Res. Technol. 234(6), 1063-1070. DOI: 10.1007/s00217-012-1711-1
Rooney, M. L. (1995). Active Food Packaging, Blackie Academic & Professional, London.
Saltveit, M. E. (1999). “Effect of ethylene on quality of fresh fruits and vegetables,” Postharvest Biol. Technol.15(3), 279-292. DOI: 10.1016/S0925-5214(98)00091-X
Sato, T., and Theologis, A. (1989). “Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants,” Proceedings of the National Academy of Sciences 86(17), 6621-6625.
Seglie, L., Martina, K., Devecchi, M., Roggero, C., Trotta, F., and Scariot, V. (2011). “β-Cyclodextrin-based nanosponges as carriers for 1-MCP in extending the postharvest longevity of carnation cut flowers: An evaluation of different degrees of cross-linking,” Plant Growth Regul. 65(3), 505-511. DOI: 10.1007/s10725-011-9621-y
Seglie, L., Spadaro, D., Trotta, F., Devecchi, M., Gullino, M. L., and Scariot, V. (2012). “Use of 1-methylcylopropene in cyclodextrin-based nanosponges to control grey mould caused by Botrytis cinerea on Dianthus caryophyllus cut flowers,” Postharvest Biol. Technol. 64(1), 55-57. DOI: 10.1016/j.postharvbio.2011.09.014
Sharma, R., Singh, D., and Singh, R. (2009). “Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review,” Biol. Control 50(3), 205-221. DOI: 10.1016/j.biocontrol.2009.05.001
Sisler, E., and Serek, M. (1997). “Inhibitors of ethylene responses in plants at the receptor level: Recent developments,” Physiol. Plantarum 100(3), 577-582. DOI: 10.1111/j.1399-3054.1997.tb03063.x
Sisler, E. C. (2006). “The discovery and development of compounds counteracting ethylene at the receptor level,” Biotechnol. Adv. 24(4), 357-367. DOI: 10.1016/j.biotechadv.2006.01.002
Sisler, E. C., and Blankenship, S. M. (1996). “Method of counteracting an ethylene response in plants,” U. S. Patent No. 5518988.
Sisler, E. C., Dupille, E., and Serek, M. (1996). “Effect of 1-methylcyclopropene and methylenecyclopropane on ethylene binding and ethylene action on cut carnations,” Plant Growth Regul. 18(1), 79-86. DOI: 10.1007/BF00028491
Trotta, F., Cavalli, R., Martina, K., Biasizzo, M., Vitillo, J., Bordiga, S., Vavia, P., and Ansari, K. (2011). “Cyclodextrin nanosponges as effective gas carriers,” J. Incl. Phenom. Macro. 71(1-2), 189-194. DOI: 10.1007/s10847-011-9926-5
Tutus et al. (2016). “Effects of using starch at size press on physical and optical properties of some packing papers,” 1st International Conference on Engineering Technology and Applied Sciences, 21-22 April, Afyon/Turkey, pp. 111-115.
Vermeiren, L., Devlieghere, F., Van Beest, M., De Kruijf, N., and Debevere, J. (1999). “Developments in the active packaging of foods,” Trends Food Sci. Tech. 10(3), 77-86. DOI: 10.1016/S0924-2244(99)00032-1
Watada, A. E. (1986). “Effects of ethylene on the quality of fruits and vegetables,” Food Technol. 40(5), 82-85. DOI: 10.1016/S0925-5214(98)00091-X
Zaman, M., Xiao, H., Chibante, F., and Ni, Y. (2012). “Synthesis and characterization of cationically modified nanocrystalline cellulose,” Carbohydr. Polym. 89(1), 163-170. DOI: 10.1016/j.carbpol.2012.02.066
Zhang, Q., Zhen, Z., Jiang, H., Li, X.-G., and Liu, J.-A. (2011). “Encapsulation of the ethylene inhibitor 1-methylcyclopropene by cucurbit [6] uril,” J. Agr. Food Chem. 59(19), 10539-10545. DOI: 10.1021/jf2019566
Ziaee, Z., Xiao, H., Guan, Y., and Fatehi, P. (2014). “Coating PHGH-modified starch on papers to induce antimicrobial properties,” BioResources 9(2), 3632-3641.
Article submitted: December 9, 2016; Peer review completed: January 28, 2017; Revised version received and accepted: January 31, 2017; Published: February 1, 2017.
DOI: 10.15376/biores.12.1.2234-2248
