Abstract
The increasing usage of petroleum-based compounds has prompted numerous environmental concerns. Consequently, there has been a steady rise in research on the synthesis of useful materials from natural sources. Paper technologists are seeking environmentally acceptable dry end and wet end additives. Among the bio-based resources available, nanocellulose is a popular sustainable nanomaterial additive in the paper industry because of its high strength, high oxygen barrier performance, low density, great mechanical properties, and biocompatibility. NC’s extensive hydroxyl groups provide a unique possibility to dramatically modify the hydrophilicity and charge of the surface in order to improve their potential applications in the paper industry. The current paper reviews two series of surface modifications, each with various subcategories, depending on why modified nanocellulose is added in the paper production: to improve barrier properties or to improve mechanical properties of packaging materials. The methods presented in this study use the minimum amount of chemically hazardous solvents to have the least impact on the environment. This review focuses on modifications of nanocellulose and their subsequent application in the papermaking. The knowledge and the discussion presented in this review will form a literature source for future use by various stakeholders and the sustainable paper manufacturers.
Download PDF
Full Article
A Comprehensive Review of Nanocellulose Modification and Applications in Papermaking and Packaging: Challenges, Technical Solutions, and Perspectives
Mozhgan Hashemzehi,a Beko Mesic,a Björn Sjöstrand,a,* and Muhammad Naqvi a,b
The increasing usage of petroleum-based compounds has prompted numerous environmental concerns. Consequently, there has been a steady rise in research on the synthesis of useful materials from natural sources. Paper technologists are seeking environmentally acceptable dry end and wet end additives. Among the bio-based resources available, nanocellulose is a popular sustainable nanomaterial additive in the paper industry because of its high strength, high oxygen barrier performance, low density, great mechanical properties, and biocompatibility. NC’s extensive hydroxyl groups provide a unique possibility to dramatically modify the hydrophilicity and charge of the surface in order to improve their potential applications in the paper industry. The current paper reviews two series of surface modifications, each with various subcategories, depending on why modified nanocellulose is added in the paper production: to improve barrier properties or to improve mechanical properties of packaging materials. The methods presented in this study use the minimum amount of chemically hazardous solvents to have the least impact on the environment. This review focuses on modifications of nanocellulose and their subsequent application in the papermaking. The knowledge and the discussion presented in this review will form a literature source for future use by various stakeholders and the sustainable paper manufacturers.
DOI: 10.15376/biores.17.2.Hashemzehi
Keywords: Nanocellulose; Modification of nanocellulose; Papermaking; Coating; Barrier; Mechanical strength
Contact information: a: Department of Engineering and Chemical Sciences, Karlstad University, Sweden; b: 2Department of Chemical Engineering, American University of Middle East, Kuwait;
* Corresponding author: Tel: +46705519303; E-mail: bjorn.sjostrand@kau.se
GRAPHICAL ABSTRACT

INTRODUCTION
Background and Motivation
Packaging is an important component, in the view of customers, to maintain the quality and safety of the goods, provide information about the product, facilitate transportation, reduce food waste during transportation and storage, allow safe storage, prevent product damage, and reduce economic losses. To achieve the above goals, packaging systems must provide a physical barrier to microbial and physicochemical damages, environmental conditions, and external stimuli (Verghese et al. 2013; Mohammadian et al. 2020). As a result, the packaging industry currently relies upon paper, aluminum, conventional petroleum-based derivatives such as polyethylene, polyvinyl chloride, polypropylene, polystyrene, waxes, and/or fluorine-based derivatives as coating that serve a variety of functions, in order to manufacture competitive packages (Ferrer et al. 2017). For instance, paper is a significant packaging material in both developed and developing countries. Paper can take that role because it is a non-toxic, non-polluting, non-radiation-emitting material for versatile use that performs exceptionally well in terms of environmental friendliness (Huang 2017).
Wood fibers in paper packaging create a porous structure that allows oil, water, and gas molecules to pass through, leading to inadequate barrier properties. Additionally, paper packaging readily absorbs moisture from the environment and/or packaged food, resulting in deterioration of its mechanical strength, which is detrimental to the integrity and quality of packaged goods (Zhang et al. 2021a). Therefore, various materials can be used to address such issue, including aluminium foil and other metalized films, which are extensively used in food packaging. Aluminum foil provides a more complete barrier against moisture, oxygen, and other gases, volatile aromas, and impact of light than that of any plastic laminate material (Lamberti and Escher 2007). However, as reported by Wang et al. (2018), the packaging systems that contain an aluminum foil layer are difficult to recycle, and therefore they have a significant environmental footprint (Wang et al. 2018).
Another way to improve the barrier properties of the paper/paperboard would be the application of synthetic polymers derived from petrochemicals, since some of them have superior mechanical properties, heat stability, and certain barrier properties against a variety of compounds such as oxygen, carbon dioxide, water vapor, and aroma compounds (Cazon and Vázquez 2020). However, plastics production and processing are energy-intensive processes; they result in increased greenhouse gas emissions of enormous magnitude, which contribute to global warming. Moreover, plastic waste has been a pressing issue for many years due to plastics’ resistance to degradation (Yogalakshmi and Singh 2020). Indeed, these fossil-based plastics are usually non-biodegradable, non-compostable, and contribute to environmental pollution (Dissanayake et al. 2021). Furthermore, when they are burned, fossil carbon disoxide as well as toxic pollutants such as carbon monoxide, chlorine, hydrochloric acid, dioxin, furans, amines, benzene, 1,3-butadiene, and acetaldehyde are released, posing a risk to the environment and human health (Dissanayake et al. 2021).
Recently, the consumption of this kind of synthetic polymer has increased massively due to the COVID-19 situation (Gorrasi et al. 2020). This pandemic has resulted in an increase in demand for certain paper products, as reflected in Fig. 1 (Liu et al. 2020).
Fig. 1. Potential increasing demand for paper products amid the COVID-19 pandemic, redrawn from (Liu et al. 2020)
Unfortunately, the total waste generated has recently accelerated globally due to ineffective waste management and inadequate segregation systems. Only about 173 million tonnes waste is collected for recycling and landfill purposes after consumer use, with the remainder being improperly discarded (Rai et al. 2021). In addition, consumer preferences and health requirements are contributing to shifts in preferences toward more natural, high-quality products (Petkoska et al. 2021). Renewable carbon resources, such as starch, cellulose, hemicellulose, lignin, and plant oil, are derived from plant and wood biomass via photosynthesis from atmospheric carbon dioxide (Iwata 2015). They are being introduced into the market as a true alternative to conventional plastic formulations in packaging, reflecting additional benefits in their commercial use, including biocompatibility, barrier properties, nontoxicity, and non-polluting characteristics (Mellinas et al. 2016). Among the carbon-renewable sources, the properties of nanocellulose (NC) are critical to its significance: it is biodegradable and renewable, has high stiffness and reinforcing capacity, and has a large specific surface area (Skočaj 2019). NCs have a high elastic modulus (110 to 220 GPa) and tensile strength (7.5 to 7.7 GPa), as well as a low density and a high concentration of hydroxyl groups on their chains, which expand their application for a variety of desired purposes (Samadani et al. 2019).
Nanocellulose (NC) has significant potential to be used in papermaking process as a sustainable packaging material. The concept of sustainable packaging involves analysis and documentation to assess the design, material selection, processing, and lifecycle of the package (Abdul Khalil et al. 2016). The Sustainable Packaging Alliance’s definition of sustainable packaging identified four principles that must be considered concurrently when evaluating or designing packaging. Packaging must meet the following criteria (Lewis et al. 2010): I) be fit for purpose (effective): NC due to the durability and flexibility, can maximize the product-to-packaging ratio and in turn, meet this requirement (Abdul Khalil et al. 2016); II) consume as little material, energy, and water as possible (efficient): cellulose-based material is expensive in terms of price, but there is a cost-effective isolation method, which results in greater availability for developing relatively inexpensive polymer-based products. For instance, manufacturing biodegradable plastics from agricultural waste may pave the way for researchers and scientists seeking cost-effective, environmentally friendly materials (Yaradoddi et al. 2020); III) generate as little waste as possible (cyclic); IV) pose the fewest possible health and safety concerns to people and ecosystems (safe): The incorporation of NC into the packaging to substitute fossil fuel-based materials reduces the impact on the environment. In particular, recycled cellulose is utilized to make transparent films for pharmaceutical and food packaging, which reduces the amount of raw materials required during the manufacturing process while also degrading in the environment (Oun et al. 2020). Based on this information, cellulose offers promising prospects for use as a sustainable packaging material, but there are some issues that will need further investigation when utilized at higher production levels. For instance, studies have reported a reduction in dewatering rates following the addition of different types of NC to papermaking feed (Rantanen and Maloney 2013). Furthermore, in humid conditions, the barrier performance is significantly reduced because of NC’s inherent hygroscopic nature (Hubbe et al. 2017; Koppolu et al. 2019). Such obstacles imply a strong need for continued research in the coming years to maximize the potential of NC in a wide variety of packaging applications. Currently, the only cellulose-based film-like material that is routinely used in packaging is cellophane. It is used as a packaging film due to its effectiveness as a gas barrier in dry conditions. However, the production of cellophane is environmentally damaging, as it produces harmful by-products and utilizes sulfur-based chemicals (Reshmy et al. 2020). To overcome these drawbacks, the NC needs to be somehow modified or combined with biopolymers or other environmentally friendly NC. Depending on the purpose of the addition of NC in papermaking, whether as coating material or wet end additive, it may require a particular type of modification. This review is particularly focused on research publications that shed light on well-known obstacles to successfully implementing modified NC products to enhance packaging performance. The review will build knowledge and discuss the potential for modified NC to be used in papermaking to address the aforementioned concerns regarding the development of gas, oil, and water-resistant coatings and enhancement of the mechanical strength while minimizing the dewatering problems. A better understanding of different NC modification processes and the parameters that must be considered will potentially provide solutions to improve the paper quality, optimize the runnability of the papermaking process, enhance barrier properties, and in turn, increase the use of this technology in packaging. Indeed, the effectiveness of packaging materials is directly related to mechanical and barrier properties. Therefore, whenever in the manuscript we discuss the positive effects of application modified nanocellulose on their mechanical or barrier properties, it means their effectiveness on the packaging material. With this objective in mind, the following outline for this review paper is provided.
Objectives, Scope, and Limitations
As shown in Table 1, numerous review articles and chapters have been published on NC modification and the application of NC in the papermaking processes. The main purpose of this article is to expand the knowledge on the use of modified NC in paper manufacturing, with an emphasis on more environmentally friendly modification methods.
A brief overview of sustainable cellulose-based packaging is outlined in Section 1. Section 2 summarizes NC for packaging applications and provides a comprehensive overview of the NC, types and characteristics, sources of NC, barrier and strength agents. In Section 3, a key overview of the challenges associated with the use of NC in papermaking is provided, including I) Technical challenges, ii) Processability and runnability challenges including dewatering retention and formation challenges, iii) Economic challenges. Section 4 contains techniques for NC modification that are divided into two parts: hydrophobization and charge functionalization. Section 5 presents modified NC which have been used in papermaking and the possibilities of using modified NC in the papermaking process are highlighted together with the discussions on future directions of research and development in the studied area.
Table 1. Review Papers Focusing on the Modification of NC and Application of NC in Packaging
NANOCELLULOSE FOR PACKAGING APPLICATIONS
Nanocellulose
Biofibers are derived from biological sources and can be divided into non-wood fibers and wood fibers (Gholampour and Ozbakkaloglu 2020). Asyraf et al. (2020) defined natural fibers by origin (animals, minerals, plants, and microorganisms) and they are classified further in Fig. 2.
Fig. 2. Classification of the origin of different biofibers, redrawn from Campilho (2015)
Certain non-wood fibers outperform wood fibers in terms of physical and mechanical properties, cellulose content, crystallinity, and weight. As a result, they are suitable for engineering applications (Gholampour and Ozbakkaloglu 2020). The fiber’s characteristics are affected by its dimensions and chemical composition. They are principally composed of cellulose, hemicellulose, starch, wax, lignin, and other substances (Vijay et al. 2019). Among these substances, cellulose is the most critical component discussed in this review due to its high mechanical strength properties, large surface area, intriguing optical and rheological properties, and ease of surface modification. In theory, the tensile strength of cellulose crystalline areas is 50 to 100 GPa, which makes them stronger than E-glass. Additionally, NC exhibits shear-thinning and anomalous behaviour such as time-dependent increase in viscosity, which are advantageous for rheology properties (Jasmania and Thielemans 2018). It’s interesting to note that cellulose is the most abundant natural polymer on the globe, since it is created by photosynthesizing green plants, which cover 30% of the planet’s land area (Oberlintner et al. 2021). Additionally, algae, bacteria, fungi, grasses, and aquatic plants, and animals can be sources of cellulose (Dhali et al. 2021). Furthermore, Amara et al. (2021) outlined the isolation of cellulose from available agro-industrial by-products and wastes, which offers numerous economic and environmental advantages (Amara et al. 2021). One has to keep in mind that the amount of cellulose, its degree of crystallinity, and crystalline size might vary between different sources of cellulose (Dhali et al. 2021).
Cellulose fibers can be converted into fibrils with a lower diameter that is eventually made up of organized linear cellulose molecular chains. Due to this hierarchical structure, as shown in Fig. 3, fibrillated cellulose has a great deal of morphological and fibril size variability, resulting in mechanical, optical, thermal, fluidic, and ionic capabilities that can be significantly superior to those of parent cellulose fibers (Li et al. 2021d).
Fig. 3. Schematic illustration of the hierarchical structure of cellulose in plant-based biomass, redrawn from Rytioja et al. (2014); Green=Cellulose, blue=hemicellulose, purple=lignin and red= pectin
Very highly fibrillated cellulose is also known as nanofibrillar cellulose, cellulose nanofiber, and cellulose nanofibril (Abdul Khalil et al. 2014). Nanocellulose (NC) types can be classified based on three major factors: i) source material for cellulose (e.g., trees, plants, algae, bacteria, tunicate), ii) method of isolation (i.e. refinement by mechanical shear or acid hydrolysis which define two classifications of NC, nanofibrillated cellulose, and cellulose nanocrystals), and iii) surface chemistry (i.e. surface chemistry might be as a result of a particular nanocellulose production method or the result of subsequent chemical modification) (Li et al. 2021c).
Nanocellulose (NC) can be isolated in a variety of ways from a wide range of lignocellulosic sources. Usually, the lignocellulosic source first undergoes some pretreatment in order to achieve superior properties for the final material (Jonoobi et al. 2015). Then, a variety of strategies have been used to produce NC, most notably acid hydrolysis and mechanical treatment, either alone or in combination with other methods to achieve the desired nanoparticles characteristics (Abdul Khalil et al. 2014). These two approaches result in obtaining particle with large differences in morphology that can be classified into two groups: nanofibrillated cellulose (NFC, also known as cellulose nanofibril) and cellulose nanocrystals (CNC). The major distinction between CNC and NFC would be the number of amorphous regions and their dimensions (Oberlintner et al. 2021; Sacui et al. 2014). CNC consists of rod-shaped nanoparticles with a diameter of 10 to 20 nm and a length as high as several hundred nanometers (Fig. 4a), depending on the biological source and isolation protocol. CNC is formed by chemically hydrolyzing the amorphous regions of cellulose (Rol et al. 2019a), whereas NFC consists of fibril networks with a fibril diameter of 10 to 20 nm. The nano-scale fibrils within typical NFC are long and flexible, as can be seen in Fig. 4b. They are formed through a process called fibrillation, which involves the separation of cellulose fibrils. There are multiple procedures for obtaining NFC, including high-pressure homogenization, TEMPO-mediated oxidation, enzymatic hydrolysis, mechanical fibrillation/grinding followed by high-intensity ultrasonication, steam explosion, and cryo-crushing (Oberlintner et al. 2021). It is worth mentioning here that NFC is highly popular among scientists, with at least 65% of publications on the subject of NC focusing on NFC, according to Rol et al. (2019a).
Fig. 4. (a) Distributions of CNC and (b) is NFC, redrawn from Kumar et al. (2021)
The two main forms of NC, namely NFC, and CNC, exhibit distinct characteristics, and these have sparked increased interest among researchers. In terms of barrier properties, as shown in Fig. 5, the web structure of NFC has a complicated diffusion path. CNC particles, on the other hand, tend to form a more organized layered structure with fewer voids and a higher density (Wang et al. 2020b).
Fig. 5. Scheme of a gas passing through CNC (left) and NFC (right) films via cross sections, redrawn from Wang et al. (2020b)
The NFC suspensions can display hard solid-like viscoelastic characteristics even at relatively low concentrations, due to the densely entangled network. The viscosity, storage modulus, and loss modulus rise progressively with the increase of NFC concentration. The rheological behaviour of the CNC suspensions has been found to be strongly influenced by the concentration and aspect ratio of CNC. Elastic gel-like behaviors are observed at high concentration levels, whereas viscous liquid-like materials are observed at low concentrations (Li et al. 2015c). This has piqued scientists’ curiosity in the rheological features of NC. In general, the percolation networks, chemical interactions, and entanglement of NC enable the achievement of highly viscous suspensions in aqueous solutions at sufficient concentrations. On the other hand, continuous shearing aligns NC with the direction of flow, leading to decrease in viscosity as the shear rate increases. Such reductions are reversible when the systems come to rest. The particular high-low shear flow behavior of the NC make it attractive to be used as rheological modifiers in a wide variety of applications (Li et al. 2021c).
Both CNC and NFC, exhibit extraordinary mechanical properties in the dry state as a result of their high crystallinity and intermolecular interactions such as hydrogen bonding between cellulose chains (Saba et al. 2017; Hivechi et al. 2019). While NC has excellent mechanical properties in the dry state, its weak wet strength is a limiting factor in many applications. This issue is caused by the intrinsic hygroscopic properties of NC because their surface is covered with hydroxyl groups (Seddiqi et al. 2021) which also cause adsorption of moist air and liquid water by NC, resulting in considerable swelling of NC (Walther et al. 2020).
Nanocellulose in Packaging
As a result of the above-mentioned issues, numerous scholars have demonstrated that NC products can be used in papermaking applications, since they are advantageous in the production of strong paper and barrier-coated packaging paper products (Ehman et al. 2020; Hu et al. 2021). They also offer significant potential due to their low weight, non-toxicity, low thermal expansion, improved mechanical properties, gas barrier properties, electrical conductivity and environmental friendliness (Kumar et al. 2021). The use of NC to enhance barrier properties and mechanical strength of paper for packaging applications is detailed in the following sections.
Barriers (gas barrier and water vapor barrier)
Packaging with high barrier properties extends the shelf-life of foods while providing safety and protection when they are transported and stored. The industry and academia are both aiming to develop high-performance packaging. They are also concerned with having high barrier layers for biodegradable materials, which will require more research. Many strategies have been proposed for improving biodegradable polymers for use in packaging barriers such as different biopolymer blends, Chemical vapor deposition (CVD) of inorganic (like metal or metal oxide), incorporation of nanoparticles crystallization/orientation and chain architecture structures (Wu et al. 2021). Due to the high aspect ratio of NFCs, they are well suited for use in the aforementioned ways to enhance gas barrier capabilities (Kwon et al. 2020). The relatively high crystallinity of NFC could be part of the reason for these barrier qualities. It could also be due to the strong hydrogen bonds between cellulose and the matrix, as well as the longer path necessary for gas molecules to diffuse (Hubbe et al. 2017).
NFC as barriers can be applied to the surface of the paper in a variety of ways, including spray, bar, roll, and size press coating, resulting in a range of coat weights (Brodin et al. 2014). Coating paper and paperboard with CNF has no discernible effect on tensile strength but significantly improves bending stiffness. This is because bending stiffness of paper and paperboard is proportional to the third power of their thickness. Covering paper with CNF thickens the papers, resulting in a stiffer product (Mazhari Mousavi et al. 2018; Ham et al. 2020). Chemin et al. (2019) fabricated multi-layered hybrid thin films with more tortuous paths by using CNC and gibbsite nanoplatelets to enhance the oxygen barrier properties (Chemin et al. 2019). Mazhari Mousavi et al. (2018) also combined CNF and carboxymethyl cellulose (CMC) to coat paper, claiming that there was a significant improvement in the barrier properties of paperboard, which was directly related to the coat weight and surface coverage (Mazhari Mousavi et al. 2018). Recently, nanofiller-NC composites have developed as a novel form of composite material with strong strength and gas barrier properties (Mirmehdi et al. 2018). Seyedmohammad Mirmehdi et al. (2018) proposed a coating layer of a hybrid composite of NFC (matrix) and nano-clay (mineral filler) on paper substrate. Indeed, the use of nano-clay alone lowered tensile strength as well as the rate of oxygen transmission rate (Cairns et al. 2019). The application of NFC, on the other hand, increased the thickness of the films, affecting their strength and barrier properties. As a result, the combination of NFC (matrix) and nano-clay can result in interesting strength and gas barrier qualities (Mirmehdi et al. 2018). NC can also be applied as a filler (NC-filler), contributing to improvement of the gas barrier properties of packaging material since (i) NC-filler material may act as physical obstacles and makes for a more tortuous journey (ii) the addition of NC-fillers also decreases the polymer’s free volume (Rigotti et al. 2020). Xu et al. (2019a) reported the great capability of NFC and nanofillers chitin whisker (CHW) as nanofillers for improving the mechanical and barrier properties of polybutylene succinate-based films for biodegradable food packaging (Xu et al. 2019a). Dhar et al. (2015) also demonstrated the production of nano-biocomposite films based on poly (3-hydroxybutyrate)/CNC with superior gas barrier properties for food packaging applications. However, there is an issue with obtaining homogeneous NC-filler dispersion and strong NC-filler-polymer interactions (Wu et al. 2021). It should be added that these bio-barriers exhibit comparable properties to those of engineering polymers, with the exception of the water vapor barrier (Chowdhury et al. 2019). Indeed, the water vapor permeability of unmodified NC does not follow the same trends due to the hydrophilicity of cellulose (Kwon et al. 2020). In order to improve the water vapor barrier property of NFC-based substances, inorganic fillers or cross-linking agents, as well as chemical modification of NFC has been suggested by Yook et al. (2020).
Strength agents
Paper’s strength is affected by three aspects: (a) fiber strength, (b) fiber bonding capability, and (c) fiber entanglement properties. The usage of strength agents mostly reflects an enhancement in fiber bonding strength due to greater hydrogen bond formation (Li et al. 2021a). The NCs as strength agents have the potential to enhance the mechanical strength, density, barrier properties/air resistance, filler retention, and flocculation (He et al. 2016). It has been demonstrated that addition modified cellulose nanofibrils to the fiber matrix improves filler retention, lead to significantly enhanced dry and wet strength of the paper (Lourenço et al. 2019).
The mechanical strength and modulus of NFC is particularly impressive, and the tensile strength of single fibers is nearly twice that of some metals (such as Carbon Steels ASTM A36, ASTM A529, ASTM 709 due to these 3 nm-wide fibrils (Zhao et al. 2018). Furthermore, NCs benefit from Young’s modulus values between 20 and 50 GPa, which enables it to be used in a wide variety of fields such as nanopapers, NC films, aerogels, and hydrogels (Usov et al. 2015; Thomas et al. 2018). In papermaking, NFC could be applied in the wet section of the papermaking process to increase the strength of paper. The strength improvement is partially accomplished by filling in voids and pores at the edges of each fiber bond, thereby increasing the available bonded area (Brodin et al. 2014). He et al. (2017) demonstrated enhancement of strength properties by filling inter-fiber spaces of the sheet with higher surface area particles, and then the contact area at the fiber to fiber joint edge is increased, allowing for more hydrogen bonding sites (He et al. 2017). Additionally, NFC can enhance the paper’s strength capabilities by establishing strong fiber-to-fiber bonds as a result of their nanostructure and huge surface area, which leads to more hydrogen bonds formed in a strong network (Das et al. 2020). Lv et al. (2017) investigated the effect of using the MFC-ground calcium carbonate (GCC) complex on the strength and optical properties of handsheets. They used an MFC dosage of 5%, based on the GCC weight. The results indicated that the modified GCC acted like fibers and had improved retention due to the effective adsorption between GCC and MFC. Better paper strength qualities were attained at the same filler quantity (Lv et al. 2017). Su et al. (2020) also premixed MFC with GCC and discovered that the MFC-modified GCC increased the opacity of the filled paper while also improving its tensile strength. It is vital to note that high NC addition does not always result in favorable reinforcement effects for paper (Yuan et al. 2016) and optimization is essential in determining the ideal amount of additive.
The second parameter to optimize is the degree of fibrillation, which has a significant effect on the mechanical properties of NCs and, more specifically, on the paper sheet. This means that MFC with a broader particle size distribution shows less improvement in mechanical properties but more efficient retention in the fiber web. Even before sheet formation, NFC with a high degree of fibrillation can be distributed more effortlessly in the bulk suspension. As a result, a more homogeneous distribution occurs, which improves the nanostructure’s strength (González et al. 2014). However, a high degree of fibrillation does not always result in a beneficial reinforced effect, owing to their shorter length and lower aspect ratio. Indeed, short fibrils are more difficult to retain on fibers, so they contribute less to the strengthening of interfibrous bonding (Hu et al. 2021).
Challenges of Nanocellulose in Papermaking
Runnability challenges
In the paper manufacturing process, runnability refers to a machine’s ability to produce high-quality paper at maximum capacity. Therefore, the maintenance of “good” operation plays a vital role in paper production (Bondoc 2018). Some researchers have investigated the influence of NC application on the runnability of the papermaking process (Kajanto and Kosonen 2012; Lu et al. 2019; Charani and Moradian 2019). Kajanto and Kosonen (2012) used a high-speed pilot paper machine to test the use of NFC as a reinforcement agent in the paper. During the trial, overall runnability was satisfactory. No web cracks are found related to the issue with paper strength or dewatering. The total retention level stayed steady and the formation remained satisfactory without the need for any further changes. Dewatering in the press and former sections was acceptable (Kajanto and Kosonen 2012). Lu et al. (2019) investigated the properties of base paper’s wet-web strength and pressability, as well as their effect on the frequency of sheet breaks and machine runnability. They reported that after applying 5% NFC to the sample, the tensile energy absorption increased from 6.32 to 10.93 J/m2. As a result, they stated that adding CNFs to the paper-making process increases wet-web strength and improves paper machine runnability (Lu et al. 2019).
Charani et al. (2019) found that combining less cationic starch (CS) (0.5%) with 3 percent NFC greatly boosted paper breaking length while lowering CS-related process challenges (Charani and Moradian 2019). Another study found that adding 3, 6, and 9 wt% NFC to handsheets increased linearly the tensile index to around 55 N m gL at 35SR, which was judged to be suitable for paper machine runnability (Vallejos et al. 2016). Ankerfors et al. (2014) used the FEX pilot paper machine to examine the impact of MFC on the runnability and paper strength of a fine-paper. During the pilot paper machine trial, no serious runnability difficulties were reported. They also demonstrated that dewatering was affected by the MFC (NC), particularly in the wire portion (Ankerfors et al. 2014). As a result of the decreased drainage rate, the machine’s speed must be decreased as well, and there would need to be strategies in place to compensate for the decreased drainage rate caused by the NC application. This means that, for example, by using retention and drainage aids, paper machine running speeds can be greatly boosted; also, the retention of fine and NC particles increases and contributes to the better runnability of paper machines (Jin et al. 2014).
Many studies have looked at the effects of NC as a coating of paper (Aulin and Ström 2013; Chowdhury et al. 2019; Lengowski et al. 2019; He et al. 2021). Regarding their runnability as the coating can be mentioned that resistance to dewatering into the substrate is a critical process parameter. Indeed, both wet film formation and process runnability are influenced by water retention or the rate at which water is discharged into the substrate. A rapid release of significant amounts of water from MFC suspensions into paper can induce fiber swelling and de-bonding, resulting in runnability issues such as web breaks (Lengowski et al. 2019). Thereby, understanding and controlling the water retention characteristics of NC suspensions is critical for a successful coating process (Kumar 2018).
Additionally, high water content in NFC coatings results in greater drying costs, which are undesirable except if outweighed by enhanced product properties (Brodin et al. 2014). Drying also contributes to increasing the concentration of NC and then would affect drying dynamics and assembly operations (Wang et al. 2019).
The next challenge with runnability is that NC suspensions are difficult to coat onto substrates using conventional coating and metering mechanisms. This is due to blockage caused by NC fiber aggregation or the NC suspensions’ extraordinarily high viscosity (Kumar 2018). NFC suspensions are well-known for their high viscosity, even when the solids content is low, contributing to the limitation of the size of the operation window (Brodin et al. 2014). It should be added that improvement and controlling the rheological features of NFC-containing coating formulations is a significant challenge for lowering water content while maintaining a uniform coating layer (Brodin et al. 2014). As a result of changes in the rheology, papermakers must vary the nip load, doctor blade angle, or pressure when introducing NFC into the composition (Sharma et al. 2020).
Processability Challenges
Dewatering
Nanocellulose (NC) has not yet been widely applied in the papermaking industry, and this has been attributed to difficulties in using it (Balea et al. 2020). Nanofibrillated cellulose (NFC) usually increases the time needed for drainage dewatering, and a short draining time is needed in the papermaking process to minimize production costs and energy (Hu et al. 2021). Numerous causes to the delayed dewatering efficiency can be summarized as:
- Dense layers are created,
- Cellulose particles clog drainage channels in the wet web,
- The reaction to vacuum is inefficient because of flocculation,
- The healing mechanism allows for the repair of thin areas of paper,
- A thin membrane forms on the surface of the paper when it comes into contact with a wet-press felt, due to rewetting of the sheet (Hubbe et al. 2020).
Besides, NFC creates a highly entangled network in aqueous dispersion, exhibiting a gel-like network at 1% solid levels. NFC surface charge appears to influence the rheological and dewatering properties of furnishes through its influence on swelling and effective binding of water. In fact, swelling of the fiber walls results in fewer fiber contacts, which reduces floc size and therefore lowers frictional forces between fibers, affecting rheology and dewatering of the fiber suspension network under flow (Dimic-Misic et al. 2013). Additionally, dewatering is closely linked to the shear-thinning behaviour of furnish. When the shear field disrupts the suspension’s microstructure, then the process can spread across the pad’s flow channels. As a result, the application of repetitive shear cycles can help the dewatering process (Dimic-Misic et al. 2013). According to some researchers, the presence of cationic NFC (CNFC) can also aid dewatering while improving the wet-web strength of paper sheets (Lu et al. 2020b). Diab et al. (2015) reported that cationic microfibrillated cellulose (CMFC) was unable to increase the pulp’s drainage. The author recommends that more trials using CMFC with a higher degree of cationization should be conducted to confirm that CMFC cannot be employed as drainage agents (Diab et al. 2015).
Lu et al. (2020b) assumed that the increased fines retention and density of paper sheets facilitated by CNFC addition are due to the factors including the following:
(1) The CNFC nanoscale dimensions provide a large specific surface area, which helps in adsorbing and “catching” the fines to create the CNFC-fines complex in the pulp;
(2) The high aspect ratio of CNFC would make it easier to twine and “lock” the fines together in order to create the CNFC-fines complex.
(3) The high surface charge density of CNFC’s quaternary ammonium ions enhances their integration with fines to create the CNFC-fines complex (Lu et al. 2020b).
Aside from the surface charge, other factors influencing the dewatering rate of NC used in paper production include the location of the NC addition and the type of retention additive used in paper manufacturing. Indeed, some researchers believe that by adding relatively small amounts of NFC directly to the pulp with appropriate retention aids, dewatering issues could be avoided (Li et al. 2021a). Ottesen et al. (2016) premixed NFC and retention chemicals with filler and predicted that the retention chemicals would enable NFC or fillers to adsorb preferentially to accessible surfaces. They reported large reductions in dewatering times through the use of retention chemicals that bind the NFC to the various components of the paper furnish. These benefits can be amplified even more for higher NFC doses by placing NFC in the filler fraction before adding it to the furnish (Ottesen et al. 2016). In general, the complicated interactions between cellulose pulp, NFC, mineral fillers, and regularly used additives should be taken into account to address this issue (Li et al. 2021a).
Retention
While a high aspect ratio of NC is critical for producing high-quality nanopapers, it is also one of the primary causes of agglomeration and flocculation problems. From the standpoint of colloid science, the most basic solution is to increase the electrostatic charge on the nanofibrils’ surface, which will cause like charges to repel each other, preventing aggregation and flocculation (Li et al. 2015c). However, from the perspective of particle retention, hydrodynamic shear and turbulence can easily wash the comparatively small negative charge particles off the web and reduce retention in the forming phase. The increase in retention appears to be due to the retention agent’s charge neutralization and aggregation properties (Ahadian et al. 2021). Therefore, there would be a trade-off between retention and flocculation in this case that must be considered.
In terms of retention, polyelectrolytes are often used by researchers to improve the retention efficiency of fillers, fines, and small particles and to reduce dewatering times in the papermaking process. These polyelectrolytes have the ability to bind fines so that they remain in wet sheets. This happens due to a flocculation effect, such that the fines are electrostatically connected to fibers. These kinds of polyelectrolytes enhance the drainage rate and retention of small particles while influencing the reinforced effect of NFC (Hu et al. 2021). The presence of retention aids was found to affect the position and association of fibrils during adsorption, and hence it affected the final handsheet characteristics. For instance, the densities of sheets formed in the presence of polyvinylamine as retention aids are lower than those formed in the absence of a retention aid. This could be explained by the fact that when retention agents are present, fibrils are more likely to attach to the fibers surface before dewatering rather than filling the pores between the fibers during dewatering (Hollertz et al. 2017). NFC-retention additive interactions and appropriate dosage of retention additive must be studied in order to select the most beneficial combination (Merayo et al. 2017b). Taipale et al. (2010) demonstrated that by selecting the right materials and operating conditions, the strength qualities could be improved without compromising the drainage. First, a thin MFC (NC) layer can develop on the adsorbed CS, and this nanonetwork can then coat the fibers instead of filling the gaps between them (Taipale et al. 2010). Merayo et al. (2017) also believed that employing the proper retention additive at the right dose could help to alleviate the dewatering issue while using NFC in paper production. So, they investigated the idea of interaction between MFC (NC) and retention agents (polyvinylamine, chitosan (CH), cationic starch (CS), and two type cationic polyacrylamide (C-PAM additive formed by poly-quaternary ammonium chloride and C-PAM-B additive formed by polyamine, PAM and hydrated bentonite clay) in order to increase the strength of recycled paper while minimizing adverse effects on the drainage process. When comparing the behavior of each additive at the lowest doses tested, C-PAM and C-PAM-B came out on top, followed by CH. Polyvinylamine also showed satisfactory drainage time results at the lowest dose; however, such dose was ten times higher than C-PAM, C-PAM-B, and CH. The CS was the least effective additive tested, especially at low and moderate doses (Merayo et al. 2017b). This result also implies that the conformation of fibrillated material onto cellulose fibers is influenced by retention aids; the final configuration indicates the extent to which the degree of strength and drainage rate have been increased (Zambrano et al. 2020). Finally, a single retention additive may not be satisfactory, and the development of two-component reagents has been recommended as a way to improve performances (Lourenço et al. 2019). In the case of NFC as retention agents, it can be mentioned that NFC l with a high specific surface area has the ability to flocculate cationic particles mostly via bridging/networking. Following the application of high shear and flocs breaking, subsequent retention agents on the flocs may help to approach flocs, resulting in reflocculation via patch or networking mechanisms. However, chemical modification of NFC is required to enhance its electrostatic interactions with other particles in the system. (Korhonen and Laine 2014).
Formation
The term “formation” (more formally “formation uniformity”) refers to the spatial variation in the density of fibers in the paper (Na and Muhlstein 2019). It can be determined by examining the sheet’s mass distribution in its plane. The goal of the fiber network formation process is to distribute the pulp as evenly as possible on the wire to ensure that the product properties remain consistent across the width of the web (Norman 2000). As shown in Fig. 6, flocculation and dispersion are two competing effects in the papermaking process.
Fig. 6. Right: Random Fiber distribution; Left: Fiber Flocculation distribution, redrawn from Dodson and Serafino (1993)
The importance of paper’s formation uniformity in terms of sheet appearance is widely acknowledged among papermakers, as the strength is controlled by fiber-to-fiber interactions that are developed during the process of paper formation, consolidation, and drying (Su et al. 2012). It has long been recognized that paper’s physical properties are somewhat dependent on its formation, with the effect being significant for tensile strength but small for elastic modulus (Anson et al. 2007). As reported by Motamedian et al. (2019), the paper formation is frequently improved by shortening fibers; this explains why the refining process might have an effect on the formation (Motamedian et al. 2019). From another perspective, increased fiber refining increases bonding formation and can somewhat offset the negative effect of filler on strength qualities (Ankerfors et al. 2014). Nanofibrillated cellulose (NFC) can bridge the filler-induced voids in the fiber-to-fiber bonding area, similar to fines, and affect the fiber network (Ämmälä et al. 2013). Two alternative processes can be used to explain the enhancing effect of NC on base paper: (1) NC promotes fiber–fiber bonding by bridging fibers together, and (2) NCs can create network architectures between relatively coarse fibers, enhancing the load-bearing capacity of papers (Li et al. 2021a). As NCs are derived from natural fibers, possessing inherent characteristics of pulp fibers, the slender filaments can form a densely entangled network structure (Li et al. 2021a). Merayo et al. (2017a) also confirm that the sheet homogeneity (formation index) improves due to a reduction in floc size by using NFC.
However, their small size makes it challenging to retain NFC during sheet formation in order to make use of their bond-forming capacity. As mentioned above, various solutions have been proposed to maintain the NC in the sheet formation process. For example, the use of a retention aid not only assists in the retention of the NC in the furnish, but it also can contribute to the formation of a homogeneous formation (Diab et al. 2015). Ahola et al. (2008) combined paper NFC with a cationic poly(amideamine epichlorohydrin) to improve the wet and dry strength of paper and they showed that when PAE first adsorbed on the fiber surface, it generated a homogeneous and viscous coating of NFC. Salminen (2010) studied the effect of (C-PAM on the short fiber fraction and CS on the long fiber fraction on the formation, retention, and strength of the paper. They claimed that adding C-PAM to short fiber fractions would prevent long fibers flocculation, resulting in the improved formation and, in turn, increased wet web strength.
Economic Challenges
Nanocellulose (NC) applications have a 6.4 million metric ton yearly market potential in the United States, and a 35 million metric ton global market potential. Currently, cellulose nanoparticles have the largest volume potential in paper and packaging applications. The automobile, construction, personal care, and textile sectors are other examples of high-volume applications (Shatkin et al. 2014). According to Nelson et al. (2016), the United States Department of Agriculture’s global NC market size projection will be reached around 2045. Commercialization of technologies for producing NC from kraft or dissolving pulp has occurred recently. Pulp is a costly raw material that necessitates rigorous, often energy-intensive chemical or mechanical processing (Blair and Mabee 2021). Additionally, chemical costs and investment in manufacturing equipment and paper machines, are other factors that influence the cost of NC manufacture (Li et al. 2021a). Due to the confidentiality issue, it is hard to provide a precise estimate of the end-use product’s cost. A rough calculation based on the raw material pricing and manufacturing costs revealed that the cost of NFC will range between USD$ 7 and 12 per kilogram of dry material (Dhali et al. 2021). Estimated CNC production costs differ widely across the literature; de Assis et al. (2017) approximated that manufacturing costs for CNC production ranged from 3632 to 4420 (dry equivalent) USD t-1. Their model process was acid hydrolysis of dissolving pulp (de Assis et al. 2017). There are currently a few pilot projects producing these nanomaterials, such as CelluForce (Canada), USDA’s Forest Products Laboratory, InnoTech Alberta (Canada), FPInnovations (Canada), and Cellulose Lab (Canada) (Rudie 2017). Numerous difficulties and obstacles remain along the path from pilot to large-scale production. For instance, CNC that is produced via acid hydrolysis presents challenges in terms of waste treatment, yield, drying, and redispersion. Numerous efforts have been made to overcome these types of problems such as the fabrication of NC using solid organic acids hydrolysis (Chen et al. 2016; Jia et al. 2017). The recovery of and the reuse of these kinds of acids is easily achievable through a conventional and commercially proven crystallization process at a low or ambient temperature. In fact, since they can be recovered using a conventional and commercially proven crystallization method, organic acids are uniquely excellent candidates for making sustainable and green cellulose nanomaterials (Chen et al. 2016; Jia et al. 2017).
In the case of NFC, the economic problem is related to the usage of mechanical methods, which involve large amounts of energy consumption (Wang and Cha 2019). Eriksen et al. (2008) calculated that a production NC by homogenizer can consume up to 70,000 kWh/t of energy, which is nearly 100 times more than the energy required to make one ton of paper (Eriksen et al. 2008; Chauve and Bras 2014). Therefore, various pre-treatment methods have been developed to significantly reduce the energy consumption associated with NC production. Even with pre-treatments, matching the cost of NC production to specific benefits such as mechanical strength performance remains a considerable difficulty (Ang et al. 2019). The economy of producing NFC materials depends largely on the pre-treatment method (e.g. enzymatic, carboxymethylation, TEMPO-modified NFC, etc.). The least costly process is likely to be the enzymatic pre-treatment process, which produces NFC from pulp integrated with a pulp mill at a cost of 0.4 €/kg and is currently used in large-scale paper manufacturing applications. The cost of non-integrated NFC in papermaking applications should be less than 2.5€/kg (Klemm et al. 2018). Indeed, integrating the production of fibrillated cellulose products with the current forest and paper industries appears to be a synergistic technique for cutting large-scale production costs (Li et al. 2021d). With the advent of low-cost commercial NC sources, there is still space for new applications and improvements to existing ones in a variety of industries that require sophisticated materials (Trache et al. 2020). In general, the economic challenges that remain in the field of NC manufacturing that need to be overcome in order to ensure rapid utilization and commercialization include the following: 1) Cost-effectively drying of NC suspensions while preserving distinct NC particle shape for optimal re-dispersion during end-use 2) costs related to the creation of international standards across the supply chain, and 3) development of low-cost, quick characterization methods for detecting product quality (Nelson et al. 2016). Additionally, in the paper industry, unmodified cellulose has few applications in packaging films. When cellulose is modified chemically, mechanically, or enzymatically, it can be used in a variety of applications (Fotie et al. 2020). Therefore, in addition to the preparation of NC, the cost of treatment should be considered, which is currently an expensive process (Nelson et al. 2016; Blair and Mabee 2021).
There is an effort underway to develop processes that will enable NC to be sold at a lower cost, for instance with increased scale production costs could be significantly reduced. Furthermore, capital costs could be reduced through government investment or infrastructure repurposing. Increased process efficiency or internal energy generation could also help to reduce energy costs (Nelson et al. 2016). Along with the successful extraction of NC via pre-treatments (Ang et al. 2019), some practices such as developing new, environmentally friendly, and inexpensive solvents as pre-treating agents are generating increased interest in academia and industry (Liu et al. 2019b).
Following the decrease in the cost of protection, additional research must be conducted to ensure the final materials’ feasibility and marketability, in particular (1) life-cycle evaluations and degradability studies, as required to demonstrate the environmental impact; (2) continuous cost-cutting optimization of the fibrillated cellulose production process; (3) a careful balance between usability and biodegradability when discarded; and (4) a recycling technique for composites incorporating fibrillated cellulose (Li et al. 2021d).
MODIFICATION TECHNIQUES OF NANOCELLULOSE
Modifying NC can help address some of the above limitations, since the hydroxyl groups in the NC chain are critical in enabling NC to be used in a variety of applications. They can be adjusted in a variety of ways, depending on the application, and NC can be impacted based on the chemical and condition utilized (Rol et al. 2019a). These modifications result in the acquisition of desirable properties, which improve their effectiveness for a particular application (Trache et al. 2020).
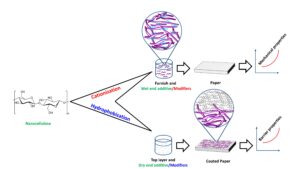
In this review paper, modification techniques are further classified into two types based on the reason for adding modified NC to the paper, as shown in Fig. 7. The first category is associated with the goal of improving the barrier properties of paper, with a particular emphasis on NC hydrophobization and its usage as a dry end additive. The next category is involved with the modification to impart stable positive or negative electrostatic charges on the surface to improve retention and mechanical properties. The factor that could determine the effectiveness of modification is the degree of substitution (DS), with the maximum value being 3, which occurs when all hydroxyl groups on anhydroglucose units react (Oberlintner et al. 2021).
Fig. 7. Various modification methods that will be discussed in this review article
Hydrophobization of Nanocellulose
The presence of a high hydroxyl group concentration on the surface of NC confers on them inherent hydrophilicity. Self-agglomeration and hydrophilicity have limited their applicability, prompting development of surface functionalization and hydrophobization in order to effectively utilize their potential (Chin et al. 2018). In general, functionalization with chemical grafting is preferred over physical grafting because the covalent bonding of grafted chains to the substrate surface prevents desorption and promotes longer chemical stability (Sun et al. 2020). However, the need for solvent-based or toxic-based systems have limited their application to industrial scale-up. As a result, several European projects have been started, such as the SUNPAP, to discover alternatives to these solvents (Missoum et al. 2013). As a consequence, researchers are increasingly concentrating their efforts on developing more environmentally friendly strategies for chemical surface modification, which can be unquestionably the most important aspect of NFC functionalization (Missoum et al. 2013). With this in mind, in the following text summarizes some of the hydrophobization methods (not totally green but a bit more “environment-friendly” methods) that use the fewest amount of solvent and toxic materials possible.
Hydrophobization: Physical adsorption of surfactants
Surfactant adsorption is a green, industrially scalable, and environmentally friendly method for engineering the NC surface (Chi and Catchmark 2017; Szlek et al. 2022). In fact, the amphiphilic nature of surfactants is ideal for modifying the particles’ surface tension and contact angle. Surfactants can reduce the interfacial tension between air and water, as well as the forces that keep water in the filter cake capillaries (Patra et al. 2016). When they are adsorbed onto the less charged regions of NC surfaces, the net Gibbs free energy is decreased, as shown in Fig. 8 (Tardy et al. 2017).
Fig. 8. Schematic illustration effect of adsorption surfactant on the Gibbs energy. Note that the details of the “hydrophilic cellulose” are not intended to represent a specific chemical structure or tubular nature.
Surfactants are made up of two parts: a hydrophilic head and a hydrophobic tail (Vaziri Hassas et al. 2014). According to the charge of their hydrophilic moiety, there are four types of surfactants: anionic, cationic, non-ionic, and amphoteric (Kurpiers et al. 2021). Two key aspects influence surfactant adsorption: the surfactant’s interaction with an electrostatic force and the surfactant’s hydrophobicity, which will cause it to interact with the surface if it is less polar than water (Aulin et al. 2008).
An anionic surfactant’s affinity for NC surfaces can be explained by hydrophobic interactions between the surfactant’s hydrophobic groups and the intrinsic hydrophobic domains found on cellulosic materials (Tardy et al. 2017). Cationic surfactants are predicted to have a high affinity for NC surfaces due to the different charges. These electrostatic interactions are enhanced by the presence of functional groups on NC. The reason for this is that electrostatic interactions are an order of magnitude more powerful than secondary interactions (Tardy et al. 2017). Ly et al. (2020) demonstrated that the nature of the surfactants used in the CNC modification can be attributed to the increasing of water contact angle (WCA) in the modified samples. All three surfactants considered (cetyltetramethylammoniumbromide with C16 single chain, dimethyldidodecylammonium bromide with C12 double chains, and dimethyldihexadecylammonium with C12 double chains), have the ability to arrange their hydrophobic tails around CNC surfaces, resulting in hydrophobicity, although the longer the surfactant’s aliphatic chain, the greater the hydrophobicity of the CNC (Ly and Mekonnen 2020). It should be added the modification of cellulose is dependent not only on the type of surfactant, but also on the amount of adsorption (Patra et al. 2016). For instance, de Lima et al. (2019) found that the surfactant had no effect on free hydroxyls. They reported that (1) the CNC has a strong physical interaction and steric stabilization, which can be seen as an increase in particle size, and (2) the surfactant does not serve as a stabilizing agent, resulting in no charge differences (de Lima et al. 2019).
It is important to notice that the use of surfactant chains makes the crystalline ordering decrease, and the spacing between the planes consequently will increase. Souza et al. (2020) modified NC during isolation with an anionic surfactant. According to their study, this in situ modification led to a decrease in the NC dimensions because of the electrostatic interactions with the surfactant which may improve the grinding process, making it more productive (Souza et al. 2020).
Hydrophobization: using oil
Vegetable oils can be used to modify the surface of cellulose nanomaterials through an esterification process or by grafting vegetable oil, as shown in Fig. 9. The majority of vegetable oils are triglycerides, which are made up of glycerol molecules and three long-chain fatty acids joined by ester bonds at the hydroxyl groups. The long-chain fatty acids are more hydrophobic than shorter fatty acids and thus more stable at high temperatures (Le and Nguyen 2020; Hashemzehi et al. 2022).
Fig. 9. Transesterification reaction between cellulose and triglycerides
Shang et al. (2013) modified CNC by using castor oil and diisocyanate as a linking agent. They believed that two of the three hydroxyl groups in castor oil should be terminated to leave only one active hydroxyl group available for covalent grafting onto CNC. Following a successful graft, the surface energy decreased and the contact angle increased significantly (Shang et al. 2013). Pourmoazzen et al. (2020) anchored successfully covalently cholesterol to CNC surfaces. It was clear that hydrophobic CNC had been achieved, because the contact angle was increased to above 115 (Pourmoazzen et al. 2020).
Transesterification results in a little increase in the crystalline index, which has been related to hydrolysis or crystallization of the amorphous portion of NC under acidic conditions and high temperatures during esterification reaction (Yoo and Youngblood 2016). Wei et al. (2017) used a transesterification reaction to study the chemical modification of CNC using canola oil fatty acid methyl ester. As compared to unmodified CNC, the trans-esterified CNC had greater thermal stability and hydrophobicity. Besides, glycerol, a by-product of the canola oil fatty acid methyl ester manufacturing process, can be used as emulsions and polymer processing aids (Wei et al. 2017). Gorade et al. (2019) developed the hydrophobic modification of microcrystalline cellulose (MCC) using rice bran oil (RBO). The WCA of AUMCC was 92.2°, with the surface energy dropping to 35.56 mN/m and water absorption of 0.9 mL/mg. AUMCC’s thermal stability and crystallinity deteriorated as treatment time progressed (Gorade et al. 2019). Dhuiege et al. (2018) present a new technique for surface functionalization of CNC in water, by transesterification of vinyl acetate. They believed that developing a water-based technique would considerably reduce the treatment’s environmental impact. As reported by Dhuiege et al. (2018), the increasing hydrophobicity contributes to the progressive change of the dispersive characteristics in various solvents (Dhuiège et al. 2018). Onwukamike et al. (2018) have done research on sustainable cellulose transesterification using the CO2-DBU solvent. They were able to achieve a DS of 1.59 and good thermal stability (95% at 360 °C) as well as high elastic moduli (up to 478 MPa), maximum stress of about 22 MPa, and the maximum elongation (strain) of about 35% (Onwukamike et al. 2018). Mokhena and John (2020) used canola vegetable oil to chemically modify NFC with and without catalyst. When NFC was esterified in the presence of a catalyst, a considerable extent of derivitization occurred, leading to relatively high DS) Modification without the addition of a catalyst resulted in a less significant change in the crystallinity index (Mokhena and John 2020). Concerning the disadvantages of this method, it should be noted that this method had a significant effect on the crystallinity, mechanical, light transmittance, and thermal properties (Mokhena and John 2020).
The next-generation materials containing oil, alkyd resins, and epoxy resin can play a key role as a coating binder because of their great flexibility in terms of construction, property changes, and their low cost (Taylor 2001). They could also be very interesting candidates for commercial application and up-scaling when combined with NC due to their hydrophobicity, biodegradability, and renewability (Aulin and Ström 2013). Their adhesive properties and excellent mechanical performance make them particularly well-suited for use in coating applications (Aziz et al. 2021). Aulin and Ström (2013) evaluated the effects of the different types of alkyd resin oil and varying the length of the oil on the unmodified NC-coated papers. Alkyd resins having longer fatty acid chains allow for the efficient formation of a hydrophobic polymer layer. Alkyd resins based on tall oil and linseed oil at the same oil length were compared. The alkyd resin based on linseed oil was shown to be the most effective in terms of lowering water vapor transmission rate (WVTR) due to the fatty acid composition and the degree of unsaturation (Aulin and Ström 2013). Xu et al. (2019b) also used epoxidized soybean oil (ESO) as a low-cost, sustainable, and environmentally friendly raw material for forming modified cellulose. The WCA of modified cellulose aerogels was 132.6° when the mass fraction of ESO was 30 wt.% (Xu et al. 2019b). Huang et al. (2017) investigated the effect of grafting ESO with varying weight percentages onto properties of the cellulose. When the ESO concentration was raised, the WCA increased to 145.1°. The modified cellulose surface became rougher, which further increased its hydrophobic properties (Huang et al. 2017). Lu et al. (2015) developed a biodegradable agent to coat the NC film with a low WVTR. To reduce the WVTR, a rod coater was used to apply a coating agent composed of AESO and 3-aminopropyltriethoxysilane (APTS). The resulting film has great potential as a green packaging material (Lu et al. 2015).
Auclair et al. (2020) reported the use of coating systems with acrylated epoxidized soybean oil and CNC. They reported that CNC should be hydrophobized to make it more compatible with the hydrophobic AESO matrix due to a polarity mismatch between AESO and CNC. Therefore, the negative or positive electrostatic charges were applied to the CNC surface, resulting in improved polymer matrix dispersion. The enhancement compatibility was also achieved by adjusting the surface energy properties of nonpolar or hydrophobic polymer matrices (Auclair et al. 2020). This means that in order to improve the adhesion between MFC and the epoxy resin polymer matrix, the surface of the MFC should be modified, changing its character from hydrophilic to hydrophobic while keeping the crystalline structure of the MFC intact (Siró and Plackett 2010). Thus, two more steps are required to modify NFC with this type of biomaterial in order to achieve the desired results, which drives up the cost of coating materials. Moreover, these materials have a relatively long cure time and require high temperatures to cure (Tambe et al. 2016). They also typically have low tensile strengths, which limits their use as structural materials. The incorporation of fillers or reinforcements has been demonstrated to be a viable strategy for increasing the mechanical strength of shape-memory polymers (Sain et al. 2020).
Hydrophobization: Silylation
The silylation method is less environmentally damaging than the esterification methods, and it can be carried out via a solid-gas reaction without the use of a solvent (Jarrah et al. 2018). The surface hydroxyl groups on hydrophilic fibers are converted to alkyl silyl ethers via silylation, resulting in hydrophobic surfaces as shown in Fig. 10 (Jarrah et al. 2018). A silylation reaction produces ammonia as a by-product, which is a significant advantage of this method (Jankauskaitė et al. 2020).
Fig. 10. Schematic diagram of the possible products of CH3Si-modified cellulose, redrawn from Jarrah et al. (2018)
Khanjanzadeh et al. (2018) modified NC with 3-aminopropyltriethoxysilane, without using hazardous solvents. The surface morphology of nanocrystals was not considerably affected by functionalization. Nie et al. (2021) also modified the hydroxyl groups of the NFC surface with triethoxy-1H,1H,2H,2H-tridecafluoro-n-octylsilane, which enhanced their triboelectric charge density and hydrophobicity. The modified NFC showed growth in contact angle from 52° to 128° (Nie et al. 2021). Jarrah et al. (2018) used a gas-solid silylation reaction with silyl chlorides (RSiCl3) to attach hydrophobic alkyl groups on the cellulose surface of cotton fibers, thereby converting them into hydrophobic materials and achieving a DS in the range of 0.10 to 0.29 per glucose unit (Jarrah et al. 2018). Yook et al. (2020) prepared various types of NFC, such as cellulose nanofibril (NFC), lignocellulose nanofibril, carboxymethylated cellulose nanofibril, silylated cellulose nanofibril (S-NFC), alkyl ketene dimer-added NFC (AKD-NFC), and coated on linerboard and wood-free paper to investigate the air, oxygen, water and grease barrier properties of these papers. The S-NFC and AKD-NFC were more water-resistant than NFC. The S-NFC had WCAs over 90 degrees. However, because of the short length of the hydrophobic alkyl groups in S-NFC, they may not inhibit effective water vapor transfer (Yook et al. 2020). It should also be emphasized that silane coupling agents have a high affinity for hydroxyl groups, even at room temperature; the NC core chains become silylated to the point of being soluble in the reaction medium, resulting in crystal disintegration and loss of the cellulose’s original morphology (Ghasemlou et al. 2021)
Hydrophobization: Using plant polyphenols
The application of plant polyphenols has recently been introduced as an approach for developing hydrophobic nanoparticles in an environmentally friendly manner, in which low-cost plant polyphenols serve as precursors for the development of multifunctional coatings (Hu et al. 2017). Tannic acid (TA) is a naturally occurring polyphenolic compound with a wide range of bonding abilities. It can complex or cross-link macromolecules at several binding sites using a variety of interactions, including hydrogen and ionic bonding as well as hydrophobic interactions (Fan et al. 2017). Hu et al. (2017) proposed a simple method for producing CNC-TA-DA (decylamine) via covalent binding to increase the water contact angle and the ability to be redispersed in organic solvents. Furthermore, they believed that this simple method is less time-consuming than polymer grafting and produces particles with minimal aggregation and morphological changes (Hu et al. 2017). Missio et al. (2018) used NFC and condensed tannins to create a durable, long-lasting packaging material. The physical and mechanical requirements were met by NFC, while the tannin was added for its antioxidant qualities. This film had a higher density and better surface hydrophobicity, resulting in a 6-fold improvement in air-barrier qualities (Missio et al. 2018). Huang et al. (2019) used layer-by-layer assembly to layer CH and TA on NFC, since TA has adhesive properties as a result of the aromatic components in its composition, allowing it to form coatings on a variety of nonporous and porous substrates. The key force behind the formation of multilayers is the electrostatic interaction between positively charged CH and negatively charged TA. Improved mechanical properties and strong antibacterial properties were found in this layer by layer structured film (Huang et al. 2019). Missio et al. (2020) used a mixture of NFC and condensed TA to fabricate functional film. They also used a non-ionic surfactant to give them even more control over the tannin-cellulose interface and, as a result, over the film properties. These tannin-containing films had a higher hydrophobic character, as shown by increased WCA (Missio et al. 2020).
Hydrophobization: Chemical vapor deposition (CVD)
Solution-based methods are commonly used to modify materials; however, in these procedures, finding an adequate solvent can be a limiting factor due to the fact that it must be compatible with both the substrate and the coating. Conformal coating of micro- or nanoscale structures is also limited by surface tension effects. As a result, methods involving the vapor phase, such as CVD, are preferred for coating complex surfaces (Cheng and Gupta 2018). Chemical vapor deposition (CVD) methods are capable of producing homogenous and conformal organic thin films without using solvents, catalysts, or separation agents (Hilt et al. 2014). CVD benefits from the excellent interface and surface morphology, growth of complex heterostructures with numerous layers, growth on patterned substrates, multiple wafer scale-up, and high layer purity (Pessoa et al. 2015). CVD procedures also are well-controlled techniques that allow the operator to quickly control the amount of material by changing the coating thickness (Hilt et al. 2014). Fluorocarbons, silicones, and organic or inorganic compounds are employed in these tactics to apply to cellulose and reduce surface energy (Leal et al. 2020). Yu et al. (2019) applied a one-step gas-solid reaction for the production of hydrophobic NC and bacterial cellulose (BC) with CVD of perfluorooctyltriethoxysilane. The results showed that perfluorooctyltriethoxysilane was successfully incorporated into the CNC and bacterial cellulose. They likewise believed that the vapor-based technique can be used to produce hydrophobic cellulosic materials on a large scale for industrial purposes (Yu et al. 2019). Rafieian et al. (2018) modified NFC via CVD of hexadecyltrimethoxylan (HDTMS). They believed that HDTMS with the longer the alkyl chain of hexadecyl (16 carbon atoms) compared to methyl (one carbon atom) contribute to making the polymer more hydrophobic, allowing the WCA of NFC to increase to 139. They found that untreated cellulose had a higher crystallinity than those treated with CVD (Rafieian et al. 2018). Cunha et al. (2018) also studied the impacts of CVD post modification on NFC filaments properties. Two organosilanes with varied numbers of methyl substituents were chosen for this purpose. Various surface structures including continuous, uniform coating layers or three-dimensional, hairy-like structures were observed, which served to decrease the surface energy and had a big impact on water interactions (Cunha et al. 2018). The decrease in surface energy was not expected and explained by the influence of the polar component in the organosilanes. A drawback of CVD is that it frequently involves extremely high temperatures and vacuum conditions. In addition, in situ monitoring of the process is restricted to specific analysis. However, Yang et al. (2020b) developed low-temperature-CVD with the goal of modifying cellulose-based to make them hydrophobic. Vacuuming has been used to allow hydrophobic gas to enter and react with the wood (Yang et al. 2020b).
Hydrophobization: Atomic layer deposition (ALD)
Atomic layer deposition (ALD) is a sub-set of the CVD process that uses a series of self-limiting surface adsorption reactions (chemisorption) carried out in a vacuum to control composition and the thickness of the deposed film with a thickness resolution of Angstroms (Wooding et al. 2020). ALD allows for higher uniformity and conformance on complex substrates as compared to standard CVD procedures, since the precursor molecules have a longer lifetime to diffuse to the cavities in complicated three-dimensional substrates (Wooding et al. 2020). Rare-earth oxide ceramics have been recognized as having strong hydrophobic surfaces, so they should find the widespread application (Azimi et al. 2013). A schematic description of the ALD process is shown in Fig. 11 (Oviroh et al. 2019).
- The first precursor is exposed in the reactor chamber to form a layer on the substrate.
- Remove any remaining initial precursors and by-products.
- The second precursor is exposed.
- Excess second precursor and by-products are purged
The procedure is repeated until the desired film thickness is obtained.
Fig. 11. A model ALD process for depositing trimethylaluminum on hydroxyl groups functionalized NC. Reproduced from Oviroh et al. (2019)
It should be added that increasing the number of cycles contributed to a rough structure, trapping more air, resulting in a more hydrophobic substrate, but the WCA decreased significantly after specific cycles of ALD coating. This may be because as the number of ALD cycles increased, more hydrophilic Al2O3 was accumulated on the surface (Lu et al. 2020a). Li et al. (2020) discovered that an increase in contact angle would be due to the hydrocarbons being drawn to the ALD-deposited metal oxide surface. The presence of hydrocarbons on the nanopaper surface was detected, but it is unclear whether this was due to accidental sources or chemical degradation of the cellulose caused by exposure to ALD precursor molecules (Li et al. 2020). Omerzu et al. (2018) evaluated the characteristics of Al2O3 coating layers formed on the surfaces of cellulose fibrous materials by using ATD. They state that a thin Al2O3 barrier coating on the surface of fibrous cellulose material effectively inhibits oxygen transport and spontaneous combustion of cellulose, but they made no mention of a water vapor barrier (Omerzu et al. 2018). Li and Losego (2021) modified NC paper (nanopapers) chemically and physically with simple gas-phase processing. They reported that longer trimethylaluminum exposure durations considerably increased the nanopaper’s tensile strength. They also showed that the NC paper became more hydrophobic after being treated with 1cy-ALD (Li and Losego 2021). It should be noted that the WVTR results obtained were not consistent with one another and that the fragile and degradation characteristics of Al2O3 films influenced the barrier properties’ stability. More importantly, the precise reasons for these disparate WVTR results remain unknown. Numerous attempts have been made to identify a critical innovation capable of significantly improving the alumina film’s protective and service characteristics. One strategy is to enhance the ALD technique by incorporating plasma pre-treatment (Fang et al. 2017). It appears that plasma-enhanced ALD requires half the number of deposition cycles as thermal ALD to achieve the same thickness of film. Additionally, no structural or protective variation in Al2O3 coatings was identified between the thermal ALD and plasma-enhanced ALD films produced (Omerzu et al. 2018).
Hydrophobization: Plasma
Plasma is usually referred to as a gas that’s been electrically charged with freely moving electrons in both the negative and positive states. Plasma treatment technology offers numerous advantages for surface modification because it is less toxic, safe, and developable technology compared to wet technologies (such as acetylation, alkylation, and carbamoylation) (Yao et al. 2021). Plasma can insert specific moieties throughout different mechanisms, such as cross-linking, deposition, etching, and functionalization, without modifying the bulk properties, as shown in Fig. 12 (Laureano-Anzaldo et al. 2020).
Fig. 12. Mechanisms of surface modification achieved with plasma treatment, redrawn from Laureano-Anzaldo et al. (2020)
Several distinct plasma approaches have been employed to hydrophobized cellulose nanomaterials: Atmospheric pressure, submerged liquid plasma, and low-pressure plasma (Oberlintner et al. 2021). Samanta et al. (2012) treated cellulosic (viscose rayon) fabric with He/1,3-butadiene (BD) plasma in a parallel-plate DBD reactor, achieving hydrophobicity with WCA of 142° by incorporation of CHx fragments on cellulose –OH sites (Samanta et al. 2012). Samanta et al. (2016) treated hydrophilic cotton (cellulosic) textile with helium-fluorocarbon (He- CF4) gases in an indigenously developed atmospheric pressure plasma reactor to impart hydrophobic functionality (Samanta et al. 2016). Due to the reaction of different CF4 species with cellulose, hydrophilic textile became hydrophobic after plasma treatment (Hubbe et al. 2015; Samanta et al. 2020). Yao et al. (2021) used oxygen plasma treatment in combination with a condensation reaction with TriSilanollsobutyl-Polyhedral oligomeric silsesquioxane to create a new hydrophobic cellulose-based organic/inorganic nanomaterial (Cellulose/TriSilanollsobutyl (TS)- Polyhedral oligomeric silsesquioxane (POSS)). The hydroxyl groups on the cellulose surface were not only activated, but the surface energy was also lowered and the surface roughness was raised, thanks to the plasma etching (TS-POSS modification) (Yao et al. 2021). Panaitescu et al. (2018) used submerged liquid plasma to surface functionalize NC. They were able to observe the effect of plasma on the morphology and surface chemistry of NC (Panaitescu et al. 2018). Matouk et al. (2020) functionalized CNC using cylindrical atmospheric pressure dielectric barrier discharge in order to minimize surface hydrophilicity and increase dispersion in polar organic solvents. They showed that using plasma with the right chemistry, such as Ar/CH4, Ar/NH3, and Ar/SiH4, can encourage hydrophobicity or hydrophilicity in CNC films by altering its elemental chemical composition and roughness (Matouk et al. 2020). Kartick et al. (2021) studied the hydrophobic functionalization of cellulosic fabric using helium/tetrafluoroethane plasma, a commercially available fluorocarbon gas (CF4), at atmospheric pressure. The highly hydrophilic cellulosic fabric became superhydrophobic after plasma treatment, with a water absorbency time of >60 minutes, a WCA of 153, and a water rolling angle of 5 (Samanta et al. 2021). As highlighted by Poodt et al. (2012) the intriguing potential of plasma has been demonstrated. However, before implementing this method on a commercial scale, further safety and risk evaluations should be conducted on cold plasma interactions (Bahrami et al. 2020).
It is worth mentioning that CF4 gases, which are commonly used for hydrophobization using plasma, are one of the most persistent greenhouse gases and should not be released into the atmosphere. Furthermore, the precise mechanisms and kinetics of the reactions that occur on the surface of cellulose nanomaterials remain unknown (Oberlintner et al. 2021).
Functionalization by Cationization
The capability of nanocellulose to adsorb on fiber surfaces is important for its use in high-strength applications (Li et al. 2021a). Accordingly, functionalized NC appears to be promising in terms of promoting bonding and subsequent mechanical strength of paper. Surface functionalization of nanoparticles is the process of joining various organic and inorganic materials together at the nanoscale via covalent or noncovalent bonds such as hydrogen bonds, the electrostatic force, or the van der Waals force (Pattnaik et al. 2020). Numerous methods of surface covalent functionalization of NC have been described, including the following: (TEMPO) oxidation (Lourenço et al. 2017), periodate oxidation (Sirviö et al. 2011), esterification (Wang et al. 2018), amidation (Pettignano et al. 2019), silylation (Nie et al. 2021), etherification (Li et al. 2021b), and other chemical modifications. Based on recent research, cationic nanocellulose has garnered considerable attention because it has the potential to provide electrostatic binding with anionic particles, which could open up more opportunities for functional NC (Sunasee and Hemraz 2018) to be used as flocculating agents and strength additions in the paper-making process. The idea of cationic fiber was established in pulp and paper production to act as a replacement for the typical cationic polymer used in the papermaking industry (Gao et al. 2016). In the pulp and paper industry, polyelectrolytes with cationic groups, such as CS and C-PAM, are used in papermaking to increase mechanical properties, promote load retention, facilitate drainage rate, and lower the biological oxygen requirement of pulp mill effluents (Li et al. 2018). Therefore, the addition of cationic fiber also can lead to improvements in filler retention, drainage, and the ability to absorb anion particles (Gao et al. 2016). Figure 13 shows the basic mechanisms through which cationic NC interacts with fibers (Abbott et al. 2006).
Fig. 13. Fibers and CNFC: electrostatic interactions upon the addition of cationic nanocellulose, redrawn from Sharma et al. (2020)
Lu et al. (2020b) demonstrated that the addition of cationic cellulose nanofiber (CNFC) slowly increased the basis weight, fines retention, and density of paper sheets since the addition of CNFC can enhance fines retention in paper sheets. Indeed, CNFC would adsorb and flocculate fines in the pulp slurry to produce the CNFC-Fines complex, and then it would attached in the wet web structure via a “bridging” effect and can pave the way for the dewatering process (Lu et al. 2020b). There are three common methods for producing cationic modified fibers. The first technique is to directly cationize the fibers in order to obtain the cellulose fiber amine compounds. The second method involves coupling a cationic prepolymer with a short chain to the fiber surface in order to achieve a fiber with a high surface charge density. Thirdly, cationic polymers are grafted onto the fiber using free-radical copolymerization; cationic monomers and neutral monomers are linked to the fiber surface via free radical copolymerization (Gao et al. 2016). In the following sections, we will compile a list of cationization processes in order to determine the one that has minimized environmental impact while still producing high-performance CNFC.
Cationization: Physical adsorption by cationic surfactant
NC consists of negatively charged particles that are expected to interact strongly with cationic surfactants electrostatically (Brinatti et al. 2016). Certain surfactants such as for example quarternary aluminium salt can change the surface on NC, and it has been shown that their zeta potential reverse from negative to positive (Xiang et al. 2019). The adsorption of cationic surfactants on cellulose exhibited a complex behaviour that varied according to the charge of the cellulose, the molecular structure of the surfactant, and the ionic strength of the solution (Alila et al. 2005). Dai and Fan (2013) demonstrated the green nano-modification in two stages: (i) cationization of natural fibers with dodecyltrimethyl-ammonium bromide and (ii) NC modification. The research used dodecyltrime-thylammonium bromide to pretreat fibers by adding cations to their surface, and then NC to change the pretreated fibers (Dai and Fan 2013). Mariano et al. (2018) modified NFC by using cationic surfactants (e.g., dodecyltrimethylammonium bromide (C12TAB), tetradecyltrimethylammonium bromide (C14TAB), and hexadecyltrimethylammonium bromide (C16TAB)) as modifying agents. Surfactants were added to NFC suspensions, causing the surface of the nanoparticles to be covered by these molecules, and lowering the ζ-potential (Mariano et al. 2018). Xiang et al. (2019) also modified CNC by electrostatic interaction of hexadecyl trimethyl ammonium bromide. The hexadecyl trimethyl ammonium bromide layer contributed a huge amount of positive charges to the CNC (Xiang et al. 2019). Kushan et al. (2020) modified CNC solutions by using cationic [C10 mim][Cl] and [C10 mim][FeCl4] surfactants as well. They showed that CNC network development is predominantly driven by hydrophobic contact of surfactant tails due to high electrostatic interaction between anionic CNC and cationic [C10 mim][Cl] surfactants. Hu et al. (2015) also modified CNC with cationic alkyl ammonium surfactants dodecyl-dimethylammonium bromide and cetyltrimethyl ammonium bromide. Both surfactants were adsorbed onto CNC with concentration-dependent morphology. Surfactant molecules adsorbed with alkyl tails pointing outward at low concentrations, resulting in hydrophobic CNC. At greater concentrations, surfactant aggregation morphologies on CNC were detected, and the hydrophobicity of CNC was diminished. Besides, CNC becomes cationic at higher surfactant concentrations since surfactant aggregates on CNC are thought to be responsible for this surface charge reversal (Hu et al. 2015).
Cationization by etherification
Etherification is a chemical process that involves grafting bifunctional monomers onto the fiber surface to facilitate the reaction between polymer chains and fibers. Currently, two commercially viable reagents, 3-chloro-2-hydroxypropyl trimethyl ammonium chloride (CHPTAC) and 2,3-epoxypropyl trimethyl ammonium chloride (EPTAC), are used to cationize NC in an alkaline medium (mostly using NaOH) (Sharma et al. 2020).
The presence of many cellulosic -OH groups on NFC allows for etherification reactions to modify NFC (Sethi et al. 2018). Depending on the substitution groups employed, a variety of features can be introduced or adjusted by selectively modifying these hydroxyl groups. The solubility and viscosity of cationized cellulose are affected by the degree of molar substitution, the distribution and type of substituents, and the molecular weight of cellulose and cellulose derivatives (O’Brien et al. 2021). Liu et al. (2019a) used cationic NFC (CNFC) as an additive in the preparation of reconstituted tobacco sheets. CNFC was created by homogenizing bleached hardwood kraft pulp that had been cationic etherized under high pressure (Liu et al. 2019a). Gu et al. (2020) stated that glycidyltrimethylammonium chloride (GTMAC)-involved etherification could be a potential method for producing well-dispersed nanoparticles, especially from parenchyma cellulose. With the help of ultrasound, quaternaryammonium groups were successfully grafted onto cellulose chains on the surface of NC (Gu et al. 2020). Luo et al. (2021) created cationic tobacco fiber by etherifying activated hydroxyl groups under alkaline circumstances using the etherifying compound EPTAC. The effect of alkali dosage on the tobacco cellulose nanofiber charge density was studied, and it was concluded that the charge density of tobacco cellulose nanofiber increased when the alkali dosage was raised since the degree of etherification of tobacco fibers was substantially increased as a result of the increased swelling and activation ability of hydroxyl groups (Luo et al. 2021). Chakrabarty and Teramoto (2021) used an alkali-activated etherification reaction with GTAC to modify the surface of NC in order to improve the ability of emulsion stabilization. It should be noted that these methods necessitate the use of specialized organic solvents, which break up the H-bonding network in cellulose. In general, the dissolution can transform cellulose I into cellulose II. These two materials differ in their crystal structure by the hydrogen-bond pattern, with cellulose I having parallel macromolecular chains while cellulose II has an antiparallel arrangement. The next difference is recognized by density functional theory optimizations, which showed that the cellulose I chain sheet models twisted to the right, while the cellulose II model twisted to the left. In general, materials made from cellulose I exhibit better mechanical properties than materials made from cellulose II. Indeed, the elastic moduli of cellulose I are about twice those of cellulose II. While industrial cellulose production technology has existed for more than 100 years, in the vast majority of cases, commercial cellulose products are still made with cellulose II (Tu et al. 2021).
Since cellulose is poorly soluble in water and most organic solvents, a substantial amount of organic solvents is required for this purpose (Tang et al. 2017). Odabas et al. (2016) showed the use of solvents such as dimethylsulfoxyde (DMSO), tetrahydrofuran (THF), or isopropanol to increase the cellulose modification (Odabas et al. 2016). Chaker and Boufi (2015) also reported DS values between 0.05 and 0.19 by using dimethylacetamide (DMAC) as solvent and potassium hydroxide (KOH) as a catalyst (Chaker and Boufi 2015). High DS is difficult to achieve and has a significant impact on energy consumption as well (Rol et al. 2019b). Therefore, the high temperature and the production of waste products severely limit the application of this method (Li et al. 2021b). The next drawback related to the etherification reaction worth mentioning would be the formation of diol and other toxic by-products. Additionally, sequential steps such as dispersing, reacting with the reagents in an alkaline medium, neutralizing the reaction mixture, separating, washing, and drying the final product are frequently required (Sharma et al. 2020). Furthermore, the traditional method for cellulose etherification reaction takes a long time, typically 8 to 80 hours (Zhang et al. 2021c), and it has low efficiency as well. Ultrasonication is widely regarded as a green mechanical method for helping modification of the surface of cellulosic materials and increasing reaction rates through a process known as cavitation. As a result, after etherification in more than 10% cellulose consistency, a higher DS could be achieved by ultrasonic processing for sonication at the frequency of 10 min per 0.5 h, indicating that a longer duration of sonication facilitated the increase in the number of cationic substituents on the PC surface (Gu et al. 2020). Microwave use is another way to aid etherification. Zhang et al. (2021c) synthesized quaternary cellulose samples with different DS ranging from 0.16 to 0.51 by etherification with the assistance of microwaves (Zhang et al. 2021c).
Cationization: Periodate oxidation and reductive amination
As previously stated, cellulose’s insolubility in most common solvents is a fundamental impediment to its use in different processes (Marcus 2019). It has been noted that the unique interactions of cellulose with solvent are largely dependent on the cellulose’s substitution sites. Due to the absence of hydrogen bonding between cellulose chains, 6-O-methylcellulose proved highly soluble in a wide range of organic solvents, but 2,3-di-O-methylcellulose is just soluble in polar aprotic solvents (Fox et al. 2011). Periodate oxidation is a highly selective oxidative fragmentation of the C2-C3 glycol bond on the glucose ring, yielding a dialdehyde NC (DAC) product (Li and Xu 2014). This DAC can be used as a reactive intermediate for further derivatization, as well as a starting point for a variety of other chemical processes (Jin et al. 2015). Due to its biocompatibility, solubility in hot water, and potential for further modification via the aldehyde groups, DAC has received considerable attention in bio-based products (Esen and Meier 2020). So, pretreatment of C2 and C3 anhydroglucose units position by periodate oxidation could be a good suggestion as shown in Fig. 14. Zhang et al. (2019) claim that it is easily modified via interfacial Schiff base coupling owing to the existence of highly reactive aldehyde groups. They also stated that the Schiff base reaction between DAC and aniline is a straightforward and easy scale-up approach of interfacial covalent assembly for producing solvent-responsive nanoparticles (Zhang et al. 2019).
Fig. 14. Periodate oxidation and reductive amination of NFC, redrawn from Xu et al. (2020)
Sirviö et al. (2011) cationized highly oxidized DAC and reported that the concentration of cationic groups increased from 2.85 to 4.27 mmol/g as the reaction time increased from 1 to 72 hours. Under these conditions, 87 percent of the aldehyde groups reacted with (2-hydrazinyl-2-oxoethyl)-trimethylazanium chloride Girard’s reagent T (GT). Huang et al. (2020) also synthesized cationic dialdehyde NFC (CDAC) with three different degrees of oxidation by oxidizing cellulose with metaperiodate and then cationizing it with (carboxymethyl) trimethylammonium chloride hydrazide as GT. They select GT, a typical grafting reagent in the paper industry that is relatively inexpensive and common, in order to introduce positive charges onto the NFC surface and make it more capable of interacting with negatively charged molecules (Huang et al. 2020). Kokol et al. (2021) also employed the two-step technique described below to functionalize NFC: sodium periodate oxidation of NFC to DAC and subsequent reaction with hexamethylene-diamine (HMDA) via a Schiff-based reaction to obtain NFC-ald-HMDA viscose yarn. They introduced this modified NFC to a ring-spun yarn as an antibacterial ingredient (Kokol et al. 2021).
Yang and van de Ven (2016) synthesized hairy cationic CNC (CNCC) by oxidizing cellulose with periodate and cationizing it with GT. They assert that this is a simple and environmentally friendly procedure that can be carried out in mild aqueous conditions. Wang et al. (2020a) synthesized CNCC via periodate oxidation and the Schiff base reaction. Periodate oxidation and disintegration were used to prepare di-aldehyde CNC from kraft pulp. Through a Schiff base reaction followed by sonication, the obtained DAC was further modified to include quaternary ammonium groups, yielding CNCC with an average length of 400 nm and a width of 15 nm. The combination of this CNCC and anionic alginate was used to make hydrogel beads (Wang et al. 2020a). Tian et al. (2017) also used this type of modification to obtain CDAC in order to be used in composite production (Tian et al. 2017). Still, these methods have a number of drawbacks including the use of large amounts of organic solvents (Yang et al. 2019b). Furthermore, the loss of crystallinity is thought to be caused by the opening of rings and the destruction of their ordered packing (Li et al. 2011).
Cationization: Using ionic liquid
The desire for more environmentally friendly functionalization procedures has resulted in significant research efforts aimed at identifying acceptable solvents. Ionic liquids (ILs) are a new class of organic molten salts with melting points less than 100 °C. They exhibit a number of desirable properties, including low vapor pressure, high thermal and chemical stability, high solvating capacities, structure tunability, ease of recycling, and nonflammability, and thus they offer the possibility for widespread industrial use. The development of ILs has created a new and versatile foundation for the widespread use of cellulose resources and the development of novel functional materials (Zhang et al. 2017). This kind of solvent (ILs) with strong hydrogen bond acceptors has demonstrated a high level of efficacy in dissolving and treating cellulose (Zhang et al. 2014).
The cellulose present in wood and plant fibers is mainly cellulose Iβ, a crystal structure with a certain shape. This shape can be changed by various treatments, such as dissolution, which weakens the hydrogen bonds between the hydroxyl groups, resulting in decreased crystallinity (Kovalenko 2010). Therefore, the solvent system that is chosen for modification needs to be carefully selected. Solvent systems have been studied for many years, and some researchers, such as Lin and Tsuchii (2022), have classified them into four groups based on their solubility. In Type A, cellulose was dissolved in large amounts, whereas Type B dissolved just slightly and Type C swelled the cellulose, but could not dissolve it at all. Type D was neither able to dissolve nor swell cellulose (Lin and Tsuchii 2022).
In general, ILs as solvent are thermally stable across a wide temperature range and can be used in a variety of reaction media for homogenous cellulose modification The considerable intermolecular and intramolecular hydrogen bonding of cellulose is anticipated to be disrupted by ILs (Zhang et al. 2014).
Abbott et al. (2006) illustrated the efficient cationic functionalization of cellulose using an IL analog composed of a mixture of a choline chloride derivative and urea that serves as both solvent and reagent. It was discovered that all of the cellulose’s available hydroxyl groups had been modified by ILs (Abbott et al. 2006). Qian et al. (2019) formulated novel poly ILs with 1-vinyl-3-aminopropyl imidazolium cations to modify cellulose. They first obtained dialdehyde cellulose aerogel via a simple oxidation process and then used sodium cyanoborohydride to prepare polyionic liquid (PIL) modified cellulose. The modified cellulose had a highly porous structure that was well-connected (Qian et al. 2019). Yang et al. (2019a) immobilized polyoxometalate (POM) supported PIL on the surface of CNC via in-situ Cu-mediated reversible deactivation radical polymerization (RDRP). CNC@PIL@POM demonstrated a nanorod-type core-shell structure with a high degree of crystallinity due to the CNC core and a PIL/POM composite shell (Yang et al. 2019a). Bernard et al. (2018) created cationic cellulose via PIL. The process began with the chemical modification of rice husk cellulose with thionyl chloride to form chlorinated cellulose (6-chloro-6-deoxycellulose, CDC), followed by surface grafting with 1-methylimidazole (Bernard et al. 2018).
The recovery of ILs is still in its early stages and is a frequent topic of discussion in the cellulose processing industry (Druel et al. 2018). Moreover, the industrial manufacturing and expense of ILs currently preclude their use in a wide range of fields. If the cost of ILs can be significantly reduced in the future, it will encourage its continued use in the pulp and papermaking industries (Li et al. 2019).
Cationization: Using deep eutectic solvent
Deep eutectic solvents (DESs) have been recognized as the most impressive environmentally friendly substitution solvents and reagents for some of the chemical and industrial processes. They are created by combining two or more chemical reagents into one solution with a lower melting point than the original reagents. It ought to be added that DESs can serve as both a reagent and a reaction medium in the fabrication of functionalized NFC and CNC (Selkälä et al. 2020). Unfortunately, the desired attention has not been received in the literature related to the use of DESs as alternative solvents for cellulose functionalization (Yang et al. 2019b).
DES should be able to disrupt the initial hydrogen-bonding networks in cellulose chains and DESs, then construct new hydrogen-bonding networks between cellulose molecules and DESs (Tang et al. 2017). Abbott et al. (2006) describe the first effective use of a DES in organic synthesis processes. Their DES containing a choline derivative and urea as both a reagent and a solvent is used in the cationic functionalization of cellulose (Abbott et al. 2006). Ren et al. (2016) propose a simple and effective DES functionalization approach for better cellulose dissolution. A novel allyl-functionalized deep eutectic solvent (DES) was created via allylic replacement of the –OH group in choline cation. As a result, substituting –OH groups in the choline cation may increase the dissolving capacity of ChCl-based DESs (Ren et al. 2016). Li et al. (2018) created a DES by combining aminoguanidine hydrochloride and glycerol (AhG) and using it as a reaction medium and reagent (aminoguanidine hydrochloride) to cationize DAC. To make cationic NC, the cationized celluloses were mechanically disintegrated. The structure of NC could be altered to produce highly cationic NFC (CNFC) or cationic CNC (CCNC) (Li et al. 2018).
Fig. 15. Cationization of oxidized cellulose pulp in aminoguanidine hydrochloride−glycerol deep eutectic solvent, redrawn from Li et al. (2018)
Deep eutectic solvents (DES) represent an economically appealing alternative, as the recycled version performs identically to the original. It has been shown that after five repetitions, the recycled DES had a similar reaction performance to the original DES (Li et al. 2018). Selkala et al. (2020) formulated CCNC by oxidizing pulp with periodate and then cationizing it in a DES in order to be used in dye removal (Selkälä et al. 2020). Jaekel et al. (2021) also used a DES to establish a sustainable approach for preparing CCNC. They formulated reactive eutectic media containing ammonium formate as the hydrogen acceptor component in combination with various organic acids, namely glycolic, lactic, and levulinic acid, which served as hydrogen bond donors. Based on the use of reactive eutectic media, they introduced a new approach for extracting CNC and functionalizing them with positively charged functional groups in a one-step procedure (Jaekel et al. 2021). Asante et al. (2020) also produced CCNC in a two-step process involving subsequent periodate oxidation and cationization in a DES and the modified cellulose was disintegrated mechanically to release CCNC. Cationization of DAC was accomplished in DES, which was formed by mixing aminoguanadine hydrochloride and glycerol in a 1:2 molar ratio (Asante et al. 2020).
Thus, the implementation of a low-cost DES opens up new possibilities for sustainable cellulose functionalization, as the reaction could be more environmentally friendly by further reducing the reaction time and recycling the DES (Selkälä et al. 2016). However, DESs have low ionic conductivity and a high viscosity. These shortcomings can be addressed by developing a novel hybrid DES with extraordinary properties (Lien et al. 2021).
APPLICATION OF MODIFIED NANOCELLULOSE IN PAPERMAKING
In general, papermakers appreciate techniques that enhance the paper’s strength and barrier characteristics, which can be accomplished through the use of NC in a variety of doses and forms (Hubbe 2014). However, as already mentioned, their usefulness as a dry end additive declines when the relative humidity surpasses 70%, because of the moisture sensitivity of NC. Additionally, NC as a wet end additive reduces drainability by blocking the pores of the wet paper web (Sharma et al. 2020). To avoid the limitations of NC in the papermaking process, a feasible approach is to modify NC and then apply it in the papermaking process. This section considers modified NC that has been used in the paper industry as dry end additive or wet end additive. Finally, the potential for using these approaches for NC modification in paper making is discussed. The goal is to be able to select certain types of environment-friendly additives/modifiers, having in mind low cost and ease of handling.
Modified NC-additive Used in Coating of Paper
The silylation of cellulose fibers has been investigated, with the goal of imparting hydrophobic characteristics of paper (Rol et al. 2019a). Yook et al. (2020) used silylation in an aqueous system to create hydrophobic NFC and coated them on linerboard and wood-free paper to evaluate their barrier properties. The introduction of hydrophobic functional groups to NFC through silylation transformed the surface chemical characteristics of NFC from hydrophilic to hydrophobic. The modified NFC coating exhibited better barrier properties than unmodified NFC coating, but the difference in functional groups induced a discrepancy in the barrier properties against water vapor and oxygen (Yook et al. 2020).
Moderate hydrophobicity properties can be created by adsorbing surfactants to the NC surface (Rol et al. 2019a). Tiitu et al. (2006) reported on the application of surfactant-to modified NC, which was then coated on paper as dry end additive. They prepared complexes of carboxymethyl cellulose and alkyltrimethylammonium surfactant to coat the paper using spray coating techniques (Tiitu et al. 2006). Xhanari et al. (2011) used cetyltrimethylammonium bromide as a surfactant to modify NFC. The mixtures were then deposited on top of filter paper to investigate the barrier properties. They observed that the wettability of filter paper coated by surfactant-NFC was slightly reduced (Xhanari et al. 2011). To promote wettability, Abou-Zeid et al. (2018) identified another task for surfactants. They prepared nano-biocomposite films of polylactic acid (PLA)/ CNC to coat onto paper. The cationic and anionic surfactants hexadecyltrimethylammonium bromide and sodium lauryl sulfate were responsible for avoiding CNC re-aggregation during drying and promoting CNC dispersion in PLA (Abou-Zeid et al. 2018).
In terms of bio-polyphenol application, the majority of research on bio-polyphenol incorporation into composites for packaging has been focused on combining tannin, NC, and cellulose film. As tannin is easily washed away due to its water solubility, so it is unsuitable for direct incorporation into the papermaking process (Ji et al. 2020). It seems that it would be more appropriate to use it in the coating process. Missio et al. (2018) developed a strong, sustainable packaging material using NFC and condensed tannins. The tannin-infused cellulose films exhibited a high density and increased surface hydrophobicity, resulting in a six-fold increase in their air-barrier properties. They concluded that NFC-tannin films are well suited for a variety of applications in packaging technology (Missio et al. 2018). Ji et al. (2020) created a novel cellulosic paper by incorporating tannin into kraft pulp for potential use in active food packaging. It was discovered that after tannin incorporation, paper became surface hydrophobic with contact angles greater than 90°, which was most likely due to covalent bonds between tannin and cellulose (Ji et al. 2020).
Gas techniques such as CVD also have shown good efficiency in nanopaper coating. Phanthong et al. (2016) used the CVD method to create amphiphobic NC-modified paper with high durability using a simple two-step method. NC-coated paper was chemically vapor redeposited with trichloro-(1H,1H,2H,2H-tridecafluoro-n-octyl)silane (FOTS). The paper obtained exhibited superhydrophobicity and oleophobicity, repelling both polar and non-polar liquids, and the contact angles of water and n-hexadecane were 156° and 144°, respectively (Phanthong et al. 2016). Another gas technique used to hydrophobise nanopaper is ALD (Shim et al. 2013; Nam et al. 2020). Li et al. (2020) modified the surface chemistry, wetting properties, and wet strength of NFC nanopaper using a few cycles of metal oxide ALD to increase the hydrophobicity of the NFC nanopaper, presumably by increasing the amount of hydrocarbons on the surface of the nanopaper (Li et al. 2020).
Modified Nanocellulose-additive Used as Wet End Additive in Papermaking
Regarding cationic additives in the paper industry, cationic cellulose can be attached to long fibers and fines via hydrogen bonding and electrostatic attraction, hence increasing wet web strength and structure tightness (Lu et al. 2020b). Aguado et al. (2017) studied the application of cationic cellulose to flocculate fillers in papermaking. It has been demonstrated that the water-soluble cationic cellulose compounds can help in the flocculation of papermaking fillers and the flocculation capacity was highly correlated with the DS of cationic cellulose. Their best results came from the flocculant with the highest degree of polymerization and charge (Aguado et al. 2017). Li et al. (2015b) carried out cellulose etherification with CHPTAC while in NaOH/urea aqueous solution. The performance of this cationic cellulose was assessed as a modifier of GCCate, as well as based on the influence of the modified fillers on paper characteristics. Their results demonstrated that surface modification of GCC fillers with cellulose-CHPTAC increased filler retention and decreased the harmful effect of filler addition on paper strength (Li et al. 2015b). Gao et al. (2016) synthesized cationic NFC with 2,3-epoxypropyl trimethyl ammonium chloride (EPTMAC) for use as an additive in the papermaking process. They demonstrated that the addition of CMCF had a positive effect on the bulk of paper sheets. This was due to the fact that fibrils can serve as binders between fibers and fines, acting as a physical filler. They also suggested that it has the potential to be used as a strength additive to increase the tensile index of paper sheets (Gao et al. 2016). The effectiveness of an oppositely charged ion can increase dramatically as the number of valence charges increases (Hubbe 2014). Lua et al. (2020) discussed this issue by comparing varying cationic charge densities of CNFC as excellent additives in the papermaking process to achieve a wet web with improved performance. They concluded that CNFC, particularly those with a higher charge density, act as effective fines flocculants and create a “bridging” effect between pulp fibers and the CNFC-Fines complex, resulting in increased wet-web strength (Lu et al. 2020b). The fact that high-charge cationic polymers typically exhibit high-affinity adsorption characteristics is one of the reasons why they are highly regarded by papermakers for a variety of applications (Wågberg 2000).
The DS is the next important aspect to consider, since recent research has shown that the DS of cellulose is substantially related to the resulting properties. It has been found that if the DS increases in the NC cationization process, the mechanical strength of fiber networks created as a result of applying high DS modified NC additives will improve (Zhang et al. 2021c). Since periodate oxidation produces reactive intermediates that are highly soluble in a wide range of solvents, this intermediate helps to boost the DS. Then, these high DS products can be used as a wet-end additive to improve mechanical strength qualities. Sirviö et al. (2011) synthesized cationic cellulose compounds by cationizing DAC obtained by periodate oxidation, since the aldehyde functionalities have high reactivity with respect to subsequent derivatization. This cationized cellulose has been used to conduct research using water-soluble CDAC as a biopolymeric flocculation agent in papermaking. Their findings indicate that it has a high potential for use as new, environmentally friendly flocculation aids (Sirviö et al. 2011). Campano et al. (2019) also synthesized CNCC through periodate oxidation and cationization. Then they investigated the ability of CNCC to flocculate the filler (kaolinite). They believed that CNCC has the potential to replace traditional wet-end retention aids, not only because it avoids the negative effects of traditional polymers on paper mechanical strength, but also because it allows for fine-tuning the characteristics of the flocs formed to meet industry needs by carefully varying the amount of CNCC added over a large number of effective dosage intervals (Campano et al. 2019).
The next suggested strategy for increasing the DS is assisted DES, which has been employed in paper manufacturing. It is critical to note that a simple and inexpensive route to cationic NC via DES can significantly expand their application range. Suopajärvi et al. (2017) reported that choline chloride-urea DES pretreatment significantly improved the nanofibrillation of cellulose board pulp grades. The properties of samples were investigated as paperboard strength-enhancing additives. Improved board handsheet qualities indicated that DES-treated NC may be used as a board strength enhancer (Suopajärvi et al. 2017). Zhang et al. (2021b) created the CCNC in two steps, starting with periodate oxidation and ending with cationization with DES, which was synthesized using aminoguanidine hydrochloride and glycerol. They achieved enhanced water resistance in self-assembling nanopaper by complexing oppositely charged CNC and NFC. They also noticed that not only did electrostatic complexation facilitate the fabrication of NC nanopapers by significantly reducing draining time, but it also improved the mechanical properties of NC sheets (Zhang et al. 2021b).
Benefits and Consequences of Various Approaches for NC in Papermaking
Reflecting on the current global environmental situation, it is critical to look for sustainable sources of raw materials to help us break our reliance on fossil fuels. Those serving the paper industry are responsible for developing substitutes for non-ecofriendly materials such as styrene-butadiene latex and C-PAM, as well as other conventional petroleum-based synthetic polymers that are commonly utilized in paper mills. As it has been already discussed, bio-based polymers including cellulose have potential to reduce environmental burdens, but substantial capital requirements and the paper industry’s inability to price above marginal cost limit their potential (Sharma et al. 2020).
On the other side, the runnability also is difficult to achieve both during cellulose manufacture and during application as a wet end or dry end additive (Yang et al. 2020a). So, it has been suggested to minimize the runnability issues by using modified NC as an additive. There are already a variety of methods for functionalizing cellulose at the lab scale, but additional research is needed to validate these technologies for real-world applications and eventual commercialization. Thus, it is critical to choose the technique of modification with an inquisitive attitude; if the method can benefit from low cost, ease of operation, absence of toxicity, and ease of access, and can be done continuously, then application and commercialization of these types of additives will be more appealing. To meet these objectives, a variety of methods have been outlined in this paper. Apart from the information provided here in earlier sections, each treatment exhibits some advantages and disadvantages that must be considered when determining the most appropriate methods to be used in the paper manufacturing process.
The following summarizes the benefit and drawbacks of the above-mentioned approaches for obtaining modified NC as an additive suitable to be use in the paper production process:
- Surfactant adsorption is a relatively environmentally friendly and cost-effective method for making cellulose more hydrophobic. While this type of NC can be used in papermaking, there are several considerations to keep in mind. To modify the NC, a large amount of surfactant is required because detectable amounts of surfactants are not absorbable (Kaboorani and Riedl 2015). As a result, if some of the surfactant goes to the furnish, it makes the foam stable and hinders inter-fiber bonding, and it reverses the effects of hydrophobic sizing agents. There is also a minor decrease in film wettability due to the process of adsorption (Hubbe 2014).
- The silylation process creates an environmentally friendly paper packaging material that is easy to manage and has a high barrier performance. However, there is a trade-off between cellulose crystallinity and silylation extent in order to get a higher degree of hydrophobization (Ghasemlou et al. 2021). Moreover, hydrophobic groups in silylation may not be effective to obstruct water vapor transport due to the short length of the hydrophobic alkyl groups. On the other hand, such a system could serve as an excellent coating for water barrier qualities (Yook et al. 2020).
- Plant oils are abundant, inexpensive, and non-toxic basic ingredients. Onwukamike et al. (2018) suggested that modified NC could be coated with trans-esterified oil or bio-epoxy oil and then used as a paper coating material over kraft paper because trans-esterified oil and bio-epoxy oil have a lower viscosity and a lower surface tension than their corresponding source oils, which results in improved wettability and the formation of a uniform film on the surface (Onwukamike et al. 2018). Linseed oil has typically been used in a wide range of industrial coating formulations; nonetheless, linseed oil-based coatings are fragile and turn yellow over time (Tambe et al. 2016). As a result, if linseed oil is used as a paper coating substance, the color of the oil-coated paper will change. The high viscosity of oil requires the use of organic diluents to be used as coating substances, which extends the curing time needed to eliminate excess solvent from the system. The use of catalysts is also a part of this method. Because of these constraints, the use of linseed oil and other highly unsaturated natural oils in the paper coating industry has been restricted. The use of bio-epoxidized oil as a coating for paper might then be recommended. As a result of the several steps required, the cost of the coating goes up significantly (Tambe et al. 2016). It is worth noting that the usage of certain epoxy derivatives (such as bisphenol-A diglycidyl ether, bisphenol-F diglycidyl ether, and novolac glycidyl ether) has been restricted in materials and objects intended to come into food by EU regulation. The epoxy derivatives mentioned above are synthetic epoxy resins, and efforts are being made to replace them with bio-based epoxy resins (Altuna et al. 2014; Islam et al. 2022).
- The modification by bio-polyphenol (tannin) and then coating of paper increase the antioxidative and UV-blocking properties of the handsheets (Ji et al. 2020). However, there are certain drawbacks to this strategy. For example, the extraction methods to obtain polyphenols necessitate complex analytical techniques and expensive equipment (Missio et al. 2017); thus, a simpler approach to fractionation must be considered to reduce the cost of cellulose modification using this kind of material.
- Chemical vapor deposit (CVD) as the next more environmentally friendly method benefits from the excellent interface and surface morphology, growth of complex heterostructures with numerous layers, growth on patterned substrates, multiple wafer scale-up, and high layer purity (Pessoa et al. 2015). Vapor deposition benefits from excellent interface and surface morphology, multiple wafer scale-up, and high layer purity. However, the major limitation of CVD is that it frequently requires a high temperature to convert precursors to reactive products capable of forming thin films (Sun et al. 2021b). So, this method is not suitable for heat-sensitive materials such as paper. In terms of ALD, it should be noted that today’s batch reactors with vacuum chambers are mostly used for paper coating. However, roll-to-roll reactors and atmospheric processes are being developed by different research groups and equipment suppliers. The drawback of this kind of reactor is that the depositions take place at high temperatures. This had a considerable impact on the topography and morphology of the extrusion coating polymers (Kääriäinen et al. 2011).
- Plasma treatment is the next low-cost, environmental-friendly and simple method to modify cellulose (Vizireanu et al. 2018). Plasma treatment of cellulose has been thoroughly investigated for a variety of applications in the textile and paper industries, and also other sectors, including cleaning, sterilization, activation, and enhancement of hydrophilicity or hydrophobicity. The “corona” method is insufficiently feasible and effective for high-speed processing of porous materials such as textiles and paper. The Diffuse Coplanar Surface Barrier Discharge plasma system has been successfully demonstrated for surface activation of paper. Due to the very high plasma power density and ambient air as working gas, the Diffuse Coplanar Surface Barrier Discharge plasma system might be applicable for in-line paper coating technology with a standardly high manufacturing speed (Černák et al. 2009). However, despite a good potential using it within papermaking, it’s worth noting that there are difficulties among researchers in interpreting and comparing the experimental results obtained through the use of plasma (Bahrami et al. 2020).
- Cationic NC derivatives were put through a variety of tests to see whether they were suitable for usage as additives. Etherification, as a common method for cationization of a surface of NC, has disadvantages including taking a long time for the reaction and difficulties to achieve a high DS (Li et al. 2021b; Rol et al. 2019a). The mechanical strength of paper and the DS of modified NC used as additives in papermaking are highly correlated. Cationic additives perform better by increasing the bonded area, which results in an increase in the sheet’s density (Lu et al. 2020b). Thus, the effect of cationic NC as a dry strength additive is enhanced when the additive contains a greater number of positions capable of bonding with the fiber in furnish.
- Cationic cellulose compounds with a high cationic charge have been found to perform better (Vuoti et al. 2018). So, periodate oxidation treatment can activate the C2-C3 position and then act as an intermediate for the further cationization to improve the charge density and DS, and when used as an additive in papermaking, it contributes more bonding, resulting in paper with superior mechanical properties. However, this method is defined on a case-by-case basis. This means that it is a combination of the starting hierarchy and oxidation scenarios, and as a result, it cannot be generalized; nonetheless, research into generalizing this method will continue (Nypelö et al. 2021).
- The next strategy for improving the performance of cationic cellulose in papermaking is to dissolve it in an ionic liquid before modification in order to activate the hydroxyl group. Ionic liquid as green solvent has a variety of appealing properties, including chemical and thermal stability, non-flammability, and immeasurable low vapor pressure. However, the industrial manufacturing and expense of ILs currently preclude their use in the paper industry (Zhu et al. 2006).
- Deep eutectic solvents (DES) is the next green solvent, and it can be used to improve the performance of cationic cellulose as a paper additive. The previously mentioned modification methods had a number of limitations, including a high cost and the need for special grade pulps. So, use of DES modified cationic cellulose promotes possibility to achieve a cost-effective modification of high consistency mixing while using industrial-grade cellulose pulp that does not require the use of harmful solvents, expensive cellulose grades, or cellulose solubilization. Many of these issues are overcome when deep eutectic solvents are used for the modification (Vuoti et al. 2018). The use of DESs could be a promising method for inducing the swelling of cellulose, which would enhance the chemical reactivity of that material. More innovation and in-depth research are unquestionably required.
CONCLUSIONS AND FUTURE PERSPECTIVES
Green chemistry involves application of processes that remove or greatly reduce harmful compound intake and output. This approach is becoming popular as a strategy for environmental sustainability. In this instance, there is a growing tendency toward developing techniques for nanocellulose (NC) modification using green technologies. These modifications allow for the introduction of novel capabilities not found in native NC, enabling them to be applied in a broader range of industries. In the paper industry, as NCs demonstrate advantageous properties such as high surface area, tailorable surface chemistry, anisotropic shape, and superior mechanical and gas barrier properties, researchers are enticed to use them to improve the mechanical and barrier properties of paper. However, the manufacture of NC-based paper has encountered a number of difficulties such as a decrease in dewatering rate in the wire section, which raises the cost of operation. There are also concerns with the wettability and water vapor barrier in a coating due to the hydrophilicity of NC. The first section of this review paper discussed surface modification of NC with the goal of improving the water barrier properties of NC using green eco-friendly methods. The procedures outlined in the in the section “Modification Techniques of Nanocellulose” have the advantage of requiring the least amount of chemically hazardous solvents and allowing for the following scenario: In the first scenario, the formation of a chemical bond reduced the surface hydroxyl groups and hydrophilicity. In the second case, non-covalent surface modification or surface deposition aids in improving surface hydrophobicity. In the final scenario, changing the morphology of the surface, such as creating an etched surface through plasma treatment, can help to change the hydrophilicity behavior of the surface. Thus, modifying the morphology, functional group, and surface with the least amount of hazardous solvents can improve the water barrier qualities of NC.
Another series of modification techniques have been summarized in this work with the aim of creating cationic sites on the surface of NC in order to overcome the dewatering rate limitation and make them more useful in the paper industry. If NC is used as a wet-end additive to improve the mechanical properties of paper, an issue with a reduction in dewatering rate will emerge. One of the major causes of this problem is the plugging of drainage channels in the wet web by NC. To address this issue, the addition of more active binding sites, particularly those with cationic charges, has been proposed. The addition of a cationically charged group to the hydrophilic backbone of NC can result in the production of amphiphilic NC, which has the potential to increase the wet-web strength through adsorption-flocculation effects on fines and fiber pulps. As the number of valence charges increases, the effectiveness of an oppositely charged ion increases dramatically. Therefore, the methods produce a greater charge density, which works as an effective fines flocculant and bridge between pulp fibers and fines, leading to increased wet web strength with a low dewatering rate impact. It should be emphasized that the majority of the treatments discussed and their effects on the paper application were carried out on a small scale by the various cited researchers. As a result, despite the benefits of these modified NC in the paper sector, scaling up is still necessary. This transition from laboratory to upper scale needs to be facilitated in order to be able to use the aforementioned approaches to track the evolution of the paper industry and identify the best answer for the technical, processability, formation, and economic issues that the paper industry faces. To our mind, future research work on surface chemical modification and application in the paper industry should be focused on five primary objectives: i) research and development of new, low-cost, and environmentally friendly methods of NC modification; ii) scale-up research from batch to continuous procedures to evaluate laboratory results and their potential commercial applications; iii) a study on the efficiency of hydrophobized/functionalized NC in the paper industry; iv) analysis of the environmental impact of NC hydrophobization/ functionalization and their application in paper production; and v) A study on cost estimation (e.g., energy consumption and building the facility on an industrial scale) and technical feasibility. The authors hope that this study will inspire researchers’ interest in improving NC characteristics, thereby expanding their industrial applications and supporting the sustainable utilization of renewable resources.
Competing Interests Declaration
There are no conflicts of interest in the submission of this manuscript, and all authors have given their approval for publication.
ACKNOWLEDGEMENTS
The authors are appreciative for the funding provided by Karlstad University for this work.
REFERENCES CITED
Abbott, A. P, Bell, T. J., Handa, S., and Stoddart, B. (2006). “Cationic functionalisation of cellulose using a choline based ionic liquid analogue,” Green Chemistry 8, 784-786. DOI: 10.1039/b605258d
Abdul Khalil, H. P. S., Davoudpour, Y., Islam, M. N., Mustapha, A., Sudesh, K., Dungani, R., and Jawaid, M. (2014). “Production and modification of nanofibrillated cellulose using various mechanical processes: A review,” Carbohydrate Polymers 99, 649-665. DOI: 10.1016/j.carbpol.2013.08.069
Abdul Khalil, H. P. S., Davoudpour, Y., Saurabh, C. K., Hossain, M. S., Adnan, A. S., Dungani, A. R., Paridah, M. T., Islam Sarker, M. Z., Nurul Fazita, M. R., Syakir, M. I., and Haafiz, M. K. M. (2016). “A review on nanocellulosic fibers as new material for sustainable packaging: Process and applications,” Renewable and Sustainable Energy Reviews 64, 823-836. DOI: 10.1016/j.rser.2016.06.072
Abou-Zeid, R. E., Diab, M. A., Mohamed, S. A. A., Salama, A., Aljohani, H. A., and Shoueir, K. R. (2018). “Surfactant-assisted poly (lactic acid)/cellulose nanocrystal bionanocomposite for potential application in paper coating,” Journal of Renewable Materials 6, 394-401. DOI: 10.7569/JRM.2017.634156
Abushammala, H., and Mao, J. (2019). “A review of the surface modification of cellulose and nanocellulose using aliphatic and aromatic mono-and di-isocyanates,” Molecules 24, article no. 2782. DOI: 10.3390/molecules24152782
Aguado, R., Lourenço, A. F., Ferreira, P. J., Moral, A., and Tijero, A. (2017). “Cationic cellulosic derivatives as flocculants in papermaking,” Cellulose 24, 3015-3027. DOI: 10.1007/s10570-017-1313-y
Ahadian, H., Zamani, E. S., Phiri, J., and Maloney, T. (2021). “Fast dewatering of high nanocellulose content papers with in-situ generated cationic micro-nano bubbles,” Drying Technology DOI: 10.1080/07373937.2021.1942898
Ahola, S., Österberg, M., and Laine, J. (2008). “Cellulose nanofibrils—Adsorption with poly (amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive,” Cellulose 15, 303-314. DOI: 10.1007/s10570-007-9167-3
Alila, S., Boufi, S., Belgacem, M. N., and Beneventi, D. (2005). “Adsorption of a cationic surfactant onto cellulosic fibers. I. Surface charge effects,” Langmuir 21, 8106-8113. DOI: 10.1021/la050367n
Altuna, F., Pettarin, V., Martin, L., Retegi, A., Mondragon, I., Ruseckaite, R., and Stefani, P. (2014). “Copolymers based on epoxidized soy bean oil and diglycidyl ether of bisphenol A: Relation between morphology and fracture behavior,” Polymer Engineering & Science 54(3), 569-578. DOI: 10.1002/pen.23588
Amara, C., El Mahdi, A., Medimagh, R., and Khwaldia, K. (2021). “Nanocellulose-based composites for packaging applications,” Current Opinion in Green and Sustainable Chemistry 31, article no. 100512. DOI: 10.1016/j.cogsc.2021.100512
Ämmälä, A., Liimatainen, H., Burmeister, C., and Niinimäki, J. (2013). “Effect of TEMPO and periodate-chlorite oxidized nanofibrils on ground calcium carbonate flocculation and retention in sheet forming and on the physical properties of sheets,” Cellulose 20, 2451-60. DOI: 10.1007/s10570-013-0012-6
Ang, S., Haritos, V., and Batchelor, W. (2019). “Effect of refining and homogenization on nanocellulose fiber development, sheet strength and energy consumption,” Cellulose 26, 4767-4786. DOI: 10.1007/s10570-019-02400-5
Ankerfors, M., Lindström, T., and Söderberg, D. (2014). “The use of microfibrillated cellulose in fine paper manufacturing – Results from a pilot scale papermaking trial,” Nordic Pulp & Paper Research Journal 29, 476-483. DOI: 10.3183/npprj-2014-29-03-p476-483
Asante, B., Sirviö, J. A., Li, P., Lavola, A., Julkunen‐Tiitto, R., Haapala, A., and Liimatainen, H. (2020). “Adsorption of bark derived polyphenols onto functionalized nanocellulose: Equilibrium modeling and kinetics,” AIChE Journal 66, article ID e16823. DOI: 10.1002/aic.16823
Asyraf, M. R. M., Ishak, M. R., Sapuan, S. M., Yidris, N., and Ilyas, R. A. (2020). “Woods and composites cantilever beam: A comprehensive review of experimental and numerical creep methodologies,” Journal of Materials Research and Technology 9, 6759-6776. DOI: 10.1016/j.jmrt.2020.01.013
Auclair, N., Kaboorani, A., Riedl, B., and Landry, V. (2020). “Effects of surface modification of cellulose nanocrystals (CNCs) on curing behavior, optical, and thermal properties of soybean oil bio-nanocomposite,” Journal of Coatings Technology and Research 17, 57-67. DOI: 10.1007/s11998-019-00237-y
Aulin, C., Andrei Shchukarev, Josefina Lindqvist, Eva Malmström, Lars Wågberg, and Tom Lindström, T. (2008). “Wetting kinetics of oil mixtures on fluorinated model cellulose surfaces,” Journal of Colloid and Interface Science 317, 556-567. DOI: 10.1016/j.jcis.2007.09.096
Aulin, C., and Ström, G. (2013). “Multilayered alkyd resin/nanocellulose coatings for use in renewable packaging solutions with a high level of moisture resistance,” Industrial & Engineering Chemistry Research 52, 2582-89. DOI: 10.1021/ie301785a
Azimi, G., Dhiman, R., Kwon, H.-M., Paxson, A. T., and Varanasi, K. K. (2013). “Hydrophobicity of rare-earth oxide ceramics,” Nature Materials 12, 315-320. DOI: 10.1038/nmat3545
Aziz, T., Zheng, J., Jamil, M. I., Fan, H., Ullah, R., Iqbal, M., Ali, A., Khan, F. U., and Ullah, A. (2021). “Enhancement in adhesive and thermal properties of bio‐based epoxy resin by using eugenol grafted cellulose nanocrystals,” Journal of Inorganic and Organometallic Polymers and Materials: 1-11. DOI: 10.1007/s10904-021-01942-1
Bahrami, R., Zibaei, R., Hashami, Z., Hasanvand, S., Garavand, F., Rouhi, M., Jafari, S. M., and Mohammadi, R. (2020). “Modification and improvement of biodegradable packaging films by cold plasma; A critical review,” Critical Reviews in Food Science and Nutrition, 1-15. DOI: 10.1080/10408398.2020.1848790
Balea, A., Monte, M. C., Merayo, N., Campano, C., Negro, C., and Blanco, A. (2020). “Industrial application of nanocelluloses in papermaking: A review of challenges, technical solutions, and market perspectives,” Molecules 25(3), article no. 526. DOI: 10.3390/molecules25030526
Bernard, F. L., Duczinski, R. B., Rojas, M. F., Fialho, M. C. C., Carreno, L. A., Chaban, V. V., Vecchia, F. D., and Einloft, S. (2018). “Cellulose based poly (ionic liquids): Tuning cation-anion interaction to improve carbon dioxide sorption,” Fuel 211, 76-86. DOI: 10.1016/j.fuel.2017.09.057
Blair, M. J., and Mabee, W. E. (2021). “Techno‐economic and market analysis of two emerging forest biorefining technologies,” Biofuels, Bioproducts and Biorefining 15(5), 1301-1317. DOI: 10.1002/bbb.2218
Bondoc, E. (2018). “Runnability-the condition monitoring perspective,” APPITA: Technology, Innovation, Manufacturing, Environment 71, 114-119.
Brinatti, C., Huang, J., Berry, R. M., Tam, K. C., and Watson Loh, W. (2016). “Structural and energetic studies on the interaction of cationic surfactants and cellulose nanocrystals,” Langmuir 32, 689-698. DOI: 10.1021/acs.langmuir.5b03893
Brodin, F. W., Gregersen, Ø. W., and Syverud, K. (2014). “Cellulose nanofibrils: Challenges and possibilities as a paper additive or coating material–A review,” Nordic Pulp & Paper Research Journal 29, 156-166. DOI: 10.3183/npprj-2014-29-01-p156-166
Cairns, M. J., Mesic, B., Johnston, J. H., and Herzog, M. B. (2019). “Use of spherical silica particles to improve the barrier performance of coated paper,” Nordic Pulp & Paper Research Journal 34, 334-342. DOI: 10.1515/npprj-2018-0066
Campano, C., Lopez-Exposito, P., Blanco, A., Negro, C., and van de Ven, T. G. M. (2019). “Hairy cationic nanocrystalline cellulose as a novel flocculant of clay,” Journal of Colloid and Interface Science 545, 153-161. 10.1016/j.jcis.2019.02.097
Campilho, R. D. S. G. (2015). Natural Fiber Composites, CRC Press. DOI: 10.1201/b19062
Cazon, P., and Vázquez, M. (2020). “Mechanical and barrier properties of chitosan combined with other components as food packaging film,” Environmental Chemistry Letters 18, 257-267. DOI: 10.1007/s10311-019-00936-3
Černák, M., Černáková, L., Hudec, I., Kováčik, D., and Zahoranová, A. (2009). “Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials,” The European Physical Journal – Applied Physics 47, article no. 22806. DOI: 10.1051/epjap/2009131
Chaker, A., and Boufi, S. (2015). “Cationic nanofibrillar cellulose with high antibacterial properties,” Carbohydrate Polymers 131, 224-232. DOI: 10.1016/j.carbpol.2015.06.003
Chakrabarty, A., and Teramoto, Y. (2021). “Cellulose nanofiber-derived efficient stabilizer for oil-in-water high-internal-phase emulsion,” Cellulose 28, 6253-6268. DOI: 10.1007/s10570-021-03948-x
Charani, P. R., and Moradian, M. H. (2019). “Utilization of cellulose nanofibers and cationic polymers to improve breaking length of paper,” Cellul. Chem. Technol. 53, 767-774. DOI: 10.35812/CelluloseChemTechnol.2019.53.75
Chauve, G., and Bras, J. (2014). “Industrial point of view of nanocellulose materials and their possible applications,” in: Handbook of Green Materials: 1 Bionanomaterials: Separation Processes, Characterization and Properties, World Scientific. DOI: 10.1142/9789814566469_0014
Chemin, M., Heux, L., Guérin, D., Crowther-Alwyn, L., and Jean, B. (2019). “Hybrid gibbsite nanoplatelet/cellulose nanocrystal multilayered coatings for oxygen barrier improvement,” Frontiers in Chemistry 7, article no. 507. DOI: 10.3389/fchem.2019.00507
Chen, L.-H., Zhu, J.-Y., Baez, C., Kitin, P., and Elder, T. (2016). “Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids,” Green Chemistry 18(13), 3835-3843. DOI: 10.1039/C6GC00687F
Cheng, C., and Gupta, M. (2018). “Roll-to-roll surface modification of cellulose paper via initiated chemical vapor deposition,” Industrial & Engineering Chemistry Research 57, 11675-11680. DOI: 10.1021/acs.iecr.8b03030
Chi, K., and Catchmark, J. M. (2017). “Enhanced dispersion and interface compatibilization of crystalline nanocellulose in polylactide by surfactant adsorption,” Cellulose 24, 4845-4860. DOI: 10.1007/s10570-017-1479-3
Chin, K.‐M., Ting, S. S., Ong, H. L., and Omar, M. (2018). “Surface functionalized nanocellulose as a veritable inclusionary material in contemporary bioinspired applications: A review,” Journal of Applied Polymer Science 135, article no. 46065. DOI: 10.1002/app.46065
Chowdhury, R. A., Nuruddin, M., Clarkson, C., Montes, F., Howarter, J., and Youngblood, J. P. (2019). “Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films,” ACS Applied Materials & Interfaces 11, 1376-1383. DOI: 10.1021/acsami.8b16897
Cunha, A. G., Lundahl, M., Ansari, M. F., Johansson, L.-S., Campbell, J. M., and Rojas, O. J. (2018). “Surface structuring and water interactions of nanocellulose filaments modified with organosilanes toward wearable materials,” ACS Applied Nano Materials 1, 5279-5288. DOI: 10.1021/acsanm.8b01268
Dai, D. S., and Fan, M.-Z. (2013). “Green modification of natural fibers with nanocellulose,” RSC Advances 3, 4659-4665. DOI: 10.1039/c3ra22196b
Das, A. K., Islam, M. N., Ashaduzzaman, M., and Nazhad, M. M. (2020). “Nanocellulose: Its applications, consequences and challenges in papermaking,” Journal of Packaging Technology and Research 4, 253-260. DOI: 10.1007/s41783-020-00097-7
De Assis, C. A., Houtman, C., Phillips, R., Bilek, E. M., Rojas, O. J., Pal, L., Peresin, M. P., Jameel, H., and Gonzalez, R. (2017). “Conversion economics of forest biomaterials: Risk and financial analysis of CNC manufacturing,” Biofuels, Bioproducts and Biorefining 11, 682-700. DOI: 10.1002/bbb.1782
De Lima, G. F., de Souza, A. G., Rodrigues Bauli, C., da Silva Barbosa, R. F., Belchior Rocha, D., and dos Santos Rosa, D. (2019). “Surface modification effects on the thermal stability of cellulose nanostructures obtained from lignocellulosic residues,” Journal of Thermal Analysis and Calorimetry: 1-15. DOI: 10.1007/s10973-019-09109-4
Dhali, K., Ghasemlou, M., Daver, F., Cass, P., and Adhikari, B. (2021). “A review of nanocellulose as a new material towards environmental sustainability,” Science of the Total Environment, article no. 145871. DOI: 10.1016/j.scitotenv.2021.145871
Dhar, P., Bhardwaj, U., Kumar, A., and Katiyar, V. (2015). “Poly (3‐hydroxybutyrate)/ cellulose nanocrystal films for food packaging applications: Barrier and migration studies,” Polymer Engineering & Science 55, 2388-2395. DOI: 10.1002/pen.24127
Dhuiège, B., Pecastaings, G., and Sèbe, G. (2018). “Sustainable approach for the direct functionalization of cellulose nanocrystals dispersed in water by transesterification of vinyl acetate,” ACS Sustainable Chemistry & Engineering 7, 187-196. DOI: 10.1021/acssuschemeng.8b02833
Diab, M., Curtil, D., El-shinnawy, N., Hassan, M. L., Zeid, I. F., and Mauret, E. (2015). “Biobased polymers and cationic microfibrillated cellulose as retention and drainage aids in papermaking: Comparison between softwood and bagasse pulps,” Industrial Crops and Products 72, 34-45. DOI: 10.1016/j.indcrop.2015.01.072
Dimic-Misic, K., Puisto, A., Gane, P., Nieminen, K., Alava, M., Paltakari, J., and Maloney, T. (2013). “The role of MFC/NFC swelling in the rheological behavior and dewatering of high consistency furnishes,” Cellulose 20, 2847-2861. DOI: 10.1007/s10570-013-0076-3
Dimic-Misic, K., Puisto, A., Paltakari, J., Alava, M., and Maloney, T. (2013). “The influence of shear on the dewatering of high consistency nanofibrillated cellulose furnishes,” Cellulose 20, 1853-1864. DOI: 10.1007/s10570-013-9964-9
Dissanayake, D. G. K., Weerasinghe, D., Perera, T. D. R., Bandara, M. M. A. L., Thathsara, S. K. T., and Perera, S. (2021). “A sustainable transparent packaging material from the arecanut leaf sheath,” Waste and Biomass Valorization 107, 2411-2502. DOI: 10.1007/s12649-021-01382-5
Dodson, C. T. J., and Serafino, L. (1993). “Flocculation, dispersion and dynamic scenarios for formation,” Nordic Pulp & Paper Research Journal 8(2), 264-272.
Druel, L., Niemeyer, P., Milow, B., and Budtova, T. (2018). “Rheology of cellulose-[DBNH][CO2Et] solutions and shaping into aerogel beads,” Green Chemistry 20, 3993-4002. DOI: 10.1039/C8GC01189C
Ehman, N. V., Felissia, F. E., Tarres, Q., Vallejos, M. E., Delgado-Aguilar, M., Mutje, P., and Area, M. C. (2020). “Effect of nanofiber addition on the physical–mechanical properties of chemimechanical pulp handsheets for packaging,” Cellulose 27, 10811-10823. DOI: 10.1007/s10570-020-03207-5
Eriksen, Ø., Syverud, K., and Gregersen, Ø. (2008). “The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper,” Nordic Pulp & Paper Research Journal 23, 299-304. DOI: 10.3183/npprj-2008-23-03-p299-304
Esen, E., and Meier, M. A. R. (2020). “Sustainable functionalization of 2,3-dialdehyde cellulose via the Passerini three-component reaction,” ACS Sustainable Chemistry & Engineering 8, 15755-15760. DOI: 10.1021/acssuschemeng.0c06153
Fan, H.-L., Wang, L., Feng, X.-D., Bu, Y.-Z., Wu, D.-C., and Jin, Z.-X. (2017). “Supramolecular hydrogel formation based on tannic acid,” Macromolecules 50, 666-676. DOI: 10.1021/acs.macromol.6b02106
Fang, M., Zhang, H., Sang, L., Cao, H., Yang, L., Ostrikov, K., Levchenko, I., and Chen, Q. (2017). “Plasma-assisted ALD to functionalize PET: Towards new generation flexible gadgets,” Flexible and Printed Electronics 2, article no. 022001. DOI: 10.1088/2058-8585/aa6add
Ferrer, A., Pal, L., and Hubbe, M. (2017). “Nanocellulose in packaging: Advances in barrier layer technologies,” Industrial Crops and Products 95, 574-582. DOI: 10.1016/j.indcrop.2016.11.012
Fotie, G., Limbo, S., and Piergiovanni, L. (2020). “Manufacturing of food packaging based on nanocellulose: Current advances and challenges,” Nanomaterials 10(9), article no. 1726. DOI: 10.3390/nano10091726
Fox, S. C., Li, B., Xu, D.-Q., and Edgar, K. J. (2011). “Regioselective esterification and etherification of cellulose: A review,” Biomacromolecules 12, 1956-1972. DOI: 10.1021/bm200260d
Gao, Y.-H., Li, Q., Shi, Y., and Cha, R.-T. (2016). “Preparation and application of cationic modified cellulose fibrils as a papermaking additive,” International Journal of Polymer Science 2016, article no. 6978434. DOI: 10.1155/2016/6978434
Ghasemlou, M., Daver, F., Ivanova, E. P., Habibi, Y., and Adhikari, B. (2021). “Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites,” Progress in Polymer Science 119, 101418-101428. DOI: 10.1016/j.progpolymsci.2021.101418
Gholampour, A., and Ozbakkaloglu, T. (2020). “A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications,” Journal of Materials Science 55, 829-892. DOI: 10.1007/s10853-019-03990-y
González, I., Alcala, M., Chinga-Carrasco, G., Vilaseca, F., Boufi, S., and Mutjé, P. (2014). “From paper to nanopaper: Evolution of mechanical and physical properties,” Cellulose 21, 2599-2609. DOI: 10.1007/s10570-014-0341-0
Gorade, V. G., Kotwal, A., Chaudhary, B. U., and Kale, R. D. (2019). “Surface modification of microcrystalline cellulose using rice bran oil: A bio-based approach to achieve water repellency,” Journal of Polymer Research 26(9), 217. DOI: 10.1007/s10965-019-1889-z
Gorrasi, G., Sorrentino, A., and Lichtfouse, E. (2020). “Back to plastic pollution in COVID times,” Environ. Chem. Lett. 19, 1-4. DOI: 10.1007/s10311-020-01129-z
Gu, H.-M., Gao, X., Zhang, H., Chen, K.-L., and Peng, L.-C. (2020). “Fabrication and characterization of cellulose nanoparticles from maize stalk pith via ultrasonic-mediated cationic etherification,” Ultrasonics Sonochemistry 66, article no. 104932. DOI: 10.1016/j.ultsonch.2019.104932
Ham, C.-H., Youn, H. J., and Lee, H.-L. (2020). “Influence of fiber composition and drying conditions on the bending stiffness of paper,” BioResources 15(4), 9197-9211. DOI: 10.15376/biores.15.4.9197-9211
Hashemzehi, M., Priouzfar, V., Nayebzadeh, H., and Su, C.-H. (2022). “Modelling and optimization of main independent parameters for biodiesel production over a Cu0. 4Zn0. 6Al2O4 catalyst using an RSM method,” Journal of Chemical Technology & Biotechnology 97(1), 111-119.
He, M., Cho, B.-U., Kyu, L. Y., and Won, J. M. (2016). “Utilizing cellulose nanofibril as an eco-friendly flocculant for filler flocculation in papermaking,” BioResources 11(4), 10296-10313. DOI: 10.15376/biores.11.4.10296-10313
He, M., Yang, G.-H., Cho, B.-U., Lee, Y. K., and Won, J. M. (2017). “Effects of addition method and fibrillation degree of cellulose nanofibrils on furnish drainability and paper properties,” Cellulose 24, 5657-5669. DOI: 10.1007/s10570-017-1495-3
He, Y.-Q., Li, H., Fei, X., and Peng, L.-C. (2021). “Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications,” Carbohydrate Polymers 252, article no. 117156. DOI: 10.1016/j.carbpol.2020.117156
Hilt, F., Boscher, N. D., Duday, D., Desbenoit, N., Levalois-Grützmacher, J., and Choquet, P. (2014). “Atmospheric pressure plasma-initiated chemical vapor deposition (AP-PiCVD) of poly (diethylallylphosphate) coating: A char-forming protective coating for cellulosic textile,” ACS Applied Materials & Interfaces 6, 18418-18422. DOI: 10.1021/am504892q
Hivechi, A., Bahrami, S. H., and Siegel, R. A. (2019). “Investigation of morphological, mechanical and biological properties of cellulose nanocrystal reinforced electrospun gelatin nanofibers,” International Journal of Biological Macromolecules 124, 411-417. DOI: 10.1016/j.ijbiomac.2018.11.214
Hollertz, R., Durán, V. L., Larsson, P. A., and Wågberg, L. (2017). “Chemically modified cellulose micro-and nanofibrils as paper-strength additives,” Cellulose 24, 3883-3899. DOI: 10.1016/j.ijbiomac.2018.11.214
Hu, F. G., Zeng, J.-S., Cheng, Z., Wang, X.-J., Wang, B., Zeng, Z.-T., and Chen, K-F. (2021). “Cellulose nanofibrils (NFC) produced by different mechanical methods to improve mechanical properties of recycled paper,” Carbohydrate Polymers 254, article no. 117474. DOI: 10.1016/j.carbpol.2020.117474
Hu, Z., Ballinger, S., Pelton, R., and Cranston, E. D. (2015). “Surfactant-enhanced cellulose nanocrystal pickering emulsions,” Journal of Colloid and Interface Science 439, 139-148. DOI: 10.1016/j.jcis.2014.10.034
Hu, Z., Berry, R. M., Pelton, R., and Cranston, E. D. (2017). “One-pot water-based hydrophobic surface modification of cellulose nanocrystals using plant polyphenols,” ACS Sustainable Chemistry & Engineering 5, 5018-5026. DOI: 10.1021/acssuschemeng.7b00415
Huang, J.-P. (2017). “Sustainable development of green paper packaging,” Environment and Pollution 6(2), 1-7. DOI: 10.5539/ep.v6n2p1
Huang, J., Cheng, Y.-X., Wu, Y., Shi, X.-W., Du, Y.-M., and Deng, H.-B. (2019). “Chitosan/tannic acid bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application,” International Journal of Biological Macromolecules 139, 191-198. DOI: 10.1016/j.ijbiomac.2019.07.185
Huang, X.-Y., Dognani, G., Hadi, P., Yang, M.-Y., Job, A. E., and Hsiao, B. S. (2020). “Cationic dialdehyde nanocellulose from sugarcane bagasse for efficient chromium(VI) removal,” ACS Sustainable Chemistry & Engineering 8, 4734-4744. DOI: 10.1021/acssuschemeng.9b06683
Huang, X.-J., Wang, A.-T., Xu, X., Liu, H., and Shang, S.-B. (2017). “Enhancement of hydrophobic properties of cellulose fibers via grafting with polymeric epoxidized soybean oil,” ACS Sustainable Chemistry & Engineering 5, 1619-1627. DOI: 10.1021/acssuschemeng.6b02359
Hubbe, M. A. (2014). “A review of ways to adjust papermaking wet-end chemistry: Manipulation of cellulosic colloidal behavior,” Lignocellulose 3, 69-107.
Hubbe, M. A, Rojas, O. J., and Lucia, L. A. (2015). “Green modification of surface characteristics of cellulosic materials at the molecular or nano scale: A review,” BioResources 10, 6095-6206. DOI: 10.15376/biores.10.3.Hubbe
Hubbe, M. A., Ferrer, A., Tyagi, P., Yin, Y.-Y., Salas, C., Pal, L., and Rojas, O. J. (2017). “Nanocellulose in thin films, coatings, and plies for packaging applications: A review,” BioResources 12, 2143-2233. DOI: 10.15376/biores.12.1.2143-2233
Hubbe, M. A., Sjöstrand, B., Nilsson, L., Koponen, A., and McDonald, J. D. (2020). “Rate-limiting mechanisms of water removal during the formation, vacuum dewatering, and wet-pressing of paper webs: A review,” BioResources 15, 9672-9755. DOI: 10.15376/biores.15.4.Hubbe
Islam, M. N., Rahman, F., Das, A. K., and Hiziroglu, S. (2022). “An overview of different types and potential of bio-based adhesives used for wood products,” International Journal of Adhesion and Adhesives 112, article no. 102992. DOI: 10.1016/j.ijadhadh.2021.102992
Iwata, T. (2015). “Biodegradable and bio‐based polymers: Future prospects of eco‐friendly plastics,” Angewandte Chemie International Edition 54, 3210-3215. DOI: 10.1002/anie.201410770
Jaekel, E. E., Sirviö, J. A., Antonietti, M., and Filonenko, S. (2021). “One-step method for the preparation of cationic nanocellulose in reactive eutectic media,” Green Chemistry 23, 2317-2323. DOI: 10.1039/D0GC04282J
Jankauskaitė, V., Balčiūnaitienė, A., Alexandrova, R., Buškuvienė, N., and Žukienė, K. (2020). “Effect of cellulose microfiber silylation procedures on the properties and antibacterial activity of polydimethylsiloxane,” Coatings 10(6), article no. 567. DOI: 10.3390/coatings10060567
Jarrah, K., Hisaindee, S., and Al-Sayah, M. H. (2018). “Preparation of oil sorbents by solvent-free grafting of cellulose cotton fibers,” Cellulose 25, 4093-4106. DOI: 10.1007/s10570-018-1846-8
Jasmania, L., and Thielemans, W. (2018). “Preparation of nanocellulose and its potential application,” International Journal of Nanomaterials, Nanotechnology and Nanomedicine 4, 014-21. DOI: 10.17352/2455-3492.000026
Ji, Y.-Z., Xu, Q.-H., Jin, L.-Q., and Fu, Y.-J. (2020). “Cellulosic paper with high antioxidative and barrier properties obtained through incorporation of tannin into kraft pulp fibers,” International Journal of Biological Macromolecules 162, 678-684. DOI: 10.1016/j.ijbiomac.2020.06.101
Jia, C., Chen, L.-H., Shao, Z.-Q., Agarwal, U. P., Hu, L.-B., and Zhu, J. Y. (2017). “Using a fully recyclable dicarboxylic acid for producing dispersible and thermally stable cellulose nanomaterials from different cellulosic sources,” Cellulose 24(6), 2483-2498.
Jin, L.-Q., Li, W.-G., Xu, Q.-H., and Sun, Q.-C. (2015). “Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes,” Cellulose 22, 2443-2446. DOI: 10.1007/s10570-015-0649-4
Jin, L.-Q., Wei, Y.-W., Xu, Q.-H., Yao, W.-R., and Cheng, Z.-L. (2014). “Cellulose nanofibers prepared from TEMPO‐oxidation of kraft pulp and its flocculation effect on kaolin clay,” Journal of Applied Polymer Science 131. DOI: 10.1002/app.40450
Jonoobi, M., Oladi, R., Davoudpour, Y., Oksman, K., Dufresne, A., Hamzeh, Y., and Davoodi, R. (2015). “Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review,” Cellulose 22, 935-969. DOI: 10.1007/s10570-015-0551-0
Kääriäinen, T. O., Maydannik, P., Cameron, D. C., Lahtinen, K., Johansson, P., and Kuusipalo, J. (2011). “Atomic layer deposition on polymer based flexible packaging materials: Growth characteristics and diffusion barrier properties,” Thin Solid Films 519, 3146-3154. DOI: 10.1016/j.tsf.2010.12.171
Kaboorani, A., and Riedl, B. (2015). “Surface modification of cellulose nanocrystals (CNC) by a cationic surfactant,” Industrial Crops and Products 65, 45-55. DOI: 10.1016/j.indcrop.2014.11.027
Kajanto, I., and Kosonen, M. (2012). “The potential use of micro-and nanofibrillated cellulose as a reinforcing element in paper,” Journal of Science & Technology for Forest Products and Processes 2, 42-48.
Khanjanzadeh, H., Behrooz, R., Bahramifar, N., Gindl-Altmutter, W., Bacher, M., Edler, M., and Griesser, T. (2018). “Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane,” International Journal of Biological Macromolecules 106: 1288-1296. DOI: 10.1016/j.ijbiomac.2017.08.136
Klemm, D., Cranston, E. D., Fischer, D., Gama, M., Kedzior, S. A., Kralisch, D., Kramer, F., Kondo, T., Lindström, T., and Nietzsche, S. (2018). “Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state,” Materials Today 21, 720-748. DOI: 10.1016/j.mattod.2018.02.001
Kokol, V., Vivod, V., Peršin, Z., Čolić, M., and Kolar, M. (2021). “Antimicrobial properties of viscose yarns ring-spun with integrated amino-functionalized nanocellulose,” Cellulose 28, 6545-6565. DOI: 10.1007/s10570-021-03946-z
Koppolu, R., Lahti, J., Abitbol, T., Swerin, A., Kuusipalo, J., and Toivakka, M. (2019). “Continuous processing of nanocellulose and polylactic acid into multilayer barrier coatings,” ACS Applied Materials & Interfaces 11, 11920-11927. DOI: 10.1021/acsami.9b00922
Korhonen, M., and Laine, J. (2014). “Flocculation and retention of fillers with nanocelluloses,” Nordic Pulp & Paper Research Journal 29, 119-128. DOI: 10.3183/npprj-2014-29-01-p119-128
Kovalenko, V. I. (2010). “Crystalline cellulose: Structure and hydrogen bonds,” Russian Chemical Reviews 79(3), 231.
Kumar, V. (2018). Roll-to-roll Processing of Nanocellulose Into Coatings, Ph.D. Dissertation, Åbo Academy University
Kumar, R., Rai, B., Gahlyan, S., and Kumar, G. (2021). “A comprehensive review on production, surface modification and characterization of nanocellulose derived from biomass and its commercial applications,” Express Polymer Letters 15(2), 104-120. DOI: 10.3144/expresspolymlett.2021.11
Kurpiers, M., Wolf, J. D., Spleis, H., Steinbring, C., Jörgensen, A. M., Matuszczak, B., and Bernkop-Schnürch, A. (2021). “Lysine-based biodegradable surfactants: increasing the lipophilicity of insulin by hydrophobic ion paring,” Journal of Pharmaceutical Sciences 110, 124-134. DOI: 10.1016/j.xphs.2020.07.024
Kushan, E., Demir, C., and Senses, E. (2020). “Surfactant driven liquid to soft solid transition of cellulose nanocrystal suspensions,” Langmuir 36, 9551-9561. DOI: 10.1021/acs.langmuir.0c01555
Kwon, G., Lee, K., Kim, D., Jeon, Y., Kim, U.-J., and You, J. (2020). “Cellulose nanocrystal-coated TEMPO-oxidized cellulose nanofiber films for high performance all-cellulose nanocomposites,” Journal of Hazardous Materials 398, article no. 123100. DOI: /10.1016/j.jhazmat.2020.123100
L’Anson, S. J., Sampson, W. W., and Sevajee, C. R. (2007). “New perspectives on the influence of formation and grammage on sheet strength,” in: Annual Meeting-Pulp and Paper Technical Association of Canada, p. 141, Pulp and Paper Technical Association of Canada.
Lamberti, M., and Escher, F. (2007). “Aluminium foil as a food packaging material in comparison with other materials,” Food Reviews International 23, 407-433. DOI: 10.1080/87559120701593830
Laureano-Anzaldo, C. M., González-López, M. E., Pérez-Fonseca, A. A., Cruz-Barba, L. E., and Robledo-Ortíz, J. R. (2020). “Plasma-enhanced modification of polysaccha-rides for wastewater treatment: A review,” Carbohydrate Polymers 252, article no. 117195. DOI: 10.1016/j.carbpol.2020.117195
Le, P. T., and Nguyen, K. T. (2020). “Hydrophobizing cellulose surfaces via catalyzed transesterification reaction using soybean oil and starch,” Heliyon 6, article ID e05559. DOI: 10.1016/j.heliyon.2020.e05559
Leal, S., Cristelo, C., Silvestre, S., Fortunato, E., Sousa, A., Alves, A., Correia, D. M., Lanceros-Mendez, S., and Gama, M. (2020). “Hydrophobic modification of bacterial cellulose using oxygen plasma treatment and chemical vapor deposition,” Cellulose 27, 10733-10746. DOI: 10.1007/s10570-020-03005-z
Lengowski, E. C., Bonfatti Júnior, E. A., Kumode, M. M. N., Carneiro, M. E., and Satyanarayana, K. G. (2019). “Nanocellulose in the paper making,” in: Sustainable Polymer Composites and Nanocomposites, Springer. DOI: 10.1007/978-3-030-05399-4_36
Lewis, H., Verghese, K., and Fitzpatrick, L. (2010). “Evaluating the sustainability impacts of packaging: The plastic carry bag dilemma,” Packaging Technology and Science: An International Journal 23, 145-160. DOI: 10.1002/pts.886
Li, A., Xu, D.-Z., Luo, L., Zhou, Y., Yan, W., Leng, X., Dai, D.-S., Zhou, Y.-H., Ahmad, H., and Jiuping Rao, J.-P. (2021a). “Overview of nanocellulose as additives in paper processing and paper products,” Nanotechnology Reviews 10, 264-281. DOI: 10.1515/ntrev-2021-0023
Li, B., Xu, C.-Q., Liu, L., Yu, J., and Fan, Y.-M. (2021b). “Facile and sustainable etherification of ethyl cellulose towards excellent UV blocking and fluorescence properties,” Green Chemistry 23, 479-489. DOI: 10.1039/D0GC02919J
Li, F., Mascheroni, E., and Piergiovanni, L. (2015a). “The potential of nanocellulose in the packaging field: A review,” Packaging Technology and Science 28, 475-508. DOI: 10.1002/pts.2121
Li, G. B., Fu, Y.-J., Shao, Z-Y., Zhang, F.-S., and Qin, M.-H. (2015b). “Preparing cationic cellulose derivative in NaOH/urea aqueous solution and its performance as filler modifier,” BioResources 10, 7782-7794. DOI: 10.15376/biores.10.4.7782-7794
Li, H.-L., Wu, B., Mu, C.-D., and Wei Lin, W. (2011). “Concomitant degradation in periodate oxidation of carboxymethyl cellulose,” Carbohydrate Polymers 84, 881-886. DOI: 10.1016/j.carbpol.2010.12.026
Li, M.-C., Wu, Q.-L., Moon, R., Hubbe, M., and Bortner, M. (2021c). “Rheological aspects of cellulose nanomaterials: Governing factors and emerging applications,” Advanced Materials 33(21), article no. 2006052. DOI: 10.1002/adma.202006052
Li, M.-C., Wu, Q.-L., Song, K.-L., Lee, S.-Y., Qing, Y., and Wu, Y.-Q. (2015c). “Cellulose nanoparticles: Structure–morphology–rheology relationships,” ACS Sustainable Chemistry & Engineering 3, 821-832. DOI: 10.1021/acssuschemeng.5b00144
Li, M.-F., Min, D.-Y., Long, X., Tu, Q.-Y., Zhang, K.-L., Wang, S.-F., and Luo, L.-X. (2019). “Effect of ionic liquid pretreatment on paper physical property and pulp refining performance,” Nordic Pulp & Paper Research Journal 34, 495-506. DOI: 10.1515/npprj-2019-0042
Li, P.-P., Sirviö, J. A., Asante, B., and Liimatainen, H. (2018). “Recyclable deep eutectic solvent for the production of cationic nanocelluloses,” Carbohydrate Polymers 199, 219-227. DOI: 10.1016/j.carbpol.2018.07.024
Li, T., Chen, C.-J., Brozena, A. H., Zhu, J.-Y., Xu, L.-X., Driemeier, C., Dai, J.-Q., Rojas, O. J., Isogai, A., and Wågberg, L. (2021d). “Developing fibrillated cellulose as a sustainable technological material,” Nature 590, 47-56. DOI: 10.1038/s41586-020-03167-7
Li, W.-G., and Xu, Q. H. (2014). “Preparation and characterization of dialdehyde nanocellulose,” in: Advanced Materials Research, pp. 79-83. Trans Tech Publ. DOI: 10.4028/www.scientific.net/AMR.988.79
Li, Y., Chen, L.-H., Wooding, J.-P., Zhang, F.-Y., Lively, R. P., Ramprasad, R., and Losego, M. D. (2020). “Controlling wettability, wet strength, and fluid transport selectivity of nanopaper with atomic layer deposited (ALD) sub-nanometer metal oxide coatings,” Nanoscale Advances 2, 356-367. DOI: 10.1039/C9NA00417C
Li, Y., and Losego, M. D. (2021). “Impact of trimethylaluminum exposure time on the mechanical properties of single-cycle atomic layer deposition modified cellulosic nanopaper,” Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 39, article no. 052407. DOI: 10.1116/6.0001198
Lien, C.-W., Vedhanarayanan, B., Chen, J.-H., Lin, J.-Y., Tsai, H.-H., Shao, L.-D., and Lin, T.-W. (2021). “Optimization of acetonitrile/water content in hybrid deep eutectic solvent for graphene/MoS2 hydrogel-based supercapacitors,” Chemical Engineering Journal 405, article no. 126706. DOI: 10.1016/j.cej.2020.126706
Lin, L. and Tsuchii, K. (2022). “Dissolution behavior of cellulose in a novel cellulose solvent,” Carbohydrate Research 511, article no. 108490.
Liu, H.-Y., Liu, Z., Liu, H.-B., Hui, L-F., Zhang, F.-D., Liu, P.-T., An, X-Y., Wen, Y.-B., and Wu, S.-J. (2019a). “Using cationic nanofibrillated cellulose to increase the precipitated calcium carbonate retention and physical properties during reconstituted tobacco sheet preparation,” Industrial Crops and Products 130, 592-597. DOI: 10.1016/j.indcrop.2019.01.021
Liu, Q., Yuan, T., Fu, Q.-J., Bai, Y.-Y., Peng, F., and Yao, C.-L. (2019b). “Choline chloride-lactic acid deep eutectic solvent for delignification and nanocellulose production of moso bamboo,” Cellulose 26, 9447-9462. DOI: 10.1007/s10570-019-02726-0
Liu, K., Wang, H., Liu, H.-Y., Nie, S.-X., Du, H.-S., and Si, C.-L. (2020). “COVID-19: Challenges and perspectives for the pulp and paper industry worldwide,” BioResources 15, 4638-4641. DOI: 10.15376/biores.15.3.4638-4641
Lourenço, A. F., Gamelas, J. A. F., Nunes, T., Amaral, J., Mutjé, P., and Ferreira, P. J. (2017). “Influence of TEMPO-oxidised cellulose nanofibrils on the properties of filler-containing papers,” Cellulose 24, 349-362. DOI: 10.1007/s10570-016-1121-9
Lourenço, A. F., Godinho, D., Gamelas, J. A. F., Sarmento, P., and Ferreira, P. J. T. (2019). “Carboxymethylated cellulose nanofibrils in papermaking: Influence on filler retention and paper properties,” Cellulose 26, 3489-3502. DOI: 10.1007/s10570-019-02303-5
Lu, J.-W., Li, Y., Song, W., Losego, M. D., Monikandan, R., Jacob, K. I., and Xiao, R. (2020a). “Atomic layer deposition onto thermoplastic polymeric nanofibrous aerogel templates for tailored surface properties,” ACS Nano 14, 7999-8011. DOI: 10.1021/acsnano.9b09497
Lu, P., Xiao, H.-N., and Yuanfeng Pan, Y.-F. (2015). “Improving water vapor barrier of green-based nanocellulose film via hydrophobic coating,” in: Proceedings of the 2014 International Conference on Materials Science and Energy Engineering (CMSEE 2014), pp. 148-153. World Scientific. DOI: 10.1142/9789814678971_0023
Lu, Z.-H., An, X.-Y., Zhang, H., Guan, M., Liu, J., Sun, Y.-W., Nie, S.-X., Cao, H.-B., Lu, B., and Liu, H.-B. (2019). “Study on the wet-web strength and pressability of paper sheet during the press process with the addition of nano-fibrillated cellulose (NFC),” Carbohydrate Polymers 210, 332-338. DOI: 10.1016/j.carbpol.2019.01.083
Lu, Z.-H., An, X.-Y., Zhang, H., Liu, L.-Q., Dai, H.-Q., Cao, H.-B., Lu, B., and Liu, H.-B. (2020b). “Cationic cellulose nano-fibers (CCNF) as versatile flocculants of wood pulp for high wet web performance,” Carbohydrate Polymers 229, article no. 115434. DOI: 10.1016/j.carbpol.2019.115434
Luo, B.-Y., An, X.-Y., Yang, J., Liu, L.-Q., Zhang, H., Hu, Q., Zhang, R.-Q., Nie, S.-X., Wu, S.-J., Cao, H.-B., Cheng, Z.-B., and Liu, H.-B. (2021). “Isolation and utilization of tobacco-based cellulose nanofiber (TCNF) for high performance reconstructed tobacco sheet (RTS),” Carbohydrate Polymers 261, article no. 117865. DOI: 10.1016/j.carbpol.2021.117865
Lv, X., YSu, Y. Q., and Liu, J. G. (2017). “Preparation and application of microfibrillated cellulose-modified ground calcium carbonate,” Paper and Biomaterials 2, 18.
Ly, M., and Mekonnen, T. H. (2020). “Cationic surfactant modified cellulose nanocrystals for corrosion protective nanocomposite surface coatings,” Journal of Industrial and Engineering Chemistry 83, 409-420. DOI: 10.1016/j.jiec.2019.12.014
Marcus, Y. (2019). “Trends and prospects for deep eutectic solvents,” in: Deep Eutectic Solvents, Springer. DOI: 10.1007/978-3-030-00608-2
Mariano, M., Wang L. H., da Silva Bernardes, J., and Strauss, M. (2018). “Microstructural characterization of nanocellulose foams prepared in the presence of cationic surfactants,” Carbohydrate Polymers 195, 153-162. DOI: 10.1016/j.carbpol.2018.04.075
Matouk, Z., Torriss, B., Rincón, R., Dorris, A., Beck, S., Berry, R. M., and Chaker, M. (2020). “Functionalization of cellulose nanocrystal films using non-thermal atmospheric-pressure plasmas,” Applied Surface Science 511, article no. 145566. DOI: 10.1016/j.apsusc.2020.145566
Mazhari Mousavi, S. M., Afra, E., Tajvidi, M., Bousfield, D. W., and Dehghani-Firouzabadi, M. (2018). “Application of cellulose nanofibril (CNF) as coating on paperboard at moderate solids content and high coating speed using blade coater,” Progress in Organic Coatings 122, 207-218. DOI: 10.1016/j.porgcoat.2018.05.024
Mellinas, C., Valdés, A., Ramos, M., Burgos, N., del Carmen Garrigos, M., and Jiménez, A. (2016). “Active edible films: Current state and future trends,” Journal of Applied Polymer Science 133. DOI: 10.1002/app.42631
Merayo, N., Balea, A., de la Fuente, E., Blanco, A., and Carlos Negro, C. (2017a). “Interactions between cellulose nanofibers and retention systems in flocculation of recycled fibers,” Cellulose 24, 677-692. DOI: 10.1007/s10570-016-1138-0
Merayo, N., Balea, A., de la Fuente, E., Blanco, A., and Carlos Negro, C. (2017b). “Synergies between cellulose nanofibers and retention additives to improve recycled paper properties and the drainage process,” Cellulose 24, 2987-3000. DOI: 10.1007/s10570-017-1302-1
Michelin, M., Gomes, D. G., Romaní, A., de Lourdes Polizeli, M., and Teixeira, J. A. (2020). “Nanocellulose production: Exploring the enzymatic route and residues of pulp and paper industry,” Molecules 25, article no. 3411. DOI: 10.3390/molecules25153411
Mirmehdi, S., Hein, P. R. G., de Luca Sarantópoulos, C. I. G., Dias, M. V., and Denzin Tonoli, G. H. (2018). “Cellulose nanofibrils/nanoclay hybrid composite as a paper coating: Effects of spray time, nanoclay content and corona discharge on barrier and mechanical properties of the coated papers,” Food Packaging and Shelf Life 15, 87-94. DOI: 10.1016/j.fpsl.2017.11.007
Missio, A. L., Tischer, B., dos Santos, P. D. B., Codevilla, C., de Menezes, C. R., Barin, J. S., Haselein, C. R., Labidi, J., Gatto, D. A., Petutschnigg, A., and Tondi, G. (2017). “Analytical characterization of purified mimosa (Acacia mearnsii) industrial tannin extract: Single and sequential fractionation,” Separation and Purification Technology 186, 218-225. DOI: 10.1016/j.seppur.2017.06.010
Missio, A. L., Mattos, B. D., Ferreira, M. F., Magalhães, W. L. E., Bertuol, D. A., Gatto, D. A., Petutschnigg, A., and Tondi, G. (2018). “Nanocellulose-tannin films: From trees to sustainable active packaging,” Journal of Cleaner Production 184: 143-51. DOI: 10.1016/j.jclepro.2018.02.205
Missio, A. L., Mattos, B. D., Otoni, C. G., Gentil, M., Coldebella, R., Khakalo, A., Gatto, D. A., and Rojas, O. J. (2020). “Cogrinding wood fibers and tannins: Surfactant effects on the interactions and properties of functional films for sustainable packaging materials,” Biomacromolecules 21, 1865-1874. DOI: 10.1021/acs.biomac.9b01733
Missoum, K., Belgacem, M. N., and Bras, J. (2013). “Nanofibrillated cellulose surface modification: A review,” Materials 6, article no. 1745-1766. DOI: 10.3390/ma6051745
Mohammadian, E., Alizadeh‐Sani, M., and Jafari, S. M. (2020). “Smart monitoring of gas/temperature changes within food packaging based on natural colorants,” Comprehensive Reviews in Food Science and Food Safety 19, 2885-2931. DOI: 10.1111/1541-4337.12635
Mokhena, T. C., and John, M. J. (2020). “Esterified cellulose nanofibers from saw dust using vegetable oil,” International Journal of Biological Macromolecules 148, 1109-1117. DOI: 10.1016/j.ijbiomac.2020.01.278
Motamedian, H. R., Armin E. Halilovic, A. E., and Kulachenko, A. (2019). “Mechanisms of strength and stiffness improvement of paper after PFI refining with a focus on the effect of fines,” Cellulose 26(6), 4099-4124.
Na, Y. J., and Muhlstein, C. L. (2019). “Relating nonuniform deformations to fracture in uniaxially loaded non-woven fiber networks,” Experimental Mechanics 59, 1127-1144. DOI: 10.1007/s11340-019-00527-x
Nam, T., Seo, S., and Kim, H. (2020). “Atomic layer deposition of a uniform thin film on two-dimensional transition metal dichalcogenides,” Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 38: article no. 030803. DOI: 10.1116/6.0000068
Nelson, K., Retsina, T., Iakovlev, M., van Heiningen, A., Deng, Y., Shatkin, J. A., and Mulyadi, A. (2016). “American process: Production of low cost nanocellulose for renewable, advanced materials applications,” in: Materials Research for Manufacturing, Springer. DOI: 10.1007/978-3-319-23419-9_9
Neves, R. M., Ornaghi Jr., H. L., Zattera, A. J., and Amico, S. C. (2020). “Recent studies on modified cellulose/nanocellulose epoxy composites: A systematic review,” Carbohydrate Polymers 255(1), article no. 117366. DOI: 10.1016/j.carbpol.2020.117366
Nie, S.-X., Fu, Q., Lin, X.-J., Zhang, C.-Y., Lu, Y.-X., and Wang, S.-F. (2021). “Enhanced performance of a cellulose nanofibrils-based triboelectric nanogenerator by tuning the surface polarizability and hydrophobicity,” Chemical Engineering Journal 404, article no. 126512. DOI: 10.1016/j.cej.2020.126512
Norman, B. (2000). “Web forming,” in: Papermaking. Part 1: Stock Preparation and Wet End, H. Paulapuro and J. Gullichsen (eds.), Papet Oy, pp. 193-250.
Nypelö, T., Berke, B., Spirk, S., and Sirviö, J. A. (2021). “Periodate oxidation of wood polysaccharides—Modulation of hierarchies,” Carbohydrate Polymers 252, article no. 117105. DOI: 10.1016/j.carbpol.2020.117105
O’Brien, C. T., Virtanen, T., Donets, S., Jennings, J., Guskova, O., Morrell, A. H., Rymaruk, M., Ruusuvirta, L., Salmela, J., Setala, H., Sommer, J.-U., Ryan, A. J., and Mykhaylyk, O. O. (2021). “Control of the aqueous solubility of cellulose by hydroxyl group substitution and its effect on processing,” Polymer 223, article no. 123681. DOI: 10.1016/j.polymer.2021.123681
Oberlintner, A., Likozar, B., and Novak, U. (2021). “Hydrophobic functionalization reactions of structured cellulose nanomaterials: Mechanisms, kinetics and in silico multi-scale models,” Carbohydrate Polymers 259, article no. 117742. DOI: 10.1016/j.carbpol.2021.117742
Odabas, N., Amer, H., Bacher, M., Henniges, U., Potthast, A., and Rosenau, T. (2016). “Properties of cellulosic material after cationization in different solvents,” ACS Sustainable Chemistry & Engineering 4, 2295-2301. DOI: 10.1021/acssuschemeng.5b01752
Omerzu, A., Saric, I., Piltaver, I. K., Petravic, M., Kapun, T., Zule, J., Stifter, S., and Salamon, K. (2018). “Prevention of spontaneous combustion of cellulose with a thin protective Al2O3 coating formed by atomic layer deposition,” Surface and Coatings Technology 333, 81-86. DOI: 10.1016/j.surfcoat.2017.10.067
Onwukamike, K. N., Grelier, S., Grau, E., Cramail, H., and Meier, M. A. R. (2018). “Sustainable transesterification of cellulose with high oleic sunflower oil in a DBU-CO2 switchable solvent,” ACS Sustainable Chemistry & Engineering 6, 8826-8835. DOI: 10.1021/acssuschemeng.8b01186
Ottesen, V., Syverud, K., and Gregersen, Ø. W. (2016). “Mixing of cellulose nanofibrils and individual furnish components: Effects on paper properties and structure,” Nordic Pulp Paper Res. J. 31(3), 441-447. DOI: 10.3183/npprj-2016-31-03-p441-447
Oun, A. A., Shankar, S., and Rhim, J.-W. (2020). “Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials,” Critical Reviews in Food Science and Nutrition 60, 435-460. DOI: 10.1080/10408398.2018.1536966
Oviroh, Ozaveshe, P., Akbarzadeh, R., Pan, D.-Q., Coetzee, R. A. M., and Jen, T.-C. (2019). “New development of atomic layer deposition: Processes, methods and applications,” Science and Technology of Advanced Materials 20, 465-496. DOI: 10.1080/14686996.2019.1599694
Panaitescu, D. M., Vizireanu, S., Nicolae, C. A., Frone, A. N., Casarica, A., Carpen, L. G., and Dinescu, G. (2018). “Treatment of nanocellulose by submerged liquid plasma for surface functionalization,” Nanomaterials 8, article no. 467. DOI: 10.3390/nano8070467
Patra, A. S., Makhija, D., Mukherjee, A. K., Tiwari, R., Sahoo, C. R., and Mohanty, B. D. (2016). “Improved dewatering of iron ore fines by the use of surfactants,” Powder Technology 287, 43-50. DOI: 10.1016/j.powtec.2015.09.030
Pattnaik, S., Surendra, Y., Rao, J. V., and Swain, K. (2020). “Carbon family nanomaterials for drug delivery applications,” Nanoengineered Biomaterials for Advanced Drug Delivery 2020, 421-445. DOI: 10.1016/B978-0-08-102985-5.00018-8
Pessoa, R. S., Fraga, M. A., Santos, L. V., Galvão, N. K. A. M., Maciel, H. S., and Massi, M. (2015). “18 – Plasma-assisted techniques for growing hard nanostructured coatings: An overview,” in: Anti-Abrasive Nanocoatings, Mahmood Aliofkhazraei (ed.), Woodhead Publishing. DOI: 10.1016/B978-0-85709-211-3.00018-2
Petkoska, A. T., Daniloski, D., D’Cunha, M. M., Naumovski, N., and Broach, A. T. (2021). “Edible packaging: Sustainable solutions and novel trends in food packaging,” Food Research International 140, article no. 109981. DOI: 10.1016/j.foodres.2020.109981
Pettignano, A., Charlot, A., and Fleury, E. (2019). “Solvent-free synthesis of amidated carboxymethyl cellulose derivatives: Effect on the thermal properties,” Polymers (11), article no. 1227. DOI: 10.3390/polym11071227
Phanthong, P., Guan, G.-Q., Karnjanakom, S., Hao, X.-G., Wang, Z.-D., Kusakabe, K., and Abudula, A. (2016). “Amphiphobic nanocellulose-modified paper: Fabrication and evaluation,” RSC Advances 6, article no. 13328-13334. DOI: 10.1039/C5RA24986D
Pourmoazzen, Z., Sadeghifar, H., Chen, J.-C., Yang, G.-H., Zhang, K., and Lucia, L. A. (2020). “The morphology, self-assembly, and host-guest properties of cellulose nanocrystals surface grafted with cholesterol,” Carbohydrate Polymers 233, article no. 115840. DOI: 10.1016/j.carbpol.2020.115840
Qian, L.-W., Yang, M.-X., Chen, H.-N., Xu, Y., Zhang, S.-F., Zhou, Q.-S., He, B., Bai, Y., and Song, W.-Q. (2019). “Preparation of a poly (ionic liquid)-functionalized cellulose aerogel and its application in protein enrichment and separation,” Carbohydrate Polymers 218, 154-162. DOI: 10.1016/j.carbpol.2019.04.081
Rafieian, F., Hosseini, M., Jonoobi, M., and Yu, Q.-L. (2018). “Development of hydrophobic nanocellulose-based aerogel via chemical vapor deposition for oil separation for water treatment,” Cellulose 25, 4695-4710. DOI: 10.1007/s10570-018-1867-3
Rai, P., Mehrotra, S., Priya, S., Gnansounou, E., and Sharma, S. K. (2021). “Recent advances in the sustainable design and applications of biodegradable polymers,” Bioresource Technology 325, article no. 124739. DOI: 10.1016/j.biortech.2021.124739
Rantanen, J., and Maloney, T. C. (2013). “Press dewatering and nip rewetting of paper containing nano- and microfibril cellulose,” Nordic Pulp & Paper Research Journal 28, 582-587. DOI: 10.3183/npprj-2013-28-04-p582-587
Ren, H.-W., Chen, C.-M., Guo, S-H., Zhao, D.-S., and Wang, Q.-H. (2016). “Synthesis of a novel allyl-functionalized deep eutectic solvent to promote dissolution of cellulose,” BioResources 11, 8457-8469. DOI: 10.15376/biores.11.4.8457-8469
Reshmy, R., Philip, E., Paul, S. A., Madhavan, A., Sindhu, R., Binod, P., Pandey, A., and Sirohi, R. (2020). “Nanocellulose-based products for sustainable applications-recent trends and possibilities,” Reviews in Environmental Science and Bio/Technology 19, 779-806. DOI: 10.1007/s11157-020-09551-z
Rigotti, D., Pegoretti, A., Miotello, A., and Checchetto, R. (2020). “Interfaces in biopolymer nanocomposites: Their role in the gas barrier properties and kinetics of residual solvent desorption,” Applied Surface Science 507, article no. 145066. DOI: 10.1016/j.apsusc.2019.145066
Rol, F., Belgacem, M. N., Gandini, A., and Bras, J. (2019a). “Recent advances in surface-modified cellulose nanofibrils,” Progress in Polymer Science 88, 241-64. DOI: 10.1016/j.progpolymsci.2018.09.002
Rol, F., Saini, S., Meyer, V., Petit-Conil, M., and Bras, J. (2019b). ‘Production of cationic nanofibrils of cellulose by twin-screw extrusion,” Industrial Crops and Products 137, 81-88. DOI: 10.1016/j.indcrop.2019.04.031
Rudie, A. (2017). “Commercialization of cellulose nanofibril (CNF) and cellulose nanocrystal (CNC): Pathway and challenges,” in: Handbook of Nanocellulose and Cellulose Nanocomposites, H. Kargarzadeh, I. Ahmad, S. Thomas, and A. Dufresne (eds.), first edition, Chapter 23, pp. 761-797. DOI: 10.1002/9783527689972.ch23
Rytioja, J., Hildén, K., Yuzon, J., Hatakka, A., De Vries, R. P., and Mäkelä, M. R. (2014). “Plant-polysaccharide-degrading enzymes from basidiomycetes,” Microbiology and Molecular Biology Reviews 78, 614-649. DOI: 10.1128/MMBR.00035-14
Saba, N., Safwan, A., Sanyang, M. L., Mohammad, F., Pervaiz, M., Jawaid, M., Alothman, O. Y., and Sain, M. (2017). “Thermal and dynamic mechanical properties of cellulose nanofibers reinforced epoxy composites,” International Journal of Biological Macromolecules 102, 822-828. DOI: 10.1016/j.ijbiomac.2017.04.074
Sacui, I. A., Nieuwendaal, R. C., Burnett, D. J., Stranick, S. J., Jorfi, M., Weder, C., Foster, E. J., Olsson, R. T., and Gilman, J. W. (2014). “Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods,” ACS Applied Materials & Interfaces 6, 6127-6138. DOI: 10.1021/am500359f
Sain, S., Åkesson, D., Skrifvars, M., and Roy, S. (2020). “Hydrophobic shape-memory biocomposites from tung-oil-based bioresin and onion-skin-derived nanocellulose networks,” Polymers 12, article no. 2470. DOI: 10.3390/polym12112470
Salminen, K. (2010). “The effects of some furnish and paper structure related factors on wet web tensile and relaxation characteristics,” Lappeenranta University of Technology.
Samadani, F., Behzad, T., and Enayati, M. S. (2019). “Facile strategy for improvement properties of whey protein isolate/walnut oil bio-packaging films: Using modified cellulose nanofibers,” International Journal of Biological Macromolecules 139, 858-66. DOI: 10.1016/j.ijbiomac.2019.08.042
Samanta, K. K., Joshi, A. G., Jassal, M., and Agrawal, A. K. (2012). “Study of hydrophobic finishing of cellulosic substrate using He/1,3-butadiene plasma at atmospheric pressure,” Surface and Coatings Technology 213, 65-76. DOI: 10.1016/j.surfcoat.2012.10.016
Samanta, K. K., Gayatri, T. N., Saxena, S., Basak, S., Chattopadhyay, S. K., Arputharaj, A., and Prasadm, V. (2016). “Hydrophobic functionalization of cellulosic substrates using atmospheric pressure plasma,” Cellulose Chemistry and Technology 50, 745-54.
Samanta, K. K., Basak, S., and Pandit, P. (2020). “Plasma and other irradiation technologies application,” in: Frontiers of Textile Materials: Polymers, Nanomaterials, Enzymes, and Advanced Modification Techniques, M. Shabbir, S. Ahmed and J. N. Sheikh (eds.), Scrivener Publ. LLC, pp. 309-333. DOI: 10.1002/9781119620396.ch13
Samanta, K. K., Joshi, A. G., Jassal, M., and Agrawal, A. K. (2021). “Hydrophobic functionalization of cellulosic substrate by tetrafluoroethane dielectric barrier discharge plasma at atmospheric pressure,” Carbohydrate Polymers 253, article no. 117272. DOI: 10.1016/j.carbpol.2020.117272
Seddiqi, H., Oliaei, E., Honarkar, H., Jin, J.-F., Geonzon, L. C., Bacabac, R. G., and Klein-Nulend, J. (2021). “Cellulose and its derivatives: Towards biomedical applications,” Cellulose 28, 1893-1931. DOI: 10.1007/s10570-020-03674-w
Selkälä, T., Sirviö, J. A., Lorite, G. S., and Liimatainen, H. (2016). “Anionically stabilized cellulose nanofibrils through succinylation pretreatment in urea–lithium chloride deep eutectic solvent,” ChemSusChem 9, 3074-3083. DOI: 10.1002/cssc.201600903
Selkälä, T., Suopajärvi, T., Sirviö, J. A., Luukkonen, T., Kinnunen, P., Braga de Carvalho, A. L. C., and Liimatainen, H. (2020). “Surface modification of cured inorganic foams with cationic cellulose nanocrystals and their use as reactive filter media for anionic dye removal,” ACS Applied Materials & Interfaces 12, 27745-27757. DOI: 10.1021/acsami.0c05927
Sethi, J., Oksman, K., Illikainen, M., and Sirviö, J. A. (2018). “Sonication-assisted surface modification method to expedite the water removal from cellulose nanofibers for use in nanopapers and paper making,” Carbohydrate Polymers 197, 92-99. DOI: 10.1016/j.carbpol.2018.05.072
Shang, W.-L., Huang, J., Luo, H., Chang, P. R., Feng, J.-W., and Xie, G.-Y. (2013). “Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil,” Cellulose 20, 179-190. DOI: 10.1007/s10570-012-9795-0
Sharma, M., Aguado, R., Murtinho, D., Valente, A. J. M., Mendes De Sousa, A. P., and Ferreira, P. J. T. (2020). “A review on cationic starch and nanocellulose as paper coating components,” International Journal of Biological Macromolecules 162, 578-598. DOI: 10.1016/j.ijbiomac.2020.06.131
Shatkin, J. A., Wegner, T. H., Bilek, E. M. T., and Cowie, J. (2014). “Market projections of cellulose nanomaterial-enabled products – Part 1: Applications,” TAPPI J. 13(5), 9-16. DOI: 10.32964/TJ13.5.9
Shim, J. H., Kang, S., Cha, S.-W., Lee, W. Y., Kim, Y. B., Park, J. S., Guer, T. M., Prinz, F. B., Chao, C.-C., and An, J. (2013). “Atomic layer deposition of thin-film ceramic electrolytes for high-performance fuel cells,” Journal of Materials Chemistry A 1, 12695-126705. DOI: 10.1039/c3ta11399j
Siró, I., and Plackett, D. (2010). “Microfibrillated cellulose and new nanocomposite materials: A review,” Cellulose 17, 459-494. DOI: 10.1007/s10570-010-9405-y
Sirviö, J., Honka, A., Liimatainen, H., Niinimäki, J., and Hormi, O. (2011). “Synthesis of highly cationic water-soluble cellulose derivative and its potential as novel biopolymeric flocculation agen,” Carbohydrate Polymers 86, 266-270. DOI: 10.1016/j.carbpol.2011.04.046
Skočaj, M. (2019). “Bacterial nanocellulose in papermaking,” Cellulose 26, 6477-6488. DOI: 10.1007/s10570-019-02566-y
Souza, A. G., Santos, D. F, Ferreira, R. R., Pinto, V. Z., and Rosa, D. S. (2020). “Innovative process for obtaining modified nanocellulose from soybean straw,” International Journal of Biological Macromolecules 165, 1803-1812. DOI: 10.1016/j.ijbiomac.2020.10.036
Su, J.-L, Mosse, W. K. J., Sharman, S., Batchelor, W., and Garnier, G. (2012). “Paper strength development and recyclability with polyamideamine-epichlorohydrin (PAE),” BioResources 7(1), 913-924.
Su, Y.-Q., Liu, J.-J., Liu, J.-G., Zhang, R,-J., Chen, J.-H., and Du, Y.-F. (2020). “Effects of MFC-modified GCC as filler on the opacity of pulp handsheets,” Paper and Biomaterials 5(3), 62-69.
Sun, L., Zhang, X.-Y., Liu, H-Y., Liu, K., Du, H.-S., Kumar, A., Sharma, G., and Si, C.-L. (2021a). “Recent advances in hydrophobic modification of nanocellulose,” Current Organic Chemistry 25, 417-436. DOI: 10.2174/1385272824999201210191041
Sun, L.-Z., Yuan, G.-W., Gao, L.-B., Yang, J.-U., Chhowalla, M., Gharahcheshmeh, M. H., Gleason, K. K., Choi, Y. S., Hong, B. H., and Liu, Z.-F. (2021b). “Chemical vapour deposition,” Nature Reviews Methods Primers 1, 1-20. DOI: 10.1038/s43586-020-00005-y
Sun, W., Liu, W.-Y., Wu, Z.-Q., and Chen, H. (2020). “Chemical surface modification of polymeric biomaterials for biomedical applications,” Macromolecular Rapid Communications 41, article no. 1900430. DOI: 10.1002/marc.201900430
Sunasee, R., and Hemraz, U. D. (2018). “Synthetic strategies for the fabrication of cationic surface-modified cellulose nanocrystals,” Fibers 6(1), article no. 15. DOI: 10.3390/fib6010015
Suopajärvi, T., Sirviö, J. A., and Liimatainen, H. (2017). “Nanofibrillation of deep eutectic solvent-treated paper and board cellulose pulps,” Carbohydrate Polymers 169, 167-175. DOI: 10.1016/j.carbpol.2017.04.009
Szlek, D. B., Reynolds, A. M., and Hubbe, M. A. (2022). “Hydrophobic molecular treatments of cellulose-based or other polysaccharide barrier layers for sustainable food packaging: A Review,” BioResources 17(2), Page numbers to be added. DOI: 10.15376/biores.17.2.Szlek
Taipale, T., Österberg, M., Nykänen, A., Ruokolainen, J., and Laine, J. (2010). “Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength,” Cellulose 17, 1005-1020. DOI: 10.1007/s10570-010-9431-9
Tambe, C., Graiver, D., and Narayan, R. (2016). “Moisture resistance coating of packaging paper from biobased silylated soybean oil,” Progress in Organic Coatings 101, 270-278. DOI: 10.1016/j.porgcoat.2016.08.016
Tang, X., Zuo, M., Li, Z., Liu, H., Xiong, C.-X., Zeng, X.-H., Sun, Y., Hu, L., Liu, S.-J., and Lei, T.-Z. (2017). “Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents,” ChemSusChem 10, 2696-2706. DOI: 10.1002/cssc.201700457
Tardy, B. L., Yokota, S., Ago, M., Xiang, W.-C., Kondo, T., Bordes, R., and Rojas, O. J. (2017). “Nanocellulose–surfactant interactions,” Current Opinion in Colloid & Interface Science 29, 57-67. DOI: 10.1016/j.cocis.2017.02.004
Taylor, S. R. (2001). “Coatings for corrosion protection: Organic,” in: Encyclopedia of Materials: Science and Technology, K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan, and P. Veyssière (eds.), Elsevier, Oxford.
Thakur, V., Guleria, A., Kumar, S., Sharma, S., and Singh, K. (2021). “Recent advances in nanocellulose processing, functionalization and applications: A review,” Materials Advances 2, 1872-1895. DOI: 10.1039/D1MA00049G
Thomas, B., Raj, M. C., Joy, J., Moores, A., Drisko, G. L., and Sanchez, C. (2018). “Nanocellulose, a versatile green platform: From biosources to materials and their applications,” Chemical Reviews 118, 11575-11625. DOI: 10.1021/acs.chemrev.7b00627
Tian, X.-Z., Yan, D.-D., Lu, Q.-X., and Jiang, X. (2017). “Cationic surface modification of nanocrystalline cellulose as reinforcements for preparation of the chitosan-based nanocomposite films,” Cellulose 24, 163-174. DOI: 10.1007/s10570-016-1119-3
Tiitu, M., Laine, J., Serimaa, R., and Ikkala, O. (2006). “Ionically self-assembled carboxymethyl cellulose/surfactant complexes for antistatic paper coatings,” Journal of Colloid and Interface Science 301, 92-97. DOI: 10.1016/j.jcis.2006.04.072
Trache, D., Tarchoun, A. F., Derradji, M., Hamidon, T., S., Masruchin, N., Brosse, N., and Hussin, M. H. (2020). “Nanocellulose: From fundamentals to advanced applications,” Frontiers in Chemistry 8, article no. 392. DOI: 10.3389/fchem.2020.00392
Tu, H., Zhu, M.-X., Duan, B., and Zhang, L.-N. (2021). “Recent progress in high‐strength and robust regenerated cellulose materials,” Advanced Materials 33(28), article no. 2000682. DOI: 10.1002/adma.202000682
Tyagi, P., Lucia, L. L., Hubbe, M. A., and Pal, L. (2019). “Nanocellulose-based multilayer barrier coatings for gas, oil, and grease resistance,” Carbohydrate Polymers 206: 281-288. DOI: 10.1016/j.carbpol.2018.10.114
Usov, I., Nyström, G., Adamcik, J., Handschin, S., Schütz, C., Fall, A., Bergström, L., and Mezzenga, R. (2015). “Understanding nanocellulose chirality and structure–properties relationship at the single fibril level,” Nature Communications 6, article no. 7564. DOI: 10.1038/ncomms8564
Vallejos, M. E., Felissia, F. E., Area, M. C., Ehman, N. V., Tarrés, Q., and Mutjé, P. (2016). “Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets,” Carbohydrate Polymers 139, 99-105. DOI: 10.1016/j.carbpol.2015.12.004
Vaziri Hassas, B., Karakaş, F., and Çelik, M. S. (2014). “Ultrafine coal dewatering: Relationship between hydrophilic lipophilic balance (HLB) of surfactants and coal rank,” International Journal of Mineral Processing 133, 97-104. DOI: 10.1016/j.minpro.2014.10.010
Verghese, K., Lewis, H., Lockrey, S., and Williams, H. (2013). “The role of packaging in minimising food waste in the supply chain of the future: Prepared for: CHEP Australia,” in.: RMIT University Report.
Vijay, R., Singaravelu, D. L., Vinod, A., Sanjay, M. R., Siengchin, S., Jawaid, M., Khan, A., and Parameswaranpillai, J. (2019). “Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens,” International Journal of Biological Macromolecules 125, 99-108. DOI: 10.1016/j.ijbiomac.2018.12.056
Vineeth, S. K., Gadhave, R. V., and Gadekar, P. T. (2019). “Chemical modification of nanocellulose in wood adhesive,” Open Journal of Polymer Chemistry 9, 86. DOI: 10.4236/ojpchem.2019.94008
Vizireanu, S., Panaitescu, D. M., Nicolae, C. A., Frone, A. N., Chiulan, I., Ionita, M. D., Satulu, V., Carpen, L. G., Petrescu, S., Birjega, R., and Dinescu, G. (2018). “Cellulose defibrillation and functionalization by plasma in liquid treatment,” Scientific Reports 8, article no. 15473. DOI: 10.1038/s41598-018-33687-2
Vuoti, S., Narasimha, K., and Reinikainen, K. (2018). “Green wastewater treatment flocculants and fixatives prepared from cellulose using high-consistency processing and deep eutectic solvents,” Journal of Water Process Engineering 26, 83-91. DOI: 10.1016/j.jwpe.2018.09.003
Wågberg, L. (2000). “Polyelectrolyte adsorption onto cellulose fibers – A review,” Nordic Pulp & Paper Research Journal 15, 586-597. DOI: 10.3183/npprj-2000-15-05-p586-597
Walther, A., Lossada, F., Benselfelt, T., Kriechbaum, K., Berglund, L., Ikkala, O., Saito, T., Wågberg, L., and Bergström, L. (2020). “Best practice for reporting wet mechanical properties of nanocellulose-based materials,” Biomacromolecules 21, 2536-2540. DOI: 10.1021/acs.biomac.0c00330
Wang, B., Ran, M., Fang, G.-G., Wu, T., Tian, Q.-W., Zheng, L.-Q., Romero-Zerón, L., and Ni, Y-H. (2020a). “Palladium nano-catalyst supported on cationic nanocellulose–alginate hydrogel for effective catalytic reactions,” Cellulose 27, 6995-7008. DOI: 10.1007/s10570-020-03127-4
Wang, J.-W., Gardner, D. J., Stark, N. M., Bousfield, D. W., Tajvidi, M., and Cai, Z.-Y. (2018). “Moisture and oxygen barrier properties of cellulose nanomaterial-based films,” ACS Sustainable Chemistry & Engineering 6, 49-70. DOI: 10.1021/acssuschemeng.7b03523
Wang, L., Chen, C., Wang, J.-W., Gardner, D. J., and Tajvidi, M. (2020b). “Cellulose nanofibrils versus cellulose nanocrystals: Comparison of performance in flexible multilayer films for packaging applications,” Food Packaging and Shelf Life 23, article no. 100464. DOI: 10.1016/j.fpsl.2020.100464
Wang, M.-Z., and Cha, R.-T. (2019). “Industrialization progress of nanocellulose in China,” Paper and Biomaterials 4, 63.
Wang, Q.-Q., Yao, Q., Liu, J., Sun, J. Z., Zhu, Q. Q., and Chen, H.-L. (2019). “Processing nanocellulose to bulk materials: A review,” Cellulose 26, 7585-7617. DOI: 10.1007/s10570-019-02642-3
Wang, Y.-G., Wang, X.-J., Xie, Y.-J., and Zhang, K. (2018). “Functional nanomaterials through esterification of cellulose: A review of chemistry and application,” Cellulose 25, 3703-3731. DOI: 10.1007/s10570-018-1830-3
Wei, L.-Q., Agarwal, U. P., Hirth, K. C., Matuana, L. M., Sabo, R. C., and Stark, N. M. (2017). “Chemical modification of nanocellulose with canola oil fatty acid methyl ester,” Carbohydrate Polymers 169, 108-116. DOI: 10.1016/j.carbpol.2017.04.008
Wooding, J. P, Li, Y., Kalaitzidou, K., and Losego, M. D. (2020). “Engineering the interfacial chemistry and mechanical properties of cellulose-reinforced epoxy composites using atomic layer deposition (ALD),” Cellulose 27, 6275-6285. DOI: 10.1007/s10570-020-03188-5
Wu, F., Misra, M., and Mohanty, A. K. (2021). “Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging,” Progress in Polymer Science 117, article no. 101395. DOI: 10.1016/j.progpolymsci.2021.101395
Xhanari, K., Syverud, K., Chinga-Carrasco, G., Paso, K., and Stenius, P. (2011). “Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants,” Cellulose 18, 257-270. DOI: 10.1007/s10570-010-9482-y
Xiang, S.-Y., Ma, X.-Z., Liao, S.-Y., Shi, H., Liu, C.-Y., Shen, Y., Lv, X., Yuan, M.-T., Fan, G.-J., Huang, J., and Sun, X. C. (2019). “Cellulose nanocrystal surface cationization: A new fungicide with high activity against Phycomycetes capsici,” Molecules 24(13), article no. 2467. DOI: 10.3390/molecules24132467
Xu, J.-F., Li, X.-Y., Liu, R., Shang, Z., Long, L., Qiu, H. Y., and Ni, Y. H. (2020). “Dialdehyde modified cellulose nanofibers enhanced the physical properties of decorative paper impregnated by aldehyde-free adhesive,” Carbohydrate Polymers 250, article no. 116941. DOI: 10.1016/j.carbpol.2020.116941
Xu, J.-W., Manepalli, P. H., Zhu, L.-J., Narayan-Sarathy, S., and Alavi, S. (2019a). “Morphological, barrier and mechanical properties of films from poly (butylene succinate) reinforced with nanocrystalline cellulose and chitin whiskers using melt extrusion,” Journal of Polymer Research 26(8), 1-10. DOI: 10.1007/s10965-019-1783-8
Xu, X., Dong, F.-H., Yang, X.-X., Liu, H., Guo, L.-Z., Qian, Y-H., Wang, A.-T., Wang, S.-F., and Luo, J.-Y. (2019b). “Preparation and characterization of cellulose grafted with epoxidized soybean oil aerogels for oil-absorbing materials,” Journal of Agricultural and Food Chemistry 67, 637-643. DOI: 10.1021/acs.jafc.8b05161
Yadav, C., Saini, A., Zhang, W.-B., You, X.-Y., Chauhan, I., Mohanty, P., and Li, X.-P. (2020). “Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting,” International Journal of Biological Macromolecules 166. 1586-1616. DOI: 10.1016/j.ijbiomac.2020.11.038
Yang, H., and van de Ven, T. G. M. (2016). “Preparation of hairy cationic nanocrystalline cellulose,” Cellulose 23, 1791-1801. DOI: 10.1007/s10570-016-0902-5
Yang, G. H., Ma, G. R., He, M., Ji, X. X., Youn, H. J., Lee, H. L. and Chen, J. C. (2020a). “Application of cellulose nanofibril as a wet-end additive in papermaking: A brief review,” Paper and Biomaterials 5, 76-84.
Yang, R., Liang, Y.-J., Hong, S., Zuo, S.-D., Wu, Y.-J., Shi, J.-T., Cai, L.-P., Li, J. Z., Mao, H. Y., and Ge, S.-B. (2020b). “Novel low-temperature chemical vapor deposition of hydrothermal delignified wood for hydrophobic property,” Polymers 12(8), article no. 1757. DOI: 10.3390/polym12081757
Yang, H.-W., Zhang, Q., Zhang, J.-H., Yang, L.-X., Ma, Z.-H., Wang, L., Li, H.-G., Bai, L.-J., Wei, D.-L., and Wang, W.-X. (2019a). “Cellulose nanocrystal shelled with poly (ionic liquid)/polyoxometalate hybrid as efficient catalyst for aerobic oxidative desulfurization,” Journal of Colloid and Interface Science 554, 572-579. DOI: 10.1016/j.jcis.2019.07.036
Yang, Z.-H., Taka-Aki Asoh, T.-A., and Uyama, H. (2019b). “Cationic functionalization of cellulose monoliths using a urea-choline based deep eutectic solvent and their applications,” Polymer Degradation and Stability 160, 126-135. DOI: 10.1016/j.polymdegradstab.2018.12.015
Yao, M. Z., Liu, Y., Qin, C. N., Meng, X. J., Cheng, B. X., Zhao, H., Wang, S. F., and Huang, Z. Q. (2021). “Facile fabrication of hydrophobic cellulose-based organic/inorganic nanomaterial modified with POSS by plasma treatment,” Carbohydrate Polymers 253, article no. 117193. DOI: 10.1016/j.carbpol.2020.117193
Yaradoddi, J. S., Banapurmath, N. R., Ganachari, S. V., Soudagar, M. E. M., Mubarak, N. M., Hallad, S., Hugar, S., and Fayaz, H. (2020). “Biodegradable carboxymethyl cellulose based material for sustainable packaging application,” Scientific Reports 10, article no. 21960. DOI: 10.1038/s41598-020-78912-z
Yogalakshmi, K. N., and Singh, S. (2020). ”Plastic waste: Environmental hazards, its biodegradation, and challenges,” in: Bioremediation of Industrial Waste for Environmental Safety, G. Saxena and R. Bharagava (eds.), Springer, Singapore. 99-133 DOI: 10.1007/978-981-13-1891-7_6
Yoo, Y., and Youngblood, J. P. (2016). “Green one-pot synthesis of surface hydrophobized cellulose nanocrystals in aqueous medium,” ACS Sustainable Chemistry & Engineering 4, 3927-3938. DOI: 10.1021/acssuschemeng.6b00781
Yook, S., Park, H., Park, H., Lee, S.-Y., Kwon, J. Y., and Youn, H. J. (2020). “Barrier coatings with various types of cellulose nanofibrils and their barrier properties,” Cellulose 27, 4509-4523. DOI: 10.1007/s10570-020-03061-5
Yu, L.-S., Zhang, Z.-M., Tang, H.-D., and Zhou, J.-P. (2019). “Fabrication of hydrophobic cellulosic materials via gas–solid silylation reaction for oil/water separation,” Cellulose 26, 4021-4037. DOI: 10.1007/s10570-019-02355-7
Yuan, J.-X., Wang, T., Huang, X.-N., and Wei, W. (2016). “Dispersion and beating of bacterial cellulose and their influence on paper properties,” BioResources 11, 9290-9301. DOI: 10.15376/biores.11.4.9290-9301
Zambrano, F., Starkey, H., Wang, Y.-H., Abbati de Assis, C., Venditti, R., Pal, L., Jameel, H., Hubbe, M. A., Rojas, O. J., and Gonzalez, R. (2020). “Using micro-and nanofibrillated cellulose as a means to reduce weight of paper products: A review,” BioResources 15, 4553-4590. DOI: 10.15376/biores.15.2.Zambrano
Zhang, Y., Li, H.-F., Li, X.-D., Gibril, M. E., and Yu, M.-H. (2014). “Chemical modification of cellulose by in situ reactive extrusion in ionic liquid,” Carbohydrate Polymers 99, 126-131. DOI: 10.1016/j.carbpol.2013.07.084
Zhang, J.-M., Wu, J., Yu, J., Zhang, X.-Y., He, J.-S., and Zhang, J. (2017). “Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends,” Materials Chemistry Frontiers 1, 1273-1290. DOI: 10.1039/C6QM00348F
Zhang, H., Liu, P.-W., Peng, X.-W., Chen, S.-L., and Zhang, K. (2019). “Interfacial synthesis of cellulose-derived solvent-responsive nanoparticles via Schiff base reaction,” ACS Sustainable Chemistry & Engineering 7, 16595-16603. DOI: 10.1021/acssuschemeng.9b03919
Zhang, J.-K., Guo, Z.-W., Chen, S., Dong, H.-L., Zhang, X., Qin, Y.-L., Yao, C.-L., and Feng Xu, F. (2021a). “High-barrier, strong, and antibacterial paper fabricated by coating acetylated cellulose and cinnamaldehyde for food packaging,” Cellulose 28, 4371-4384. DOI: 10.1007/s10570-021-03778-x
Zhang, K. T., Ismail, M. Y., and Liimatainen, H. (2021b). “Water-resistant nanopaper with tunable water barrier and mechanical properties from assembled complexes of oppositely charged cellulosic nanomaterials,” Food Hydrocolloids 120, article no. 106983. DOI: 10.1016/j.foodhyd.2021.106983
Zhang, X.-Y., Zhu, W.-Y., Guo, J.-Q., Song, J.-L., and Xiao, H.-N. (2021c). “Impacts of degree of substitution of quaternary cellulose on the strength improvement of fiber networks,” International Journal of Biological Macromolecules 181, 41-44. DOI: 10.1016/j.ijbiomac.2021.03.121
Zhao, M.-C., Ansari, F., Takeuchi, M., Shimizu, M., Saito, T., Berglund, L. A., and Isogai, A. (2018). “Nematic structuring of transparent and multifunctional nanocellulose papers,” Nanoscale Horizons 3, 28-34. DOI: 10.1039/C7NH00104E
Zhu, S.-D., Wu, Y.-X., Chen, Q.-M., Yu, Z.-N., Wang, C.-W., Jin, S.-W., Ding, Y.-G., and Gang Wu, G. (2006). “Dissolution of cellulose with ionic liquids and its application: A mini-review,” Green Chemistry 8, 325-327. DOI: 10.1039/b601395c
Article submitted: February 16, 2021; Peer review completed: March 26, 2022; Revised version received and accepted: April 8, 2022; Published: April 8, 2022.
DOI: 10.15376/biores.17.2.Hashemzehi
