Abstract
The dissolving properties of softwood in an aqueous solution of the ionic liquid tetra-n-butylphosphonium hydroxide ([P4,4,4,4]OH) were studied. Cedar wood meal and 40% [P4,4,4,4]OH aqueous solution were mixed in a glass tube and treated at 121 °C and 2 atm in an ordinary autoclave. As the treatment time increased, the residue content decreased to 68.7%, 49.4%, and 37.0% after 1, 5, and 20 h, respectively. Following the 20-h treatment time, 97.0% of the residue was determined to be holocellulose and 96.3% of the constituent monosaccharide in the residue was glucose. UV analysis showed that most of the lignin had dissolved in the [P4,4,4,4]OH solution. Phenolic hydroxyl group analysis of the dissolved lignin suggested that the macromolecular structures of the lignin were considerably degraded. The results of this study suggest the possibility of developing a simple multi-sample analysis method for lignin content using a single-step autoclave treatment in a glass tube using an aqueous solution of [P4,4,4,4]OH.
Download PDF
Full Article
A Simple Method for Separating Lignin and Carbohydrates from Softwood Biomass in a Glass Tube using Tetra-n-Butylphosphonium Hydroxide
Hajime Yamada,a Hisashi Miyafuji,b Hiroyuki Ohno,c and Tatsuhiko Yamada a,*
The dissolving properties of softwood in an aqueous solution of the ionic liquid tetra-n-butylphosphonium hydroxide ([P4,4,4,4]OH) were studied. Cedar wood meal and 40% [P4,4,4,4]OH aqueous solution were mixed in a glass tube and treated at 121 °C and 2 atm in an ordinary autoclave. As the treatment time increased, the residue content decreased to 68.7%, 49.4%, and 37.0% after 1, 5, and 20 h, respectively. Following the 20-h treatment time, 97.0% of the residue was determined to be holocellulose and 96.3% of the constituent monosaccharide in the residue was glucose. UV analysis showed that most of the lignin had dissolved in the [P4,4,4,4]OH solution. Phenolic hydroxyl group analysis of the dissolved lignin suggested that the macromolecular structures of the lignin were considerably degraded. The results of this study suggest the possibility of developing a simple multi-sample analysis method for lignin content using a single-step autoclave treatment in a glass tube using an aqueous solution of [P4,4,4,4]OH.
Keywords: Aqueous ionic liquid; Tetra-n-butylphosphonium hydroxide; Softwood; Lignin; Multi-sample analysis
Contact information: a: Department of Biomass Chemistry, Forestry and Forest Products Research Institute, 1 Matsunosato, Tsukuba, Ibaraki 305-8687, Japan; b: Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Hangi-cho, Shimogamo, Sakyo-ku, Kyoto 606-8522, Japan; c: Department of Biotechnology, Tokyo University of Agriculture and Technology, Naka-cho, 2-24-16, Koganei, Tokyo 184-8588, Japan; *Corresponding author: yamadat@affrc.go.jp
INTRODUCTION
Ionic liquids are defined as molten state salts at ambient temperatures. They are known as “green” solvents because of their low vapor pressure, thermal stability, and recyclability. Because ionic liquids consist of cations and anions, they can be made to display a wide variety of solvent properties by designing their cations and anions. Recently, ionic liquids have received attention because of their novel cellulosic biomass-dissolving abilities. Imidazolium-based ionic liquids can dissolve cellulose (Swatloski et al. 2002; Remsing et al. 2006) and lignocellulose (Fort et al. 2007; Kilpeläinen et al. 2007). Those studies suggested that chloride anions with imidazolium cations in ionic liquids promote cellulose dissolution efficiently.
In previous studies, the authors have found that cellulose was dissolved in a series of low-polarity ionic liquids containing formate or methylphosphonate anions (Fukaya et al. 2006, 2008; Abe et al. 2010). These low-polarity ionic liquids can extract polysaccharides from plant biomass under mild conditions. They are relatively viscous and require a long time to dissolve cellulose. These low-polarity ionic liquids are highly hygroscopic and readily absorb water from the atmosphere, similar to polar molecules. However, a small addition of water to the ionic liquids causes a drastic decrease in their cellulose-dissolving abilities (Mazza et al. 2009). In addition, raw plant biomass typically contains a considerable amount of moisture. For this reason, both the ionic liquid and the plant biomass need a pretreatment of drying before ionic liquids can be used as solvents for lignocellulosic biomass. A new system of plant biomass dissolution in ionic liquid without a drying pretreatment is therefore a desirable objective.
With the aim of avoiding the drying pretreatment, we focused on the use of hydroxide anions in the solvent system. Ionic pairs of hydroxide anions with onium cations, such as tetra-n-butylphosphonium hydroxide ([P4,4,4,4]OH, Fig. 1) and tetra-n-butylammonium hydroxide ([N4,4,4,4]OH), have been reported as novel solvents for cellulose (Abe et al. 2012, 2014; Ema et al. 2014). In particular, [P4,4,4,4]OH has strong proton-accepting ability, even in the presence of water. We have reported that [P4,4,4,4]OH containing 30% to 60% water readily dissolves cellulose (Abe et al. 2012), and some woody biomass (Abe et al. 2014) without heating. However, the [P4,4,4,4]OH aqueous solution exhibited a low ability to dissolve softwoods such as cedar and pine, compared to hardwoods such as poplar (Abe et al. 2014, 2015). This difference was considered to be caused by the properties of lignin, including its content, chemical structures, constituent monomers, and bonding patterns. Lignin is decomposed by conventional pulping methods such as the kraft or the soda–anthraquinone cooking processes (Marton 1971; Lin and Dence 1992). These processes require severe conditions for softwood cooking, usually at over 160 °C in a high-pressure stainless-steel reactor. In these conventional lignin decomposition processes it is well known that the dissolution of softwood is more difficult than that of hardwood. This was also observed in the [P4,4,4,4]OH dissolution treatment (Abe et al. 2015) described above.
In the present study, a simple method was evaluated for separating lignin and carbohydrates from softwood biomass in a glass tube. Wood meal with an aqueous solution of [P4,4,4,4]OH was placed in a glass tube and treated in an autoclave at 121 °C and 2 atm. After autoclaving, the glass tube was used for separating the residue in a centrifuge. This process can be applied using a general-purpose apparatus such as a screw-top glass tubes and an ordinary autoclave. In a general-purpose autoclave, more than 100 glass tubes can be treated at once. We plan to apply this simple separating method for the chemical analysis of multiple samples.
Fig. 1. Chemical formula of tetra-n-butylphosphonium hydroxide ([P4,4,4,4]OH)
EXPERIMENTAL
Materials
A [P4,4,4,4]OH aqueous solution (40%) was purchased from Hokko Chemical Industry (Tokyo, Japan). Other chemicals were purchased from Tokyo Chemical Industry (Tokyo, Japan), Wako Pure Chemical Industries (Osaka, Japan), and Junsei Chemical (Tokyo, Japan). All chemicals were used as received without further purification.
Japanese cedar (Cryptomeria japonica D. Don) chips were used as softwood chips. The chips used contained a mixture of heartwood and sapwood and did not contain any bark. Air-dried softwood chips were crushed with a rotor mill (Pulverisette 14, Fritsch Japan, Yokohama, Japan), and wood meal passing a 0.5-mm mesh sieve was collected. The wood meal was extracted with an ethanol:benzene solution (v/v 1:2) in a Soxhlet extractor. The extractive-free wood meal was vacuum-dried at 40 °C for 24 h and used for the [P4,4,4,4]OH dissolution test.
Softwood Dissolution Test
For the dissolution test at room temperature (RT), 0.35 g of wood meal and 7.0 g of 40% [P4,4,4,4]OH aqueous solution were placed in a 30-mL glass vial. The mixture was stirred with a magnetic stirrer at 200 rpm for 1 h, 24 h, and 7 d at RT.
For the dissolution test in an autoclave (AC), 0.1 g of wood meal and 2.0 g of 40% [P4,4,4,4]OH aqueous solution were placed in a screw-top glass test tube (NR-10, 12-mL volume, Maruemu, Osaka, Japan). The screw caps were tightly closed. The glass tubes were placed in an autoclave (STH364FA, 50.2 L, Advantec, Tokyo, Japan) and treated at 121 °C and 2 atm for 1, 3, 5, 10, 15, and 20 h. The autoclave temperature was controlled as follows: warming at 121 °C for 30 min, holding at 121 °C for 1 to 20 h, and cooling for 90 min to room temperature.
After the RT or AC treatment, the supernatant liquid and residue were separated by centrifugation. The residue was filtered and washed with more 40% [P4,4,4,4]OH aqueous solution, followed by methanol and water. After the washing and drying, the weight of the residue was determined.
Chemical Composition Analysis of [P4,4,4,4]OH Residue
The holocellulose content in the [P4,4,4,4]OH residue was determined by the chlorine dioxide method (Dahlman et al. 1999), and the lignin content was determined by the Klason method (Lin and Dence 1992). The lignin content obtained was the sum of the acid-insoluble and -soluble lignin contents.
The monosaccharide content in the [P4,4,4,4]OH residue was determined using the alditol acetate method (Blakeney et al. 1983) with a gas chromatograph (GC 6890N, Agilent Technologies, Santa Clara, USA). The GC was equipped with a flame ionization detector and a fused silica capillary column (SP-2380, Supelco, Bellefonte, USA). Myo-inositol was used as an internal standard. Standard curves were obtained using the alditiol acetate methods with preparations of authentic D-glucose, L-arabinose, D-mannose, D-xylose, and D-galactose in 3% sulfuric acid aqueous solution.
Lignin Properties in the [P4,4,4,4]OH Solution
The lignin content in the [P4,4,4,4]OH solution was determined by spectrophotometry (Lin and Dence 1992) with an ultraviolet-visible (UV-Vis) spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The [P4,4,4,4]OH solution sample was diluted with 1,4-dioxane:0.2 M NaOH solution (v/v 1:1). For the reduction pretreatment, NaBH4 was added to the sample solution, which was kept at RT for 1 week. The pH of the NaBH4-reduced and unreduced sample solution was adjusted to 5.0 with acetic acid, and the UV absorbance at 280 nm was measured. The lignin content was estimated (Lin and Dence 1992) using the absorption coefficients of 26.9 L g-1 cm-1 for reduced lignin and 30.3 L g-1 cm-1 for unreduced lignin (Takahashi et al. 2014).
The phenolic hydroxyl group (Phe-OH) content in the [P4,4,4,4]OH-dissolved lignin was determined by the ionization difference (Δεi) method (Lin and Dence 1992). A previously prepared NaBH4-reduced sample solution was diluted with 1,4-dioxane:0.2 M HCl (v/v 1:1) to make the solution acidic. The difference in UV absorbance at 300 nm of lignin solution between alkaline and acidic conditions was measured. The Phe-OH content was estimated on the basis of the molar extinction coefficient 4100 L mol-1 cm-1 (Lin and Dence 1992).
RESULTS AND DISCUSSION
Dissolution of Softwood without Heating
The softwood dissolving ability in a 40% [P4,4,4,4]OH aqueous solution in the RT treatment was examined. Table 1 shows the relationship between the treatment time and [P4,4,4,4]OH residue contents in the RT treatment. The residue contents decreased with increasing treatment time, but were greater than 90% even after the 7-d treatment. Only a small amount of softwood was dissolved in the 40% [P4,4,4,4]OH aqueous solution in the RT treatment.
It has been reported that [P4,4,4,4]OH in the presence of water dissolved microcrystalline cellulose completely at 25 °C (Abe et al. 2012) and that a 40% [P4,4,4,4]OH aqueous solution dissolved more than 30% of poplar (Abe et al. 2014). These reports suggested that 40% [P4,4,4,4]OH aqueous solution had sufficient dissolving ability for hardwood and cellulose. In contrast, the data in Table 1 show that softwood was dissolved with difficulty in the RT treatment.
Dissolution of Softwood at the Autoclave Treatment
The dissolving ability of 40% [P4,4,4,4]OH aqueous solution in the AC treatment was tested. Table 2 shows the relationships between the treatment time and [P4,4,4,4]OH residue contents in the AC treatment. The residue contents decreased with increasing treatment time, finally reaching 37.0% with the 20-h treatment. They decreased rapidly in the early stages of the treatments. These results showed that AC treatment strengthened the softwood-dissolving ability of 40% [P4,4,4,4]OH aqueous solution in comparison with the RT treatment. It was suggested that 121 °C heating accelerated the dissolution of softwood.
Table 1. Treatment Time and [P4,4,4,4]OH Residue Contents in the Room Temperature (RT) Treatment
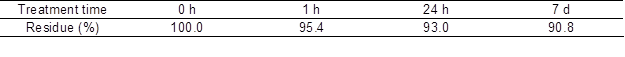
Table 2. Treatment Time and [P4,4,4,4]OH Residue Contents in the Autoclave (AC) Treatment
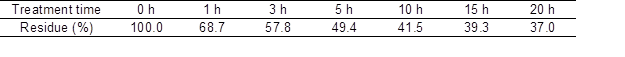
Change in Chemical Composition
The chemical composition of the [P4,4,4,4]OH residue after the RT and AC treatments was determined. Figure 2 shows the relationship between treatment time and compositional ratios of holocellulose, lignin, and the [P4,4,4,4]OH-soluble fraction in the RT treatment. The holocellulose content decreased from 69.1% to 67.2% and 66.0% at 0, 1, and 24 h, respectively, and the lignin content decreased from 30.9% to 28.1% and 27.0% at 0, 1, and 24 h, respectively.
Figure 3 shows the relationship between the treatment time and compositional ratio in the AC treatment. The holocellulose content decreased from 69.1% to 49.1%, 38.4%, and 35.9% at 0, 1, 10, and 20 h, respectively, and the lignin content decreased from 30.9% to 19.6%, 3.1%, and 1.1% at 0, 1, 10, and 20 h, respectively.
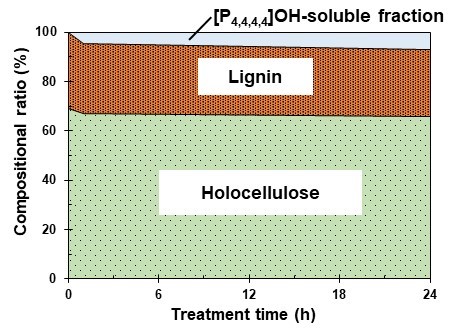
Fig. 2. The relationship between treatment times and compositional ratios of holocellulose, lignin, and the [P4,4,4,4]OH-soluble fraction in the RT treatment
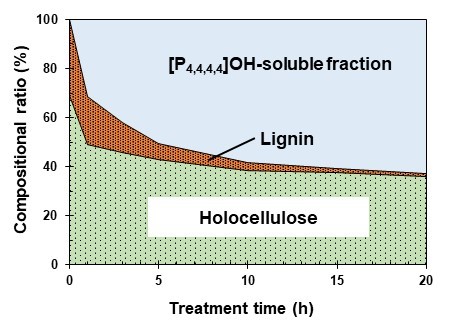
Fig. 3. The relationship between treatment time and compositional ratio of holocellulose, lignin, and the [P4,4,4,4]OH-soluble fraction in the AC treatment
Figure 4 shows the relationship between the treatment time and holocellulose and lignin proportions, relative to the original contents. The AC treatment showed higher decreasing proportions than the RT treatment for both the holocellulose and lignin. The lignin content decreased faster than the holocellulose proportion in both RT and AC treatments. After the 20-h AC treatment, the residue consisted of holocellulose (97.0%) and lignin (3.0%). Most of the lignin dissolved in [P4,4,4,4]OH in the long-time AC treatment, and the residue consisted primarily of holocellulose.
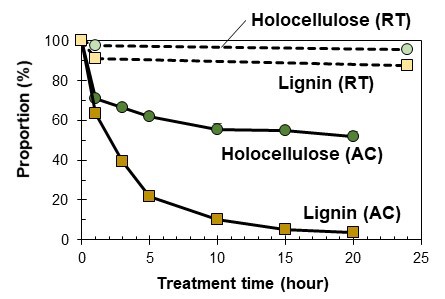
Fig. 4. The relationship between the treatment times and holocellulose and lignin proportions, relative to the original content of each component
Change in the Monosaccharide Composition in the Residue
The change in the monosaccharide composition of the residues after AC treatment was investigated using the alditol acetate method (Blakeney et al. 1983). Table 3 shows the relationship between treatment time and monosaccharide composition of glucose, arabinose, xylose, mannose, and galactose. The amount of glucose increased with treatment time, from 75.7% at 0 h to 83.7% at 1 h, 90.7% at 5 h, 93.9% at 10 h, and 96.3% at 20 h. The other monosaccharides tended to decrease as the treatment time increased. The glucose composition increased, exceeding 95% after more than 15 h of treatment time. Glucose contents based on untreated softwood weight were estimated as 52.3%, 41.1%, 38.7%, 36.1%, and 34.6% at 0, 1, 5, 10, and 20 h, respectively.
Table 3. Treatment Time and Monosaccharide Compositions of the Solid Residue from the AC Treatment
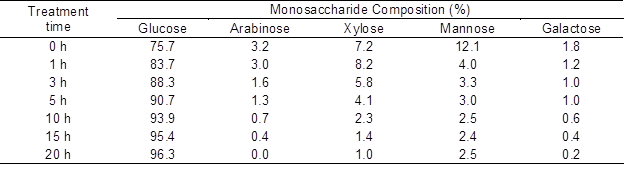
When the treatment time was more than 5 h, these glucose contents converged at approximately 35% to 39%. In contrast, the alpha-cellulose content in the untreated softwood sample was 39.9%. These data indicate that glucose was the main monosaccharide constituent of the residue following long-time treatment and may have originated from cellulose.
UV Spectrum of the [P4,4,4,4]OH Solution
As shown by the results above, the lignin content in the residue decreased to 3.6% after 20 h of AC treatment of the wood meal. This suggested that the lignin was dissolved in the [P4,4,4,4]OH solution. To check the content of dissolved lignin, the UV spectra of the [P4,4,4,4]OH solutions were determined. Figure 5 shows the UV spectrum of the [P4,4,4,4]OH solution after the 5 h AC treatment.
As a control, 40% [P4,4,4,4]OH aqueous solution was diluted with 1,4-dioxane:0.2 M NaOH solution (v/v 1:1), and the UV spectrum was obtained. The UV spectrum of [P4,4,4,4]OH showed zero absorbance from 250 to 400 nm. In contrast, the UV spectrum of softwood dissolved in [P4,4,4,4]OH showed the typical pattern for lignin (Lin and Dence 1992) absorbance. According to these results, we estimated the lignin content in the [P4,4,4,4]OH solution using absorbance at 280 nm, which is conventionally applied in quantitative lignin determination (Lin and Dence 1992). In this method, NaBH4 reduction is usually applied in order to eliminate the effect of furan derivatives that have UV absorption at 280 nm. Sodium borohydride degrades part of lignin as well. This decreasing of lignin’s UV absorbance is called a hypsochromic effect (Lin and Dence, 1992). Sodium borohydride reduction is thought to cause the degradation of the lignin chromophoric structure of quinoids or conjugated carbonyls (Ehara et al. 1988).
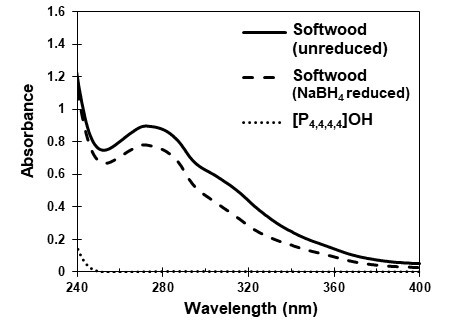
Fig. 5. The UV spectra of the [P4,4,4,4]OH dissolved softwood, following the 5-h AC treatment
Determination of Dissolved Lignin Concentration
The lignin concentration in the [P4,4,4,4]OH solution was determined using the UV absorbance at 280 nm with lignin absorption coefficients (Lin and Dence 1992) of 30.3 L g-1 cm-1 for the unreduced sample and 26.9 L g-1 cm-1 for the NaBH4-reduced sample (Takahashi et al. 2014). Figure 6 shows the relationship between the treatment time and the calculated lignin concentrations in the [P4,4,4,4]OH solutions. In the reduced and unreduced samples, almost the same lignin concentrations were obtained. These data suggest that the influence of furans at 280 nm was limited, and the reduction step could be eliminated for this UV-lignin determination approach.
These estimated lignin concentrations were supported by the data of residual lignin concentrations in Fig. 3. These two lignin concentrations were almost equal at the same treatment time. For example at the 5h AC treatment, lignin concentrations of 12.9 g/L and 12.1 g/L were shown by the UV method and the residual lignin from Fig.3, respectively. These results indicated that since most of the lignin dissolved in 20 h of the AC treatment (Fig. 4), the lignin content of the softwood sample can be determined by this UV method.
If this process of AC treatment with [P4,4,4,4]OH were applied as a lignin content analysis method, this new method would have many advantages compared with the conventional Klason method (Lin and Dence 1992). All of the steps can be completed in a glass tube, and hundreds of biomass samples can be treated in one AC treatment involving multiple glass tubes.
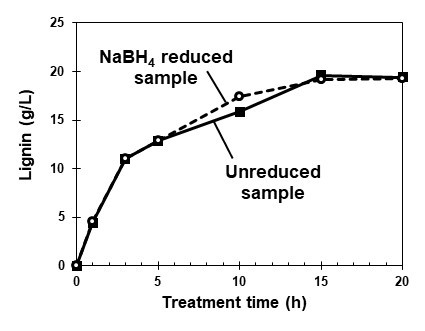
Fig. 6. The relationship between treatment time and calculated lignin concentration in the [P4,4,4,4]OH solution
Phenolic Hydroxyl Group Content in the Dissolved Lignin
The Phe-OH content of the [P4,4,4,4]OH dissolved lignin was determined by the Δεi method (Lin and Dence 1992). Generally, when the Phe-OH content increases, lignin’s solubility increases. Table 4 shows the relationship between the treatment time and the Phe-OH contents of the [P4,4,4,4]OH-dissolved lignin. The contents of Phe-OH were calculated to be 2.37 to 2.72 mmol/g lignin. The treatment time did not affect the Phe-OH content much as compared with the difference between the treatment methods. Given the molecular weight of a lignin unit as 180 g/mol, the contents of Phe-OH were calculated to be 0.43 to 0.49 mol/unit lignin. The Phe-OH content of softwood lignin was 0.26 mol/unit lignin by the milled-wood method (Lin and Dence 1992), 0.45 mol/unit lignin by the soda-anthraquinone method (Takahashi et al. 2014), and 0.65 mol/unit lignin by the kraft method (Månsson 1983). The Phe-OH content of the [P4,4,4,4]OH soluble lignin was higher than that of the milled-wood lignin and close to that of the soda-anthoraquinone lignin. These data suggested that the macromolecular structures of softwood lignin were degraded to be soluble in the [P4,4,4,4]OH solution by the AC treatment.
Table 4. Treatment Time and Phe-OH Contents of the [P4,4,4,4]OH-Dissolved Lignin
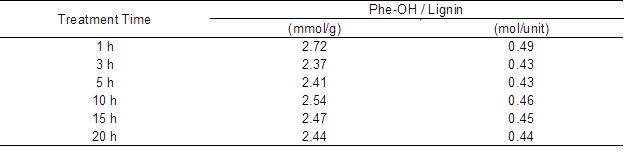
CONCLUSIONS
- The new method was successful in separating the lignin and carbohydrates from softwood using 40% [P4,4,4,4]OH aqueous solution in a glass tube.
- Softwood meal was dissolved smoothly in 40% [P4,4,4,4]OH aqueous solution by AC treatment. The residue content decreased to 37.0% after 20 h of AC treatment.
- Lignin dissolved faster than holocellulose, and the residue of the 20-h AC treatment consisted primarily of holocellulose (97.0%). In that residue, glucose was the main constituent monosaccharide (96.3%).
- Most of the lignin was dissolved in the [P4,4,4,4]OH solution, and the determination of the lignin content using UV analysis was demonstrated.
- It is proposed that this separation method offers many advantages for chemical compositional analysis. All of the steps can be completed in a glass tube, using only a general-purpose apparatus, and hundreds of biomass samples can be analyzed in one AC treatment carried out with several glass tubes simultaneously.
ACKNOWLEDGMENTS
This study was supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (26052A).
REFERENCES CITED
Abe, M., Fukaya, Y., and Ohno, H. (2010). “Extraction of polysaccharides from bran with phosphonate or phosphinate-derived ionic liquids under short mixing time and low temperature,” Green Chem. 12(7), 1274-1280. DOI: 10.1039/C003976D
Abe, M., Fukaya, Y., and Ohno, H. (2012). “Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water,” Chem. Commun. 48(12), 1808-1810. DOI: 10.1039/c2cc16203b
Abe, M., Yamada, T., and Ohno, H. (2014). “Dissolution of wet wood biomass without heating,” RSC Adv. 4(33), 17136-17140. DOI: 10.1039/C4RA01038H
Abe, M., Yamanaka, S., Yamada, H., Yamada, T., and Ohno, H. (2015). “Almost complete dissolution of woody biomass with tetra-nbutylphosphonium hydroxide aqueous solution at 60 °C,” Green Chem. (in press). DOI: 10.1039/C5GC00646E
Blakeney, A. B., Harris, P. J., Henry, R. J., and Stone, B. A. (1983). “A simple and rapid preparation of alditol acetates for monosaccharide analysis,” Carbohydr. Res. 113(2), 291-299. DOI: 10.1016/0008-6215(83)88244-5
Dahlman, O., Mörck, R., and Knuutinen, J. (1999). “Analysis of bleach liquors,” in: Analytical Methods in Wood Chemistry, Pulping, and Papermaking, E. Sjöström and R. Alén (eds.), Springer-Verlag, Berlin, pp. 233-268. DOI: 10.1007/978-3-662-03898-7_8
Ehara, K., Tsutsumi, Y., and Nishida, T. (1988). “Structural changes of residual lignin in softwood kraft pulp treated with manganese peroxidase,” J. Wood Sci. 44(4), 327-331. DOI: 10.1007/BF00581315
Ema, T., Komiyama, T., Sunami, S., and Sakai, T. (2014). “Synergistic effect of quaternary ammonium hydroxide and crown ether on the rapid and clear dissolution of cellulose at room temperature,” RSC Adv. 4(5), 2523-2525. DOI: 10.1039/C3RA45888A
Fort, D. A., Remsing, R. C., Swatloski, R. P., Moyna, P., Moyna, G., and Rogers, R. D. (2007). “Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride,” Green Chem. 9(1), 63-69. DOI: 10.1039/B607614A
Fukaya, Y., Sugimoto, A., and Ohno, H. (2006). “Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formate,” Biomacromolecules 7(12), 3295-3297. DOI: 10.1021/bm060327d
Fukaya, Y., Hayashi, K., Wada, M., and Ohno, H. (2008). “Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions,” Green Chem. 10(1), 44-46. DOI: 10.1039/B713289A
Kilpeläinen, I., Xie, H., King, A., Granstrom, M., Heikkinen, S., and Argyropoulos, D. S. (2007). “Dissolution of wood in ionic liquids,” J. Agric. Food Chem. 55(22), 9142-9148. DOI: 10.1021/jf071692e
Lin, S. Y., and Dence, C. W. (eds.) (1992). Methods in Lignin Chemistry, Springer-Verlag, Berlin. DOI: 10.1007/978-3-642-74065-7
Månsson, P. (1983). “Quantitative determination of phenolic and total hydroxyl groups in lignins,” Holzforschung 37(3), 143-146. DOI: 10.1515/hfsg.1983.37.3.143
Mazza, M., Catana, D. A., Vaca-Garcia, C., and Cecutti, C. (2009). “Influence of water on the dissolution of cellulose in selected ionic liquids,” Cellulose 16(2), 207-215. DOI: 10.1007/s10570-008-9257-x
Remsing, R. C., Swatloski, R. P., Rogers, R. D., and Moyna, G. (2006). “Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems,” Chem. Commun. 12, 1271-1273. DOI: 10.1039/B600586C
Marton, J. (1971). “Reactions in alkaine pulping,” in: Lignins: Occurrence, Formation, Structure and Reactions, K. V. Sarkanen and C. H. Ludwig (eds.), John Wiley & Sons, New York, pp. 639-694.
Swatloski, R. P., Spear, S. K., Holbrey, J. D., and Rogers, R. D. (2002). “Dissolution of cellose with ionic liquids,” J. Am. Chem. Soc. 124(18), 4974-4975. DOI: 10.1021/ja025790m
Takahashi, S., Hattori, M., Morimoto, M., Uraki, Y., and Yamada, T. (2014). “Performance of softwood soda-anthraquinone lignin as water-reducing chemical admixture in concrete,” J. Wood Chem. Technol. 34(1), 31-38. DOI: 10.1080/02773813.2013.820322
Article submitted: July 29, 2015; Peer review completed: September 30, 2015; Revised version received and accepted: November 11, 2015; Published: November 30, 2015.
DOI: 10.15376/biores.11.1.839-849