Abstract
As biomass has become increasingly important, wood pellets are becoming more widely used, and the storage of wood flour, which is the raw material of wood pellets, has become inevitable. The purpose of this study was to reduce the economic losses from fires during storage of porous combustible materials. To achieve this purpose, the authors analyzed and compared the wood flour loss rate between the use of water and the use of wetting agents to extinguish a deep-seated fire through a scale model experiment. To do this, the authors measured the penetration amount of the water and dilute solutions of wetting agent, the weight change of the wood flour holder, and the emissions on a real time basis when that spray amount was the same. Furthermore, the authors analyzed the calorific value and combustion gas to examine the reusability of the wood flour with the added wetting agent. This study quantitatively demonstrated that the active use of wetting agents in wood flour storage fires dramatically reduced the fire loss rate of raw materials and resulted in early fire extinguishing, which minimizes companies’ economic loss.
Download PDF
Full Article
A Study on Fire Risk Reduction of Porous Combustible Storage
Nam Kyun Kim,a and Dong Ho Rie b,*
As biomass has become increasingly important, wood pellets are becoming more widely used, and the storage of wood flour, which is the raw material of wood pellets, has become inevitable. The purpose of this study was to reduce the economic losses from fires during storage of porous combustible materials. To achieve this purpose, the authors analyzed and compared the wood flour loss rate between the use of water and the use of wetting agents to extinguish a deep-seated fire through a scale model experiment. To do this, the authors measured the penetration amount of the water and dilute solutions of wetting agent, the weight change of the wood flour holder, and the emissions on a real time basis when that spray amount was the same. Furthermore, the authors analyzed the calorific value and combustion gas to examine the reusability of the wood flour with the added wetting agent. This study quantitatively demonstrated that the active use of wetting agents in wood flour storage fires dramatically reduced the fire loss rate of raw materials and resulted in early fire extinguishing, which minimizes companies’ economic loss.
Keywords: Deep-seated fire; Wetting agent; Porous combustibles; Fire risk reduction; Scale model test
Contact information: a: Graduate School, Incheon National University, Incheon 22012, Republic of Korea.; b: Fire Disaster Prevention Research Center, Incheon National University, Incheon 22012, Korea; *Corresponding author: riedh@inu.ac.kr
INTRODUCTION
Today, fossil fuels are a major energy source, accounting for more than 80% of global energy demand (IEA 2013). However, fossil fuels are not regenerable and have limited reserves. In recent years, as an effort to replace fossil fuels, biomass has become more important (Williams et al. 2012; Guo et al. 2015). As a result, the market for wood flour pellets has grown (Toscano et al. 2013). The EU’s bioenergy policies and rise in oil prices have led to a significant increase in wood pellet boilers for home heating (Kristöfel et al. 2016). Wood pellets are also considered high quality biomass material suitable for many industrial and household applications (Li et al. 2012; Yu and Chen 2016). Many reports on the increasing consumption of wood pellets forecast that the consumption of wood pellets will increase steadily in the future (Williams et al. 2012; Toscano et al. 2014; Proskurina et al. 2015; Kristöfel et al. 2016; McKechnie et al. 2016; Nabavi et al. 2016; Nunes et al. 2016; Simpson et al. 2016). Therefore, the production and storage of wood flour and the raw material for making wood pellets will become inevitable (Alakoski et al. 2016). The stored wood flour, consisting of fine particles, has a larger surface area and more interparticle voids per weight unit than wood. These characteristics facilitate oxygen inflow and increase the possibility of smoldering, even at a low external oxygen concentration, due to a high internal oxygen content. At the same time, the particles serve as an insulating material, thereby reducing heat loss and enabling continuous combustion despite a low heat emission rate. Furthermore, the carbides that are not fully degraded by smoldering are likely to ignite if there is rapid oxygen ingress at relatively low temperatures (≥ 670 K) (SFPE 2002). A phenomenon in which a fire generated on the surface of porous materials is transferred to the deep part of porous materials, leading to continuous smoldering, is called a “deep-seated fire”. In the case of a deep-seated fire, it is difficult to judge whether or not the fire is extinguished completely, and the use of general water, which has a low surface penetration performance due to high surface tension, is not effective for extinguishing a deep-seated fire. Also, if the cooling surface formed during the extinguishing of the surface fire is lost due to the external force, there is a high possibility of reignition due to rapid oxygen supply (SFPE 2002; Toscano et al. 2014). In a recent deep-seated fire case, “Woodchip fire at the South Wales recycling site”, the fire occurred in Heol Llan, Coity, Bridgend at 03:30 BST on September 19, 2016. The store had about 3000 tonnes of wood chips, and the fire lasted eight days until it was completely extinguished on September 26. In case of a deep-seated fire, the high temperature inside the porous materials due to the fire is maintained for a long time, so the penetration performance of the water used for extinguishing the fire should be excellent and should be kept on the fire for a long time (National Fire Protection Association 2005). Therefore, it is important to cope effectively with fire extinguishing by changing the physical and chemical properties of fire water through the addition of wetting agents (Kim and Rie 2015).
Torero and Fernandez-Pello (1995), Palmer (1957), and Kim and Rie (2013) focused on the implementation of deep-seated fires and the transfer rate. Kong et al. (2013) studied the effect of the surfactant on the penetration performance, and Lazghab et al. (2016) described the limitations as well as the merits and demerits of the methods of evaluating the wettability of the solid powders reported in the literature, providing the basis for obtaining appropriate data and selecting system specifications. The previous studies conducted to date have been focused on the implementation of deep-seated fires and the penetration performance in non-fires, but not many studies have been conducted on the extinguishing of deep-seated fires. Kim and Rie (2016) conducted a study on the development and validation of a scale model apparatus and the fire extinguishing performance evaluation of the deep-seated fire using the existing agents.
In this study, the authors tried to confirm the reduction in economic losses from deep-seated fires by the use of a wetting agent. To accomplish this, the authors implemented deep-seated fires in wood flour and conducted fire extinguishing experiments according to the addition of wetting agents through a scale model experiment, and then analyzed the wood flour loss rate. Furthermore, to examine the reusability of residual wood flour after fire extinguishing, the changes in the caloric value and combustion gas were analyzed through a cone calorimeter and FT-IR.
EXPERIMENTAL
Materials
The sample used in the experiment was wood flour, which is the main raw material of wood pellets (Woodtech, Sejong-si, Republic of Korea). This was the wood flour of the radiata pine (Pinus radiata), which comprises 75% of the wood used in Korea (Kim 2012b). The wood flour was imported from New Zealand.
The sample size ranged from 1.0 to 2.0 mm. That was sieved using a sieve shaker (CG-211-8) and standard sieves (US standard sieves no. 18 and 10) (Chunggyesanggongsa, Seoul, Republic of Korea) to conduct the experiment with uniform particle size.
The water content of the wood flour was measured repeatedly at intervals of 4 h after drying the sample in a dryer at 80 °C for 48 h until the weight change was ± 1 g. The water content was calculated using Eq. 1. The results showed that the water content of the wood flour used in the experiment was 10.6% (Simpson 1987).
![]() (1)
(1)
In Eq. 1, MC is moisture content (%), Wm is weight of sample before oven-drying (g), and Wd is weight of sample after oven-drying (g).
Wetting agents
The three agents used in this study were certified agents currently on the market in Korea and overseas (Hanjung Lube, Gimhea-si, Republic of Korea). Table 1 shows the physical properties of the agents used in this study. The concentration of the agents was set at the concentration recommended by the manufacturer. The median value of the recommended use concentration of the agent whose use concentration was specified within the range value was used to minimize experimental errors due to the concentration. The average dilution concentration of diluted agent was 0.77% ± 0.23%, the mean surface tension was 31.3 mN/m ± 2.3 mN/m, and the mean viscosity was 1.027 cP ± 0.01 cP, and the mean pH was 7.01 ± 0.05.
Table 1. Physical Properties of Wetting Agents Used in the Experiment
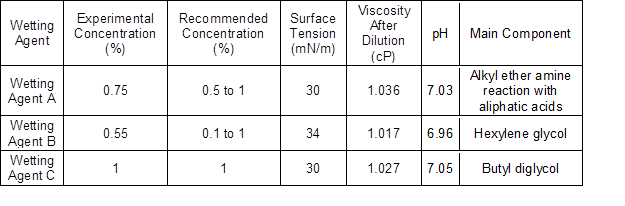
Methods
Figure 1 shows a flow chart for this study, which consisted of two parts. Part 1 was the analysis of the wood flour loss rate through a scale model experiment. Part 2 was the analysis of the calorific value and combustion gas of residual wood flour through a cone calorimeter experiment to determine the reusability of residual wood flour as raw pellet material. The economic loss reduction rate when using each of the wetting agents was analyzed through these experiments.
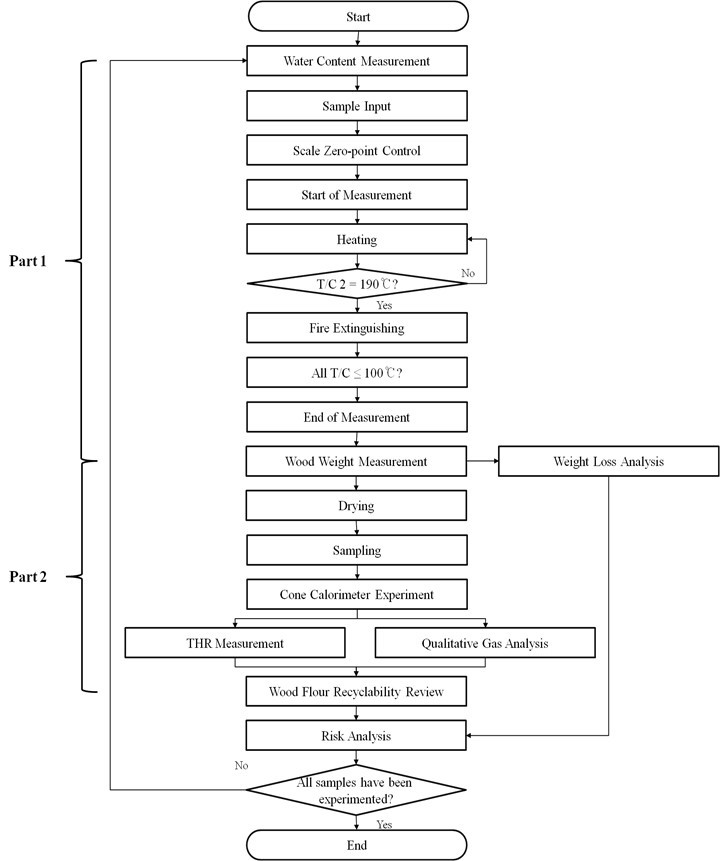
Fig. 1. Flow chart of study
Scale model experiment
A scale model experiment was conducted to measure the change in weight loss of the wood flour according to the change in water. This experiment was conducted using the scale model experimental apparatus developed by Nam Kyun Kim in his study (Kim and Rie 2016). Figure 2 below shows a scale model experiment apparatus.
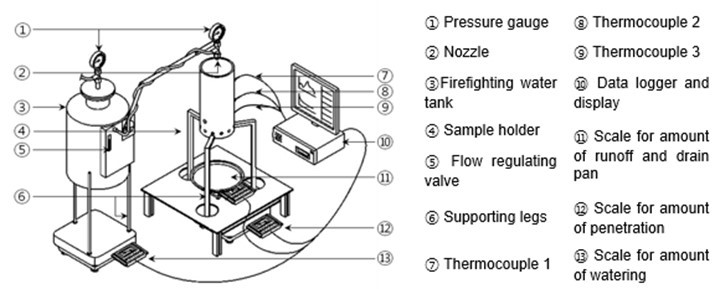
Fig. 2. Schematic diagram of experimental apparatus (Kim and Rie 2016)
The combustion reduction and penetration amount of wood flour were measured in real time using three load cells. In Load Cell 1, the spray amount and penetration amount were measured. In Load Cell 2, the loss on combustion and penetration amount of wood flour were measured. In Load Cell 3, emissions were measured.
Table 2 shows the experimental conditions of the scale model experiment. The initial temperature of the laboratory was set at 278 K ± 3 K, the humidity was set at 50% ± 5%, and the air-pressure was set at 1 atm.
Table 2. Experimental Conditions of the Scale Model Experiment

The experimental results were obtained by repeating the experiment three times under the same conditions. The sample was heated using a methane burner at a distance of 10 cm from the bottom of the sample, and the output pressure of the methane was 2.5 bar. The bottom of the sample was heated for 5 min using a methane burner to implement a deep-seated fire, and the water was sprayed when the temperature of the thermocouple (T/C) 2 reached 493 K for the heat transfer and fire spread. The ejection angle of the water spray nozzle was 45°, and the water spray height was calculated through Eq. 2 to uniformly spray water onto the surface of the sample:
![]() (2)
(2)
where θ is the spray angle of the nozzle (°), h is the spray height (cm), and r is the radius of the sample holder (cm). The water was sprayed at a rate of 0.5 L/min and a height of 24 cm, and was calculated using 0.75 L water in the agent tank. The weight and height of the wood flour in the holder were maintained at 400 g and 13 cm, respectively, and the filling density was maintained at 0.096 g/cm3 to ensure the same conditions.
Analysis of calorific value and combustion gas of wood flour after fire extinguishing
To examine the reusability of wood flour after fire extinguishing, the calorific value and combustion gas of wood flour were analyzed after the scale model experiment. The unburned wood flour was sampled and dried for the experiment. Samples could not be taken when water was used because of the high combustion rate. Therefore, wood flour was dried before the experiment for a control group. A cone calorimeter (Fire Testing Technology, East Grinstead, UK) and FT-IR (Midac Co., MA, USA) were used for the analysis of calorific value and combustion gas. The cone calorimeter is widely used to measure the calorific value of samples (Seo et al. 2016; Liang et al. 2017), and FT-IR is mainly used to look at the chemical bonding of solids (Cal et al. 2016; Li et al. 2017), but in this study, it was used for a combustion gas analysis. Calorific value measurement was conducted in accordance with ISO 5660-1 (2015), and the combustion gas analysis was conducted in accordance with the test method specified in ISO 19702 (2015). The FT-IR spectra were recorded in the spectral region of 4400 cm-1 to 400 cm-1 with eight scans at a 4 cm-1 resolution. Figure 3 shows the schematic of the sampling system specified in ISO 19702 (2015).
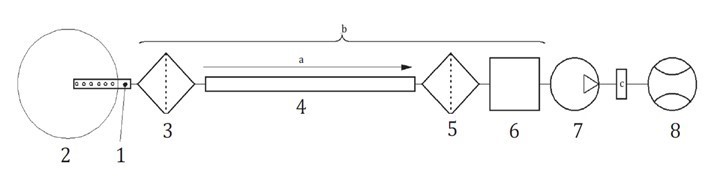
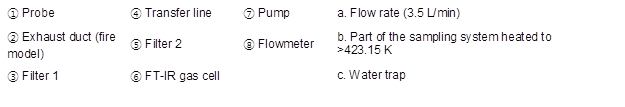
Fig. 3. Schematic diagram for a “pulling” sampling system on ISO 19702 (2015)
RESULTS AND DISCUSSION
Scale Model Experiment
The criterion used to determine extinguishing in the scale model experiment was the phase change temperature of water at 373 K (Kim and Rie 2016). The sample was deemed extinguished when the temperature was kept below that for more than 15 min. The wetting agent experiment was terminated at 1500 s and the water experiment was terminated at 7500 s, based on relevant standards.
Figure 4 shows the temperature change of T/C 1. In the wetting agent experiment, the temperature did not rise above 373 K from the time of spraying to the end of the experiment. However, in the water experiment, the temperature continuously rose after it reached 373 K at 590 s, it showed 831.4 K at 5232 s, and the temperature dropped below the fire extinguishing standard at 6467 s.
Figure 5 shows the temperature change of T/C 2. In the wetting agent experiment, the average temperature of the three types of agents dropped below the fire extinguishing standard at 90 s after being sprayed. However, in the water experiment the temperature decreased slightly immediately after spraying, and then continuously rose to 761.6 K at 1122 s, and then dropped. Thereafter, the temperature rose again starting at 2883 s to 748.8 K at 5055 s, and the temperature dropped below the fire extinguishing standard at 6267 s.
Figure 6 shows the temperature change of T/C 3. In the wetting agent experiment, the average temperature of the three types of agents dropped below the fire extinguishing standard at 189 s after spraying. However, in the water experiment, the temperature rose starting at 113 s, after the temperature drop immediately after spraying, to 736.9 K at 336 s, and then dropped. Thereafter, the temperature rose again starting at 2882 s to 662.6 K at 5143 s, and then dropped below the fire extinguishing standard at 6114 s.
The results of analyzing the internal temperature of wood flour showed the reignition point where the temperature suddenly rose again at around 4900 s, as shown in Figs. 4, 5, and 6. Table 3 shows the time to reach the fire extinguishing point by T/C (≤ 373 K). The results showed that the fire extinguishing time was effectively reduced compared to water.
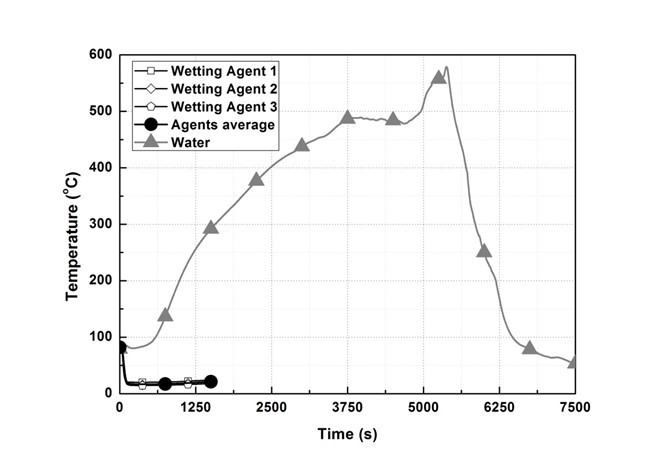
Fig. 4. T/C 1 temperature change
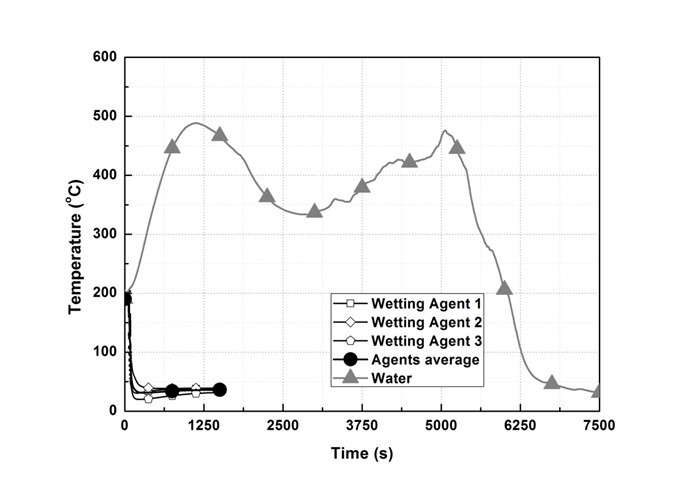
Fig. 5. T/C 2 temperature change
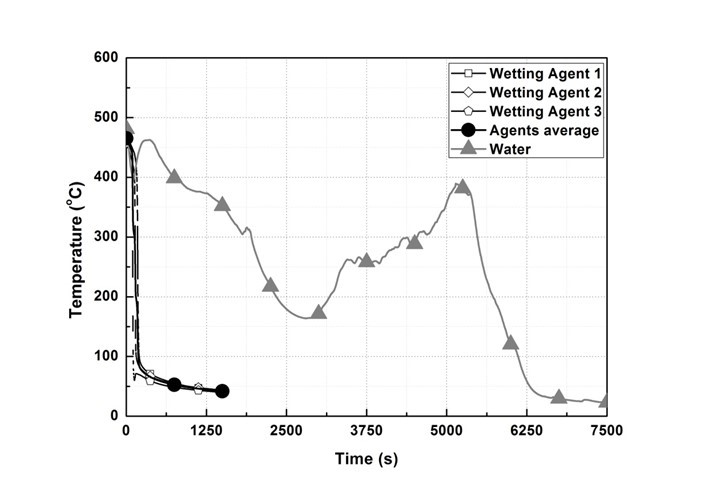
Fig. 6. T/C 3 temperature change
Table 3. Time to Reach Fire Extinguishing Point for each T/C
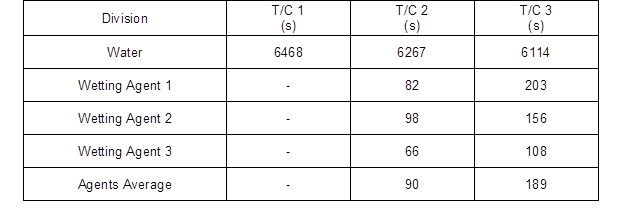
Figure 7 shows the emission measurement results, while Fig. 8 shows the weight measurement results of the sample holder. In the wetting agent experiment, it was confirmed that 750 g of sprayed water penetrated into the wood flour, whereas in the water experiment, 354.2 g of water did not penetrate into the inside, and remained outside. Furthermore, it was confirmed that the weight within the sample holder continuously decreased due to internal smoldering and was reduced rapidly from the re-ignition point.
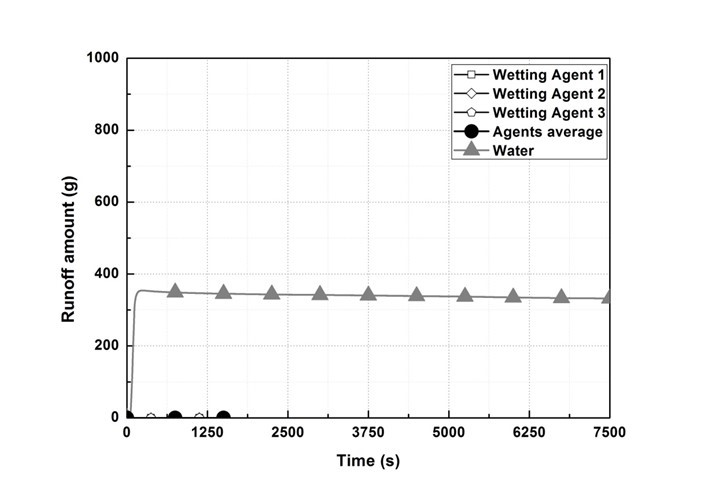
Fig. 7. Emission measurement results
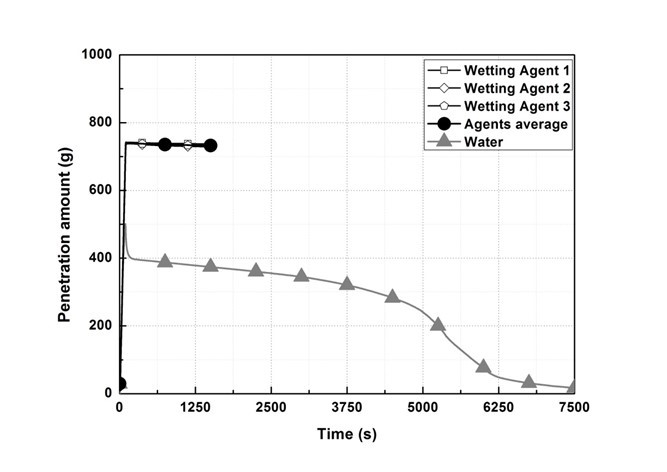
Fig. 8. Sample holder weight measurement results
The residual wood flour was dried and weighed to examine the weight loss before and after the experiment. Table 4 shows the weight change of wood flour before and after the experiment. As shown in the above table, the weight of the wood flour was reduced 73.1% after the use of water and 3.9% after the use of the wetting agents.
Table 4. Weight Change Before and After the Experiment
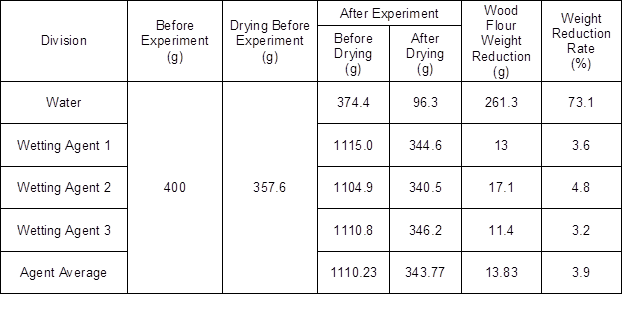
Analysis of Calorific Value and Combustion Gas of Wood Flour after Fire Extinguishing
This experiment was conducted to examine the reusability of wood flour after fire extinguishing. For that reason, the authors examined whether heat emission was reduced and whether toxic gases were generated during combustion with the use of agents. Figure 9 shows the total heat release (THR) of each sample. Experiment results confirmed that the addition of agents did not cause a decrease in the heat emission rate or the decrease of the combustion rate, whereas THR increased approximately 4% by the addition of the agent.
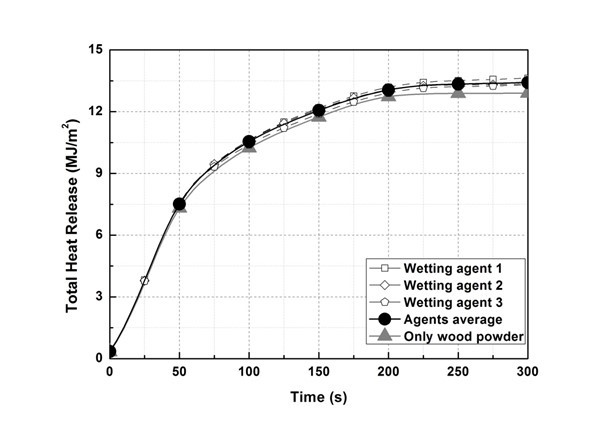
Fig. 9. THR of respective samples
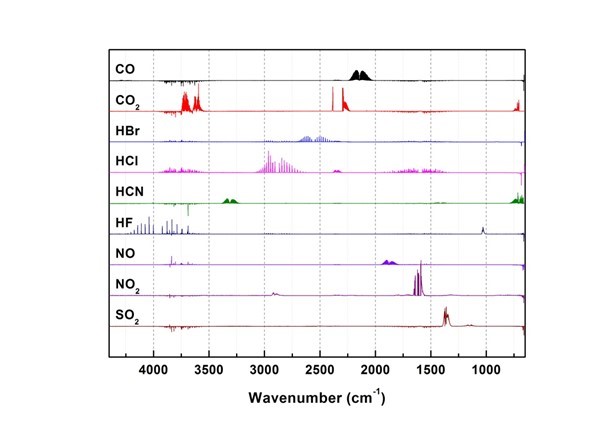
Fig. 10. Toxic gas (9 types) spectrum (Kim 2012a)
Generated gas was qualitatively analyzed according to the peak correspondence with each gas database. Figure 10 shows the standard gas spectra of nine types of toxic gases that can be generated during combustion (Kim 2012). Figures 11 through 16 show the results of the spectrum analysis of combustion gases generated during combustion according to time. A qualitative analysis of nine types of gases confirmed that no gases other than CO and CO2 were generated in all combustion gases from the four types of samples.
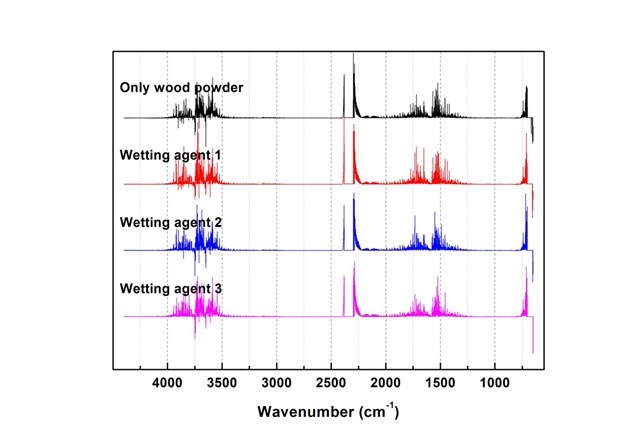
Fig. 11. Results of combustion gas spectrum analysis (40 s)
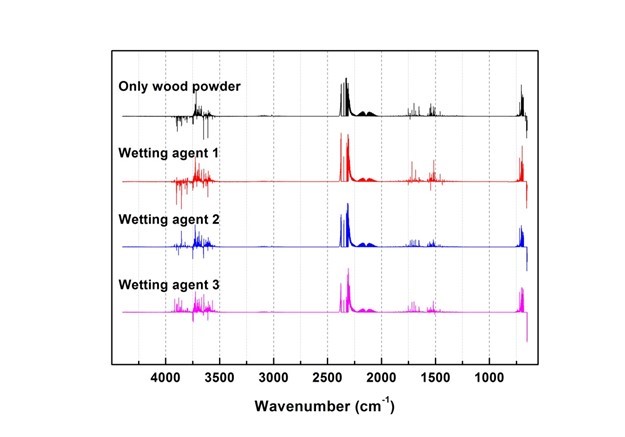
Fig. 12. Results of combustion gas spectrum analysis (80 s)
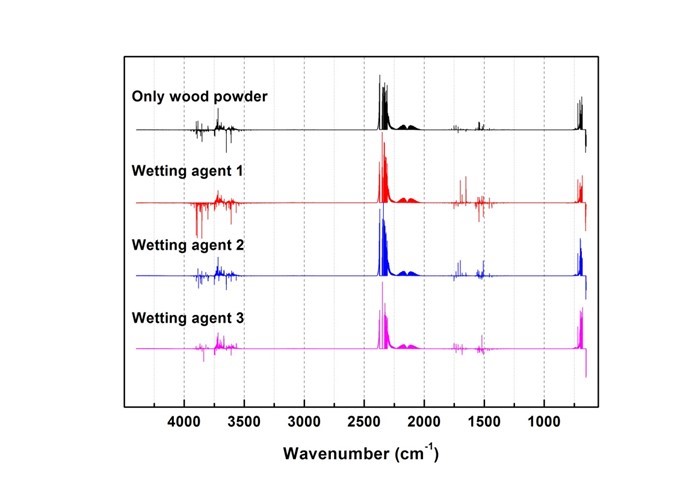
Fig. 13. Results of combustion gas spectrum analysis (120 s)
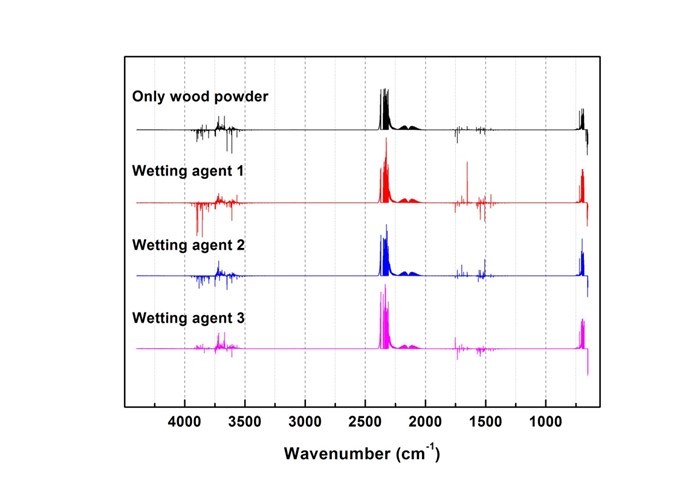
Fig. 14. Results of combustion gas spectrum analysis (160 s)
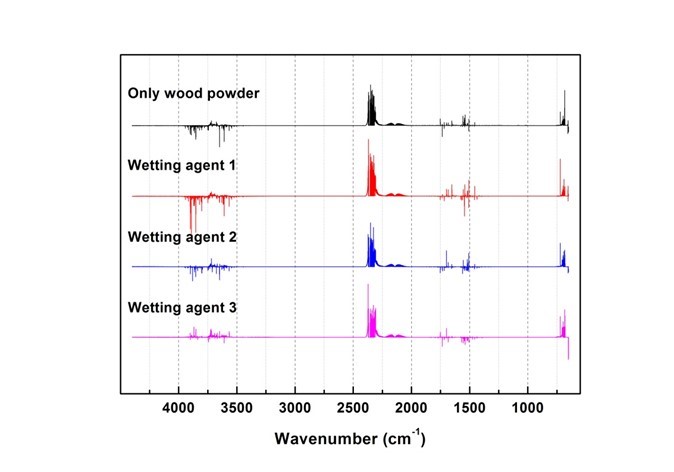
Fig. 15. Results of combustion gas spectrum analysis (200 s)
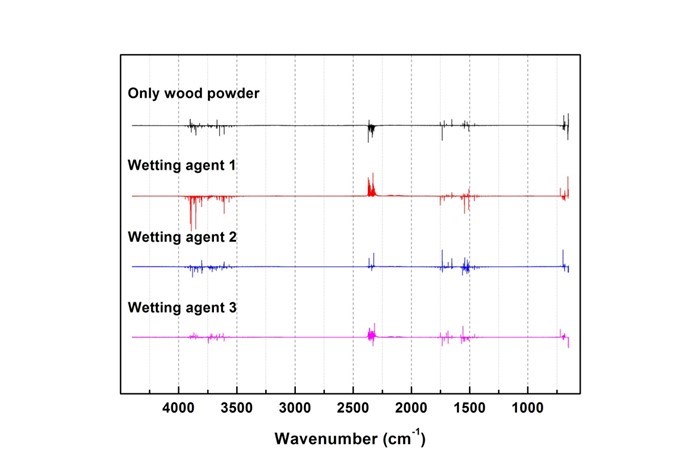
Fig. 16. Results of combustion gas spectrum analysis (240 s)
Table 5 shows the percentage of wood pellet production activity by process (Mani et al. 2006). As shown in the table, the percentage of the raw material was higher than any other material.
Table 5. Percentage of Wood Pellet Production Activity by Process (McKechnie et al. 2016)
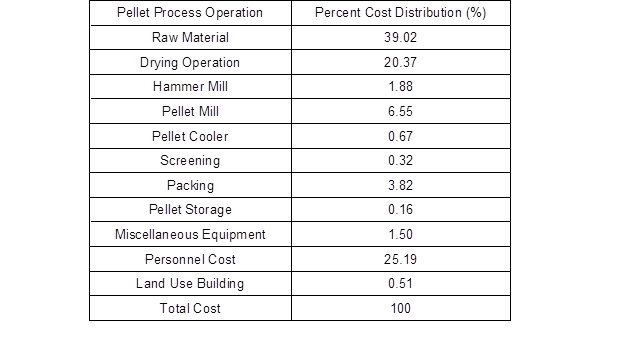
McKechnie et al. (2016) reported that the cost of raw materials can exceed 50% of the cost of producing pellets. Kristöfel et al. (2016) showed that the cost of raw materials is directly related to the price of wood pellets. As such, the loss of raw materials due to fires greatly affects companies’ economic loss. Furthermore, disruption in business due to disasters causes huge losses to companies (Sung 2014). Fire is a serious risk to the business continuity; thus disaster prevention plans must be established in advance based on a quantitative evaluation of fire risk. Therefore, the importance of ensuring business continuity is growing compared to the past years when economic profit alone had the highest value. In addition, business growth is the interest in resilient corporate operations enabling flexible response to the shocks caused by disasters and crisis situations and recovering from them quickly (Lee 2014). Hence, one way to minimize the economic loss when a fire occurs in a wood flour storage facility, of which the size and number increases with demand, is by extinguishing a fire quickly.
This study found that dilute aqueous solutions of wetting agents had much higher fire extinguishing performance than the water used for fire extinguishing, and could reduce wood flour loss and fire extinguishing time in the event of a deep-seated fire. The results of the scale model experiment showed that 73.1% of the wood flour was lost when water was used, and 3.9% of the wood flour was lost when the wetting agent was used under the same conditions. The fire extinguishing time was also reduced 97.5% from around 6250 s to around 150 s, with wetting agents. The results of analyzing the calorific value and the combustion gas of the wood flour with added wetting agent through cone calorimeter showed a slight increase in the caloric value, while the qualitative analysis results of the nine analyzed toxic gases also showed a slight increase in calorific value. The results of the qualitative analysis of the toxic gases from the nine samples showed that there was no additional toxic gas generation due to the wetting agent, which indicated that unburned wood flour can be reused.
The results of this study were based on a scale model experiment; thus there are some limitations in applying the results directly to actual fires.
Firstly, in an actual fire, there are other variables, such as the particle size of the wood raw material in the storage, the particle size of the water to be sprayed, water pressure, and water flow rate per unit area. In an actual fire, the particle size of the wood raw material is larger than that of the experimental conditions in this study, and the particles of the water to be sprayed also tend to be large and uneven with a spray flow rate per unit area that cannot be maintained evenly.
Secondly, in an actual fire situation, the surface tension of the sprayed water is high, so the water does not penetrate into the wood flour and flows along the slope surface, and therefore is not transferred inside the wood flour. However, in the scale model experiment, the water that did not penetrate the wood flour traveled along the holder wall, which substantially increased the contact area with the sample, and increased the penetration amount into the wood flour.
These limitations could cause larger loss rate deviations based on the use of the wetting agent in actual fires. Furthermore, it was found qualitatively that, considering the prevention of fire spread by early fire extinguishing and the reduction of secondary water damage by the reduction of the amount of water used, the active use of a wetting agent can minimize the economic loss of a company in the event of a fire in wood flour storage.
CONCLUSIONS
- The results of the scale model experiment showed that the use of water when extinguishing a deep-seated fire under the same conditions led to reignition, whereas the use of the wetting agent reduced the wood flour loss by approximately 69.2%.
- A deep-seated fire which was not extinguished by the use of water was extinguished within about 150 s by the use of the wetting agent; thus the use of wetting agent can be expected to improve companies’ business continuity.
- Cone calorimeter experiment results showed that the total heat emission of the wood flour with added wetting agent was approximately 4% higher than that of the control group. A qualitative analysis of combustion gas found no additional toxic gas generation compared with the control group. These results demonstrated that wood flour with the added wetting agent can be reused as raw pellet material.
- The authors found qualitatively in this study that the active use of the wetting agent during fires in wood flour storage allowed drastic reduction in the fire loss rate of raw materials and minimization of economic losses by facilitating early fire extinguishing.
ACKNOWLEDGMENTS
This work was supported by the Incheon National University (International Cooperative) Research Grant in 2014.
REFERENCES CITED
Alakoski, E., Jämsén, M., Agar, D., Tampio, E., and Wihersaari, M. (2016). “From wood pellets to wood chips, risks of degradation and emissions from the storage of woody biomass–A short review,” Renewable and Sustainable Energy Reviews 54, 376-383. DOI: 10.1016/j.rser.2015.10.021
Cal, L., Zhuang, B., Huang, D., Wang, W., Niu, M., Xie, Y., Chen, T., and Wang, X. (2016). “Ultra-low density fibreboard with improved fire retardance and thermal stability using a novel fire-resistant adhesive,” BioResources 11(2), 5215-5229. DOI: 10.15376/biores.11.2.5215-5229
Guo, M., Song, W., and Buhain, J. (2015). “Bioenergy and biofuels: History, status, and perspective,” Renewable and Sustainable Energy Reviews 42, 712-725. DOI: 10.1016/j.rser.2014.10.013
IEA (2013). World Energy Outlook 2013, International Energy Agency, Paris, France.
ISO 5660-1 (2015). “Reaction-to-fire tests — Heat release, smoke production and mass loss rate — Part 1: Heat release rate (cone calorimeter method) and smoke production rate (dynamic measurement),” International Organization for Standardization, Geneva, Switzerland.
ISO 19702 (2015). “Guidance for sampling and analysis of toxic gases and vapours in fire effluents using Fourier transform infrared (FTIR) spectroscopy,” International Organization for Standardization, Geneva, Switzerland.
Kim, N. K., and Rie, D. H. (2015). “A study of fire extinguishment characteristic for the real scale deap-seated fire,” Journal of Korean Institute of Fire Science and Engineering 29(2), 13-19. DOI: 10.7731/KIFSE.2015.29.2.013
Kim, N. K., and Rie, D. H. (2016). “A study on the fire extinguishing characteristics of deep-seated fires using the scale model experiment,” Fire Safety Journal 80, 38-45. DOI: 10.1016/j.firesaf.2016.01.003
Kim, J. S., and Rie, D. H. (2013). “Downward smoldering fire characteristics of wood chips and wood flour,” Journal of KOSHAM 13(1), 269-274. DOI: 10.9798/KOSHAM.2013.13.1.269
Kim, S. S. (2012a). A Research of Calculating Toxicity Index and Quantitative Analyzing for Fire Gas Using FT-IR, Master’s Thesis, University of Inchoen, Inchoen, South Korea.
Kim, Y. S. (2012b). A Study on the Explosion Efficiency and Risk Assessment of Combustible Wood Dust by Using the Cone Calorimeter, Master’s Thesis, University of Inchoen, Inchoen, South Korea.
Kong, I. C., Park, I. G., Lim, K. B., and Rie, D. H. (2013). “A study for characteristics of water that penetrates wood flour due to changes of concentration of BDG,” Journal of the Korean Society of Safety 28(3), 74-79. DOI: 10.14346/JKOSOS.2013.28.3.074
Kristöfel, C., Strasser, C., Schmid, E., and Morawetz, U. B. (2016). “The wood pellet market in Austria: A structural market model analysis,” Energy Policy 88, 402-412. DOI: 10.1016/j.enpol.2015.10.039
Lazghab, M., Saleh, K., Pezron, I., Guigon, P., and Komunjer, L. (2016). “Wettability assessment of finely divided solids,” Powder Technology 157(1-3), 79-91. DOI: 10.1016/j.powtec.2005.05.014
Lee, K. J. (2014). A Study on the Optimal Disaster Reduction Program for Industrial Disaster Early Response, Master’s Thesis, University of Seoul, Seoul, South Korea.
Li, C., Li, J. B., Lei, C. Y., and Liu, P. H. (2017). “Preparation and in vitro release mechanisms of modified pectin matrix tablets for colon-targeted drug delivery,” BioResources 12(1), 1491-1505. DOI: 10.15376/biores.12.1.1491-1505
Li, H., Liu, X., Legros, R., Bi, X. T., Lim, C. J., and Sokhansanj, S. (2012). “Pelletization of torrefied sawdust and properties of torrefied pellets,” Applied Energy 93, 680-685. DOI: 10.1016/j.apenergy.2012.01.002
Liang, S., Zhang, L., Chen, Z., and Fu, F. (2017). “Flame retardant efficiency of melamine pyrophosphate with added Mg-Al-layered double hydroxide in medium density fiberboards,” BioResources 12(1), 533-545. DOI: 10.15376/biores.12.1.533-545
Mani, S., Sokhansanj, S., Bi, X., and Turhollow, A. (2006). “Economics of producing fuel pellets from biomass,” Applied Engineering in Agriculture 22(3), 421-426. DOI: 10.13031/2013.20447
McKechnie, J., Saville, B., and MacLean, H. L. (2016). “Steam-treated wood pellets: Environmental and financial implications relative to fossil fuels and conventional pellets for electricity generation,” Applied Energy 180, 637-649. DOI: 10.1016/j.apenergy.2016.08.024
Nabavi, V., Azizi, M., and Tarmian, A. (2016). “Costs of wood pellet production in Iran,” iBusiness 8(03), 37. DOI: 10.4236/ib.2016.83005
NFPA 12 (2005). “Standard on carbon dioxide extinguishing systems,” National Fire Protection Association, Quincy, MA.
Nunes, L. J. R., Matias, J. C. O., and Catalao, J. P. S. (2016). “Wood pellets as a sustainable energy alternative in Portugal,” Renewable Energy 85, 1011-1016. DOI: 10.1016/j.renene.2015.07.065
Palmer, K. N. (1957). “Smouldering combustion in dusts and fibrous materials,” Combustion and Flame 1(2), 139-154. DOI: 10.1016/0010-2180(57)90041-X
Proskurina, S., Heinimö, J., Mikkilä, M., and Vakkilainen, E. (2015). “The wood pellet business in Russia with the role of North-West Russian regions: Present trends and future challenges,” Renewable and Sustainable Energy Reviews 51, 730-740. DOI: 10.1016/j.rser.2015.06.051
Seo, H. J., Park, J. E., and Son, D. W. (2016). “Combustion and thermal characteristics of Korean wood species,” BioResources 11(3), 7537-7550. DOI: 10.15376/biores.11.3.7537-7550
SFPE (2002). The SFPE Handbook of Fire Protection Engineering (3rd edition), National Fire Protection Association, Quincy, MA.
Simpson, A. T., Hemingway, M. A., and Seymour, C. (2016). “Dangerous (toxic) atmospheres in UK wood pellet and wood chip fuel storage,” Journal of Occupational and Environmental Hygiene 13(9), 699-707. DOI: 10.1080/15459624.2016.1167279
Simpson, W. T. (1987). “Drying and control of moisture and dimensional changes,” Wood Handbook – Wood as an Engineering Material, Forest Product Laboratory US Department of Agriculture, Forest Products Laboratory, Madison, WI, pp. 1-12.
Sung, J. H. (2014). A Study on the Error of Risk Assessment for BCM in the Enterprise, Master’s Thesis, Seoul National University of Science and Technology, Seoul, South Korea.
Torero, J. L., and Fernandez-Pello, A. C. (1995). “Natural convection smolder of polyurethane foam, upward propagation,” Fire Safety Journal 24(1), 35-52. DOI: 10.1016/0379-7112(94)00030-J
Toscano, G., Duca, D., Amato, A., and Pizzi, A. (2014). “Emission from realistic utilization of wood pellet stove,” Energy 68, 644-650. DOI: 10.1016/j.energy.2014.01.108
Toscano, G., Riva, G., Pedretti, E. F., Corinaldesi, F., Mengarelli, C., and Duca, D. (2013). “Investigation on wood pellet quality and relationship between ash content and the most important chemical elements,” Biomass and Bioenergy 56, 317-322. DOI: 10.1016/j.biombioe.2013.05.012
Williams, A., Jones, J. M., Ma, L., and Pourkashanian, M. (2012). “Pollutants from the combustion of solid biomass fuels,” Progress in Energy and Combustion Science 38(2), 113-137. DOI: 10.1016/j.pecs.2011.10.001
Yu, D., and Chen, M. (2016). “Oxygen enriched co-combustion of biomass and bituminous coal,” Energy Sources Part A: Recovery, Utilization, and Environmental Effects 38(7), 994-1001. DOI: 10.1080/15567036.2013.860506
Article submitted: March 5, 2017; Peer review completed: May 22, 2017; Revised version received: June 8, 2017; Accepted: June 9, 2017; Published: June 16, 2017.
DOI: 10.15376/biores.12.3.5550-5568
