Abstract
The concentrates of coking effluents are toxic and technically challenging to treat. Environmental protection demands an efficient and cost-effective technique for coking wastewater treatment. The intertidal marine macro alga Ulva prolifera has a high tolerance to various environmental stresses. In this study, U. prolifera was collected from an intertidal field and tested in a laboratory-scale photobioreactor for potential bioremediation of coking effluent concentrate. Algal physiology and water quality were measured. During treatment, the quantum yield Fv/Fm and effective quantum yield Y(II) values showed 12% and 15% decreases in 24 h, respectively, and a complete recovery in 168 h. The algal physiological characteristics indicated that light irradiance provided in a sine pattern of enhanced algal growth by 53% in the coking concentrate. The removal efficiency for total nitrogen and total phosphorus was up to 26.1% and 68.5% in 24 h, respectively. It also showed high removal efficiencies for some heavy metals, chemical oxygen demand, biological oxygen demand, and nutrients within 24 h. The feasibility of using U. prolifera for coking concentrate treatment was also considered. This study provided a possible bioremediation technique for wastewater treatment, especially for concentrates of coking effluent, in the coking industry.
Download PDF
Full Article
Absorptive Process and Biological Activity of Ulva prolifera and Algal Bioremediation of Coking Effluent
Wenhui Gu a,b,c and Guangce Wang a,b,c,*
The concentrates of coking effluents are toxic and technically challenging to treat. Environmental protection demands an efficient and cost-effective technique for coking wastewater treatment. The intertidal marine macro alga Ulva prolifera has a high tolerance to various environmental stresses. In this study, U. prolifera was collected from an intertidal field and tested in a laboratory-scale photobioreactor for potential bioremediation of coking effluent concentrate. Algal physiology and water quality were measured. During treatment, the quantum yield Fv/Fm and effective quantum yield Y(II) values showed 12% and 15% decreases in 24 h, respectively, and a complete recovery in 168 h. The algal physiological characteristics indicated that light irradiance provided in a sine pattern of enhanced algal growth by 53% in the coking concentrate. The removal efficiency for total nitrogen and total phosphorus was up to 26.1% and 68.5% in 24 h, respectively. It also showed high removal efficiencies for some heavy metals, chemical oxygen demand, biological oxygen demand, and nutrients within 24 h. The feasibility of using U. prolifera for coking concentrate treatment was also considered. This study provided a possible bioremediation technique for wastewater treatment, especially for concentrates of coking effluent, in the coking industry.
Keywords: Ulva prolifera; Photobioreactor; Coking wastewater; Algal bioremediation; Biosorption; Marine algae
Contact information: a: Center for Ocean Mega-Science, Chinese Academy of Sciences, 7 Nanhai Road, Qingdao, 266071, P. R. China; b: Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, P. R. China; c: Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, P. R. China;
* Corresponding author: gcwang@qdio.ac.cn
INTRODUCTION
The coking (the carbonization of raw coal and subsequent refinery processes) and steel industries generate coking wastewater that contains highly toxic or mutagenic substances, including cyanide (CN−), polycyclic aromatic hydrocarbons (PAHs), and pyridine (Wei et al. 2012; Song et al. 2013; Zhang et al. 2013; Yu et al. 2016), which require advanced treatment before water reuse or discharge. China, which has the world’s largest coking industry, generated 0.18 billion cubic meter of coking effluent in 2005 (Wei et al. 2007). This represents more than half of the world’s coke production, and this number is continuously increasing (Babich and Senk 2019). In addition, progressively tightening environmental protection regulations are requiring greater removal efficiencies to meet the recently issued discharge standard in China. Consequently, coking and its related industries are facing environmental challenges, leading to higher production costs. Thus, an advanced treatment method for coking wastewater using conventional or emerging techniques that has a low cost is required.
The composition of coking wastewater is complex and characterized by highly toxic components. Biological degradation, filtration, and membrane separation are currently the most widely applied techniques for the treatment of coking wastewater (Jin et al. 2013). Filtration and osmotic processes inevitably generate concentrates with increased concentrations of pollutants that could lead to severe environmental disasters if not treated properly. Moreover, the concentrate produced by filtration and reverse osmosis can equal 25% to 50% of the feeding water (Dialynas et al. 2008; Jin et al. 2013). However, the discharge of the produced concentrate is strictly prohibited and the advanced treatment of highly toxic concentrates remains challenging. Concentrates produced by filtration and reverse osmosis processes contain high concentrations of inorganic nutrients and organic pollutants, most notably, heavy metals and biologically toxic substances. Conventional biological effluent treatment techniques, such as activated sludge, are not applicable due to their inhibitory effects on growth (Wu and Zhu 2012).
The use of algal species for various wastewater treatments has been investigated (Boelee et al. 2011; Christenson and Sims 2011), and algal biomass has been shown to be effective for removal of toxic elements (Bilal et al. 2018). Both Chlorella vulgaris and Arthrospira maxima have been comprehensively studied by scientists and demonstrated technically to be applicable and efficient for industrial and domestic wastewater treatment (López-Pacheco et al. 2019a,b). Especially, the bioreactor-based strategies were shown to be efficient (Tolboom et al. 2019). Marine intertidal areas are habitats for many marine algal species. The periodic rising tide leads to fluctuating environmental conditions. In addition, the nearshore and inland waterways bring nutrients and pollutants to the intertidal area. These natural conditions have conferred many stress-tolerant characteristics, especially salt and pollutant resistance, to intertidal algae (Davison and Pearson 1996). Consequently, the intertidal algal species, dominated by green and brown macro-algae, are potential candidates for effluent bioremediation. Efforts focusing on bioremediation using marine algae have been made, mostly for the treatment of aquatic (Bartoli et al. 2005; Del Río et al. 1996) and municipal effluents (Huan et al. 2018; Noemí Gil et al. 2005). Their potential applications for remediating highly toxic effluents, such as concentrates of coking effluent, have not yet been evaluated. The widely distributed intertidal macro-alga Ulva. prolifera showed tolerance to a wide range of salinity levels (Taylor et al. 2001). Notably, its rapid biomass accumulation in coastal China has made the material economically feasible for the bioremediation of the concentrate of coking effluent.
For algal-based biosorption techniques, the disposal and subsequent process should be taken into consideration. Traditionally, U. prolifera could be used as raw material for fertilizer, feed additives, or refinery chemistry (Moral et al. 2019; Trivedi et al. 2016). Even though it is economically feasible to implement this scheme for the treatment of coking effluent concentrates, there is a need for further safety evaluation. Direct landfill after composting will result in land pollution and obviously is not applicable. As a hazardous waste, the resultant U. prolifera biomass, if economically advantageous, can be exploited as raw materials for sustainable industries.
In the current study, the marine intertidal macro-alga U. prolifera was tested for its effects on coking effluent concentrate treatment. In particular, the absorptive process was monitored in real time. Its toxic tolerance to coking effluent concentrates, especially the carbon fixation and photosynthetic activity, was evaluated. The removal efficiencies of heavy metals and nutrients, as well as chemical oxygen demand (COD) and 5-day biological oxygen demand (BOD5), were also measured. The aim of this study was to evaluate the possibility of using U. prolifera to treat concentrates of coking effluents.
EXPERIMENTAL
Materials
The coking wastewater used in this research was collected from a coking plant in a steel company in Shanghai (China). It was the combined concentrates produced from the processes of ultrafiltration, nanofiltration, and reverse osmosis. The suspended solid (SS) content was approximately 40 ± 2 mg/L, the total CN– was 30 ± 1 mg/L, F− was 180 ± 7 mg/L, and the Cl– concentration was 5500 ± 192 mg/L. The salinity levels of the coking concentrate and natural seawater used were 15‰ and 30‰, respectively. Wild U. prolifera was collected from the intertidal zone at Huiquan Bay (36°03′11.60″N, 120°20′16.61″E) in Qingdao, China. The algal thalli were rinsed using sterilized seawater and then cultivated at 22 °C under 100 μE irradiance for further use.
Methods
Adsorption process using U. prolifera
Every 700 mL of coking effluent concentrate was treated with 7.00 g of fresh cultivated U. prolifera, which was fixed in a flat position and completely immersed in the water for treatment. The mixture of coking concentrate and alga was cultivated in a panel photobioreactor (FMT-1000; PSI Instruments, Brno, Czech Republic). The temperature was controlled at 22 ± 0.1 °C and continuously bubbled with air (flow rate 350 mL/min). Air was humidified by being passed through sterilized distilled water and filtered with a 0.22-μm membrane. The continuous bubbling of filtered air enabled water mixing and sufficient contact with U. prolifera thallus. Light was provided by a white LED panel (LED spectrum provided in Fig. S1) either under constant or sine pattern light. For the sine pattern light, the sine amplitude was 800 μE, and the constant light irradiance was 400 μE. Thus, they yielded approximately the same light quantum. The light period lasted for 14 h, while the dark lasted for 10 h. The setup for water treatment using U. prolifera biomass was shown in Fig. 1.
Measurement of algal photosynthetic activity during the bioremediation process
The photosynthetic activity of U. prolifera was measured using a pulse-amplitude modulated fluorometer (Dual-PAM 100; Heinz Walz, Effeltrich, Germany) as described in previous research (Gu et al. 2015). Briefly, the samples were dark adapted for 5 min prior to recording the inductive fluorescence curve. Photosynthetic parameters, including maximal photosystem (PSII) quantum yield (Fv/Fm), the photochemical quantum yield of PSI [Y(I)], and the effective quantum yield of PSII [Y(II)] were achieved or calculated based on the acquisition of the inductive fluorescence curve as described (Baker 2008).
Water-treatment process monitoring
To determine whether the water quality correlated with algal growth during bioremediation, the pH, dissolved CO2 (dCO2), and dissolved oxygen (dO2) levels were monitored using Mettler probes. The Fv/Fm values under light and dark periods were both measured using the online fluorometer installed on the photobioreactor. Data were recorded hourly for evaluation of algal photosynthetic activity during the bioremediation process.
Fig. 1. Schematic figure shows the setup for coking effluent treatment. Air was provided by air pump at flow rate of 350 mL/min, creating airlift bubbling to enable sufficient mix of coking wastewater for direct contact with algal biomass. Light was provided with LED panel. Water pH, O2 and CO2 level were monitored with online probes.
Determination of pollutants
The coking effluent concentrate before and after algal treatment was collected and used for pollutant measurements, without filtration. China National Standard methods GB/T 11893 (1989) and HJ/T 636 (2012) were used for the determination of the total amounts of P and N, respectively. The China National Standard method HJ/T 132 (2003) was used for the COD measurement of coking effluent containing high levels of Cl− ions. China National Standard method HJ/T 505(2009) was used for the BOD5 (5-day biological oxygen demand) measurement. Heavy metals, including arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), and zinc (Zn) were measured before and after algal treatment using an atomic absorption method according to China National Standard method GB/T 17378.4 (2007).
Algal growth measurement
After each cultivation batch, the alga was removed from the bioreactor, and the surface water was absorbed using filter paper. Fresh weight was measured, and three independent replicates were conducted.
Statistical analysis
All statistical analysis was conducted by SAS 9.1 for Windows software (SAS Institute Inc., Cary, NC, USA). A one-way analysis of variance was used to determine the significant difference at p < 0.05.
RESULTS AND DISCUSSION
Algal Photosynthetic Activity During the Bioremediation Process
Both the short-term (24 h) and long-term (168 h) effects of coking concentrate effluent on algal physiology were studied. After 24 h, which was a cycle of one light and one dark period, under constant light, the Fv/Fm was not significantly different when the alga was cultivated with a mixture of 50% coking effluent and 50% seawater, compared with cultivation in 100% seawater (Fig. 2-A). However, the alga cultivated in 100% coking effluent concentrate showed a 12% decrease (Fig. 2-A). Similarly, for Y(II), there was no difference between 50% coking effluent and 100% seawater, but there was a significant decrease (15%) in 100% coking concentrate (Fig. 2-C). In contrast, Y(I) was slightly increased when subjected to 100% coking effluent (Fig. 2-C), but no significant difference was observed when subjected to a 50% mixture of coking effluent compared with seawater (Fig. 2-E).
As for long-term cultivation, the Fv/Fm increased when the alga was cultivated in 100% coking concentrate (Fig. 2-B) compared with in seawater and 50% coking concentrate. Moreover, Y(II) showed slight increases in both 50% and 100% coking water compared with in seawater (Fig. 2-D). For Y(I), no significant difference was observed among the three groups (Fig. 2-F).
Unlike some microalgae that are sensitive to hazardous compounds during wastewater treatment, U. prolifera showed high tolerance to coking waste concentrate. Both the short-term and long-term stress tolerance of U. prolifera to coking concentrate were evaluated in this study.
In photosynthetic organisms, both Fv/Fm and Y(II) are considered indicators of stress in U. prolifera (Huan et al. 2018; Zhao et al. 2016). Their decreased values indicated that U. prolifera was stressed in the coking water in the first 24 h. Additionally, the increase in Y(I) observed in this study (Fig. 2-E) was also consistent with the previously reported stress responses of U. prolifera (Gao et al. 2011). Although U. prolifera showed stress within 24 h of cultivation, its photosynthetic activity had completely recovered. In addition, the increased value of Fv/Fm after cultivation in 100% coking concentrate for 168 h, indicated a better adaptive growth in coking effluent. This may have resulted from the high concentrations of N and P in the coking concentrate, which provided nutrients for algal growth.
The rapid recovery of photosynthetic activity is a prerequisite for algal survival and makes the subsequent bioremediation possible. Many refractory substances, such as phenols and cyanides, make the biodegradability of the coking wastewater extremely low (Kim et al. 2008). U. prolifera could adapt to the inhibitory effects of toxic organics within 24 h. Additionally, due to its other tolerance capabilities, U. prolifera is a potential candidate for coking concentrate treatment.
Fig. 2. Photosynthetic activity indicated by algal chlorophyll fluorescence: A and B, Fv/Fm of U. prolifera cultivated for 24 h and 168 h, respectively; C and D, Y(II) of U. prolifera cultivated for 24 h and 168 h, respectively; E and F, Y(I) of U. prolifera cultivated for 24 h and 168 h, respectively; SW- seawater and CCW- coking concentrate water. Different letters on the bars represent significant differences between groups (p<0.05).
Variations in pH, dO2, and dCO2 Under Constant and Sine Lights
To mimic natural light during the water treatment, constant and sine lighting were used independently for the algal bioremediation of coking effluent. Under constant light, the pH increased from 9.02 to 9.37 during the light period and decreased to 9.03 at the end of the dark period (Fig. 3-A). When cultivated under constant light, the dO2 in the coking effluent increased from 19.9% to 20.8% in 30 min and then gradually decreased to 20.1% at the end of light period (14 h). During the dark period, dO2 decreased to as low as 18.3% due to algal respiration. The dCO2 level gradually decreased from 6.60% to 4.85% during the light period due to photosynthesis and then increased to 6.22% in the dark period due to respiration.
Under sine light, as shown in Fig. 3-B, the light irradiance increased from 0 μE to 800 μE in 7 h. The light irradiance was 650 μE at 5 h and the dO2 in coking effluents increased up to 21.5%. The dO2 was stabilized at 18.7% during the dark period. The dCO2 was more rapidly consumed under sine light, as indicated by the rapid decrease in dCO2 from 6.55% to 3.86%. Additionally, the pH of the coking effluent increased to 9.35 during the light period and decreased to 9.02 during the dark period, which was similar to the constant light treatment.
Fig. 3. The changes in light, dissolved CO2 (dCO2), dissolved O2 (dO2), and the pH of coking con-centrate during algal bioremediation under a constant (A) and sine (B) white light regime in 24 h
Light Impact on the Physiology of Algal Cells in Coking Concentrate
The quantum yield (Qy) of U. prolifera during the light and dark periods was monitored. The photosynthetic Qy showed different patterns under constant and sine lighting. When cultivated in coking concentrate under constant light, the Qy values during the light and dark periods were both relatively stable at 0.25 and 0.65, respectively (Fig. 4). Comparatively, when sine lighting was applied in the first 7 h, Qy gradually decreased as the light intensity increased, with a minimum Qy of 0.1 when the irradiance level reached 800 μE. With a decrease in light from 7 h to 14 h, Qy gradually recovered to 0.71 and stabilized during the dark period. This was significantly higher than under constant light.
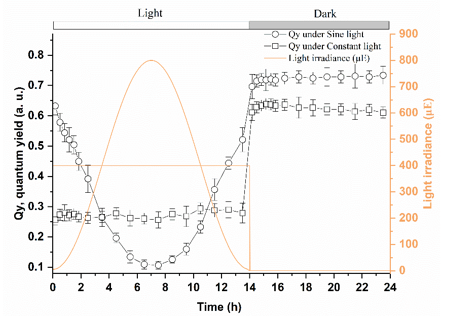
Fig. 4. Quantum yield (Qy) of algae cultivated in coking concentrate under constant and sine lighting in 24 h
In a natural environment, U. prolifera either floats on the surface or grows attached to the intertidal habitat, where it is exposed to natural light in a sine pattern on a daily basis. Light provides energy for algal photosynthesis, and thus, the bioremediation. Excess light might lead to photoinhibition and low light could lead to inefficient photosynthesis. In this study, when sine light was used, U. prolifera showed higher biomass and a relatively greater recovery efficiency, as indicated by the elevated Qy during the dark period, which equaled the maximum quantum efficiency (Fig. 4). Additionally, sine lighting also led to a greater dO2 and lower dCO2 (Fig. 3), which indicated a higher photosynthetic oxygen production and likely a greater CO2 fixation rate, respectively. Those results were consistent with the higher Qy, and thus led to a higher amount of biomass, compared with those of algae treated with constant light. The greater biomass accumulation and rapid recovery of photosynthetic activity indicated that sine light was beneficial for algal growth in coking concentrate. Thus, the results showed that sine light should be chosen over constant light to support algal growth as a coking concentrate treatment.
Under constant and sine light, the U. prolifera cultivated in coking concentrate showed different growth characteristics. Approximately 7 g of fresh alga was used for 700 mL of coking concentrate treatment under both constant and sine lighting. After 24 h, the fresh weight under constant light had increased 32%, while the alga biomass grown under sine light increased 53% (Fig. 5).
During the coking water treatment process using photosynthetic organisms, the use of artificial light or natural sunlight needs to be optimized (Muñoz and Guieysse 2006). Constant light has shown effects on microalgae’s ability to absorb PAHs from coking water (Lei et al. 2007). However, in many cases, constant light at a high irradiance might cause photodamage, especially when exposed to a highly toxic environment, such as coking concentrate. Based on the findings of the current study, the algae could benefit from a lower light during adaption in early stages. In industrial bioremediation applications, it would be economically important to utilize either natural light or artificial light in a sine pattern.
Fig. 5. Algal fresh weight increased in coking concentrate after 24 h treatment. Different letters on the bars represent significant differences between groups (p<0.05).
Removal Efficiency of Nitrogen (N), Phosphorus (P), BOD5, COD, and Heavy Metals from Coking Concentrates
When sine light was provided, N and P in coking concentrate were significantly reduced by 24 h, with removal efficiencies of 26.1% and 68.5%, respectively (Fig. 6-A and B). In addition, both COD and BOD5 were also significantly reduced after cultivation, with removal efficiencies of 13.9% and 50.1%, respectively (Fig. 6-C and D).
Fig. 6. Removal efficiencies of total nitrogen (A), total phosphorus (B), COD (C), and BOD5 (D) from coking concentrates under sine light conditions in 24 h. Different letters on the bars represent significant differences between groups (p<0.05).
After 24 h of cultivation using sine light, some heavy metals were significantly reduced. Heavy metals, including As, Cd, Cr, Cu, Pb, and Zn, were greatly reduced, and their removal ratios, defined as (initial-residual/initial value×100%), were 56.0%, 42.4%, 3.7%, 83.4%, 65.8%, and 11.7%, respectively (Fig. 7). The results showed that U. prolifera had low absorption of chromium in coking wastewater.
Fig. 7. Removal efficiencies of heavy metals by U. prolifera under sine light: A, arsenic (As), B, cadmium (Cd), C, chromium (Cr), D, copper (Cu), E, lead (Pb), and F, zinc (Zn). Different letters on the bars represent significant differences between groups (p<0.05).
Efficiency and Economic Feasibility
To date, the blooming of a green tide caused by U. prolifera in northern China’s coastal area, has been one of the world’s largest documented green tides (Liu et al. 2013; Zhao et al. 2018), and it has occurred every year from 2007 to 2018. Unfortunately, the occurrence of green tides caused by U. prolifera is now a global issue that is continuously increasing and the biomass accumulated during a green tide can reach millions of tons (Smetacek and Zingone 2013). Thus far, the most effective remediation method is still clearing using human labor, which requires a large labor resource. Another issue is the storage and processing of the fresh algae. Although many alternative methods for U. prolifera biomass utilization, including biogas and animal food additive production, have been tested, the results have been far from cost effective (Charlier et al. 2008). Using green tide-forming U. prolifera for heavy metal absorption has been investigated (Huan et al. 2018; Suzuki et al. 2005). The results were encouraging but their economic effectiveness of the process needs to be further evaluated.
Based on the data obtained in this study, 10 kg of fresh algae was required for every ton of coking concentrate treatment, and the process could be completed within 24 h. The use of microalgae alone or in combination with other methods has been tested and shown to be technically applicable, with the exception of biomass harvesting, which greatly increased the energy cost (Muñoz and Guieysse 2006). However, the macro-algae U. prolifera could be directly harvested without relying on energy-costing techniques, such as centrifugation, making it energy efficient. Photobioreactors showed economic and efficient advantages (Tolboom et al. 2019), and they could be designed and scaled up for pilot applications of coking effluent treatment. Additionally, based on the photosynthetic activity, as indicated by recovery of Fv/Fm at 168 h, the U. prolifera showed potential for recycling and subsequent wastewater treatment. Its binding capacity for multiple biosorption cycles needs further study.
The most prominent advantage of algal absorption in coking wastewater treatment is that hazardous materials could be stabilized in solid biomass instead of aqueous solutions. However, for algal biomass collected after wastewater treatment, especially for the case of coking wastewater, conventional recycling and utilization processes including landfill or direct combustion, were not applicable due to non-biodegradable toxic compounds or heavy metals contained in the mixture. The utilization of wild field collected U. prolifera has been reviewed by Moral et al. (2019), and it was shown to be promising raw material for papermaking, mineral feed additives for livestock (Michalak et al. 2011), and biorefinery products (Glasson et al. 2017). The disposal or subsequent processing of contaminated U. prolifera must, therefore, be conducted with due consideration of their potential hazards. The pyrolysis of the dried biomass has been shown to be applicable for recycling of heavy metals and bioenergy (Han et al. 2018) from hyperaccumulators plants. Those of the successful applications were possible be used in the U. prolifera case. The collected algal biomass could be treated as heavy metals hyperaccumulators and used for heavy metal recycling.
CONCLUSIONS
- Marine intertidal algae showed high stress tolerance capabilities toward coking effluent concentrate. By subjecting naturally grown U. prolifera to coking effluent concentrate, this study demonstrated a possible bioremediation technique. U. prolifera could tolerate the coking concentrate, although the alga underwent physiological stress as indicated by the decline of photosynthesis.
- The laboratory-scale photobioreactor-based cultivation showed that the green alga U. prolifera could adapt to and grow in a coking effluent concentrate environment. Additionally, providing light irradiance in a sine pattern significantly improved the photosynthesis of U. prolifera. This might be because the low light irradiance in the beginning provided a better environment for algal adaption and acclimation to the coking effluent concentrate.
- The production of oxygen by U. prolifera in the coking effluent might be beneficial biodegradation of hazardous pollutants.
ACKNOWLEDGMENTS
This work was financially supported by the Key Deployment Project of Centre for Ocean Mega-Research of Science (COMS2019Q02), Natural Science Foundation of China (41506188), the China Agriculture Research System-50, Key R&D Program of Shandong Province (2018GHY115019). We would like to thank Dr. Jianzhong Xia from Shanghai Baosteel Chemical Co. Ltd. and Mr. Liangping Ni for providing the coking effluent.
REFERENCES CITED
Babich, A., and Senk, D. (2019). “Coke in the iron and steel industry,” in: New Trends in Coal Conversion, I. Suárez-Ruiz, M. A. Diez, and F. Rubiera (eds.), Woodhead Publishing, Cambridge, MA, pp. 367-404.
Baker, N. R. (2008). “Chlorophyll fluorescence: A probe of photosynthesis in vivo,” Annual Review of Plant Biology 59(1), 89-113. DOI: 10.1146/annurev.arplant.59.032607.092759
Bartoli, M., Nizzoli, D., Naldi, M., Vezzulli, L., Porrello, S., Lenzi, M., and Viaroli, P. (2005). “Inorganic nitrogen control in wastewater treatment ponds from a fish farm (Orbetello, Italy): Denitrification versus Ulva uptake,” Marine Pollution Bulletin 50(11), 1386-1397. DOI: 10.1016/j.marpolbul.2005.06.011
Bilal, M., Rasheed, T., Sosa-Hernández, J. E., Raza, A., Nabeel, F., and Iqbal, H. M. N. (2018). “Biosorption: An interplay between marine algae and potentially toxic elements—A review,” Marine Drugs 16(2), 65. DOI: 10.3390/md16020065
Boelee, N. C., Temmink, H., Janssen, M., Buisman, C. J. N., and Wijffels, R. H. (2011). “Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms,” Water Research 45(18), 5925-5933. DOI: 10.1016/j.watres.2011.08.044
Charlier, R. H., Morand, P., and Finkl, C. W. (2008). “How Brittany and Florida coasts cope with green tides,” International Journal of Environmental Studies 65(2), 191-208. DOI: 10.1080/00207230701791448
Christenson, L., and Sims, R. (2011). “Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts,” Biotechnology Advances 29(6), 686-702. DOI: 10.1016/j.biotechadv.2011.05.015
Davison, I. R., and Pearson, G. A. (1996). “Stress tolerance in intertidal seaweeds,” Journal of Phycology 32(2), 197-211. DOI: 10.1111/j.0022-3646.1996.00197.x
Del Río, M. J., Ramazanov, Z., and García-Reina, G. (1996). “Ulva rigida (Ulvales, Chlorophyta) tank culture as biofilters for dissolved inorganic nitrogen from fishpond effluents.” Hydrobiologia 326(1), 61-66. DOI: 10.1007/bf00047787
Dialynas, E., Mantzavinos, D., and Diamadopoulos, E. (2008). “Advanced treatment of the reverse osmosis concentrate produced during reclamation of municipal wastewater,” Water Res 42(18), 4603-8. DOI: 10.1016/j.watres.2008.08.008
Gao, S., Shen, S., Wang, G., Niu, J., Lin, A., and Pan, G. (2011). “PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions,” Plant Cell Physiol. 52(5), 885-93. DOI: 10.1093/pcp/pcr038
GB/T 11893 (1989). “Water quality – Determmination of total phosphorus–ammonium molybdate spectrophotometric method,” Standardization Administration of China, Beijing, China.
GB/T 17378.4 (2007). “The specification for marine monitoring – Part 4: Seawater analysis,” Standardization Administration of China, Beijing, China.
Glasson, C. R. K., Sims, I. M., Carnachan, S. M., de Nys, R., and Magnusson, M. (2017). “A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva ohnoi.” Algal Research, 27, 383-391. DOI: 10.1016/j.algal.2017.07.001
Gu, W., Huan, L., Yu, R., Pan, G., and Wang, G. (2015). “Enhancement of cytochrome b 559 indicates its possible involvement in long-term high light stress tolerance in intertidal macroalgae,” Plant Molecular Biology Reporter 33(6), 1918-1927. DOI: 10.1007/s11105-015-0885-7
HJ/T 132 (2003). “High-chlorine wastewater – Determination of chemical oxygen demand – Potassium iodidle alkaline permanganate method,” State Bureau of Environment Protection, Beijing, China.
HJ/T 505 (2009). “Water quality – Determination of biochemical oxygen demand after 5 days (BOD5) for dilution and seeding method,” State Bureau of Environment Protection, Beijing, China.
HJ/T 636 (2012). “Water quality – Determination of total nitrogen-alkaline potassium persulfate digestion UV spectrophotometric method,” State Bureau of Environment Protection, Beijing, China.
Han, Z., Guo, Z., Zhang, Y., Xiao, X., and Peng, C. (2018). “Potential of pyrolysis for the recovery of heavy metals and bioenergy from contaminated Broussonetia papyrifera biomass,” 13(2), 2932-2944. DOI: 10.15376/biores/13.2.2932-2944
Huan, L., Su, H. x., Duan, C. x., Gao, S., Xie, X. j., and Wang, G. c. (2018). “Ulva prolifera (Chlorophyta): A suitable material to remove Cd2+ from aquatic environments,” Water and Environment Journal 32(1), 26-33. DOI: 10.1111/wej.12286
Jin, X., Li, E., Lu, S., Qiu, Z., and Sui, Q. (2013). “Coking wastewater treatment for industrial reuse purpose: Combining biological processes with ultrafiltration, nanofiltration and reverse osmosis,” Journal of Environmental Sciences 25(8), 1565-1574. DOI: 10.1016/S1001-0742(12)60212-5
Kim, Y. M., Park, D., Lee, D. S., and Park, J. M. (2008). “Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment,” Journal of Hazardous Materials 152(3), 915-921. DOI: 10.1016/j.jhazmat.2007.07.065
Lei, A.-P., Hu, Z.-L., Wong, Y.-S., and Tam, N. F.-Y. (2007). “Removal of fluoranthene and pyrene by different microalgal species,” Bioresource Technology 98(2), 273-280. DOI: 10.1016/j.biortech.2006.01.012
Liu, F., Pang, S., Chopin, T., Gao, S., Shan, T., Zhao, X., and Li, J. (2013). “Understanding the recurrent large-scale green tide in the Yellow Sea: Temporal and spatial correlations between multiple geographical, aquacultural and biological factors,” Marine Environmental Research, 83, 38-47. DOI: 10.1016/j.marenvres.2012.10.007
López-Pacheco, I. Y., Carrillo-Nieves, D., Salinas-Salazar, C., Silva-Núñez, A., Arévalo-Gallegos, A., Barceló, D., Afewerki, S., Iqbal, H. M. N., and Parra-Saldívar, R. (2019a). “Combination of nejayote and swine wastewater as a medium for Arthrospira maxima and Chlorella vulgaris production and wastewater treatment,” Science of The Total Environment 676, 356-367. DOI: 10.1016/j.scitotenv.2019.04.278
López-Pacheco, I. Y., Salinas-Salazar, C., Silva-Núñez, A., Rodas-Zuluaga, L. I., Donoso-Quezada, J., Ayala-Mar, S., Barceló, D., Iqbal, H. M. N., and Parra-Saldívar, R. (2019b). “Removal and biotransformation of 4-nonylphenol by Arthrospira maxima and Chlorella vulgaris consortium,” Environmental Research 179, 108848. DOI: 10.1016/j.envres.2019.108848
Michalak, I., Chojnacka, K., Dobrzański, Z., Górecki, H., Zielińska, A., Korczyński, M., and Opaliński, S. (2011). “Effect of macroalgae enriched with microelements on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings,” Journal of Animal Physiology and Animal Nutrition 95(3), 374-387. DOI: 10.1111/j.1439-0396.2010.01065.x
Moral, A., Aguado, R., Castelló, R., Tijero, A., and Ballesteros, M. (2019). “Potential use of green alga Ulva sp. for papermaking,” BioResources 14(3), 6851-6862. DOI: 10.15376/biores.14.3.6851-6862
Muñoz, R., and Guieysse, B. (2006). “Algal–bacterial processes for the treatment of hazardous contaminants: A review,” Water Research 40(15), 2799-2815. DOI: 10.1016/j.watres.2006.06.011
Noemí Gil, M., Iadran Torres, A., and Luis Esteves, J. (2005). “Uptake of sewage derived nitrogen by Ulva rigida (Chlorophyceae) in Bahía Nueva (Golfo Nuevo, Patagonia, Argentina),” Hydrobiologia 532(1), 39-43. DOI: 10.1007/s10750-004-8770-7
Smetacek, V., and Zingone, A. (2013). “Green and golden seaweed tides on the rise,” Nature 504, 84. DOI: 10.1038/nature12860
Song, G., Zhu, C., Hu, Y., Chen, J., and Cheng, H. (2013). “Determination of organic pollutants in coking wastewater by dispersive liquid–liquid microextraction/GC/MS,” Journal of Separation Science 36(9-10), 1644-1651. DOI: 10.1002/jssc.201201151
Suzuki, Y., Kametani, T., and Maruyama, T. (2005). “Removal of heavy metals from aqueous solution by nonliving Ulva seaweed as biosorbent,” Water Research 39(9), 1803-1808. DOI: 10.1016/j.watres.2005.02.020
Taylor, R., Fletcher, R. L., and Raven, J. A. (2001). “Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate,” Botanica Marina 327. DOI: 10.1515/BOT.2001.042
Tolboom, S. N., Carrillo-Nieves, D., de Jesús Rostro-Alanis, M., de la Cruz Quiroz, R., Barceló, D., Iqbal, H. M. N., and Parra-Saldivar, R. (2019). “Algal-based removal strategies for hazardous contaminants from the environment – A review,” Science of The Total Environment, 665, 358-366. DOI: 10.1016/j.scitotenv.2019.02.129
Trivedi, N., Baghel, R. S., Bothwell, J., Gupta, V., Reddy, C. R., Lali, A. M., and Jha, B. (2016). “An integrated process for the extraction of fuel and chemicals from marine macroalgal biomass,” Scientific Reports 6, 30728. DOI: 10.1038/srep30728
Wei, C., He, M., Ren, Y., Li, G., and Chen, J. (2007). “Pollution characteristics of coking wastewater and control strategies: biological treatment process and technology,” Acta Scientiae Circumstantiae 27(7), 1083-1093. DOI: 10.13671/j.hjkxxb.2007.07.003
Wei, X.-x., Zhang, Z.-y., Fan, Q.-l., Yuan, X.-y., and Guo, D.-s. (2012). “The effect of treatment stages on the coking wastewater hazardous compounds and their toxicity,” Journal of Hazardous Materials 239-240, 135-141. DOI: 10.1016/j.jhazmat.2012.08.042
Wu, Z., and Zhu, L. (2012). “Removal of polycyclic aromatic hydrocarbons and phenols from coking wastewater by simultaneously synthesized organobentonite in a one-step process,” Journal of Environmental Sciences 24(2), 248-253. DOI: 10.1016/S1001-0742(11)60780-8
Yu, X., Xu, R., Wei, C., and Wu, H. (2016). “Removal of cyanide compounds from coking wastewater by ferrous sulfate: Improvement of biodegradability.” Journal of Hazardous Materials, 302, 468-474. DOI: 10.1016/j.jhazmat.2015.10.013
Zhang, W., Wei, C., Yan, B., Feng, C., Zhao, G., Lin, C., Yuan, M., Wu, C., Ren, Y., and Hu, Y. (2013). “Identification and removal of polycyclic aromatic hydrocarbons in wastewater treatment processes from coke production plants,” Environmental Science and Pollution Research 20(9), 6418-6432. DOI: 10.1007/s11356-013-1697-7
Zhao, J., Jiang, P., Qiu, R., Ma, Y., Wu, C., Fu, H., Chen, H., and Li, F. (2018). “The Yellow Sea green tide: A risk of macroalgae invasion.” Harmful Algae 77, 11-17. DOI: 10.1016/j.hal.2018.05.007
Zhao, X., Tang, X., Zhang, H., Qu, T., and Wang, Y. (2016). “Photosynthetic adaptation strategy of Ulva prolifera floating on the sea surface to environmental changes,” Plant Physiology and Biochemistry 107, 116-125. DOI: 10.1016/j.plaphy.2016.05.036
Article submitted: September 6, 2019; Peer review completed: November 23, 2019; Revised version received: February 15, 2020; Accepted: February 18, 2020; Published: February 25, 2020.
DOI: 10.15376/biores.15.2.2605-2620
APPENDIX
Supplementary Information
Fig. S1. Spectrum of white LED used in the photobioreactor during algal cultivation
