Abstract
Trunks and stumps of various deciduous species act as natural habitats for Ganoderma lucidum. The chemical composition of their cell wall affects the development of fungal ligninolytic enzyme system as well as its ability to degrade lignin from the plant cell wall. Additionally, numerous compounds structurally similar to lignin can be degraded by the G. lucidum enzyme system which could take important roles in various biotechnological processes. The laccases, which are the dominant enzymes synthesized by G. lucidum, have been studied more extensively than the Mn-oxidizing peroxidases. Therefore, this study aimed to create the dynamics profile of Mn-oxidizing peroxidases activities in four G. lucidum strains, classifying and determining their properties depending on the cultivation type and plant residue as a carbon source in the medium, as well as to establish whether intraspecific variety exists. The findings suggest that submerged cultivation appeared to be a more appropriate cultivation type for enzyme activities compared with solid-state cultivation, and oak sawdust was a better carbon source than wheat straw. Under the optimum conditions, on day 14, G. lucidum BEOFB 431 was characterized by the highest levels of both Mn-dependent and Mn-independent peroxidase activities (4795.5 and 5170.5 U/L, respectively). Strain, cultivation type, and carbon source were factors that affected the profiles of Mn-oxidizing peroxidases isoenzymes.
Download PDF
Full Article
Activity of Mn-Oxidizing Peroxidases of Ganoderma lucidum Depending on Cultivation Conditions
Jasmina Ćilerdžić,* Mirjana Stajić, and Jelena Vukojević
Trunks and stumps of various deciduous species act as natural habitats for Ganoderma lucidum. The chemical composition of their cell wall affects the development of fungal ligninolytic enzyme system as well as its ability to degrade lignin from the plant cell wall. Additionally, numerous compounds structurally similar to lignin can be degraded by the G. lucidum enzyme system which could take important roles in various biotechnological processes. The laccases, which are the dominant enzymes synthesized by G. lucidum, have been studied more extensively than the Mn-oxidizing peroxidases. Therefore, this study aimed to create the dynamics profile of Mn-oxidizing peroxidases activities in four G. lucidum strains, classifying and determining their properties depending on the cultivation type and plant residue as a carbon source in the medium, as well as to establish whether intraspecific variety exists. The findings suggest that submerged cultivation appeared to be a more appropriate cultivation type for enzyme activities compared with solid-state cultivation, and oak sawdust was a better carbon source than wheat straw. Under the optimum conditions, on day 14, G. lucidum BEOFB 431 was characterized by the highest levels of both Mn-dependent and Mn-independent peroxidase activities (4795.5 and 5170.5 U/L, respectively). Strain, cultivation type, and carbon source were factors that affected the profiles of Mn-oxidizing peroxidases isoenzymes.
Keywords: Fermentation; Ganoderma lucidum; Mn–dependent peroxidases; Mn–independent peroxidases; Oak sawdust; Wheat straw
Contact information: University of Belgrade, Faculty of Biology, Takovska 43, 11000 Belgrade, Serbia; *Corresponding author: simonicj@bio.bg.ac.rs
INTRODUCTION
In addition to its well-documented health benefits, Ganoderma lucidum is attracting increasing attention because of its ligninolytic potential (Silva et al. 2005). Because of the type of the substrate on which this species naturally develops (trunks and stumps of oak, maple, elm, willow, etc.), its lignocellulolytic enzyme system is well-developed and thus capable of degrading plant cell wall components, classifying it in the group of white rot fungi (Hammel 1997; Kersten and Cullen 2007). Ligninolytic enzymes are characterized by a broad substrate specificity such that they can depolymerize both lignin and numerous structurally similar organic compounds, which opens up the possibility for applications in various biotechnological processes (Baldrian 2008; Janusz et al. 2013).
Lignocellulosic biomass, generated as a by-product of expanding agricultural and industrial production, is the most abundant potential renewable energy and fiber source in the world (Sánchez 2009; Alvira et al. 2010). Although this resource could be used in the production of food, feed, paper, and energy production, only a small fraction (3%) of the available lignocellulosic biomass is used, and the rest remains as environmental ballast because of relatively low degradation rates (Zechendorf 1999; Pahkala and Pihala 2000; Tabka et al. 2006; Yusoff 2006; Rodriguez et al. 2008; Dias et al. 2010). Wheat straw is one of the most abundant and cheapest crop residues capable of being used as a substrate for the commercial cultivation of numerous mushrooms. It is a good substrate for obtaining nutritionally valued fruiting bodies and can be utilized in the production of high-quality and more digestible animal feed (Villas-Bôas et al. 2002; Dias et al. 2010; Shrivastava et al. 2012; Ćilerdžić et al. 2014a). Lignocellulosic wastes could also be an excellent resource for paper pulp and biofuel productions, leading to the protection of autochthonous forests, reduction of fossil fuels usage and CO2 emissions, and production of many economic benefits (González et al. 2009; Sánchez 2009; Stajić et al. 2009; Nigam and Singh 2011). The delignification of lignocellulosics is a crucial step in the process. Considering that removing lignin via physical and chemical methods is expensive, inefficient, and not environmentally friendly, white rot fungi and their ligninolytic enzymes have attracted the special attention of scientists and technologists.
Ganoderma lucidum (Curt.: Fr.) Karst. synthesizes laccases (EC 1.10.3.2), Mn-oxidizing peroxidases (Mn-dependent peroxidase (EC 1.11.1.13; MnP) and Mn-independent peroxidase (EC 1.11.1.6; MnIP)), and lignin peroxidases (EC 1.11.1.14) (D’Souza et al. 1996; 1999; Ko et al. 2001; Silva et al. 2005; Stajić et al. 2010). In previous studies, laccases were the main ligninolytic enzymes studied because they are the dominant, and sometimes the solely produced, enzymes (D’Souza et al. 1999; Ko et al. 2001; Ćilerdžić et al. 2014b). On the contrary, Mn-oxidizing peroxidases of the same species have not been sufficiently researched, despite their high potential in applications such as biopulping, biobleaching, and bioremediation (Janusz et al. 2013). Therefore, the present study aimed to create the dynamics profile of MnP and MnIP activities in G. lucidum and determine their properties depending on cultivation type and plant residue as a carbon source in the medium, as well as to establish if an intraspecific variety exists.
EXPERIMENTAL
Materials
The cultures of studied G. lucidum strains were obtained from fruiting bodies, originating from Serbia, Montenegro, and China (Table 1). The cultures were maintained on a malt agar medium in the culture collection of the Institute of Botany, Faculty of Biology, University of Belgrade, Serbia (BEOFB).
Table 1. Studied Ganoderma lucidum Strains

Methods
Growth conditions
The inoculum was prepared by inoculating 100.0 mL of the synthetic medium (glucose, 10.0 g/L; NH4NO3, 2.0 g/L; K2HPO4, 1.0 g/L; NaH2PO4·H2O, 0.4 g/L; MgSO4·7H2O, 0.5 g/L; yeast extract, 2.0 g/L; pH 6.5) with 25 mycelial discs (Ø 0.5 cm, using a 7-day-old culture from malt agar). The incubation was conducted at room temperature (22 ± 2 °C) on a rotary shaker (160 rpm) for seven days. Sterile distilled water (dH2O) was used to wash the obtained biomass (three times) and biomass homogenization was conducted with 100.0 mL of sterile dH2O in a laboratory blender.
Solid-state cultivation was carried out at 25 °C in 100-mL flasks, containing 5.0 g of wheat straw or oak sawdust as the carbon source and 10.0 mL of the modified synthetic medium (without glucose). Submerged fermentation of the ground tested plant raw materials (5.0 g) was carried out in 250-mL flasks, with 50.0 mL of the modified synthetic medium, on a rotary shaker and at room temperature. The suspension obtained after inoculum homogenization was used for medium inoculation (3.0 mL per flask for solid-state and 5.0 mL for submerged fermentation).
Assays of enzyme activity and total protein production
Samples from the flasks were harvested after 7, 10, and 14 days of cultivation, with the aim of following the dynamics of the enzyme’s activity. The extracellular enzymes produced during solid-state cultivation were extracted by stirring the samples containing 50.0 mL dH2O with a magnetic stirrer at 4 °C for 10 min, and the extracts were separated by centrifugation (at 4 °C and 5000 rpm, for 15 min). After submerged cultivation, the samples were prepared by centrifugation. The obtained supernatants were used for the determination of Mn-oxidizing peroxidase activities and total protein content spectrophotometrically (BioQuest CECIL CE2501, UK).
The Mn-oxidizing peroxidases activities were determined according to methods used by Stajić et al. (2006), who used 3mM phenol red (ε610 = 22 000 M-1cm-1) as a substrate in a buffer composed of succinic acid disodium salt, bovine serum albumin, and DL-lactic acid sodium salt (pH 4.5). The reaction mixture (Vtot = 1 mL) contained: buffer, sample, 2 mM H2O2, phenol red, and 2 mM MnSO4 (for MnP and MnIP, respectively). The reaction was stopped by 2 M NaOH.
Enzymatic activity of 1 U was defined as the amount of enzyme that transforms 1 μmol of substrate per min. The amount of total proteins was determined according to the Bradford method (1976), which utilizes bovine serum albumin as the standard. The reaction mixture was composed of Coomassie brilliant blue G-250 (CBB), the sample, and distilled water, and its absorbance was measured at 595 nm (Silva et al. 2005). The total protein content (mg/mL) was used to determine the specific enzyme activity (U/mg), an indicator of the enzyme purity.
The assays were carried out as three replicates, and the results are expressed as mean ± standard error.
Electrophoresis
Screening of the MnP and MnIP isoforms was performed for all analyzed G. lucidum strains by cultivation under the previously defined and optimum conditions (Ćilerdžić et al. 2014b). Tests were also conducted to determine the best producer of the enzymes under all cultivation conditions.
Mn–oxidizing peroxidases isoforms and their isoelectric points (pI) were determined by isoelectric focusing (IEF) using a Mini IEF cell 111 (Bio-Rad, USA). The IEF was carried out in a 7.5% polyacrylamide gel with 5% ampholyte on a pH gradient of 3.0 to 10.0. Bands with Mn–oxidizing peroxidases activities were located via incubation of the gel in a mixture composed of 10% 4-chloro-naphthol, 0.03% H2O2, and 100 mM K-phosphate buffer (pH 6.5), with or without 100 mM MnSO4. After completion of focusing, the gel was fixed in trichloroacetic acid, and protein bands were detected by staining using CBB. An IEF marker of the pI range from 3.6 to 9.3 (Sigma-Aldrich, USA) was used.
RESULTS AND DISCUSSION
The activities of Mn-oxidizing peroxidases were detected after both submerged and solid-state cultivation on tested plant residues for all measurement points was conducted, excluding day 14 of wheat straw solid-state fermentation by G. lucidum BEOFB 431 (Figs. 1, 2). Generally, submerged cultivation appeared as the more appropriate cultivation type for MnP and MnIP activities compared with solid-state cultivation, and oak sawdust was a better carbon source than wheat straw.
During wheat straw solid-state fermentation, MnP activity was uniform, except in strain BEOFB 431, where no activity was detected on day 14, and in strain BEOFB 432, where extremely high activity was noted on day 7 (5602.5 ± 362.0 U/L). The activity decreased throughtout the remaining duration of the cultivation period and reached the minimum on day 14 (281.5 ± 2.0 U/L). However, during submerged fermentation, the same substrate activity level was generally increased, and the maximum was noted in strain BEOFB 434 on day 14 (3327.0 ± 25.5 U/L). Comparison of wheat straw fermentation types showed that submerged conditions were more favorable for all studied strains except BEOFB 432 (Fig. 1).
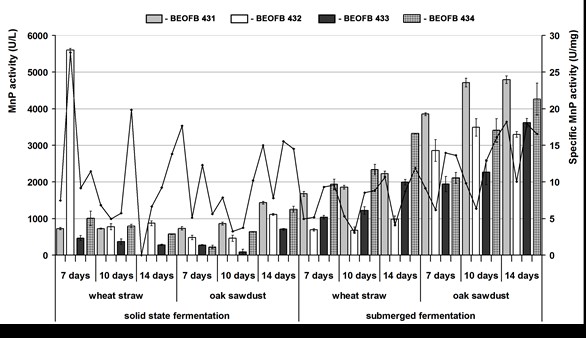
Fig. 1. Mn-dependent peroxidase activity of G. lucidum strains, depending on type and period of fermentation of tested plant raw materials
A gradual increase in MnP activity during oak sawdust solid-state fermentation was obtained for all tested strains, except BEOFB 433, where reduction of the activity was noted on day 10 (88.0 ± 2.5 U/L). Under these cultivation conditions, the maximum was observed after 14 days of fermentation for strain BEOFB 431 (1433.5 ± 82.0 U/L). On the other hand, the obtained results showed that submerged cultivation in oak sawdust medium was noticeably better for MnP activity for all strains, excluding strain BEOFB 432. Namely, G. lucidum BEOFB 431 stood out, with the highest activity levels for all measurement points, exhibiting a peak of 4795.5 ± 4.5 U/L on day 14. Meanwhile, BEOFB 433 showed the lowest values and a minimum of 1946.0 ± 77.0 U/L on day 7. This demonstrated that the maximum was five times higher than that obtained for solid-state fermentation. Additionally, the minimum was higher than the maximum noted for solid-state fermentation (Fig. 1).
In the case of MnIP, the picture was similar, i.e., submerged cultivation was the optimum type, oak sawdust was a more favorable carbon source for the activity, and the noted minimum level was still higher than the maximum observed during solid-state cultivation (Fig. 2). Both the maximum and minimum activities were measured after oak sawdust fermentation, but a maximum of 5170.5 ± 106.5 U/L was noted on day 14 after submerged cultivation for strain BEOFB 431 and the minimum (240.5 ± 1.9 U/L) after 7-day old solid-state cultivation of BEOFB 433. In the case of wheat straw, submerged fermentation was also better, with an activity peak of 3366.5 ± 7.0 U/L for strain BEOFB 434 (on day 14), which was aproximately 3.5 times higher than the maximum obtained on day 7 of solid-state cultivation of the same strain.
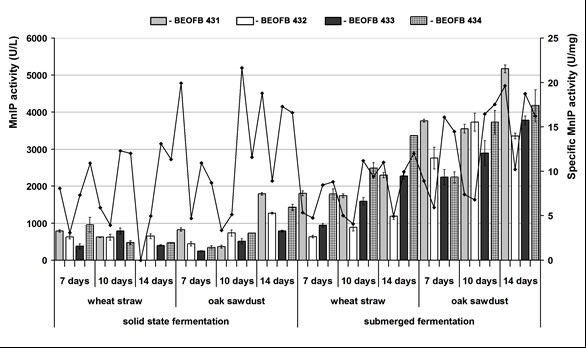
Fig. 2. Mn-independent peroxidase activity of G. lucidum strains depending on type and period of fermentation of tested plant raw materials
The production of total proteins was also higher during the submerged cultivation of the strains. Thus, the maximum concentration was noted after 10 days of oak sawdust submerged fermentation by G. lucidum BEOFB 432 (548.0 ± 6.37 mg/mL), and the minimum of 22.0 ± 2.32 mg/mL after solid-state cultivation of BEOFB 433 on the same substrate. This profile of protein production reflected specific Mn-oxidizing peroxidases activities (Figs. 1, 2).
The position and intensity of Mn-oxidizing peroxidases isoforms were different in tested G. lucidum strains and depended on plant residues. Thus, after 14 days of oak-sawdust submerged fermentation, one acidic MnP isoform (pI 3.7) was detected in all studied strains (Fig. 3A). In the same fermentation of wheat straw by strains BEOFB 431, 433, and 434, one strong (pI 3.7) and one weak band (pI 3.6) were observed. Meanwhile, in strain BEOFB 432, only one weak band of pI 3.7 was vizualized (Fig. 3A). Two acidic MnIP isoforms (pI 3.6 and 3.7) were detected after 14 days of submerged fermentation for both plant raw materials in strain BEOFB 431, and only one (pI 3.8) for the other tested strains (Fig. 3B).
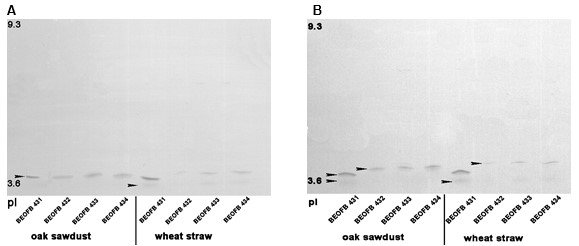
Fig. 3. Isoelectric focusing pattern of (A) Mn-dependent peroxidases and (B) Mn–independent peroxidases in studied G. lucidum strains after 14 days of submerged fermentation of tested plant raw materials
The profiles of MnP and MnIP isoforms in G. lucidum BEOFB 431, where enzyme activities were the highest, differed during the cultivation period depending on the cultivation type (Fig. 4).

Fig. 4. Isoelectric focusing profiles of (A) Mn-dependent peroxidases and (B) Mn–independent peroxidases in G. lucidum BEOFB 431, depending on the type and period of fermentation of tested plant raw materials
After 7 and 10 days of solid-state cultivation on oak sawdust-enriched medium, two acidic MnP isoforms (pI 3.6 and 3.8) were detected, while on day 14, only one band (pI 3.8) was visualized (Fig. 4A). During the solid-state fermentation of wheat straw, G. lucidum BEOFB 431 synthetized one MnP isoenzyme at all measurement points. The difference in pI was notable: isoforms produced after 7 days of cultivation had a pI of 3.7, and after 10 and 14 days, the pI was equal to 3.8. Likewise, one MnP band of pI 3.8 was visualized on all measurement points during submerged fermentation for both plant residues (Fig. 4A). During the first seven days of solid-state fermentation for both plant residues, G. lucidum BEOFB 431 synthetized two MnIP isoforms (with pI levels ranging from 3.6 to 3.8), while at other measurement points, only one band was visualized.
This study clearly demonstrated the great potential of G. lucidum to synthesize Mn-oxidizing peroxidases. Contrary to the data found by Elisashvili et al. (2009), in which numerous fungal species produced more active MnP during solid-state lignocellulose fermentation, results obtained for the selected G. lucidum strains showed that submerged conditions were optimal for the synthesis and related activities of Mn-oxidizing peroxidases. This can primarily be explained by the higher availability of soluble carbohydrates, aromatic compounds, and microelements, which act as promoters of the production of enzymes. The fact that more active enzymes were obtained in the submerged fermentation of abundant lignocellulosic wastes could have special significance for their pretreatment. For example, it is extremely enticing to have the possibility of more effective and rapid production of the enzymes under strictly controlled conditions. Numerous studies have demonstrated that the chemical composition of plant residue is an important factor that influences the production and properties of ligninolytic enzymes, and consequently the effectiveness of the ligninolysis process. For example, D’Souza et al. (1999) and Songulashvili et al. (2007) reported that G. lucidum synthetized more active MnP during the submerged fermentation of poplar sawdust, wheat, soy brans, and tangerine peels than pine sawdust, corn bran, kiwi, and banana peels. Based on the results, it can be concluded that markedly different effects can be obtained when using wheat straw and oak sawdust for Mn-oxidizing peroxidases activities because of their chemical compositions (Nishimura and Matsuyama 1989; Rakić et al. 2006).
If different capacities of species and strains (to produce active enzymes) are added to the effects of cultivation conditions, a complete picture of delignification regulation can be obtained. The existence of significant inter- and intraspecific variabilities in ligninolytic enzymes activities within the generas Pleurotus, Ganoderma, Cerrena, and Trametes have been demonstrated in several studies (Camarero et al. 1996; Stajić et al. 2004; Silva et al. 2005; Songulashvili et al. 2007; Simonić et al. 2010; Ćilerdžić et al. 2014b). However, cultivation types, plant wastes, plant species, and strain characteristics also affect the fungal profiles Mn-oxidizing peroxidases. In laymen’s terms, the number of MnP and MnIP isoforms vary depending on conditions of fermentation of certain lignocellulosic materials (Coelho et al. 2010; Simonić et al. 2010; Knežević et al. 2013).
According to the aforementioned information, it can be concluded that selected G. lucidum strains (especially strain BEOFB 431) are effective producers of active Mn-oxidizing peroxidases, which could potentially be used in the production of fungal biomass and ligninolytic enzymes. Additionally, the bioconversion of various abundant residues into fiber and energy could result, as well as the bioremediation of polluted soil and water.
CONCLUSIONS
- Submerged cultivation was preferable for Mn-oxidizing peroxidases activity compared to solid-state cultivation.
- Oak sawdust was a better carbon source than wheat straw for Mn-oxidizing peroxidases activity.
- Mn-oxidizing peroxidases properties exhibited intraspecific diversity within Ganoderma lucidum.
- The profiles of MnP and MnIP isoforms depended on both the cultivation conditions and strain.
ACKNOWLEDGEMENTS
This study was conducted with the financial support of the Ministry of Education, Science, and Technological Development of the Republic of Serbia, Project No. 173032.
REFERENCES CITED
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresource Technol. 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093.
Baldrian, P. (2008). “Wood-inhabiting ligninolytic basidiomycetes in soils: Ecology and constraints for applicability in bioremediation,” Fungal Ecol. 1(1), 4-12. DOI: 10.1016/j.funeco.2008.02.001.
Bradford, M. M. (1976). “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding,” Analyt. Biochem. 72, 248–254.
Camarero, S., Böckle, B., Martínez, J. M., and Martínez, T. A. (1996). “Manganese-mediated lignin degradation by Pleurotus pulmonarius,” Appl. Environ. Microb. 62(3), 1070-1072.
Coelho, J. S., Oliveira, L., Souza, C. G. M., Bracht, A., and Peralta, R. M. (2010). “Effect of the herbicides bentazon and diuron on the production of ligninolytic enzymes by Ganoderma lucidum,” Int. Biodeter. Biodegr. 64(2), 156-161. DOI: 10.1016/j.ibiod.2009.12.006
Ćilerdžić, J., Vukojević, J., Stajić, M., Stanojković, T., and Glamočlija, J. (2014a). “Biological activity of Ganoderma lucidum basidiocarps cultivated on alternative and commercial substrate,” J. Ethnopharmacol. 155(1), 312-319. DOI: 10.1016/j.jep.2014.05.036.
Ćilerdžić, J., Vukojević, J., Stajić, M., and Lončar, N. (2014b). “Intraspecific diversity in the production and characterization of laccase within Ganoderma lucidum,” BioResources 9(3), 5577-5587. DOI: 10.15377/biores.9.3.5577-5587
D’Souza, T. M., Boominathan, K., and Reddy, C. A. (1996). “Isolation of laccase gene–specific sequences from white rot and brown fungi by PCR,” Appl. Environ. Microb. 62(10), 3739-3744.
D’Souza, T. M., Merritt, C. S., and Reddy, C. A. (1999). “Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum,” Appl. Environ. Microb. 65(12), 5307-5313.
Dias, A. A., Freitas, G. S., Marques, G. S. M., Sampaio, A., Fraga, I. S., Rodrigues, M. A. M., Evtugin, D. V., and Bezerra, R. M. F. (2010). “Enzymatic saccharification of biologically pre-treated wheat straw with white-rot fungi,” Bioresource Technol. 101(15), 6045-6050. DOI: 10.1016/j.biortech.2010.02.110.
Elisashvili, V., Kachlishvili, E., Tsiklauri, N., Metreveli, E., Khardziani, T., and Agathos, S. N. (2009). “Lignocellulose-degrading enzyme production by white-rot Basidiomycetes isolated from the forests of Georgia,” World J. Microb. Biot. 25(2), 331-339. DOI: 0.1007/s11274-008-9897-x.
González, A. M., Tejado, A., Blanco, M., Mondragon, I., and Labidi, J. (2009). “Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process,” Chem. Eng. J. 148(1), 106-114. DOI: 10.1016/j.cej.2008.08.008
Hammel, E. H. (1997). “Fungal degradation of lignin,” in: Driven by Nature: Plant Litter Quality and Decomposition, Cadisch, G., and Giller, K. E. (eds.), CAB International, UK, pp. 33-45.
Janusz, G., Kucharzyk, K. H., Pawlik, A., and Staszczak, M. (2013). “Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation,” Enzyme Microb. Tech. 52(1), 1-12. DOI: 10.1016/j.enzmctec.2012.10.003.
Kersten, P. and Cullen, D. (2007). “Extracellular oxidative systems of the lignin-degrading basidiomycete Phanerochaete chrysosporium,” Fungal Genet. Biol. 44(2), 77-87. DOI: 10.1016/j.fgb.2006.07.007
Knežević, A., Milovanović, I., Stajić, M., Lončar, N., Brčeski, I., Vukojević, J., and Ćilerdžić, J. (2013). “Lignin degradation by selected fungal species,” Bioresource Technol. 138, 117-123. DOI: 10.1016/j.biortech.2013.03.182.
Ko, E. M., Leem, Y. E., and Choi, H. T. (2001). “Purification and characterization of laccase isozymes from the white–rot basidiomycete Ganoderma lucidum,” Appl. Microbiol. Biot. 57(1-2), 98-102.
Nigam, P. S., and Singh, A. (2011). “Production of liquid biofuels from renewable resources,” Prog. Energ. Combust. 37(1), 52-68. DOI: 10.1016/j.pecs.2010.01.003.
Nishimura, K., and Matsuyama, R. (1989). “Maturation and maturation chemistry,” in: The Science and Technology of Whiskey, Piggot, J. R., Sharp, R., and Duncan, R. E. B. (eds.), Longman, New York, pp. 235.263.
Pahkala, K. and Pihala, M. (2000). “Different plant parts as raw material for fuel and pulp production,” Ind. Crops Prod. 11(2-3), 119-128. DOI: 10.1016/S0926-6690(99)00050-3.
Rakić, S., Povrenović, D., Tešević, V., Simić, M., and Maletić, R. (2006). “Oak acorn, polyphenols and antioxidant activity in functional food,” J. Food Eng. 74(3), 416-423. DOI: 10.1016/j.jfoodeng.2005.03.057.
Rodriguez, A., Serrano, L., Moral, A., Pérez, A., and Jiménez, L. (2008). “Use of high-boiling point organic solvents for pulping oil palm empty fruit bunches,” Bioresource Technol. 99(6), 1743-1749. DOI: 10.1016/j.biortech.2007.03.050.
Sánchez, C. (2009). “Lignocellulosic residues: Biodegradation and bioconversion by fungi,” Biotechnol. Adv. 27(2), 185-194. DOI: 10.1016/j.biotechadv.2008.11.001.
Shrivastava, B., Nandal, P., Sharma, A., Jain, K. K., Khasa, Y. P., Das, T. K., Mani, V., Kewalramani, N. J., Kundu, S. S., and Kuhad, R. C. (2012). “Solid state bioconversion of wheat straw into digestible and nutritive ruminant feed by Ganoderma sp. rckk02,” Bioresource Technol. 107(1), 347-351. DOI: 10.1016/j.biortech.2011.12.096.
Silva, C. M. M. S., Melo, S. I., and Oliveira, R. P. (2005). “Ligninolytic enzyme production by Ganoderma spp.,” Enzyme Microb. Tech. 37(3), 324-329. DOI: 10.1016/j.enzmictec.2004.12.007
Simonić, J., Vukojević, J., Stajić, M., and Glamočlija, J. (2010). “Intraspecific diversity within Ganoderma lucidum in the production of laccase and Mn-oxidizing peroxidases during plant residues fermentation,” Appl. Biochem. Biotech. 162(2), 408-415. DOI: 10.1007/s12010-009-8833-3.
Songulashvili, G., Elisashvili, V., Wasser, S. P., Nevo, E., and Hadar, Y. (2007). “Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes,” Enzyme Microb. Tech. 41(1-2), 57-61. DOI: 10.1016/j.enzmictec.2006.11.024.
Stajić, M., Persky, L., Cohen, E., Hadar, Y., Brčeski, I., Wasser, S. P., and Nevo, E. (2004). “Screening of the laccase, manganese peroxidase, and versatile peroxidase activities of the genus Pleurotus in media with some raw plant materials as carbon sources,” Appl. Biochem. Biotech. 117(3), 15-164. DOI: 10.1385/ABAB:117:3:155
Stajić, M., Persky, L., Friesem, D., Hadar, Y., Wasser, S. P., Nevo, E., and Vukojević, J. (2006). “Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species,” Enzyme Microb. Tech. 38(1-2), 65-73. DOI: 10.1016/j.enzmictec.2005.03.026
Stajić, M., Vukojević, J., and Duletić-Laušević, S. (2009). “Biology of Pleurotus eryngii and role in biotechnological processes: A review,” Crit. Rev. Biotechn. 29(1), 55-66.
Stajić, M., Kukavica, B., Vukojević, J., Simonić, J., Veljović-Jovanović, S., and Duletić-Laušević, S. (2010). “Wheat straw conversion by enzymatic system of Ganoderma lucidum,” BioResources 5(4), 2362-2373. DOI: 10.15376/biores.5.4.2362-2373
Tabka, M. G., Herpoel-Gimbert, I., Monod, F., Asther, M., and Sigoillot, J. C. (2006). “Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment,” Enzyme Microb. Tech. 39(4), 897-902. DOI: 10.1016/j.enzmictec.2006.01.021
Villas-Bôas, S. G., Esposito, E., and Mitchell, D. A. (2002). “Microbial conversion of lignocellulosic residues for production of animal feeds,” Anim. Feed Sci. Technol. 98(1), 1-12. DOI: 10.1016/S0377-8401(02)00017-2
Yusoff, S. (2006). “Renewable energy from palm oil – Innovation on effective utilization of waste,” J. Clean. Prod. 14(1), 87-93. DOI: 10.1016/j.jclepro.2004.07.005
Zechendorf, B. (1999). “Sustainable development: How can biotechnology contribute?” Trends Biotechnol. 17(6), 219-225. DOI: 10.1016/S0167-7799(98)01297-9
Article submitted: August 3, 2015; Peer review completed: October 16, 2015; Revised version received: October 19, 2015; Accepted: October 20, 2015; Published: November 9, 2015.
DOI: 10.15376/biores.11.1.95-104
