Abstract
shell (), a abundant and inexpensive material, is currently being investigated as an adsorbent to remove cobalt(II) from water. Before the adsorption experiments, QCS was subjected to chemical treatment to provide maximum surface area. Then, the kinetics and adsorption mechanism of Co(II) ions on QCS were studied using different parameters such as adsorbent dosage, initial concentration, temperature, contact time, and solution pH. The loaded metals could be desorbed effectively with dilute hydrochloric acid, nitric acid, and 0.1 M EDTA. The Langmuir and Freundlich models were used to describe the uptake of cobalt on QCS. The equilibrium adsorption data were better fitted to Langmuir adsorption isotherm model. The maximum adsorption capacity (qm) of QCS for Co(II) was 33 mg g-1. Various kinetic models were used to describe the adsorption process. The adsorption followed pseudo second-order kinetic model. The intraparticle diffusion was found to be the rate-limiting step in the adsorption process. The diffusion coefficients were calculated and found to be in the range of 3.11×10−6 to 168.78×10−6 cm2s-1. The negative DH* value indicated exothermic nature of the adsorption.
Download PDF
Full Article
ADSORPTION CHARACTERIZATION OF Co(II) IONS ONTO CHEMICALLY TREATED QUERCUS COCCIFERA SHELL: EQUILIBRIUM, KINETIC, AND THERMODYNAMIC STUDIES
M. Hamdi Karaoğlu,* Mehmet Uğurlu, and İbrahim Kula
Quercus coccifera shell (QCS), a relatively abundant and inexpensive material, is currently being investigated as an adsorbent to remove cobalt(II) from water. Before the adsorption experiments, QCS was subjected to chemical treatment to provide maximum surface area. Then, the kinetics and adsorption mechanism of Co(II) ions on QCS were studied using different parameters such as adsorbent dosage, initial concentration, temperature, contact time, and solution pH. The loaded metals could be desorbed effectively with dilute hydrochloric acid, nitric acid, and 0.1 M EDTA. The Langmuir and Freundlich models were used to describe the uptake of cobalt on QCS. The equilibrium adsorption data were better fitted to Langmuir adsorption isotherm model. The maximum adsorption capacity (qm) of QCS for Co(II) was 33 mg g-1. Various kinetic models were used to describe the adsorption process. The adsorption followed pseudo second-order kinetic model. The intraparticle diffusion was found to be the rate-limiting step in the adsorption process. The diffusion coefficients were calculated and found to be in the range of 3.11×10−6 to 168.78×10−6 cm2s-1. The negative H* value indicated exothermic nature of the adsorption.
Keywords: Adsorption; Divalent cobalt; Quercus coccifera shell; Desorption; Kinetics
Contact information: Department of Chemistry, Faculty of Science, Muğla University, Muğla 48000, Turkey, Corresponding author: fahamdi1972@hotmail.com
INTRODUCTION
Environmental pollution by toxic metals arises as a result of many activities, largely industrial, although sources such as agriculture and sewage disposal also contribute (Matheickal and Yu 1997; Karaoğlu et al. 2010a; Sarı et al. 2007). Cobalt-containing compounds are widely used in many industrial applications such as mining, metallurgical, electroplating, paints, pigments, and electronics (Bhatnagar et al. 2010). Cobalt is also present in the wastewater of nuclear power plants. The increased use of Co(II) in nuclear power plants and in many industries such as mining, metallurgical, electroplating, paints, pigments, and electronic industries has resulted in Co(II) finding its way into natural bodies of water. The tolerance limit for Co(II) in potable water has been fixed as 0.05 mg L−1 (Manohar et al. 2006). Its high doses cause paralysis, diarrhoea, low blood pressure, lung irritations, and bone defects.
With a better awareness of the problems associated with cobalt, research studies related to the methods of removing cobalt from wastewater have drawn attention increasingly (Rengaraj and Moon 2002). In this context, the recovery of heavy metals from the wastewater is a major topic in water research, and several methods have been commonly used for this purpose (chemical precipitation, electrochemical reduction, evaporation, reverse osmosis, membrane filtration, co-precipitation, electrodialysis, adsorption, biosorption, etc.) (Gupta et al. 2005; Gupta and Rastogi 2009; Sarı and Tuzen 2008a). Precipitation, ion exchange, solvent extraction, and adsorption on oxides are the conventional methods for the removal of heavy metal ions from aqueous solutions, but due to high maintenance cost these methods do not suit the needs of developing countries (Karaoğlu et al. 2010a; Uluozlu et al. 2008).
In this study, the biosorbent Quercus coccifera shell (QCS) was used as a biosorbent due to its low cost and high efficiency. Quercus coccifera trees are abundant in the Mediterranean countries, and both this tree and its shell are usually burnt. To the best of our knowledge, this material was not used before for this kind of application. Utilization of Quercus coccifera shell not only provides a low cost and easily available sorbent for the removal of heavy metals such as Co(II), but also contributes to the prevention of environmental pollution.
EXPERIMENTAL
Reagents
All reagents used in present study were of analytical grade. CoCl26H2O, concentrated HCl, concentrated HNO3, and NaOH were obtained from Merck (Germany), while Co(II) atomic absorption spectrometer standard solution (1000 mgL-1) was purchased from GBC Company chemicals (Australia). All glassware and polypropylene flasks used were overnight immersed in 10% (v/v) HNO3 and rinsed several times with ultrapure water (18.3 mohm). For adjusting the pH of the medium 0.1N solutions of NaOH and HCl were used.
Preparation of Formaldehyde-Treated QCS
QCS was collected from a local Quercus coccifera tree near Muğla, Turkey and washed repeatedly with deionized water to remove the water-soluble impurities and other surface adhered particles. For treatment with formaldehyde (FA) (Carlo Erba, Italy) to obtain FA-treated biomass, a mixture of 17 mL formalin 30% and 33 mL HCl 0.1 M was added to 2.5 g of smoothly crushed biomass. The mixture was left at room temperature with gentle mixing. After 1 h, the biomass was filtered and washed with distilled water. Subsequently, the biosorbent was incubated with 50 mL sodium carbonate solution (0.2 M) for 15 min, filtered, washed with distilled water and dried overnight at 80 °C (Jalali-Rad et al. 2004; Ebrahimi et al. 2009). Dried powder was crushed in a rotary crusher and sieved with 355 μm sieve (Retsch, Germany; No: 45) and stored in desiccator.
Batch Adsorption Studies
A batch equilibrium method was used to determine the sorption of cobalt by QCS. A set of 250 mL Erlenmeyer flasks containing 50 mL of metal solution was used in the experiments. QCS (0.2 g) was contacted with the metal solutions (50 mL) for 90 minutes
on an incubator shaker (Zhwy-200D, South Korea) at 175 rpm at room temperature (25 °C). The contents of the flasks were filtered, and the filtrates were analyzed for residual metal concentration using a GBC atomic absorption spectrometer (GBC Avanta, Australia) with deuterium background corrector. All measurements were carried out in an air/acetylene flame. The operating parameters for the working element were set as recommended by the manufacturer. WTW model pH-meter (Germany) equipped with a combination pH electrode was used to measure the pH of all solutions. All the chemicals were of analytical grade. Reference solutions were prepared as required by further dilution with ultrapure water.
Desorption Experiment
Desorption studies were carried out with hydrochloric acid, nitric acid, and ethylenedinitrilotetraacetic acid disodium salt (Na2EDTA, C10H14N2Na2O82H2O) (Merck, Germany). These desorption solutions were adjusted to a molarity of 0.1 M. To determine the most effective desorption solution, 0.2 g of QCS and 50 mL of desorption solution (without any pH adjustment) was kept in contact in an incubator shaker (Zhwy-200D) at 175 rpm and 25 °C for 90 min. The mixture was filtered after desorption, and the filtrate was analyzed for the residual Co(II) concentration by Flame Atomic Absorption Spectrophotometry (FAAS).
RESULTS AND DISCUSSION
Characterization of QCS
FT-IR spectra for QCS in its natural and chemically treated forms are presented in Fig. 1. A strong peak at 3330 cm−1 represents the –OH stretching of the phenol group of cellulose and lignin (Karaoğlu et al. 2010a). A strong and sharp band at 2921 cm−1 is attributed to the C–H stretching vibration from CH2 group of cellulose and hemicellulose. The peak at 1731 cm−1 is due to C–O stretching of carbonyl groups (>C=O) in hemicellulose (Weng et al. 2009).
The peaks at 1608 and 1505 cm− 1 are the indication of C=C aromatic stretch vibration (Senturk et al. 2010). The band in the 1233 cm−1 is due to the bending modes of O–C–H, C–C–H, and C–O–H. The band at 1033 cm−1 was assigned to C–O stretching, which also confirmed the presence of lignin on the QCS (Pavan et al. 2008). The FT-IR spectrum of chemically treated QCS indicates that the peaks, due to the above functional groups, were affected in their position and intensity.
QCS used in this study was analyzed by scanning electron microscopy (SEM) in order to examine its morphology. SEM image of porous surface of natural and chemically treated biomass is illustrated in Fig. 2. The image also reveals that the external surface was full of cavities, which suggest that chemically treated QCS material exhibits a high surface area.
Similar morphology was already seen for a treated olive stone with sodium hydroxide (Aziz et al. 2009).
| Fig. 1. FTIR spectra of (a) raw QCS and (b) chemically treated QCS |
Fig. 2. SEM micrographs of (a) raw QCS; (b) chemically treated QCS
Adsorption Experiments
Adsorption of Co(II) onto QCS was systematically investigated by parameters such as adsorbent dosage, initial concentration, temperature, contact time, and natural pH. The experimental results and the relevant observations are discussed in the following sections.
Effect of initial concentration
The metal uptake mechanism was especially dependent on the initial heavy metal concentration (Co), and at low concentrations metals were adsorbed by specific sites. Nevertheless, by increasing metal concentrations the specific sites become saturated (Saeed et al. 2005). The Co(II) biosorption capacity of QCS is presented as a function of metal ion concentration in Fig. 3. Initial concentrations of Co(II) ions were studied by varying the concentrations from 25 to 100 mg L−1. The amount of Co(II) ions adsorbed per unit mass of the biosorbent increased with the increase in initial concentration of metal ions. The maximum amount of Co(II) adsorbed on this biosorbent was 17.72 mg g−1.
Effect of adsorbents dosage
The influence of QCS dosage on cobalt biosorption was examined by varying dosages from 1.0 to 4.0 gL-1 (Fig. 4). The optimum biosorbent dose selected was 4.0 gL-1 for the rest of the experimental studies. From the analysis of experimental data obtained for cobalt ion, it was shown that the removal efficiency increased with the increase in biosorbent dosage. An increase in biomass concentration generally increases the biosorbed metal ions because of an increase in surface area of the biosorbent, which in turn increases the binding sites (Vijayaraghavan et al. 2006).
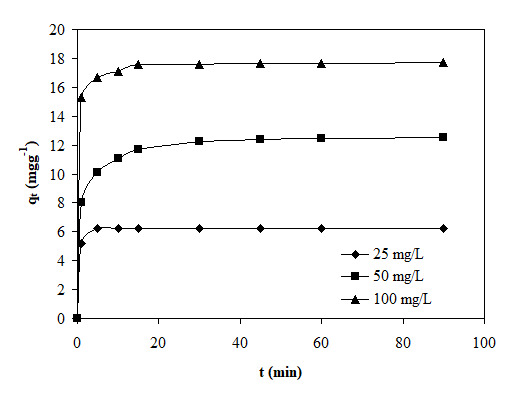
Fig. 3. Influence of initial concentration on cobalt biosorption by chemically treated QCS (biosorbent dossage: 4 g/L; pH: natural; temperature: 298 K; contact time: 90 min).
Effect of pH on adsorption process
Industrial wastewaters are characterized by substantial variations in pH values, and hence the initial pH of the solution is an important factor to be considered during adsorption studies (Suhasini et al. 1999). The effect of pH on Co(II) ions biosorption is shown in Fig. 5. The pH range studied was between 3.0 and 11.0. At low pH values, the high hydrogen ions adsorbed at the interface may repel positively charged metal ions electrostatically and prevents their approach to QCS surface (Coşkun et al. 2006). The lowest cobalt sorption capacity (7.33 mg g−1) was found at an initial pH solution (pH: 3.0). As the initial pH increased to 5, cobalt sorption capacity increased to 12.46 mg g−1. Cobalt adsorption capacity varied little and the sorption capacity was kept constant (about 12.26 mg g−1) in the initial pH 7.0. When the initial pH was 9, the sorption capacity was increased to 12.50 mg g−1, which could be attributed to the precipitation of Co(OH)2. Similar results have also been reported by Kara et al. (2003) for the adsorption of Co(II) ions onto sepiolite.
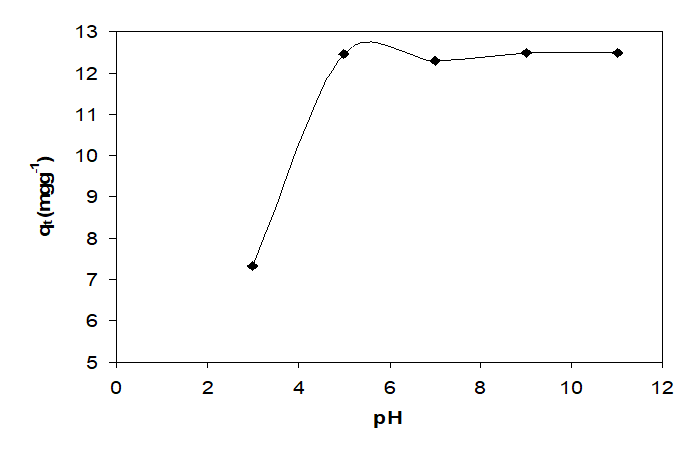
Fig. 5. Effect of pH on adsorption Co(II) onto chemically treated QCS (Co concentration:50 mgL−1; biosorbent dossage: 4.0gL-1; temperature: 298K, time: 90 min.)
Effect of Temperature
Temperature is one of the important parameters for successful biosorption application (Sarı and Tuzen 2008b). The effect of temperature on Co(II) ions biosorption is shown in Fig. 6. Over the range investigated (288 to 308 K) temperature-related effects were not significant. For economic considerations, room temperature was chosen as the optimum for the adsorption of Co(II) ions by QCS.
Fig. 6. Influence of temperature on cobalt biosorption by chemically treated QCS (Co concentration: 50 mg L−1; biosorbent dosage: 4.0 g L−1; pH: natural; contact time: 90 min) |
Desorption experiment
The results of desorption experiments are presented in Fig. 7, which clearly indicates that effective desorption of Co(II) was observed with mineral acids (HCl and HNO3) and chelating agent EDTA after 30 min. As shown in Fig.7, desorption ratio of cobalt ions using HCl approached to about 98%, while EDTA and HNO3 showed about 90% and 86% of the adsorbed cobalt ions, respectively. Consequently, HCl was chosen as the best desorbent for cobalt ions.
| Fig. 7. Cobalt(II) recovery by different desorbents | |
Sorption Isotherms
The equilibrium adsorption isotherms are one of the most important forms of information by which one can understand the mechanism of the adsorption systems. The adsorption equilibrium data were further analyzed using two well-known isotherm models, the Langmuir and Freundlich models.
The Langmuir isotherm theory assumes monolayer coverage of adsorbate over a homogenous adsorbent surface (Karaoğlu et al. 2009; Anayurt et al. 2009). The Langmuir isotherm is given by Eqs. 1 and 2,
(1)
(2)
where qe (mgg-1) and Ce (mgL-1) are the amount of adsorbed Co(II) per unit weight of adsorbent and the un-adsorbed Co(II) concentration in solution at equilibrium, respectively, qmis the maximum amount of the Co(II) bound per unit weight of adsorbent to form a complete monolayer on the surface at high Ce, and K is the equilibrium constant or Langmuir constant related to the affinity of binding sites (Lmg-1). qm and K were calculated from the slope and intercept of the straight lines of the plot Ce/qe vs. Ce (Tahir and Rauf 2006; Karaoğlu et al. 2010b).
The empirical Freundlich model can be applied for non-ideal sorption on heterogeneous surfaces and multilayer sorption. The Freundlich equation may be written as (Oh and Tshabalala 2007; Ghassabzadeh et al. 2010),
(3)
(4)
where KF is a Freundlich constant that shows both the adsorption capacity of an adsorbent and the strength of the relationship between adsorbate and adsorbent.
The slope 1/n, ranging between 0 and 1, is a measure of adsorption intensity or surface heterogenity, becoming more heterogeneous as its value gets closer to zero. In general, KFincreases the adsorption capacity of an adsorbent for a given adsorbate increases.
KF and (1/n) can be determined from the linear plot of lnqe vs. lnCe (Eren and Acar 2006; Karaoğlu et al. 2009). Values of K, qm, KF, and n were calculated from the intercept and slope of the plots. The values for qm and K are presented in Table 1.
Table 1. Characteristic Parameters of Sorption Process of Co(II) on QCS
The results given in Table 1 show that the Langmuir isotherm described cobalt biosorption by QCS better than the Freundlich isotherm. From Table 1, it was seen that the Freundlich isotherm was not suitable (R2 < 0.63). In additon, the maximum adsorption capacities for Co(II) onto the biosorbent surface was found to be in the range of 33 mg g-1 (Table 1).
The essential characteristics of the Langmuir equation can be expressed in term of a dimensionless separation factor, RL, defined as:
(5)
The value of RL indicates the type of the isotherm to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0). The RL values are reported in Table 1, which shows the adsorption behaviour of QCS. The value of RL was found to be in the range of 0 to 1, indicating that the adsorption process is favourable for Co(II).
Adsorption Kinetic Studies
In order to examine the controlling mechanism of the adsorption process, pseudo first, pseudo second-order equations and intra-particle diffusion model were used to test the experimental data (Chairat et al. 2005). The Lagergren pseudo first-order equation is given as follows (Sarı and Tuzen 2009):
(6)
The straight line of the plot of ln(qe-qt) versus time suggests the applicability of the Lagergren equation for the present system. The values of R2 were determined from the slope of the plots and are given in Table 2. The pseudo-second-order model can be represented in the following form (Ho and McKay 1999; Prasad and Abdullah 2010),
(7)
where k2 is the adsorption rate constant (g mg-1min-1). The plot of t/qt versus time is similar as shown in Weber and Morris. The values of k2 were determined by the slope of the plot and are given in Table 2.
Table 2. First, Pseudo-second Order Kinetics, and Intra-particle Diffusion Model Parameters for the Adsorption Systems in the Study
The coefficients of determination for the pseudo-first-order model were in the range of 0.661 to 0.968. The results indicated that the adsorption of Co(II) onto QCS did not follow the pseudo-first-order kinetics. As a result, the kinetic adsorption data were further fitted the pseudo-second-order kinetic model (R2: 0.999) (Table 2).
The half-adsorption time, t1/2, is defined as the time required for the adsorption to take up half as much QCS as its equilibrium value. This time is often used as a measure of the adsorption rate (Doğan et al. 2009).
(8)
In Eq. 8 k2 is the second-order adsorption rate constant (g mg-1min-1). The values of t1/2 determined for the tested parameters are given in Table 2. A further verification of the exothermic nature of the process was done by calculating the half-life of process at each temperature, which was found to increase with increasing temperatures. An empirically found functional relationship, common to the most adsorption processes, is that the uptake varies almost proportionally with t1/2, the Weber–Morris plot, rather than with the contact time t(Weber et al. 1963; Alkan et al. 2007).
(9)
In Eq. 9 qt is the amount of Co(II) adsorbed at time t (mgg-1), C is the intercept, and kdif is the intra-particle diffusion rate constant (mg s-1/2 g-1). According to Eq. (9), a plot of qtversus t1/2 should be a straight line with a slope kdif and intercept C when the adsorption mechanism follows the intra-particle diffusion process. The intra-particle diffusion plots are given in Figs. 8 through 10 for the effect of particle size, initial Co(II) concentration, and temperatures on biosorption rate, respectively. The linearity of the plots demonstrated that intra-particle diffusion played a significant role in the uptake of Co(II) by QCS. In this study no plot passed through the origin. This indicates that although intra-particle diffusion was involved in the adsorption process, it was not the sole rate-controlling step. This also confirms that adsorption of Co(II) on the biosorbent was a multi-step process, involving adsorption on the external surface and diffusion into the interior (Doğan et al. 2009). From Figs. 8 through 10, at all conditions, the sorption process tended to take place in two phases. It was found that an initial linear portion ended with a smooth curve, followed by second linear portion. The two phases in the intra-particle diffusion plot suggests that the sorption process proceeds first through surface sorption and then intra-particle diffusion. The initial curved portion of the plot indicates a boundary layer effect, while the second linear portion is due to intra-particle or pore diffusion. Since ki,1 values for first part of plot were high, this step is not rate limiting step. The slope of second linear portion of the plot has been defined as the intra-particle diffusion parameter ki,2 (mg/(g min0.5). On the other hand, the intercept of the plot reflects the boundary layer effect. Values of intercept give an idea about the thickness of boundary layer. The larger the intercept, the greater is the contribution of the surface sorption in the rate-limiting step (Alkan et al. 2008). The calculated intra-particle diffusion coefficient ki,1 and ki,2 values at different conditions are given in Table 2. The ki,1 and ki,2 express diffusion rates of the different stages in the adsorption. At the beginning, Co(II) was adsorbed by the exterior surface of QCS particle, so the adsorption rate was very fast. When the adsorption of the exterior surface reached saturation, Cobalt(II) ion entered into the QCS particle through the pore within the particle and was adsorbed by the interior surface of the particle. The rate-limiting step in adsorption process is intra-particle diffusion due to low ki,2 values.
The values of diffusion coefficient largely depend on the surface properties of adsorbents. The diffusion coefficients for the intra-particle transport of Co(II) within the pores of QCS particles have been calculated under various conditions by following the equation,
(10)
where t1/2 is the half life in seconds as calculated from Eq. (10), r0 is the radius of the adsorbent particle in centimetres, and D is the diffusion coefficient value in cm2s-1. In these calculations, it has been assumed that the solid phase consists of spherical particles with an average radius between the radii corresponding to upper- and lower-size fractions. The value of r0 was calculated as 1.775×10−2 cm for QCS samples. Calculated values of t1/2 and D are given in Tables 2. D values for the adsorption of Co(II) under different conditions are in the range of 3.11×10−6 to 168.78×10−6 cm2s-1. Similar results were found for Cr(III) on vineyard pruning waste (Karaoğlu et al. 2010a).
| Fig. 8. Intraparticle diffusion plots for different particle sizes |
| Fig. 9. Intraparticle diffusion plots for different initial cobalt(II) |
| Fig. 10. Intraparticle diffusion plots for different temperatures |
Thermodynamic Study
Thermodynamic parameters were calculated to confirm the adsorption nature of the present study. The thermodynamic constants, free energy change (G*), enthalpy change (H*), and entropy change (S*) were calculated to evaluate the thermodynamic feasibility of the process. The activation energy of adsorption was also calculated from the linearized Arrhenius equation (Bulut and Aydın 2006),
(11)
where Ea is the Arrhenius activation energy, and k0 is the Arrhenius factor. To extract k0 and Ea from kinetic data, we plot the series of rate constants measured at different temperatures in a graph of ln k versus 1/T. The corresponding activation energy was determined from the slope of the linear plot. The result obtained was -22.92 kJ mol-1 for the biosorption of Co(II) onto QCS. The thermodynamic parameters such as change in standard enthalpy (H*) and entropy (S*) were determined by using Eyring equations,
(12)
where kb and h are Boltzmann’s and Planck’s constants, respectively. According to Eq. (12), a plot of ln(k/T) versus 1/T should be a straight line with a slope -(ΔH*/Rg) and intercept [ln(kb/h)+(ΔS*/Rg)]. H* and S* were calculated from the slope and intercept of line. Gibbs energy of activation may be written in terms of entropy and enthalpy of activation:
(13)
G* was calculated at 298 K from Eq. (13). It was determined that the values of the free energy G*, enthalpy (H*), and entropy (S*) of activation were 81.21 kJ mol-1, -25.40 kJmol-1, and -357.76 jmol-1K-1, respectively. The negative H* value indicated exothermic nature of the adsorption.
Comparison of the Adsorption Capacity of QCS
Adsorption capacity of the adsorbent, QCS, was compared with that of the capacities of other nonconventional low-cost adsorbents. It can be seen from Table 3 that the adsorption capacity of QCS is significant and comparable to that of other adsorbents used for the removal of Co(II). The results show the applicability of QCS for the removal of Co(II) from aqueous solutions.
Table 3. The Comparison of Co2+ Sorption Capacities of Various Sorbents
CONCLUSIONS
- Adsorption studies with Quercus coccifera shell (QCS) as biosorbent revealed the ability of plant biomaterials to remove Co(II) from the aqueous phase.
- QCS is an effective adsorbent for the removal of Co(II) from aqueous solutions.
- The Langmuir and Freundlich adsorption models were used to express the sorption phenomenon of the sorbate. The equilibrium data were well described by the Langmuir Model.
- Various kinetic models were used to describe the adsorption process. The kinetic data showed that the pseudo-second-order kinetic model was obeyed better than pseudo-first-order kinetic model.
- The negative H* value indicated an exothermic nature of the adsorption.
REFERENCES CITED
Ahmadpour, A., Tahmasbi, M., Rohani Bastami, T., and Amel Besharati, J. (2009). “Rapid removal of cobalt ion from aqueous solutions by almond green hull,” J. Hazard. Mater. 166, 925-930.
Alkan, M., Demirbaş, Ö., and Doğan, M. (2007). “Adsorption kinetics and thermody-namics of an anionic dye onto sepiolite,” Micropor. Mesopor. Mater. 101, 388-396.
Alkan, M., Doğan, M., Turhan, Y., Demirbaş, Ö., Turan, P. (2008). “Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solutions,” Chem. Eng. J. 139, 213-223.
Anayurt, R. A., Sari, A., Tuzen, M. (2009). “Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass,” Chem. Eng. J. 151, 255-261.
Aziz, A., Ouali, M. S., Elandaloussi, E. H., Menorval, L. C. D., and Lindheimer, M. (2009). “Chemically modified olive stone: A low-cost sorbent for heavy metals and basic dyes removal from aqueous solutions,” J. Hazard. Mater. 163, 441-447.
Bhatnagar, A., Minocha, A. K., and Sillanpaa, M. (2010). “Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent,” Biochem. Eng. J. 48, 181-186.
Bulut, Y., and Aydın, H. (2006). “A kinetics and thermodynamics study of methylene blue adsorption on wheat shells,” Desalination, 194, 259-267.
Chairat, M., Rattanaphani, S., Bremner, J. B., and Rattanaphani, V. (2005). “An adsorption and kinetic study of lac dyeing on silk,” Dyes and Pigments, 64, 231-241.
Coşkun, R., Soykan, C., and Saçak, M. (2006). “Adsorption of copper(II), nickel(II) and cobalt(II) ions from aqueous solution by methacrylic acid/acrylamide monomer mixture grafted poly(ethylene terephthalate) fiber,” Sep. Purif. Technol. 49, 107-114.
Dahiya, S., Tripathi, R. M., and Hegde, A. G. (2008). “Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca green hull biomass,” J. Hazard. Mater. 150, 376-386.
Doğan, M., Abak, H., Alkan, M. (2009). “Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters,” J. Hazard. Mater. 164, 172-181.
Ebrahimi, M., Panahi, R., and Dabbagh, R. (2009). “Evaluation of native and chemically modified Sargassum glaucescens for continuous biosorption of Co(II),” Appl. Biochem. Biotechnol. 158, 736-746.
Eren, Z., and Acar, F.N. (2006). “Adsorption of reactive black 5 from an aqueous solution; equilibrium and kinetic studies,” Desalination, 194, 1–10.
Ghassabzadeh, H., Mostaedi, M. T., Mohaddespour, A., Maragheh, M. G., Ahmadi, S. J., and Zaheri, P. (2010). “Characterizations of Co (II) and Pb (II) removal process from aqueous solutions using expanded perlite,” Desalination, 261, 73-79.
Gupta, V. K., and Rastogi, A. (2009). “Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions,” J. Hazard. Mater. 163,396-402.
Gupta, V. K., Saini, V. K., and Jain, N. (2005). “Adsorption of As(III) from aqueous solutions by iron oxide-coated sand,” J. Colloid and Interf. Sci. 288, 55-60.
Güzel, F., Yakut, H., and Topal, G. (2008). “Determination of kinetic and equilibrium parameters of the batch adsorption of Mn(II), Co(II), Ni(II) and Cu(II) from aqueous solution by black carrot (Daucus carota L.) residues,” J. Hazard. Mater. 153, 1275-1287.
Ho, Y. S., and McKay, G. (1999). “Pseudo-second order model for sorption processes,” Process Biochem. 34, 451-465.
Jalali-Rad, R., Ghafourian, H., Asef, Y., Dalir, S. T., Sahafipour, M. H., and Gharanjik, B.M. (2004). “Biosorption of cesium by native and chemically modified biomass of marine algae: Introduce the new biosorbents for biotechnology applications,” J. Hazard. Mater. 116, 125-134.
Kara, M., Yuzer, H., Sabah, E., and Celik, M.S. (2003). “Adsorption of cobalt from aqueous solutions onto sepiolite,” Water Res. 37, 224-232.
Karaoğlu, M. H., Doğan, M., and Alkan, M. (2010b). “Removal of reactive blue 221 by kaolinite from aqueous solutions,” Ind. Eng. Chem. Res. 49, 1534-1540.
Karaoğlu, M. H., Doğan, M., and Alkan, M. (2009). “Removal of cationic dyes by kaolinite,” Micropor. Mesopor. Mater. 122, 20-27.
Karaoğlu, M. H., Zor, Ş., and Uğurlu, M. (2010a). “Biosorption of Cr(III) from solutions using vineyard pruning waste,” Chem. Eng. J. 159, 98-106.
Manohar, D. M., Noeline, B. F., and Anirudhan, T. S. (2006). “Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase,” Appl. Clay Sci. 31, 194-206.
Matheickal, J. T., and Yu, Q. (1997). “Biosorption of lead(II) from aqueous solutions by phellinus badius,” Minerals Eng. 10(9), 941-957.
Oh, M., and Tshabalala, M. A. (2007). “Pelletized ponderosa pine bark for adsorption of toxic heavy metals from water,” BioResources 2(1), 66-81.
Parab, H., Joshi, S., Shenoy, N., Lali, A., Sarma, U. S., and Sudersanan, M. (2006). “Determination of kinetic and equilibrium of Co(II), Cr(III), and Ni(II) onto coir pith,” Process Biochem. 41, 609-615.
Pavan, F. A., Lima, E. C., Dias, S. L. P., and Mazzocato, A. C. (2008). “Methylene blue biosorption from aqueous solutions by yellow passion fruit waste,” J. Hazard. Mater. 150, 703-712.
Prasad, A. G. D., and Abdullah, M. A. (2010). “Biosorption of Cr(VI) from synthetic wastewater using the fruit shell of gulmohar (Delonix regia): Application to electroplating wastewater,’’ BioResources 5(2), 838-853.
Rengaraj, S., and Moon, S. H. (2002). “Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins,” Water Res. 36, 1783-1793.
Saeed, A., Akhter, M. W., and Iqbal, M. (2005). “Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent,” Sep. Purif. Technol. 45, 25-31.
Sarı, A., Tuzen, M., Uluözlü, Ö.D., Soylak, M. (2007). “Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass,” Biochem. Eng. J. 37, 151-158.
Sarı, A., and Tuzen, M. (2008a). “Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies,” J. Hazard. Mater. 157, 448-454.
Sarı, A., and Tuzen, M. (2008b). “Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass,” J. Hazard. Mater. 152, 302-308.
Sarı, A., and Tuzen, M. (2009). “Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass,” J. Hazard. Mater. 164, 1004-1011.
Senturk, H. B., Ozdes, D., and Duran, C. (2010). “Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent,” Desalination252, 81-87.
Suhasini, I. P., Sriram, G., Asolekar, S. R., and Sureshkumar, G. K. (1999). “Biosorptive removal and recovery of cobalt from aqueous systems,” Process Biochem. 34, 239-247.
Tahir, S. S., and Rauf, N. (2006). “Removal of a cationic dye from aqueous solutions by adsorption on to bentonite clay,” Chemosphere 63, 1842-1848.
Uluozlu, O. D., Sarı, A., Tuzen, M., and Soylak, M. (2008). “Biosorption of Pb(II) and Cr(III) from aqueous solution by lichen (Parmelina tiliaceae) biomass,” Bioresour. Technol.99, 2972-2980.
Vijayaraghavan, K., Jegan, J., Palanivelu, K., and Velan, M. (2005a). “Biosorption of copper, cobalt and nickel by marine green alga Ulva reticulata in a packed column,” Chemosphere, 60, 419-426.
Vijayaraghavan, K., Palanivelu, K., and Velan, M. (2006). “Biosorption of copper(II) and cobalt(II) from aqueous solutions by crab shell particles,” Bioresour. Technol. 97, 1411-1419.
Vijayaraghavan, K., Thilakavathi, M., Palanivelu, K., and Velan, M. (2005b). “Continuous sorption of copper and cobalt by crab shell particles in a packed column,” Environ. Technol.26, 267-276.
Weber, W. J., and Morris, J. C. (1963). “Kinetics of adsorption on carbon from solution,” J. San. Eng. Div. ASCE 89, 31-59.
Weng, C. H., Lin, Y. T., and Tzeng, T. W. (2009). “Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder,” J. Hazard. Mater. 170, 417-424.
Article submitted: February 17, 2011; Peer review completed: March 27, 2011; Revised version received and accepted: April 13, 2011; Published: April 18, 2011.
