Abstract
This study presents the adsorption behavior of the methylene blue (MB) dye onto the activated carbon produced from soybean oil cake by chemical activation with KOH at 800 °C. The adsorption isotherms, kinetic models, and thermodynamic parameters of the adsorption were studied. The Langmuir isotherm showed a better fit than the Freundlich isotherm. The adsorption rate was described by pseudo-second-order kinetics. The negative values of ΔG° and the positive values of ΔH° indicate that the adsorption of MB was favored and endothermic.
Download PDF
Full Article
ADSORPTION OF METHYLENE BLUE FROM AQUEOUS SOLUTION ON ACTIVATED CARBON PRODUCED FROM SOYBEAN OIL CAKE BY KOH ACTIVATION
Turgay Tay,a,* Murat Erdem,a Burak Ceylan,a and Selhan Karagöz b
This study presents the adsorption behavior of the methylene blue (MB) dye onto the activated carbon produced from soybean oil cake by chemical activation with KOH at 800 °C. The adsorption isotherms, kinetic models, and thermodynamic parameters of the adsorption were studied. The Langmuir isotherm showed a better fit than the Freundlich isotherm. The adsorption rate was described by pseudo-second-order kinetics. The negative values of ΔG° and the positive values of ΔH° indicate that the adsorption of MB was favored and endothermic.
Keywords: Adsorption; Activated carbon; Kinetics; Dye; Isotherm
Contact information: a: Department of Chemistry, Faculty of Science, Anadolu University, 26470- Eskisehir-Turkey; b: Department of Chemistry, Faculty of Science, Karabük University, 78050-Karabük- Turkey; *Corresponding author: ttay@anadolu.edu.tr
INTRODUCTION
The textile industry produces large volumes of highly colored wastewaters containing dyestuffs and other organic compounds. Colored wastewaters are the major environmental issue of the textile industries, as most of the dyes are not biodegradable. The processes of dyestuff removal from industrial wastewaters include adsorption, oxidation–ozonation, biological processes, coagulation–flocculation, and membrane processes (Santos and Boaventura 2008). Among these processes, adsorption appears to be a key process for the removal of dyestuff (Weber 1978; Mckay et al. 1999).
Activated carbon is one of the most widely used adsorbents due to its high adsorption capacity and the fact that it can be produced from renewable (Yagmur et al.
2008) and non-renewable resources (Izquierdo et al. 2001). Because of economic and environmental reasons, there has been a plethora of research on the efforts to employ various lignocellulosic materials for the production low cost activated carbon (Kumagai et al. 2007; Gergova and Eser 1996; Guo and Lua 2000). Activated carbons are produced by either physical or chemical activation. Chemical activation is carried out using chemical reagents such as ZnCl2, H3PO4, HCl, H2SO4, KOH, K2CO3, NaOH, Na2CO3, and so on.
Activated carbons have been tested for the removal of methylene blue (MB) from aqueous solutions. MB is one of the model compounds used to investigate the adsorption process of organic contaminants from aqueous solutions and the adsorption characteristics of adsorbents (Hameed et al. 2007; Kavitha and Namasivayam 2007). T he maximum MB monolayer adsorption capacities of the activated carbons from renewable sources depend on several factors, i.e. carbonization temperature, activation time, and biomass type (Aygun et al. 2003; Thirumalisamy and Subbian 2010). In our previous works, we have produced activated carbons from waste biomass by chemical activation with sulfuric acid (Karagoz et al. 2008) and with alkali hydroxide and salt (Tay et al. 2009).
The present investigation reports adsorption studies of MB onto the activated carbon produced from soybean oil cake by impregnation with KOH at the carbonization temperature of 800 °C. The effects of initial MB concentration, pH, kinetics and thermodynamic studies were carried out to evaluate the adsorption capacity of the activated carbon.
EXPERIMENTAL
Materials
Soybean oil cake was obtained from Altinyag Oil Company, Izmir, Turkey. The details about the raw material can be found in the literature (Tay et al. 2009). The impregnation ratio of 1.0 was employed in the preparation of the activated carbon from soybean oil cake by KOH activation. The details about the characteristics of the activated carbon can be found in the literature (Tay et al. 2009). All chemicals used in the investigation were of analytical grade.
Adsorption Experiments
The adsorption experiments were carried out with 50 mL of a 250 mg L-1 MB solution and 50 mg of the activated carbon. The pH of the solution was carefully adjusted between 2 and 10 by adding a small amount of dilute HCl or NaOH solution and using a pH meter (Hanna Instruments HI 8314). The MB solutions in 100 mL Erlenmeyer flasks were stirred using a magnetic stirrer, and the flasks were sealed with parafilm to avoid evaporation. The optimum adsorption pH was determined as 6 and used throughout further adsorption experiments conducted at various contact times (from 0 min to 240 min) and temperatures (20, 30, and 40 °C) to determine the adsorption equilibrium time and the maximum amount of removal of MB. After the adsorption process, the solutions were centrifuged and then subjected to quantitative analyses. The MB concentrations in the solutions were determined from the absorbance as determined with a spectrophotometer (Shimadzu UV-2450PC). The amount of MB adsorbed onto the activated carbon was determined by the difference between the initial and remaining concentrations of MB. The amount of adsorbed MB at equilibrium, qe (mg g-1) was calculated using Eq1,
(1)
where Co and Ce (mg L-1) are the liquid-phase initial and equilibrium concentrations of MB, respectively. V is the volume of the solution (L), and W is the mass of dry adsorbent used (g).
The adsorption of MB onto the activated carbon was also evaluated at a constant temperature of 20 °C for the adsorption isotherms.
The procedures for the kinetic experiments were basically identical to those of equilibrium tests. The aqueous samples were taken at present time intervals, and the concentrations of MB were similarly measured. The amount of adsorption at time t, qt (mg g-1), was calculated by,
(2)
where Co and Ct (mg L-1) are the liquid-phase concentrations of MB at initial and any time t, respectively. V is the volume of the solution (L), and W is the mass of dry adsorbent used (g).
RESULTS AND DISCUSSION Effect of pH
The effect of the pH of the solution for the adsorption of MB onto the activated carbon was studied at a constant initial concentration of 250 mg L-1 MB, 50 mg adsorbent, and with a 150 rpm agitation speed at 20 °C. The range of the pH was varied between 2 and 10 (Fig. 1). It can be clearly seen from Fig. 1 that the pH of solution affects the adsorption characteristics of MB onto the activated carbon. As the pH of the solution increased from 2 to 6, an increase in the adsorption capacity of the activated carbon was observed. Beyond pH 6, the adsorption capacity gradually decreased. The amphoteric nature of carbon depends on the determination of the isoelectric point (pH IEP) or point of zero charge (pHPZC) of the carbon (Radovic et al. 1997).
Fig. 1. Effect of pH for the adsorption of MB onto the activated carbon (Co = 250 mg L-1, mAC = 50 mg, t = 24 h, V = 50 mL, T = 20 oC)
The isoelectric point of our activated carbon was found to be 5.36. With increase in the pH value, the surface of the activated carbon was charged negatively to a greater degree. Thus, the adsorption of the MB with positive charge reached a maximum at higher pH conditions. This implies that the adsorption of MB could be enhanced at higher pH conditions. In our previous work (Karagoz et al. 2008), we have prepared activated carbons from sunflower oil cake by chemical activation with H2SO4 and used them for MB adsorption in which the maximum adsorption capacity was observed at a solution of pH 6. In another study, the activated carbon produced from a biomass namely, Euphorbia rigida by chemical activation with H2SO4 was used for MB adsorption (Gercel et al. 2007), and the maximum uptake of MB was observed at pH 6. Our maximum adsorption result is in good agreement with the results of those works (Karagoz et al. 2008; Gercel et al. 2007).
Effect of Contact Time
A series of contact time experiments for the MB adsorption from 0 to 240 min were carried out at 250 mg L-1 initial concentration of MB and temperatures of 20, 30, and 40 °C. The maximum MB adsorption capacities of the activated carbon were observed at 240 min contact time for all tested temperatures.
Effect of Temperature
The equilibrium adsorption capacities for the MB adsorption onto the activated carbon was studied at the temperatures of 20, 30, and 40°C. The experimental results indicated that the adsorption process depended on the temperature and the adsorption capacity increased with temperature.
Effect of Initial MB Concentration
The effect of initial MB concentrations varying from 50 to 500 mg L-1 was studied on the adsorption of MB onto the activated carbon. The MB concentration provides a powerful driving force to overcome all mass transfer resistances of the dye between the aqueous and solid phases. Thus, the adsorption capacity for MB increased with increasing initial MB concentration. For example, the amount of adsorbed MB was increased from 150 to 200 mg g-1 as the initial MB concentration was increased from 150 to 200 mg L-1. The maximum adsorption capacity was 281 mg g-1, and it was obtained at the initial MB concentration of 500 mg L -1.
Adsorption Kinetics
To define the adsorption kinetics of MB onto the activated carbon, the kinetic parameters of the adsorption process (the pseudo-first-order, pseudo-second-order, and intraparticle diffusion) were investigated. The plot of the kinetic models for MB adsorption onto the activated carbon are shown in Figs. 2 and 3, and the results of kinetic parameters are given in Table 1. The R2 values for the pseudo-second-order kinetic model were higher than those of the pseudo-first-order kinetic model for all tested temperatures. This indicates that the kinetic modeling of the MB adsorption onto the activated carbon adsorbent fitted better to the pseudo-second-order kinetic model.
The first order kinetic model equation (Lagergen 1898) is:
(3)
where q1 and qt are the amounts of the MB adsorbed at equilibrium at time t, in mg g-1 , and k1 is the first-order rate constant (min-1). Values of k1 from the slope of the plots of ln(q1-qt) versus t (Fig. 2) are given in Table 1.
The pseudo–second–order kinetic model (Ho and McKay 1998) is expressed as,
(4)
where q2 is the maximum adsorption capacity (mg g-1) for the pseudo-second-order adsorption, and k2 is the equilibrium rate constant for the pseudo-second-order adsorption (g mg-1 min-1). Values of k2 and q2 were calculated from the plot of t/q1 against t (Fig. 3).
Fig. 2. Pseudo-first-order kinetic plots for the adsorption of MB onto the activated carbon
The intraparticle diffusion (Crank 1933) can be expressed by the following equation,
(4)
where C is the intercept and kp is the intraparticle diffusion rate constant (mg g-1 min -1/2). According to this model, the plot of the uptake, qt, versus the square-root of time, t1/2 is linear. If intraparticle diffusion is involved in the adsorption process and if the line passes through the origin, then intraparticle diffusion is the rate-controlling step (Kannan and Sundaram 2001; Bhattacharyya and Sharma 2004; Chen et al. 2003).
Fig. 3. Pseudo-second-order kinetic plots for the adsorption of MB onto the activated carbon
As can be seen from Fig. 4, the adsorption onto the activated carbon was found to be consistent with intraparticle diffusion for the temperature of 20°C.
Fig. 4. Intraparticle diffusion plots for the adsorption of MB onto the activated carbon
However, the line does not pass through the origin for the temperature of 30 and 40 °C, which shows that activated carbon is not consistent with intraparticle diffusion for the temperatures of 30 and 40°C. The correlation coefficients (R 2) for the intraparticle diffusion model is presented in Table 1. As can be seen from Table 1, the correlation coefficient (Rp ) for the intraparticle diffusion model was lower than that of the pseudo- second-order kinetic model for all tested temperatures. In our previous study, we have found similar results (Karagoz et al. 2008).
Table 1. Kinetic Parameters for the Adsorption of MB onto the Activated Carbon
Adsorption Isotherms
The results obtained on the adsorption of MB onto the activated carbon were analyzed by well-known models given by Langmuir and Freundlich (Adamson 1960). The Langmuir isotherm can be expressed as,
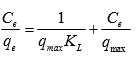 (6)
(6)
where qe is the equilibrium MB concentration on the activated carbon (mg g-1), Ce is the equilibrium MB concentration in the solution (mg L-1), qmax is the monolayer adsorption capacity of the activated carbon (mg g-1), and KL is the Langmuir adsorption constant (L mg-1).
The plot of Ce/qe versus Ce for the adsorption of MB onto the activated carbon is shown in Fig. 5. The Freundlich isotherm can be expressed as follows,
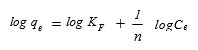 (7)
(7)
where qe is the equilibrium MB concentration on the activated carbon (mg g-1), Ce is the equilibrium MB concentration in the solution (mg L-1), KF (L g-1), and n are the Freundlich adsorption isotherm constants.
Fig. 5. Langmuir plot for the adsorption of MB onto the activated carbon at 20 ºC
The plot of log qe versus log Ce for the adsorption of MB onto the activated carbon is shown in Fig. 6.
Fig. 6. Freundlich plot for the adsorption of MB onto the activated carbon at 20 ºC
The Langmuir and Freundlich isotherm parameters for the MB adsorption onto the activated carbon are given in Table 2. The results indicate that the Langmuir isotherm fit better than the Freundlich isotherm. Thus, according to a linear regression method, the MB uptake is due to monolayer coverage of solute particles onto the surface of activated carbon. A Langmuir isotherm assumes monolayer adsorption onto a surface containing a finite number of adsorption sites (Adamson 1960). A Freundlich isotherm model assumes heterogeneous surface energies (Adamson 1960).
Table 3 shows a comparison for the maximum monolayer adsorption capacities of the activated carbons from different biomass sources for the MB adsorption at different operating conditions. The maximum monolayer adsorption capacities of all activated carbons reported in the literature (Aygun et al. 2003; Attia et al. 2003; Karagoz et al. 2008; Gercel et al. 2007; Nunes et al. 2009) were much lower than that of the present work.
Table 2. Adsorption Isotherms Constants for the Adsorption of MB onto the Activated Carbon at 20°C
Table 3. Comparison of the Maximum Monolayer Adsorption of MB onto Activated Carbons from Various Sources
Adsorption Thermodynamics
Thermodynamic parameters such as free energies, enthalpies, and entropies of the adsorption of MB onto the activated carbon were evaluated (Table 4). The negative values of ΔG° and the positive values of ΔH° indicate that the adsorption of MB was spontaneous and endothermic. Due to the relatively weak adsorbent-adsorbate interactions, the MB adsorption process should be regarded as physical adsorption. A plot of ln KL versus 1/T for estimation of thermodynamic parameters is shown in Fig. 7. Thermodynamic parameters including Gibbs free energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) were calculated from the following equation,
ΔG = -RT lnKL (8)
where R is the universal gas constant (8.314 J/mol K), T is the temperature (K), and the KL value was calculated using the following equation,
(9)
where qe and Ce are the equilibrium concentration of MB onto the activated carbon (mg g−1) and in the solution (mg L−1), respectively.
The enthalpy change (ΔH°) and entropy change (ΔS°) of the adsorption were estimated from the following equation;
(10)
The enthalpy change (ΔH°) and entropy change (ΔS°) can be obtained from the slope and intercept of a Van’t Hoff equation of lnKL versus 1/T,
ΔG = ΔH° – T ΔS° (11)
where ΔG° is the Gibbs free energy change (J), R the universal gas constant (8.314 J
mol−1 K−1), and T is the absolute temperature (K).
Figure 7. Plot of log k2 vs. 1/T for estimation of activation energy for the adsorption of MB onto the activated carbon
The activation energy (Ea) value of MB adsorption onto adsorbent was calculated from Arrhenius equation expressed by the following equation,
(12)
where Ea is the activation energy , k2 is the equilibrium rate constant for the pseudo- second-order adsorption, and A is the preexponential factor. Values of A and Ea were calculated from the plot of lnk2 against 1/T.
Table 4. Thermodynamic Parameters Calculated from the Langmiur Isotherm
Constant (KL) for the Adsorption of MB onto the Activated Carbon
CONCLUSIONS
1. The adsorption of MB from aqueous solution onto the activated carbon fits Langmuir equation based on the formation of a monomolecular layer. The maximum monolayer adsorption capacity of the activated carbon was found to be 278 mg g-1.
2. The adsorption capacity of activated carbon for MB increased while the temperature of solution was increased.
3. The adsorption capacity of activated carbon for MB increased with increasing in the initial concentration of MB.
4. The pseudo second order kinetic equation of MB adsorption is better obeyed than the pseudo first order.
5. MB adsorption process is endothermic and should be regarded as physical adsorption.
REFERENCES CITED
Adamson, A. W. (1960). Physical Chemistry of Surfaces, Interscience Publication, New York.
Attia, A. A., Girgis, B. S., and Khedr, S. A. (2003). “Capacity of activated carbon derived from pistachio shells by H3PO4 in the removal of dyes and phenolics,” J. Chem. Technol. Biotechnol. 78, 611-619.
Ahmed, M. J., and Dhedan, K. S. (2012). “Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons,” Fluid Fhase Equilibr. 317, 9-14.
Altenor, S., Carene, B., Emmanuel, E., Lambert, J., Ehrhardt, J. J., and Gaspard S. (2009) “Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation,” J. Hazard. Mater. 165, 1029-1039.
Aygun, A., Yenisoy-Karakas, S., and Duman, I. (2003). “Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties,” Micropor. Mesopor. Mat. 66, 189-195.
Bhattacharyya, K. G., and Sharma, A. (2004). “Azadirachta indica leaf powder as an effective biosorbent for dyes: A case study with aqueous Congo red solutions,” J. Environ. Manage. 71, 217-229.
Chen, J. P., Wu, S., and Chong, K. H. (2003). “Surface modification of a granular activated carbon by citric acid for enhancement of copper adsorption,” Carbon 41, 1979-1986.
Crank, G. (1933). The Mathematics of Diffusion, Clarendon Press, London, New York. Gercel, O., Ozcan, A., Ozcan, A. S., and Gercel, H. F. (2007). “Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions,” Appl. Surface Sci. 253, 4843-4852. Gergova, K., and Eser, S. (1996). “Effects of activation method on the pore structure of activated carbons from apricot stones,” Carbon 34(7), 879-888.
Guo, J., and Lua, A. C. (2000). “Effect of surface chemistry on gas-phase adsorption by activated carbon prepared from oil-palm stone with pre-impregnation,” Separat. Purificat. Technol. 18, 47-55.
Hameed, B. H., Ahmad, A. L., and Latif, K. N. A. (2007). “Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust,” Dyes Pigments 75, 143–149.
Ho, Y. S., and McKay, G. (1998). “Kinetic models for the sorption of dye from aqueous solution by wood,” Process Safety Environ. Protec. 76, 183-191.
Izquierdo, M. T., Rubio, B., Mayoral, C., and Andrés, J. M. (2001). “Modifications to the surface chemistry of low-rank coal-based carbon catalysts to improve flue gas nitric oxide removal,” Appl. Catal. B-Environ. 33, 315-324.
Kannan, N., and Sundaram, M. M. (2001). “Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-A comparative study,” Dyes Pigments. 51, 25-40.
Karagoz, S., Tay, T., Ucar, S., and Erdem, M. (2008). “Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption,” Bioresour. Technol. 99, 6214-6222.
Kavitha, D., and Namasivayam, C. (2007). “Experimental and kinetic studies on methylene blue adsorption by coir pith carbon,” Bioresour. Technol. 98, 14-21.
Kumagai, S., Noguchi, Y., Kurimoto, Y., and Takeda, K. (2007). “Oil adsorbent produced by the carbonization of rice husks,” Waste Manage. 27, 554-561.
Lagergen, S. (1898). “Zur Theorie der sogenannten Adsorption geloster Stoffe,” Kunglinga Svenska Vetenskapsakademiens, Hand Lingar. 24, 1-39.
Mckay, G., Porter, J. F., and Prasad, G.R. (1999). “The removal of dye colours from aqueous solutions by adsorption on low-cost materials,” Water Air Soil Poll. 114, 423-438.
Nunes, A. A., Franca, A. S., and Oliveira, L. S. (2009). “Activated carbons from waste biomass: An alternative use for biodiesel production solid residues,” Bioresour. Technol. 100(5), 1786-1792.
Radovic, L. R., Silva, I. F., Ume, J. I., Menendez, J. A., Leon, C. A., Leon, Y., and Scaroni, A. W. (1997). “An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron donating functional groups by chemically modified activated carbons,” Carbon 35(9), 1339-1348.
Santos, S. C. R., and Boaventura, R. A. R. (2008). “Adsorption modelling of textile dyes by sepiolite,” Appl. Clay. Sci. 42, 137-145.
Tay, T., Ucar, S., and Karagöz, S. (2009). “Preparation and characterization of activated carbon from waste biomass,” J. Hazard. Mater. 165, 481-485.
Thirumalisamy, S., and Subbia, M. (2010). “Removal of methylene blue from aqueous solution by actıvated carbon prepared from the peel of Cucumıs satıva fruit by adsorption,” BioResources 5(1), 419-437.
Weber, W. J. (1978). Physico-Chemical Methods of Treatment of Water and Wastewater, John Wiley & Sons, Inc.
Yagmur, E., Ozmak, M., and Aktas, Z. (2008). “A novel method for production of activated carbon from waste tea by chemical activation with microwave energy,” Fuel 87, 3278-3285.
Article submitted: February 27, 2012; Peer review completed: April 22, 2012; Revised version received and accepted: May 15, 2012; Published: June 4, 2012.
