Abstract
Mushroom products have been used as a biotechnological tool for many applications. Particularly, thermostable chitinase plays a vital role in biowaste management and biological control. In the present investigation, Amanita sp. was recorded in the subtropical region of Saudi Arabia, therefore, it was utilized for chitinase production using substrates chitin and dead fungal mycelia (DFM). Compared with the DFM, chitin was more suitable for chitinase activity at different temperatures and pH. Amanita sp. produced chitinase up to 70 °C, but the optimum was 50 °C. The chitinase activity was 4.98, 3.5, and 0.9 U.mg-1 with the use of chitin, while it was 4.6, 3.1, and 0.6 U.mg-1 with the use of DFM at 50, 60, and 70 °C, respectively. Chitinase activity was stable up to 60 °C, then it began to decrease at 70 °C. The chitinase activity was better at pH 4 and 5 than pH 8 and 9. The antifungal effect of the produced chitinase at 50 °C was more effective than at 60 °C. For instance, the Alternaria alternata colony radius was 3.50 cm and 2.26 cm at 50 °C while it was 4.35 cm and 4.13 cm at 60 °C when using DFM and chitin, respectively.
Download PDF
Full Article
Amanita sp. from Subtropical Region of Saudi Arabia as a Source of Chitinase Enzyme and its Antifungal Activity
Aisha M. H. Al-Rajhi,a Mohamed M. Alawlaqi,b Tarek M. Abdel Ghany c,* and Hanan Moawad,b,d
Mushroom products have been used as a biotechnological tool for many applications. Particularly, thermostable chitinase plays a vital role in biowaste management and biological control. In the present investigation, Amanita sp. was recorded in the subtropical region of Saudi Arabia, therefore, it was utilized for chitinase production using substrates chitin and dead fungal mycelia (DFM). Compared with the DFM, chitin was more suitable for chitinase activity at different temperatures and pH. Amanita sp. produced chitinase up to 70 °C, but the optimum was 50 °C. The chitinase activity was 4.98, 3.5, and 0.9 U.mg-1 with the use of chitin, while it was 4.6, 3.1, and 0.6 U.mg-1 with the use of DFM at 50, 60, and 70 °C, respectively. Chitinase activity was stable up to 60 °C, then it began to decrease at 70 °C. The chitinase activity was better at pH 4 and 5 than pH 8 and 9. The antifungal effect of the produced chitinase at 50 °C was more effective than at 60 °C. For instance, the Alternaria alternata colony radius was 3.50 cm and 2.26 cm at 50 °C while it was 4.35 cm and 4.13 cm at 60 °C when using DFM and chitin, respectively.
DOI: 10.15376/biores.18.2.2928-2939
Keywords: Amanita sp.; Saudi Arabia; Chitinase; Thermostable; Antifungal activity
Contact information: a: Department of Biology, College of Science, Princess Nourah bint Abdulrahman University P.O. Box 84428, Riyadh 11671, Saudi Arabia; b: Biology Department, Faculty of Science, Jazan University, Jazan, Saudi Arabia; c: Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo 11725, Egypt; d: Plant Department, Faculty of Science, Fayoum University, Faiyum, Egypt; *Corresponding author: tabdelghany.201@azhar.edu.eg
GRAPHICAL ABSTRACT

INTRODUCTION
The genus Amanita is one of the biggest genera of basidiomycetous with around 500 taxa identified globally. Some of its most toxic species include Amanita phalloides (Fr.) Link, A. verna (Bull.: Fr.) Lam., A. virosa (Fr.) Bertillon, and A. fuliginea. One of the dangerous Amanita species has been linked to more than 90% of the fatal occurrences of mushroom poisoning in humans. The death cap, A. phalloides, is the most notorious member of this genus and has attracted a lot of media attention (Enjalbert et al. 1996). Bioremediation of heavy metals was successful using Amanita spp. According to Podlasińska et al. (2015), A. citrina collected Pb at the greatest amount. According to Yang et al. (2018), the Amanitaceae family has 1000 species globally, including roughly 50 species in tropical Africa. According to descriptions, the genus Amanita (Amanitaceae) is an ECM genus that forms symbiotic relationships with its neighboring plants (Onguene and Kuyper 2012). In Saudi Arabia, Manzelat (2019) reported the occurrence of numerous mushrooms in the Jizan and Abha regions, including Amanita spp., Morchella spp., Agrocybe spp., Boletus spp., Coprinus spp., Podaxis spp., Lepiota spp., Pleurotus spp., and Agaricus spp.
The capacity of numerous ECM fungi to enzymatically hydrolyze different components of plant and fungal cell walls is becoming increasingly clear. This suggests that certain nutrients may be obtained from refractory materials as well as simple organic substrates (Leake et al. 2002). Because the majority of the Amanita species in this genus participate in ECM connections with higher plants, cultivating an Amanita species is often a challenging process. Despite this, more than 10 different Amanita species have been successfully cultivated (Pringle et al. 2009).
The second most prevalent biopolymer on this planet next cellulose is chitin, a linear polymer formed of 1,4-N-acetylglucosamine. In addition to the interior structures of various invertebrates, chitin may be found in the external skeletons of crabs, algae, shrimp, yeasts, insects, lobsters, and fungi (Shahidi and Abozaytoun 2005). Sizes of chitinases, which are glycosyl hydrolases, range from 20 kDa to approximately 90 kDa (Bhattacharya and Gupta 2007). Both live and dead fungi have been found to have their cell walls lysed by chitinase. Numerous biotechnological uses for chitinases include the production of pharmaceutically remarkable chitooligosaccarides and acetyl-D-glucosamine (Sørbotten et al. 2005), controlling pathogenic fungus (Bakri et al. 2022; Ekundayo et al. 2022), treating chitinous waste (Dahiya et al. 2005), isolating protoplasts from fungi and yeasts (Vyas and Deshpande 1991), making single-cell proteins (Krishnaveni and Ragunathan 2014), and dye removal (Abdel Ghany et al. 2019). Chitinases have crucial physiological and ecological roles in a variety of species, including fungi, bacteria, insects, higher plants, and mammals (Mathew et al. 2021; Al-Rajhi et al. 2022a,b). Previously, Mucha et al. (2006) studied the creation of chitinase by Amanita muscaria, Laccaria laccata, and Suillus bovinus with the use of various substrates. They found that monitored that Amanita muscaria was the highest producer of chitinase. The advantage of thermostable chitinases have an advantage over mesophilic chitinases is that Thermostable chitinases can break down the substrate at relatively high temperatures and display decreased viscosity, significantly lower risk of contamination, thermal and chemical stability and better solubility (Akram et al. 2021). Thermostable as well as thermophilic chitinases are increasing popularity in current years, as they can tolerate high temperatures and preserve the stability of chitinases for longer periods.
The Jazan region in Saudi Arabia is characterized with hot climate conditions; thus, these environments were thriving with thermophilic microorganisms and its thermostable products such as enzymes. Most previous investigations are focused on a newly discovered species of mushrooms in Saudi Arabia but have not considered their biotechnological. Therefore, the production of chitinase under stress conditions of temperatures and its antifungal activities were the target of the present investigation.
EXPERIMENTAL
Materials and Methods
Isolation and Culturing of Pure Fungus
The chitinase producer was isolated from Subtropical Region of Saudi Arabia known Abu Arish, Jazan governorate (16°96′26.5′′N, 42°83′55.6′′E). In a rich home garden with grasses and wood residues, mature basidiocarps of basidiomycetes were observed. Under appropriate conditions they were collected for identification according to Yang (2015), Kounbo et al. (2019), and Vizzini et al. (2020). Macroscopic examination was recorded utilizing standard manuals and keys for identification as well as structure of gills and spores. Additionally, stipe width and length, spacing of the lamellae, and existence of ring and volva were remarked. Structure of the collected fruiting bodies was documented via different sections of the sample of collected mushroom. Moreover, a spore print illustrating the internal construction of the collected mushroom gills was taken. Pure fungus culture was obtained from young of fruiting body, a primary culture was prepared by eliminating a small piece from gill , then placing it in tube containing appropriate volume medium containing the following ingredients per 100 mL distilled water (Dextrose 1.5 g, peeled potato 20 g, MgSO4 0.1 g, KH2PO4 0.1 g, KNO3 0.1 g, (NH4)2HPO4 0.05 g, CaCl2 0.025 g, thiamin–HCl 0.02 mg, malt extract 12 mL and 2 g agar) adjusted at pH 5.6 .Fungus culture was incubated at 30 °C for 15 days. The appeared mycelial masses were purified several times using the same growth medium to obtain pure culture.
Chitinase Production and its Thermal Stability at Different Temperatures
Chitinase production was estimated at a range of incubation temperatures from10 to 70 °C. The flasks containing growth media with colloidal chitin or dead fungal biomass (Aspergillus niger via autoclaving) were inoculated with the tested fungus, followed by incubation for 12 days. Once the period of incubation finished, the mycelia that had appeared were detached from the metabolized growth medium, then via 0.5 μM bacteria-proof filter the broth was filtrated, which was followed by centrifugation for 5 min at 8,000 rpm. The obtained supernatant was precipitated using ammonium sulphate at 0-100% ranges at 4 °C and continuously stirred up to 12 h. To obtain the precipitate, the supernatant was re-centrifuged at 9,000 rpm at 4 °C for a period of 30 min. The obtained precipitate was dissolved within 0.2 M of phosphate buffer (pH 9), then dialyzed against the same buffer to obtain crude chitinase, then the activity of produced chitinase at each incubation temperature was estimated. The thermal stability of chitinase was assessed, where the obtained enzyme was incubated at separate temperature from 10 °C up to 70 °C for 60 min, and then utilized a water bath (WBL-10LC-SSD1, LW Scientific, Lawrenceville, GA, USA). Activity of enzyme was detected as mentioned previously (Al Abboud et al. 2022).
Chitinase Production at Different pH
Chitinase production was estimated within a range of pH from 3 to 9. The flasks containing growth media with colloidal chitin or dead fungal biomass were adjusted at different pH, then inoculated with the tested fungus, followed by incubation for 12 days. Once the incubation period finished, the activity of chitinase was estimated.
Chitinase Activity Assessment
The filtrate metabolized medium of fungus growth (100 µL) was fused with colloidal chitin (200 µL) and mixed with sodium acetate buffer (10 mM) that adapted at pH 5.2. For 1 h at 50 °C, the reaction mixture was reserved, then using dimethylamino benzaldehyde (DMAB) reagent (Sigma-Aldrich Chemical Co., Ltd., St. Louis, MO, USA), the liberated N-acetylglucosamine was quantified. A certain. Certain volume of the mixture reaction (0.5 mL) was mixed in the test tube with the prepared 120 mM potassium borate buffer (0.5 mL) that equilibrated at pH 8.9 followed by warming for 3 min in boiling water via water bath. After that, it was cooled, and 3 mL DMAB were added and subsequently kept at 38 °C for 20 min, re-cooled at 20 °C, then at 544 nm the absorbance was recorded spectrophotometry (Jasco, Japan). The liberated N-acetylglucosamine was estimated via calibration of stock curve of N-acetylglucosamine in borate buffer (Reissig et al. 1955), and the quantity of liberated protein was evaluated according to Bradford (1976) using bovine serum albumin.
Fungal Inactivation by Chitinase
Under sterilized conditions,100 U/mL of the produced chitinase using the two substrates (Chitin and dead fungal biomass) were added to the autoclaved Czapek Dox Agar medium (Sigma-Aldrich Chemical Co., Ltd ,St. Louis, MO, USA). Then, the growth medium was poured into sterilized Petri plates, followed by inoculation with 0.6 mm of active colonies of tested fungi (Fusarium oxysporum, Aspergillus flavus, Aspergillus terreus, Curvularia lunata, Cladosporium cladosporioides, and Alternaria alternata) in the center of plates. They were incubated at 30 °C for 7 days. Once the incubation time finished, the inhibitory potential of chitinase was measured by measuring the colony radius.
Statistical Analysis
Standard deviation of all results were recorded from calculation of the mean three obtained results.
RESULTS AND DISCUSSION
Typical mature ascocarps (Fig. 1) were observed during a dry period after 14 days of rains in Abu Arish rejoin. The collected ascocarps (Fig. 2) were subjected for characterization and identification. The isolate was identified for level of genus as Amanita sp.
Fig. 1. Mature ascocarps of Amanita sp. growing in soil richened with grasses
Fig. 2. Basidiocarp of Amanita sp. annulus and the scales on the cap
In Jazan rejoin, nine genera of mushroom were observed including Amanita, Agaricus, Boletus, Pleurotus, Podaxis, Lepiota, Coprinus, and Agrocybe (Manzelat 2019), the highest frequency was associated to Lepiota procera, L. rhacodes, and Agaricus bisporus. However, in previous study (Abou-Zeid and Altalhi 2006), Pleurotus ostreatus, Lepiota cristata, and Boletus rarely occurred. Some species of mushrooms were firstly recorded by Jamal et al. (2019) in Saudi Arabia including A. pediades, C. scabella, P. conopilea, T. fimbriatum, and P. demidoffii from Al-Baha, while C. palaeotropicum and C. macrospora were collected from Jeddah and Al-Taif, respectively. The collected isolate was recorded previously as mentioned but not subjected for biotechnological application, particularly chitinase production. Therefore, it was tested for enzyme production at different conditions using different substrates.
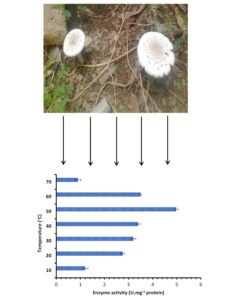
From Fig. 3, two parameters including different temperatures up to 70 °C and different substrates (namely dead fungal biomass and chitin) were effective for chitinase production. It is clear that chitinase activity increased with increased temperature up to 50 °C, . Then it decreased and continued in activity until 70 °C. It is also evidenced from the Fig. 3 that the chitinase activity at 60 °C was higher than its activity at 20 and 30 °C. These results reflected growth of the producing fungus in the climatic condition of the Jazan region, which is characterized by high temperatures. In contrast, chitin encouraged the chitinase activity compared with fungal dead biomass at all tested temperatures but at a narrow spectrum. The activity was 1.2, 2.76, 3.2, 3.4, 4.98, 3.5, and 0.9 U.mg-1 using chitin, whereas the activity was 0.9, 2.1, 2.98, 3.2, 4.6, 3.1, and 0.6 U.mg-1 using dead fungal biomass at 10, 20, 30, 40, 50, 60, and 70 °C, respectively. Thermostable and denaturation-resistant chitinase was produced by A. niveus at 65 °C and pH 5.0 (Alves et al. 2018). Its stability was recorded at 60 °C for up to 2 h. According to a previous report, chitin induced the fungal chitinase with maximum activity at 1% chitin after 8 days (Alves et al. 2018). Fungal mycelia were utilized for chitinase production in numerous examinations. Mucha et al. (2006) mentioned that during mycoparasitism, chitinase production was observed by a number of ECM fungi including Amanita muscaria, Laccaria laccata, Suillus bovinus, and S. luteus using different enzyme substrates including colloidal chitin and different mycelia of fungi including Mucor hiemalis, Trichoderma harzianum, and T. virens. Earlier investigation detected that the activities of root chitinase was encouraged by ECM Pisolithus tinctorius against phytopathogenic Phytophthora cinnamomi (Albrecht et al. 1993).
Thermal stability results indicated that chitinase produced using the two substrates was stable up to 60 °C, and then it gradually lost its activity (Fig. 4). There was no relationship among optimum temperature of enzyme activity and thermal stability. For instance in the current study, 50 °C yielded optimal enzyme activity, while thermal stability continued up to 60 °C. The property of enzyme thermal stability may depend on the fungus producer as previously mentioned and exposure time to temperature (Xia et al. 2001; Farag et al. 2016). Thermal stability studies indicated that chitinase of C. cladosporioides (Al Abboud et al. 2022), Trichoderma viride (Ekundayo et al. 2016), Penicillium chrysogenum (Atalla et al. 2020), and A. flavus (Beltagy et al. 2018) was stable up to 50 ºC. The high activity of thermostable chitinase at higher temperatures, in agreement with Guo et al. (2008), gave it remarkable benefits over industrial catalysis. For instance, the creation of chito-oligosaccharides from the waste of chitin conducted at elevated temperature can decrease the viscosity and contamination of the medium. Additional probable utilization is in control agents involving chitinase to fight plant infections by molds as well as insects that should be applied in the fields for long times at high temperatures (Shahidi and Abozaytoun 2005; Bakri et al. 2022). The conservation of the enzymatic activity beneath adverse environments is vital to achieve affirmative effects in the protection of plant from pathogens invasion.
Fig. 3. Chitinase production at different temperatures using two substrates chitin and dead fungal mycelia
Fig. 4. Chitinase thermal stability produced using two substrates at different temperatures
Activity of chitinase at different pH was visualized in Fig. 5 using chitin and fungal dead biomass. The results showed that pH 6 was optimal for chitinase activity using the two substrates. As can be seen from the results, acidic conditions at pH 4 and 5 were preferable than alkaline conditions at pH 8 and 9 for enzyme activity. Additionally, chitin compared with dead fungal biomass was more efficient for chitinase production. This may be because of the occurrence of other components in dead fungal mycelia that interfere with the secretion of enzyme. Some investigators reported that pH 5 and 6 were optimum for fungal hydrolases enzymes (Abd El Monssef et al. 2016; Bhamare et al. 2018).
Fig. 5. Chitinase production at different pH using two substrates chitin and dead fungal mycelia
In this work, the impact of chitinase on the growth of Fusarium oxysporum, Aspergillus flavus, Aspergillus terreus, Curvularia lunata, Cladosporium cladosporioides, and Alternaria alternata was studied (Table 5). The obtained results indicated that all growth of the tested fungi decreased remarkably according to substrate used. In addition, the decrease in the growth was variable according to species. All results of fungal growth inhibition by chitinase were compared to fungal growth for control that were without treatment with chitinase. Antifungal activity of produced chitinase at 50 ºC was more than at 60 ºC, where the colony radius was less at 50 ºC than at 60 ºC. For instance, Alternaria alternata colony radius was 3.50 ± 0.07 and 2.26 ± 0.05 cm at 50 ºC, while it was 4.35 ± 0.07 and 4.13 ± 0.05 cm at 60 ºC using dead fungal mycelia and chitin as as substrate of enzyme, respectively. These results indicated that the chitinase has thermophilic properties. Although the chitinase activity towards the tested fungi decreased at 60 ºC, it did not lose activity. These results confirmed earlier results about the fungistatic activity of chitinase (Al-Rajhi et al. 2022a). Additionally, for example, Pandya and Saraf (2015) reported that the mechanism of chitinase activity against plant disease-causing fungi, such as Macrophomina phaseolina (60% inhibition) and Rhizoctonia solani (73% inhibition), via lysis of cell wall construction. Different levels of the antifungal potential of chitinase produced by Aspergillus niveus were observed with different values of MIC against different fungi (21 µg/mL for A. fumigatus and Paecilomyces variotii), (24 µg/mL for A. flavus and A. phoenicis), (84 µg/mL for A. niger) (Alves et al. 2018). A deficiency of A. niger chitinase activity was recorded after 3 h at 60 °C (Brzezinska and Jankiewicz 2012). In another investigation (Beltagy et al. 2018), the activity of A. flavus chitinase was stable for 15 min at 50 °C, while the activity of Penicillium chrysogenum chitinase was stable for 1 h at 50 °C (Atalla et al. 2020).
Table 1. Effect of Chitinase at Different Temperatures for 30 min on Fungal Growth
CONCLUSIONS
- Amanita sp. was able to produce thermostable chitinase at 60 °C using chitin and dead fungal mycelia as enzyme substrates.
- The produced chitinase at high temperature play a critical role in control of phytopathogenic fungi.
- Acidic conditions at pH 4 and 5 were more effective in increasing chitinase production.
ACKNOWLEDGMENTS
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R217), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia are acknowledged.
REFERENCES CITED
Abd El Monssef, R. A., Hassan, E. A., and Ramadan, E. M. (2016). “Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment,” Annals of Agricultural Sciences 61(1), 145-154. DOI: 10.1016/j.aoas.2015.11.007
Abdel Ghany, T. M., Alawlaqi, M. M., Shater, A. R. M., and Abboud, M. A. A. (2019). “Congo red biosorption with live and dead biomass of thermophilic Aspergillus fumigatus,” The Egyptian Journal of Experimental Biology (Botany) 15(1), 1-6. DOI: 10.5455/egyjebb.20181206084342
Abou-Zeid, A. M., and Altalhi, A. E. (2006). “Survey of some mushrooms in Al-Taif Governorate of Saudi Arabia,” Survey of Some Mushrooms in Al-Taif Governorate of Saudi Arabia 2(1), 1-5
Al Abboud, M. A., Al-Rajhi, A. M. H., Shater, A.-R. M., Alawlaqi, M. M., Mashraqi, A., Selim, S., Al Jaouni, S. K., and Abdelghany, T. M. (2022). “Halostability and thermostability of chitinase produced by fungi isolated from salt marsh soil in subtropical region of Saudi Arabia,” BioResources 17(3), 4763-4780. DOI: 10.15376/biores.17.3.4763-4780
Albrecht, C., Asselin, A., Piche, Y., and Lapeyrie, F. (1993). “Chitinase activities are induced in Eucalyptus globulus roots by ectomycorrhizal or pathogenic fungi during early colonization,” Physiologia Plantarum 91, 104-110.
Al-Rajhi, A. M. H., Asmaa, A. A., Reham, Y., and Abdel Ghany, T. M. (2022a). “Induction of hydrolytic enzyme production and antibiosis via a culture of dual fungal species isolated from soil rich with the residues of woody plants in Saudi Arabia,” BioResources 17(2), 2358-2371. DOI: 10.15376/biores.17.2.2358-2371
Al-Rajhi, A. M. H., Yahya, R., Alawlaqi, M. M., Fareid, M. A., Amin, B. H., and Abdelghany, T. M. (2022b). “Copper oxide nanoparticles as fungistat to inhibit mycotoxins and hydrolytic enzyme production by Fusarium incarnatum isolated from garlic biomass,” BioResources 17(2), 3042-3056. DOI: 10.15376/biores.17.2.3042-3056
Akram, F., Akram, R., Haq, I. U., Nawaz, A., Jabbar, Z., and Ahmed, Z. (2021). “Biotechnological eminence of chitinases: A focus on thermophilic enzyme sources, production strategies and prominent applications,” Protein and Peptide Letters 28(9):1009-1022. DOI: 10.2174/0929866528666210218215359.
Alves, T. B., de Oliveira, P. H., de Oliveira, A. H. C., Jorge, J. A., and Guimarães L. H. S. (2018). “Production and characterization of a thermostable antifungal chitinase secreted by the filamentous fungus Aspergillus niveus under submerged fermentation,” 3 Biotech 8(8), Article Number 369. DOI: 10.1007/s13205-018-1397-6
Bakri, M. M., Al-Rajhi, A. M. H., Abada, E., Salem, O. M. A., Shater, A.-R., Mahmoud, M. S., and Abdel Ghany, T. M. (2022). “Mycostimulator of chitinolytic activity: Thermodynamic studies and its activity against human and food-borne microbial pathogens,” BioResources 17(3), 4378-4394. DOI: 10.15376/biores.17.3.4378-4394
Beltagy, E. A., Rawway, M., Abdul-Raouf, U. M., Elshenawy, M. A., and Kelany, M. S. (2018). “Purification and characterization of thermohalophilic chitinase producing by halophilic Aspergillus flavus isolated from Suez Gulf,” The Egyptian Journal of Aquatic Research 44(3), 227-232. DOI: 10.1016/j.ejar.2018.08.002
Bhamare, H. M., Jadhav, H. P., and Sayyed, R. Z. (2018). “Statistical optimization for enhanced production of extracellular laccase from Aspergillus sp. HB_RZ4 isolated from bark scrapping,” Environmental Sustainability 1(2), 159-166. DOI: 10.1007/s42398-018-0015-1
Bhattacharya, D. A., and Gupta, R. K. (2007). “Bacterial chitinases: Properties and potential,” Critical Reviews in Biotechnology 27(1), 21–28. DOI: 10.1080/07388550601168223
Bradford, M. M. (1976). “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding,” Analytical Biochemistry 72(1-2), 248-254. DOI: 10.1016/0003-2697(76)90527-3
Brzezinska, M. S., and Jankiewicz, U. (2012). “Production of antifungal chitinase by Aspergillus niger LOCK 62 and its potential role in the biological control,” Current Microbiology 65(6), 666-672. DOI: 10.1007/s00284-012-0208-2
Dahiya, N., Tewari, R., Tiwari, R. P., and Hoondal, G. S. (2005). “ Production of an antifungal chitinase from Enterobacter sp. NRG4 and its application in protoplast production,” World Journal Microbiology and Biotechnology 21, 1611–1616 . DOI: 10.1007/s11274-005-8343-6
Ekundayo, F. O., Folorunsho, A. E., Ibisanmi, T. A., and Olabanji, O. B. (2022). “Antifungal activity of chitinase produced by Streptomyces species isolated from grassland soils in Futa Area, Akure,” Bulletin of the National Research Centre 46, Article Number 95. DOI: 10.1186/s42269-022-00782-4
Ekundayo, E. A., Ekundayo, F. O., and Bamidele, F. (2016). “Production, partial purification and optimization of a chitinase produced from Trichoderma viride, an isolate of maize cob,” Mycosphere 7(6), 786-793. DOI: 10.5943/mycosphere/7/6/9
Enjalbert, F., Cassanas, G., Guinchard, G., and Chaumont, J. P. (1996). “Toxin composition of Amanita phalloides tissues in relation to the collection site,” Mycologia 88(66), 909–921. DOI: 10.1080/00275514.1996.12026731
Farag, A. M., Abd-Elnabey, H. M., Ibrahim, H. A. H., and El-Shenawy, M. (2016). “Purification, characterization and antimicrobial activity of chitinase from marine derived Aspergillus terreus,” Egypt Journal of Aquatic Research 42, 185–192. DOI: 10.1016/j.ejar.2016.04.004
Guo, R.-F., Shi, B.-S., Li, D.-C., Ma, W., and Wei, Q. (2008). “Purification and characterization of a novel thermostable chitinase from Thermomyces lanuginosus SY2 and cloning of its encoding gene,” Agricultural Sciences in China 7(12), 1458–1465. DOI: 10.1016/S1671-2927(08)60403-4
Jamal, M. K., Naiyf, S. A., Ramzi, A. M., Shine, K., and Ahmed, S. A. (2019). “First report on morphological and molecular characteristics of eight Agaricomycotina mushrooms in Saudi Arabia,” Journal of King Saud University – Science 34(8), Article ID 102345. DOI: 10.1016/j.jksus.2022.102345
Kounbo, D., Elise, S. K., Marie, L. G., Samson, N., Elisabeth, Z., and Philippe, S. (2019). “Taxonomical study of the genus Amanita from Western Burkina Faso,” Journal of Yeast and Fungal Research 10(2), 45-54. DOI: 10.5897/JYFR2019.0193
Krishnaveni, B., and Ragunathan, R. (2014). “Chitinase production from marine wastes by Aspergillus terreus and its application in degradation studies,” International Journal of Current Microbiology and Applied Sciences 3(1), 76-82.
Leake, J. R., Donnelly, D. P., and Boddy, L. (2002). “Interactions between ecto-mycorrhizal and saprotrophic fungi,” in: Mycorrhizal Ecology, Vol. 157, M. G. A. van der Heijden, and I. R. Sanders (eds.), Springer, Berlin, Heidelberg, Germany, pp. 345-372. DOI: 10.1007/978-3-540-38364-2_14
Mathew, G. M., Madhavan, A., Arun, K. B., Sindhu, R., Binod, P., Singhania, R. R., Sukumaran, R. K., and Pandey, A. (2021). “Thermophilic chitinases: Structural, functional and engineering attributes for industrial applications,” Applied Biochemistry and Biotechnology 193(1):142-164. DOI: 10.1007/s12010-020-03416-5.
Manzelat, S. M. (2019). “Mushrooms in Saudi Arabia (local and imported),” International Journal of Current Research in Biosciences and Plant Biology 6(8), 8-12. DOI: 10.20546/ijcrbp.2019.608.002
Mucha, J., Hanna D., Edmund S., and Antoni, W. (2006). “Synthesis of enzymes connected with mycoparasitism by ectomycorrhizal fungi,” Archives of Microbiology 185(1), 69–77. DOI: 10.1007/s00203-005-0068-2
Onguene, N. A., and Kuyper, T. W. (2012). “Habitat and diversity of ectomycorrhizal fungi in forests of South Cameroon,” Cameroon Journal of Experimental Biology 8(1), 26-34.
Pandya, U., and Saraf, M. (2015). “Purification and characterization of antifungal chitinase from Bacillus safensis MBCU6 and its application for production of chito-oligosaccharides,” Biologia 70, 863–868. DOI: 10.1515/biolog-2015-0112
Podlasińska, J., Mazurkiewicz, N., and Szymańska, A. (2015). “Content of Pb, Hg, Zn, Mn, Cu, and Fe in macrofungi collected from Wkrzanska Forest in Northwestern Poland,” Polish Journal of Environmental Studies 24(2):651-656. DOI: 10.15244/pjoes/26959.
Pringle, A., Adams, R. I., Cross, H. B., and Bruns, T. D. (2009). “ The ectomycorrhizal fungus Amanita phalloides was introduced and is expanding its range on the west coast of North America,” Molecular Ecology, 18(5):817-833. DOI: 10.1111/j.1365-294x.2008.04030.x.
Reissig, J. L., Strominger, J. L., and Lefloir, L. F. (1955). “A modified colorimetric method for the estimation of N-acetyl amino sugars,” The Journal of biological Chemistry 217(2), 959-966.
Sørbotten, A., Horn, S. J., Eijsink, V. G., and Vårum, K. M. (2005). “Degradation of chitosans with chitinase B from Serratia marcescens. Production of chito-oligosaccharides and insight into enzyme processivity,” FEBS Journal 272(2), 538-549. DOI: 10.1111/j.1742-4658.2004.04495.x
Shahidi, F., and Abozaytoun, R. (2005). “Chitin, chitosan and co-products: Chemistry, production, applications and health effects,” Advances in Food and Nutrition Research 49, 93–135. DOI: 10.1016/S1043-4526(05)49003-8
Vizzini, A., Cingarlini, C., Sartori, D., Maraia, G. L., Setti, L., Poumarat, S., Kudzma, L., and Dovana, F. (2020). “Assessing the taxonomic status of Amanita citrina var. intermedia (Basidiomycota, Agaricales),” Phytotaxa 440(1), 055-068. DOI: 10.11646/phytotaxa.440.1.3
Vyas, P., and Deshpande, M. V. (1991). “Enzymatic hydrolysis of chitin by Myrothecium verrucaria chitinase complex and its utilization to produce SCP,” Journal of General and Applied Microbiology, 37, 267-257 DOI:10.2323/JGAM.37.267
Xia, G., Jin, C. Z., Hou, J. Y., Ang, S. Z., Hang, S., and Jin, C. (2001). “A novel chitinase having a unique model of action from Aspergillus fumigatusYJ-407,” European Journal of Biochemistry 208, 4079-4085. DOI: 10.1046/j.1432-1327.2001.02323.x
Yang, Z. L., Cai, Q., and Cui, Y. Y. (2018). “Phylogeny, diversity and morphological evolution of Amanitaceae,” Biochemical Systematics and Ecology 34, 359–380.
Yang, Z. L. (2015). Atlas of the Chinese Species of Amanitaceae, Science Press, Beijing, China.
Article submitted: January 28, 2023; Peer review completed: February 22, 2023; Revised version received and accepted: February 22, 2023; Published: February 28, 2023.
DOI: 10.15376/biores.18.2.2928-2939
