Abstract
Wood veneer/biopolyethylene (bio-PE) biocomposite materials were produced by using poly(N-vinylformamide-co-vinylamine) (PVFA-co-PVAm) copolymers as a phase-mediating reagent. In a preliminary step, PVFA-co-PVAm was adsorbed onto the wood veneer component from aqueous solution. In its adsorbed form, it served as an adhesion promoter and improved the compatibility between both the highly polar wood veneer and weakly polar bio-PE surface. Structural parameters and their effect on the adsorption process, such as the degree of hydrolysis (DH) of poly(N-vinylformamide) (PVFA) (30, 50, and > 90%), the molecular weight of PVFA-co-PVAm (Mw 10,000, 45,000, or 340,000 g/mol), and the pH value (4, 7, and 11) influenced the resulting wetting behavior of the PVFA-co-PVAm-modified wood veneer surface. Thus, the hydrophobizing effect of the PVFA-co-PVAm was clearly detectable because the contact angle with water was considerably increased up to 116° by adsorption of PVFA-co-PVAm 9095 at pH 11. The adsorbed amount of PVFA-co-PVAm was determined by energy-dispersive X-ray (EDX) spectroscopy and X-ray photoelectron spectroscopy (XPS). The PVFA-co-PVAm-coated wood veneers were consolidated with bio-PE in a hot press process. The modified composite materials showed remarkably improved Young’s moduli (552 MPa) and tensile strengths (4.5 MPa) compared to former composite materials produced without PVFA-co-PVAm modification.
Download PDF
Full Article
Aqueous Poly(N-Vinylformamide-co-Vinylamine) as a Suitable Adhesion Promoter for Wood Veneer/Biopolyethylene Composite Materials
Rico John,a Katja Trommler,a Katja Schreiter,a Carolin Siegel,b Frank Simon,c André Wagenführ,b and Stefan Spange a,*
Wood veneer/biopolyethylene (bio-PE) biocomposite materials were produced by using poly(N-vinylformamide-co-vinylamine) (PVFA-co-PVAm) copolymers as a phase-mediating reagent. In a preliminary step, PVFA-co-PVAm was adsorbed onto the wood veneer component from aqueous solution. In its adsorbed form, it served as an adhesion promoter and improved the compatibility between both the highly polar wood veneer and weakly polar bio-PE surface. Structural parameters and their effect on the adsorption process, such as the degree of hydrolysis (DH) of poly(N-vinylformamide) (PVFA) (30, 50, and > 90%), the molecular weight of PVFA-co-PVAm (Mw 10,000, 45,000, or 340,000 g/mol), and the pH value (4, 7, and 11) influenced the resulting wetting behavior of the PVFA-co-PVAm-modified wood veneer surface. Thus, the hydrophobizing effect of the PVFA-co-PVAm was clearly detectable because the contact angle with water was considerably increased up to 116° by adsorption of PVFA-co-PVAm 9095 at pH 11. The adsorbed amount of PVFA-co-PVAm was determined by energy-dispersive X-ray (EDX) spectroscopy and X-ray photoelectron spectroscopy (XPS). The PVFA-co-PVAm-coated wood veneers were consolidated with bio-PE in a hot press process. The modified composite materials showed remarkably improved Young’s moduli (552 MPa) and tensile strengths (4.5 MPa) compared to former composite materials produced without PVFA-co-PVAm modification.
Keywords: Biocomposite material; Adhesion promoter; Compatibilizer; Biopolyethylene; Wood veneer; Polyvinylamine; Adsorption; Natural fiber reinforced thermoplastics
Contact information: a: Department of Polymer Chemistry, Chemnitz University of Technology, 09107 Chemnitz, Germany; b: Institute of Wood and Paper Technology, Technische Universität Dresden, 01062 Dresden, Germany; c: Leibniz Institut für Polymerforschung Dresden e.V., Hohe Strasse 6, 01069 Dresden, Germany; *Corresponding author: stefan.spange@chemie.tu-chemnitz.de
INTRODUCTION
Ecological and economical sustainability concerns play an important role in the construction of lightweight materials, especially in mechanical engineering and building trades (Hanselka 1998; Herrmann et al. 1998; Fowler et al. 2006). Natural fiber reinforced thermoplastics have gained greater attention, among other things, in automobile body construction because of their excellent properties, such as high stiffness, high strength, and low density (Woodhams et al. 1984; Sain and Panthapulakkal 2006; Ashori 2008, Carus et al. 2008). Natural fibers are mainly obtained from flax, hemp, jute, sisal, and wood, whereas the majority of thermoplastics, such as polyolefins, polyvinyl chloride, and polyamide, are produced from crude oil. A more environmentally friendly approach for thermoplastics is the utilization of biopolymers, which are made from renewable materials (Sain and Panthapulakkal 2004). Additionally, composite materials produced from biopolymers are completely made of green materials.
The incompatible surface polarities of the polar natural fibers and the nonpolar thermoplastic matrix of polyolefins frequently causes poor mechanical properties during the compounding process (Kazayawoko et al. 1999; Torres and Cubillas 2005). The most common approach to obtain a homogeneous and compatible composite material is the modification of a thermoplastic component. Usually, polyolefins have been grafted with maleic anhydride to promote adhesion (Woodhams et al. 1984; Dalväg et al. 1985; Raj et al. 1989; Felix and Gatenholm 1991; Kazayawoko et al. 1999; Colom et al. 2003; Lai et al. 2003).
The production of biocomposite materials requires the use of natural reinforcing materials, which are embedded in the biopolymers. In order to avoid a modification of the biopolymers, it seems possible to instead modify the surfaces of the reinforcing materials. Biopolyethylene (bio-PE), which is less expensive than biopolyamide, provides several benefits, such as lower processing temperatures. However, little studies have been done to the production of veneers/bio-PE in thin bio-based wooden laminates (Buchelt et al. 2015). Unidirectional wood veneer processed with bio-PE and bio-PA were fabricated. The tensile strength and the Young’s modulus of these produced composite materials are considerably higher compared to the common wood-plastic-composites (WPC). Short wood fibers and particles, respectively, which are used in WPC do not exploit the full mechanical spectrum of the wood fibers (Ashori 2008; Wagenführ 2008). In order to utilize the structural anisotropy of the wood, it has to be processed to orientated unidirectional wooden layers (wood veneer). It is assumed, that an adhesion promoter is necessary to make the largely non-polar bio-PE compatible with cellulose-based reinforcing materials.
Wood mainly consists of cellulose, hemicellulose, and lignin. Hydroxyl and carbonyl groups situated on the surface endow wood materials with a pronounced surface polarity (Fengel and Grosser 1975). The presence of negatively charged surface sites supports the entropy-driven adsorption of cationic polyelectrolytes by attractive electrostatic interactions (Sandermann and Rothkamm 1959; Gindl and Tschegg 2002; Jung and Roffael 2002).
Poly(N-vinylformamide-co-vinylamine) (PVFA-co-PVAm) is partially ionic over a wide pH range, and it seems to be a suitable cationic polyelectrolyte because it can be spontaneously adsorbed onto negatively charged surfaces from an aqueous solution (Pelton 2014). Two primary routes for the synthesis of PVFA-co-PVAm have been established, the radical polymerization of N-vinylformamide with consecutive acid- or base-catalyzed hydrolysis of poly(N-vinylformamide) (PVFA) (Pinschmidt Jr. et al. 1997; Gu et al. 2002) and the Hoffmann rearrangement of polyacrylamide (Mullier and Smets 1957; Achari et al. 1993; Pelton 2014). The hydrolysis of PVFA does not completely convert formamide groups into primary amino groups, but the degree of hydrolysis (DH) and the resulting content of amino groups, as well as the residual formamide groups in the PVFA-co-PVAm, can be controlled. PVFA-co-PVAm compounds with high degrees of hydrolyzed formamide groups are characterized by high charge densities, which can be precisely adjusted with the pH of the aqueous solvent. For DHs below 50%, the resulting charge densities do not correlate with the pH value (BASF SE 2004).
The adsorption of PVFA-co-PVAm onto cellulose (Geffroy et al. 2000; Shulga et al. 2003a,b; Miao et al. 2007), silica (Voigt et al. 2000, 2001; Spange et al. 2004), carbon black (Piasta et al. 2009), glass (Poptoshev et al. 1999, 2000), metal, and metal oxides have been studied extensively (Seifert et al. 2011, 2012). However, the adsorption behaviors of PVFA-co-PVAm onto wood-based substrates and their interactions on the manifold surface structure of wood have been barely investigated (Khabbaz et al. 2008). The cited authors reported a method of gluing wood-based materials by using different adhesive compositions, e.g. poly(vinyl amine) in combination with various starch samples, such as corn starch. According to previous studies (Voigt et al. 2001; Piasta et al. 2009; Seifert et al. 2011), it is expected that the adsorption of PVFA-co-PVAm onto wood will have an effect on the surface polarity. It is assumed, that the adaption of the surface polarities improves the adhesion strength with polyolefins because the dispersion forces could operate effectively.
The adsorbed PVFA-co-PVAm endows the wood substrate with a large number of primary amino groups, which can be used as surface sites to carry out polymer analogous reactions (Badesso et al. 1993; Fischer and Heitz 1994; Pinschmidt Jr. et al. 1997). The attached amino groups can also be crosslinked with bi- or multi-functional agents, such as diisocyanates, bis-epoxides, or electrophilic carbonyl compounds, to fix the groups irreversibly on the substrate surface (Voigt et al. 2000). In this sense, PVFA-co-PVAm can be considered a polymeric adhesion promoter that is able to compatibilize a reinforcing solid surface with a polymer matrix and allows the adaption of the solid/polymer interphase to the desired adhesive, wetting, and mechanical properties.
The aim of the current work was the development of novel biocomposites by the combination of wood veneer and bio-PE. For this purpose, the adsorption of PVFA-co-PVAm onto wood veneer surfaces was studied by varying the DH of the PVFA, molecular weight of the PVFA-co-PVAm copolymers, weight concentration, pH value of the aqueous PVFA-co-PVAm solutions, and adsorption time. In order to produce biocomposite materials, the PVFA-co-PVAm-coated wood veneers were processed, and their tensile strength and Young’s modulus were determined.
EXPERIMENTAL
Materials
Wood veneers with a thickness of 0.55 mm (sliced veneer) were made from European beech wood veneer (Fagus sylvatica L.). Pieces of bio-PE foil (Green PE SHC 7260) with a 100-µm thickness were purchased from Braskem, São Paulo, Brazil. Bio-PE is produced from ethanol, which is obtained from sugarcane. Aqueous PVFA-co-PVAm (Lupamin®) (Mw = 340,000 g/mol [9030], [9050], [9095]) solutions and low molecular weight PVFA-co-PVAm solutions (Mw ≈ 10,000 g/mol [1595], and Mw = 45,000 g/mol [4595]) were provided by BASF SE (Ludwigshafen, Germany). The PVFA-co-PVAm was abbreviated as [90xx], where xx indicated the DH of the PVFA, namely 30%, 50%, and greater than 90%. To remove the formate ions, which were released during the hydrolysis process of PVFA, PVFA-co-PVAm solutions with a DH greater than 90% were purified by microfiltration. High-purity water (TKA-Pacific-UPW, σ = 0.078 μS/cm) was used to dilute the PVFA-co-PVAm solutions. The stock concentrations of the various aqueous PVFA-co-PVAm solutions may be found in the appendix (Table S1).
Table 1. Supplements to the Aqueous PVFA-co-PVAm Solutions
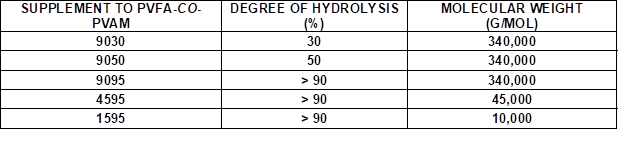
Methods
Adsorption of PVFA-co-PVAm onto the wood veneer surfaces
Adsorption of PVFA-co- PVAm onto the wood veneer surfaces were performed at room temperature. The standard adsorption time was 10 min in order to ensure reproducibility of the various adsorption experiments. The weight concentrations of the PVFA-co-PVAm solutions were adjusted by diluting the polymer stock solution with deionized water. The adsorption procedures were conducted at different pH values. The pH values were obtained with 0.1 mol/L solutions of HCl or NaOH. The pH measurements were carried out using a Vario pH meter (WTW, Weilheim, Germany), which was equipped with a glass electrode and an AgCl/KCl reference electrode. The accuracy of the measurements was within 0.01 pH unit.
The wood veneers were soaked with the aqueous PVFA-co-PVAm solutions for different lengths of time, and were subsequently dried at room temperature for 2 h. Afterwards, the coated wood veneers were finally dried at 40 °C in a vacuum drying oven for 3 d and stored under inert conditions.
Preparation of the wood veneer/biopolyethylene composites by hot pressing
Two coated wood veneers were hot pressed at 1 MPa and 170 °C for 1 min with a piece of bio-PE foil between them. The freshly pressed composites were kept in the press (universal test machine TIRAtest 2810, Tira, Schalkau, Germany) at 1 MPa and cooled down. Then, they were stored in a conditioned atmosphere with 65% relative humidity at 20 °C for 1 week.
Fig. 1. Preparation of the wood veneer/biopolyethylene composite material by hot pressing
Instrumentation
Contact angle measurements
Static contact angle (CA) measurements were carried out as sessile-drop experiments by employing a Contact Angle Measuring System G2 (Krüss, Hamburg, Germany). A droplet of deionized water (surface tension γsl = 72.75 mN/m at 20 °C) was placed with a motor-controlled syringe onto the surface of the sample. In order to measure the advancing CA values (θadv) and characterize the wetting process, the droplet volume was increased. For measuring the receding CA values (θrec), the wetted surface was de-wetted by reducing the volume of the droplet. Drop Shape Analysis Software DSA II (Krüss) was used to analyze the shape of the droplet and calculate the corresponding CA values. The reported CA values were the mean values of three individual measurements carried out at different locations on the surface of the sample. The contact angle hysteresis (CAH, Δθ) was computed as the difference between the advancing and receding CA (Δθ = θadv – θrec).
Scanning electron microscopy (SEM)
With a BAL-TEC SCD 050 sputter coater (Capovani Brothers, Scotia, NY, USA), all of the samples were coated with platinum at 40 mA for 120 s. Scanning electron microscopy (SEM) images were taken with a Nova NanoSEM (FEI, Hillsboro, OR, USA). Energy-dispersive X-ray (EDX) spectroscopy was performed for the overall mapping and three-point analysis with the same instrument. The spectra recorded at 1000-fold magnification from the three-point measurements at three different positions for every sample were averaged and quantified (TU Chemnitz, Department of Solid Surfaces Analysis).
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectroscopy (XPS) studies were carried out with an Axis Ultra photoelectron spectrometer (Kratos Analytical, Manchester, UK). The spectrometer was equipped with a monochromatic Al Kα (hν = 1486.6 eV) X-ray source that was operated at 300 W and 20 mA. The kinetic energy of the photoelectrons was determined with a hemispheric analyzer set to pass energy of 160 eV for the wide-scan spectra and 20 eV for the high-resolution spectra. During all of the measurements, the electrostatic charging of the sample was overcompensated by a low-energy electron source working in combination with a magnetic immersion lens. Later, all of the recorded peaks were shifted by the same amount, which was necessary to set the C 1s peak equal to 285.00 eV for the saturated hydrocarbons. The quantitative elemental compositions (atom percentages, e.g., N% and C%) were determined from the peak areas using experimentally determined sensitivity factors and the spectrometer transmission function. The spectrum background was subtracted according to Shirley (1972). The high-resolution XPS spectra were deconvoluted by means of the Kratos spectra deconvolution software. The free parameters of the component peaks were the binding energy, height, full width at half-maximum, and Gaussian-Lorentzian ratio.
Tensile tests
Tensile tests were performed by employing a Hegewald and Peschke Inspekt 10 test machine (Hegewald & Peschke, Nossen, Germany) according to DIN EN ISO 527-4 (1997) with a testing speed of 2 mm/s (DIN 52188 (1979)). Further information of the testing conditions can be seen in the appendix. The load cell of the machine was 500 N. The tensile properties of the conditioned composite materials (65 % relative humidity at 20 °C), which were cut into strips (10 x 100 mm), were determined perpendicular to the fiber direction.
RESULTS AND DISCUSSION
Influence of the Adsorption Parameters on the Resulting Contact Angle of the PVFA-co-PVAm Modified Wood Veneer Surface
The hydrophilic wood veneer was treated with different aqueous PVFA-co-PVAm solutions to obtain a modified material. Analyzing the CA and CAH provided the opportunity to obtain information about the polarities of the coated wood veneer. A 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution was adsorbed onto beech wood veneer at different pH values for 10 min. The experimental procedure is illustrated in Fig. 2. After drying the samples, the CA and CAH values were determined. Table 2 shows the results of the adsorption experiments at different pH values, which affected the CA strongly. The greater the pH value was, then the higher the contact angle. This result was likely because of the higher hydrophilicity of the protonated amino groups compared with the non-charged amino groups (–NH2). In Brønsted-acid solutions, the protonation equilibrium is shifted to the side of the protonated amino groups, –NH3+ (Kuo et al. 2001). However, there was a higher amount of protonated amino groups because of the lower pH value, which seemed unnecessary for improving the electrostatic interactions with the wood surface. This assumption was confirmed by smaller CA values. A successful hydrophobization was achieved for the adsorption of PVFA-co-PVAm at a pH of 7 and 11 because the CA was higher than 90°. The CAH of all of the modifications were approximately 12°. The CA of the native wood veneer could not be measured because water completely wetted the surface.
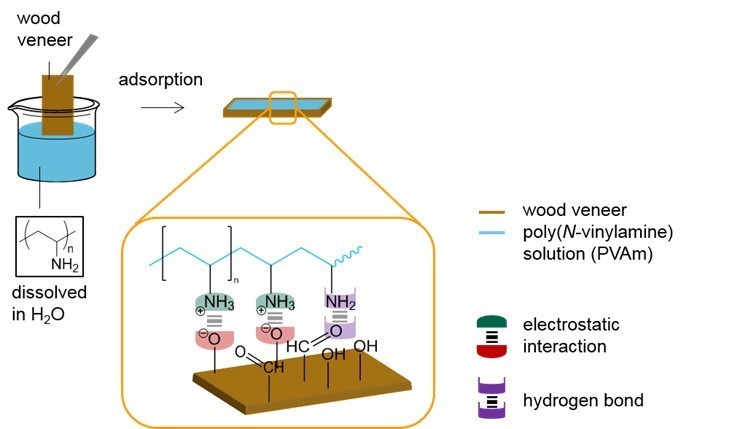
Fig. 2. Suggested adsorption model of PVFA-co-PVAm on the wood veneer surface caused by electrostatic interactions and hydrogen bonds. For reasons of simplification, the formamide groups were omitted, and only the amino and ammonium groups of the copolymer were sketched out.
Table 2. Advancing Contact Angle (θadv) and Contact Angle Hysteresis (Δθ) Measurements of the PVFA-co-PVAm-Modified Wood Veneer as a Function of the pH Value
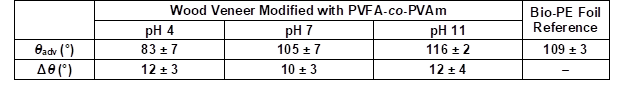
The wood veneer was coated with 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution and the adsorption time was 10 min.
The adsorption of the cationically charged PVFA-co-PVAm polyelectrolytes was mainly driven by adsorption entropy and the formation of strong electrostatic interactions between the polar wood veneer surface and protonated amino groups. Additionally, the formation of hydrogen bonds between the wood veneer and amino groups of the PVFA-co-PVAm contributed to the enthalpy term of the adsorption free energy. As a result of the specific interactions (electrostatic interactions and formation of hydrogen bonds), the coated surfaces had a stronger hydrophobicity (Table 2). Thus, the PVFA-co-PVAm-modified wood veneers achieved CA values similar to that of the bio-PE. These findings can be considered to be the fundamental requirements for compatibilizing a wood material and biopolymer. The amino groups align themselves along the wooden surface while the polymer backbone turns away from the wood veneer surface. This results in a compensation of the polar groups and the polymer backbone increasingly determines the resulting modified surface to external partners such as water drop or PE. It is proposed here hydrophobicity can be attributed to the polymer backbone, which mostly contains -CH2-CH- repeating units. Fig. 2 illustrates the suggested interaction mechanism between the PVFA-co-PVAm and wood veneer after the adsorption process.
Furthermore, the DH of the N-vinylformamide sequences in the PVFA-co-PVAm molecule correlates with the number of primary amino groups along the polymer chain. Because only the amino groups can be protonated, the DH provided the molecular basis for controlling the charge density of the polyelectrolytes by the pH value. A high DH causes a high number of amino groups and a potentially high charge density. Therefore, PVFA-co-PVAm with a DH of 30% (9030), 50% (9050), and greater than 90% (9095) were investigated. The approximate charge densities of the used PVFA-co-PVAm as a function of the pH value are shown in Table 3. The 0.5 wt.% aqueous polymer solution was adsorbed for 30 s.
Table 3. Approximate Charge Densities of the Used PVFA-co-PVAm Copolymers as Function of the pH Values (BASF SE 2004)
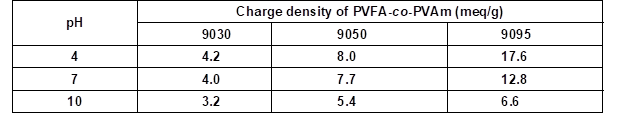
Figure 3 (and Table S2) shows that the wood veneer coated with PVFA-co-PVAm 9030 had contact angles smaller than 90° over the entire pH range. Thus, the desired hydrophobization, which is a necessary condition to compatibilize the wood surface and bio‑PE, was not achieved. The adsorption of PVFA-co-PVAm with a higher DH changed the wetting behavior of the wood surface from hydrophilic to hydrophobic. The highest degree of hydrophobization was obtained by the adsorption of PVFA-co-PVAm 9095 from an aqueous solution with a pH of 11.
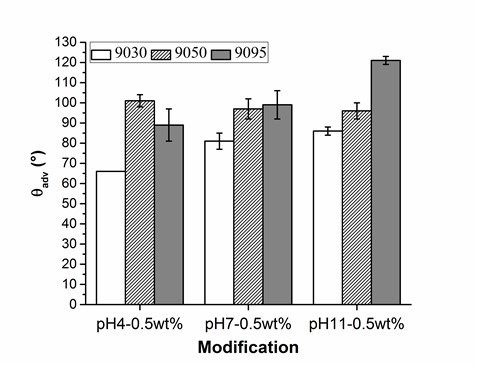
Fig. 3. Results of the advancing contact angle (θadv) measurement on the wood veneer coated with 0.5 wt.% aqueous solutions of PVFA-co-PVAm 9030 (white bars), 9050 (striped bars), and 9095 (grey bars), as a function of the pH value. The adsorption time was 30 s.
The experiment underlined the role of amino groups in the adsorption process of PVFA-co-PVAm onto wood surfaces. If the DH of PVFA-co-PVAm is high, then the amount of formamide groups within the polymer chain is low. The formamide groups cannot be bonded to the substrate surface, but they can polarize the coated surface, which results in a smaller CA. A high number of amino groups can increase the charge density along the polymer, which enables stronger electrostatic interactions with the wood surface. Hence, the sample coated with PVFA-co-PVAm 9050 showed CA values higher than 90° independently of the pH of the aqueous polyelectrolyte solution. However, a high charge density within the PVFA-co-PVAm increased the intra- and intermolecular electrostatic repulsions. The polymer chain has a stiffer structure, which affects the adsorption process onto the wood veneer surface (Kirwan et al. 2004). Thus, the adsorption from strong or moderate acidic solutions is accompanied by lower adsorption amounts and causes smaller CA values, which was observed for the samples that were coated with the basic PVFA-co-PVAm solutions. Reduced CAH values were determined on the sample surfaces, which were prepared by PVFA-co-PVAm adsorption from basic solutions. The lower values were considered to be a clear indication of a more homogeneous distribution of the polyelectrolytes on the wood surface (Fig. 4, Table S3).
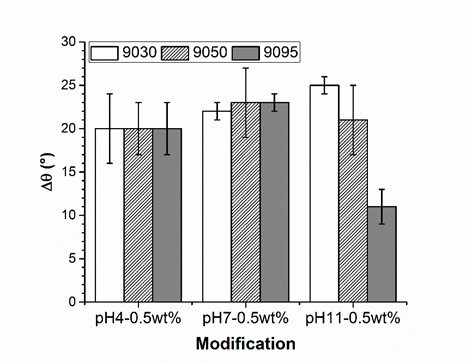
Fig. 4. Contact angle hysteresis measurement (Δθ) of wood veneer, coated with 0.5 wt.% aqueous solution of PVFA-co-PVAm 9030 (white bars), 9050 (striped bars) and 9095 (grey bars), as function of the pH value. The adsorption time was 30 s.
For potential industrial uses, short adsorption times of PVFA-co-PVAm are desirable. Therefore, the influence of the adsorption time during the coating experiment was investigated. The wood veneer samples were coated with 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution at a pH of 11. The adsorption times were 30 s, 1, 2, 5, 7, and 10 min. The experiments clearly showed that the adsorption time had no remarkable influence on the adsorption process. The CA and CAH results demonstrated that an adsorption time of 30 s was sufficient to obtain homogenously coated materials. Longer adsorption times affected neither the CA nor the CAH values (Fig. 5, Table S4).
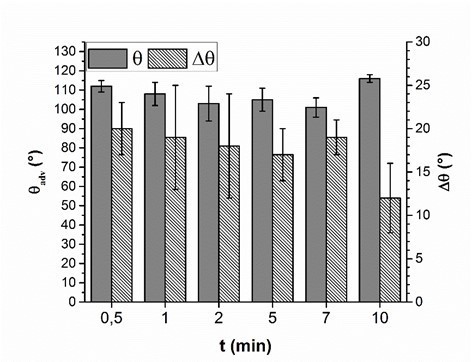
Fig. 5. Advancing contact angle measurement (θadv, grey bars) and contact angle hysteresis (Δθ, striped bars) of wood veneer, coated with 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution at pH 11, as function of the adsorption time.
The weight concentration of the polymer solution, as another parameter that could influence the adsorption procedure, was examined. For economic and ecological reasons, PVFA-co‑PVAm solutions should have weak concentrations. The weight concentrations were 0.5, 1.0, 2.0, and 5.0 wt.%. The adsorption experiments were carried out at a pH of 11 with aqueous PVFA-co‑PVAm 9095 solutions and an adsorption time of 30 s. The CA values measured on the wood veneer samples were not noticeably different (Fig. 6, Table S5). Neither the adsorption time nor weight concentration had a considerable effect on the adsorption of PVFA-co-PVAm onto the wood veneer. The CAH decreased with weight concentrations higher than 0.5 wt.%, which was because of a higher amount of adsorbed PVFA-co-PVAm (see SEM and EDX investigations). As a result, the surface was more homogeneous compared with the surface that adsorbed the 0.5 wt.% polymer solution.
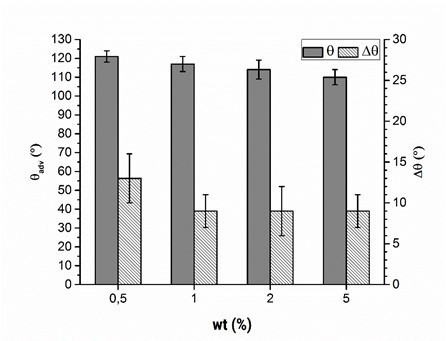
Fig. 6. Advancing contact angle measurement (θadv, grey bars) and contact angle hysteresis (Δθ, striped bars) of wood veneer, coated with aqueous PVFA-co-PVAm 9095 solution at pH 11 for 30 s, as function of the mass concentration.
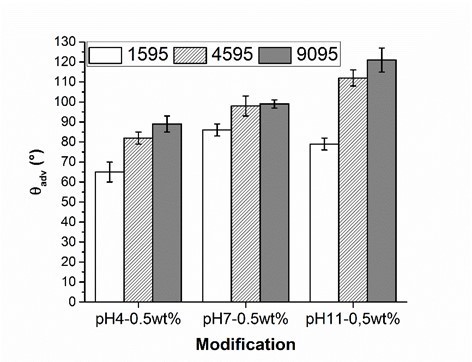
Fig. 7. Advancing contact angle measurement (θadv) of wood veneer, coated with 0.5 wt.% aqueous solution of PVFA-co-PVAm 1595 (white bars), 4595 (striped bars) and 9095 (grey bars), as function of the pH value. The adsorption time was 30 s.
The influence of the molecular weight of PVFA-co-PVAm (DH > 90%) on the adsorption onto wood surfaces was investigated. PVFA-co-PVAm solutions with a molecular weight smaller than 10,000 (PVFA-co-PVAm 1595), 45,000 (PVFA-co-PVAm 4595), and 340,000 g/mol (PVFA-co-PVAm 9095) were used. As was discussed above, the CA values of each wood veneer modification rose with higher pH values of the aqueous solution. Furthermore, the CA values also became higher with an increased molecular weight of the PVFA-co-PVAm solution. The highest CA was achieved with PVFA-co-PVAm 9095 at a pH of 11 (Fig. 7, Table S6).
The adsorption of PVFA-co-PVAm 1595 caused similar CAH values over the entire pH range. The other two modifications had the highest CAH values at a pH of 7 and lowest values at a pH of 11 (Fig. 8, Table S7).

Fig. 8. Contact angle hysteresis measurement (Δθ) of wood veneer, coated with 0.5 wt.% aqueous solution of PVFA-co-PVAm 1595 (white bars), 4595 (striped bars) and 9095 (grey bars), as function of the pH value. The adsorption time was 30 s.
It is assumed that the CA results reflected the interplay between the adsorption driving forces (entropy and formation of specific interactions) and steric hindrances of the more or less charged PVFA-co-PVAm molecules during the adsorption process onto the wood veneer surface. To obtain the desired surface properties of the wood veneer after the adsorption of PVFA-co-PVAm, as well as for later processing, economic, and ecological reasons, the following experimental parameters were classified as ideal: adsorption of a 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution with a pH of 11 for 30 s.
SEM and EDX Investigations
SEM images and EDX spectra were obtained to examine the homogeneity of the PVFA-co-PVAm coating on the wood veneer surface. Moreover, the EDX spectra of the various coatings were recorded to quantify and compare the local elemental composition of the samples at different topological positions.
Figure 9 shows the lateral distribution of carbon (C), oxygen (O), and nitrogen (N) on a PVFA-co‑PVAm 9095-coated wood veneer sample (1.0 wt.%, pH 11, 30 s). The distribution of nitrogen (green) revealed that the polymer was adsorbed homogeneously over the entire wood veneer surface. Similar results were obtained for each adsorption process, whereby agglomerations on the micrometer scale did not occur. Furthermore, the SEM images (Fig. 9) showed that the lumens of the wood veneers were only partially filled in the case of the adsorption of PVFA-co-PVAm at a pH of 11.
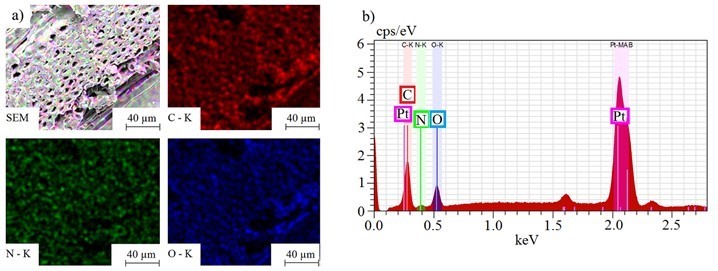
Fig. 9. SEM images (a) and EDX spectra (b) of a wood veneer sample coated with 1.0 wt.% aqueous PVFA-co-PVAm 9095 solution at a pH of 11 (30 s adsorption time). The EDX images show the elemental distribution of carbon (red), nitrogen (green), and oxygen (blue).
Table 4 summarizes the elemental ratios determined by the quantified EDX spectra. The nitrogen to carbon ([N]:[C]) ratios allowed for estimations of the amount of coating. The native wood veneer exhibited only traces of nitrogen. The EDX measurements showed that the [N]:[C] ratio only increased when increasing the weight concentration from 0.5 to 1.0 wt.% (Table 4). Thus, the nitrogen content increased, and consequently the amount of adsorbed PVFA-co-PVAm increased, as well. The adsorption of highly concentrated polymer solutions did not increase the nitrogen content at a pH of 11 (Table 5). As was mentioned before, the adsorption of moderate amounts of PVFA-co-PVAm were adequate to equip the hydrophilic wood surface with the desired hydrophobic property. Furthermore, the CA measurements verified that a higher weight concentration did not influence the hydrophobic nature of the coated wood veneer surface.
Table 4. Quantitative Determination of the [N]:[C] Ratios of the PVFA-co-PVAm-Modified Wood Veneer Samples
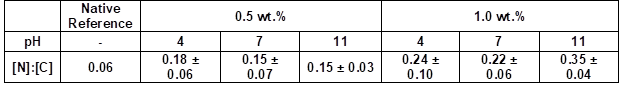
Note: The samples were coated with an aqueous PVFA-co-PVAm 9095 solution (30 s adsorption time) and measured by EDX at 1000-fold magnification.
The influence of the molecular weight of the PVFA-co‑PVAm and DH on the elemental distribution on the wood veneers was also analyzed by SEM and EDX. The [N]:[C] ratios increased with higher DH values. The pH value did not strongly affect the amount of adsorbed PVFA-co‑PVAm, but the weight concentration clearly influenced the [N]:[C] ratio, as well as the adsorption behavior. The results of the molecular weight investigations were similar to the DH studies. A higher molecular weight for the PVFA-co‑PVAm at the same weight concentration led to increased [N]:[C] ratios. According to the results of the effect of DH, the pH did not remarkably affect the [N]:[C] ratio (Table S8, S9). These findings were in agreement with the results of the CA studies and supported the conclusions drawn above.
Table 5. Quantitative Determination of the [N]:[C] ratio by EDX Measurements at 1000-fold Magnification *
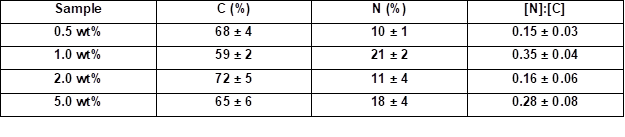
* The samples were coated 30 s with an aqueous PVFA-co-PVAm solution 9095 at pH 11.
XPS Studies
The spectrum on the left in Fig. 10, part a, shows the wide-scan spectrum recorded from an untreated native beech wood veneer sample. In addition to carbon (C 1s peak) and oxygen (O 1s and O 2s peaks, and O KLL Auger series), small amounts of nitrogen (N 1s peak), silicon (Si 2p and Si 2s peaks), and calcium (Ca 2p peak; the other calcium element peaks were too small to be seen in the spectrum) were detected (Table 6 summarizes the elemental quantification). The N 1s peak indicated the presence of proteins, and the traces of silicon and calcium resulted from mineral substances. The shape of the high-resolution C 1s spectrum (Fig. 10a, middle column) was characteristic of polysaccharides, such as cellulosic materials. The spectrum had five distinguishable component peaks, A, B, C, D, and E, which were used to discuss the different binding states of carbon. The intensive component peak A at 285 eV represented the saturated and unsaturated hydrocarbons. For pure cellulose, the presence of hydrocarbons is not expected. However, the investigated samples were natural wood products, where lignin penetrated the hemicellulose.

Fig. 10. XPS Spectra of the wide-scan (left column) and high-resolution scans of C 1s (middle column) and N 1s (right column) recorded from the native wood veneer (a) and wood veneer sample that was coated with an aqueous 0.5 wt.% PVFA-co-PVAm 9095 solution at a pH of 11 (10 min adsorption time) (b)
Table 6. [N]:[C] Ratios Determined by XPS from the Surfaces of PVFA-co-PVAm Modified Wood Veneer Samples *
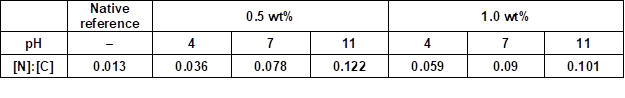
* All samples were coated with aqueous PVFA-co-PVAm solutions 9095 at different pH values for 10 min adsorption time.
Hydrocarbons from the other extractives, such as waxes, fatty acids, resin acids, and terpenes, also contributed to component peak A. Component peak C (at 286.5 eV) contained C–OH groups. It is assumed, that the majority of these groups were constituents of cellulose, but phenolic C-OH groups of lignin were also analyzed as component peak C. Carbon atoms of the β-1,4-glycoside bonds also contributed to component peak C. Carbon atoms of the hemiacetal groups (O–C–O) were observed to be part of component peak D at 288.1 eV.
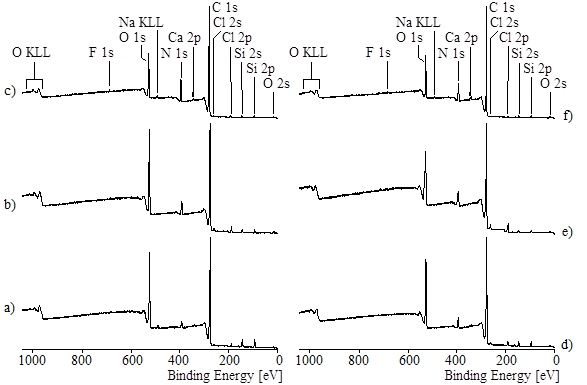
Fig. 11. Wide-scan XPS spectra recorded from wood veneer samples modified with aqueous PVFA-co-PVAm solutions of different pH values for 10 min adsorption time: (a) 0.5 wt.% PVFA-co-PVAm solution of pH = 4; (b) 0.5 wt.% PVFA-co-PVAm solution of pH = 7; (c) 0.5 wt.% PVFA-co-PVAm solution of pH = 11; (d) 1.0 wt.% PVFA-co-PVAm solution of pH = 4; (e) 1.0 wt.% PVFA-co-PVAm solution of pH = 7; (f) 1.0 wt.% PVFA-co-PVAm solution of pH = 11.
The contribution of proteins to this component peak was rather small because only traces of nitrogen were found in the wide-scan spectrum. Component peak E (at 289.2 eV) contained carbonyl carbon atoms from carboxylic acid and carboxylate ester groups. These groups were constituents of the extractives and oxidation products of saccharides. The application of PVFA-co-PVAm introduced considerable amounts of nitrogen ([N]:[C] = 0.122) to the surface region of the veneer sample (Fig. 10b and 11). The amino groups of the PVFA-co-PVAm molecules were analyzed as component peak B (at 285.74 eV), which resulted from the C–N bonds of the amine-bonded carbon atoms. Because the relative intensity of component peak B equaled the [N]:[C] ratio, which was separately determined from the wide-scan spectrum, it was concluded that primary amino groups were present on the sample surface. Carbonyl carbon atoms of moieties of formamide groups contributed to component peak D. The corresponding amine-sided carbon atoms were observed to be a fraction of component peak B. From the noticeably decreased intensities of the component peaks, which represented the chemical structure of the cellulose materials (component peaks C and D), it was concluded that the wood substrate was almost homogeneously covered by a PVFA-co-PVAm layer. The N 1s spectrum (Fig. 10b, right column) of the PVFA-co-PVAm-coated samples showed two different binding states for nitrogen, peaks K and L. Component peak K (at 399.26 eV) resulted from the nitrogen atoms of the primary amino groups (C–NH2), as well as amide moieties (O=C–NH–C). Component peak L at 401.54 eV showed the presence of protonated amino groups (C–NH3+). Chlorine ions, identified as the Cl 2p and Cl 2s peaks, were typical counter ions of these protonated nitrogen species.
Tensile Tests of the Wood Veneer/Bio-PE Composites
The CA, EDX, and XPS results showed that wood veneer modifications with PVFA-co-PVAm are promising for the production of wood veneer/bio-PE composite materials. According to the findings of the optimized adsorption process, wood veneers were coated with differently concentrated solutions of PVFA-co-PVAm 9095 with pH values of 7 (0.5 wt.%) and 11 (0.5 and 1.0 wt.%). As mentioned above, the adsorption of PVFA-co-PVAm 9095 under these conditions caused the hydrophilic native wood veneer samples to become hydrophobic, and compatibilized the wood surface and bio-PE foil. The wood veneers were coated with each of the modification formulations, and afterwards they were consolidated in a hot press with bio-PE. For the first tests, the adsorption time was 10 min, instead of the adequate 30 s. In order to perform preliminary mechanical tests, the first product was designed as a three-layer veneer-biopolymer-veneer (VPV) composite. Furthermore, a VPV composite material with an unmodified wood veneer was produced as a reference. The Young’s modulus and tensile strength were determined perpendicular to the fiber direction.
Table 7 summarizes the results of the Young’s moduli investigations for the native wood veneer, unmodified VPV, and modified VPV samples. Compared with the native wood veneer, the unmodified VPV composite had a higher Young’s modulus with less scattered measuring values. The pretreatment of the wood veneers with PVFA-co-PVAm further improved the Young’s modulus but also increased the scattering of the measured values. The best result was achieved by the adsorption of PVFA-co-PVAm 9095 with a weight concentration of 1.0 wt.% at a pH of 11. The Young’s modulus appeared to be enhanced by the adsorption of the polymer solution at higher pH values. In comparison to the native and unmodified VPV samples, the PVFA-co-PVAm 9095 polymer clearly contributed to the mechanical stability of the VPV composites. In addition to the increased Young’s moduli, the tensile strengths were increased by the modification of the wood veneer samples with PVFA-co-PVAm 9095 (Table 7). The VPV composite materials had a greater tensile strength than the native wood and unmodified VPV samples. In particular, the VPV composite made from wood veneers, which were modified with an aqueous 0.5 wt.% PVFA-co-PVAm 9095 solution at a pH of 7, showed the highest tensile strength. A direct influence of the pH value and weight concentration was not observed.
Table 7. Determination of the Young’s Moduli (E) and Tensile Strengths (Rm) Perpendicular to the Fiber Direction of the VPV Composite Materials and Native Wood Veneer
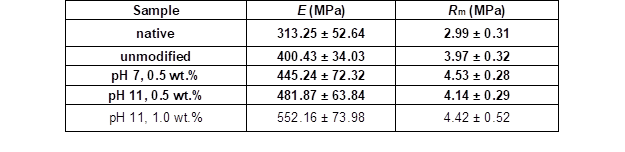
Note: The wood veneers were coated partially with an aqueous PVFA-co-PVAm 9095 solution for 10 min at various pH values and weight concentrations.
It is suggested that the improvement of the mechanical properties was because of the dispersion forces between the hydrophobic wood veneer surface and nonpolar bio-PE. The adsorption of PVFA-co-PVAm onto the wood veneer by electrostatic interactions and hydrogen bonds led to a hydrophobic wood surface. Additionally, during the adsorption of PVFA-co-PVAm, other polar substances, such as water, were displaced from the wood surface. The hydrophobic wood surface and spatial closeness to the bio-PE layer enabled effective dispersion forces. The interactions within the VPV composite materials are illustrated in Fig. 12.
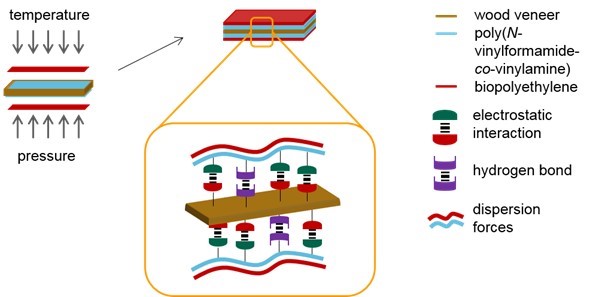
Fig. 12. Production of the composite materials by the hot pressing process. Adhesion between the PVFA-co-PVAm-coated wood veneer and bio-PE was promoted because of the dispersion forces.
Scanning electron microscopy was performed to obtain information about the interface between the wood veneer and bio-PE. The interface of the VPV composite made from the unmodified wood veneer should have been inhomogeneous because of the incompatible surface polarities. In contrast, the treatment with PVFA-co-PVAm should have improved the homogeneity along the interface because of the previously mentioned interactions. The EDX measurements of the cross-section images clearly showed the interface of the VPV composite. In both cases, voids between the composite materials were identified, which are demonstrated in Fig. 13, 14, and 15. However, the number and length of the voids was larger in the case of the composite, which was made from the unmodified wood veneer samples. SEM images of the fracture surface can be seen in Figs. 16 and 17. Even if the detection of nitrogen in the interface failed, there was no doubt of the compatibilizing and adhesion-promoting effect of the PVFA-co-PVAm polymer on the consolidated polar wood materials with nonpolar polyolefins.
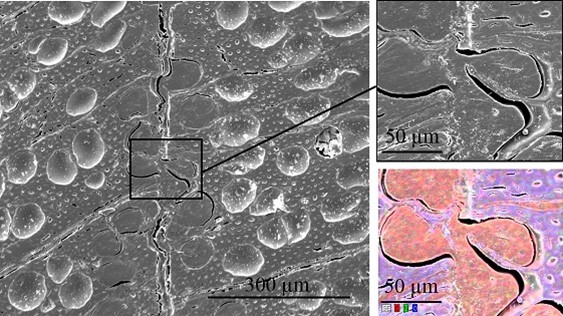
Fig. 13. SEM cross-section image of an unmodified VPV composite material. The EDX image shows the elemental distribution of carbon (red), nitrogen (green), and oxygen (blue).
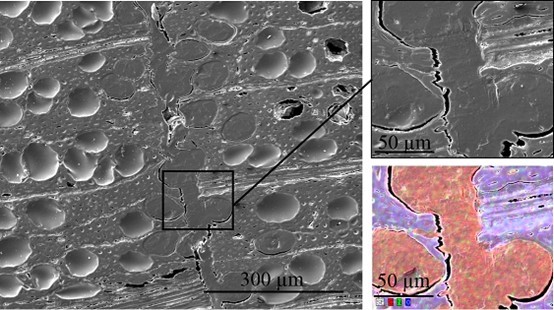
Fig. 14. SEM cross-section image of a PVFA-co-PVAm-modified VPV composite material. The wood veneer was coated with a 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution at a pH of 11 for 10 min. The EDX image shows the elemental distribution of C (red), N (green), and O (blue).
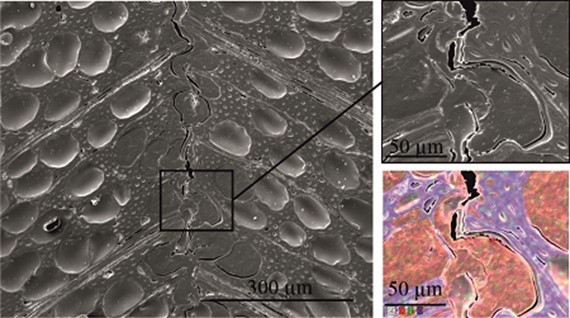
Fig. 15. SEM cross section image of a PVFA-co-PVAm-modified VPV composite material. The wood veneer was coated with a 1.0 wt% aq. PVFA-co-PVAm 9095 solution at pH 11 for 10 min. The EDX image shows the elemental distribution of C (red), N (green), and O (blue).
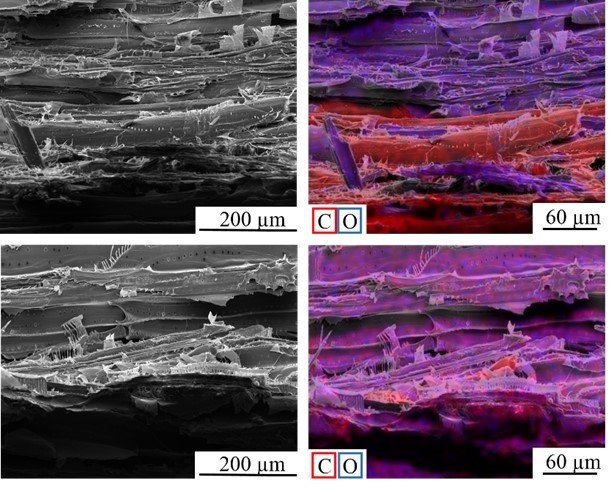
Fig. 16. SEM images of the fracture surface of the PVFA-co-PVAm-modified VPV composite material after the tensile test perpendicular to the fiber direction. The wood veneer was coated with a 1.0 wt.% aqueous PVFA-co-PVAm 9095 solution at pH 11 for 10 min. The EDX image shows the elemental distribution of carbon (red), and oxygen (blue).

Fig. 17. SEM images of the fracture surface of the unmodified VPV composite material after the tensile test perpendicular to the fiber direction. The EDX image shows the elemental distribution of carbon (red), and oxygen (blue).
CONCLUSIONS
- The adsorption of the polar wood surface with a 0.5 wt.% aqueous PVFA-co-PVAm 9095 solution for 30 s was suitable to strongly improve the mechanical properties of wood/polyolefin composite materials. In this regard, PVFA-co-PVAm can be considered to be a new adhesion promoter and compatibilizer system.
- The excellent mechanical properties of the wood veneer/bio-PE composite materials were a result of the hydrophobic treatment of the wood veneer surfaces with PVFA-co-PVAm, whereby a better adhesion to the bio-PE was promoted.
- The Young’s moduli and tensile strengths of VPV composite materials were determined to represent the mechanical properties of the composites. Compared with the unmodified VPV test samples, the PVFA-co-PVAm-modified wood veneer samples were characterized by improved Young’s moduli and tensile strengths.
- The SEM cross-section images taken from the PVFA-co-PVAm-modified VPV composites showed a reduced number of voids in the wood veneer/bio-PE interface.
- The adsorption experiments showed that the pH value had an important influence on the formation of the adsorption layer, and thus on converting the hydrophilic wood surface to a hydrophobic surface.
- The DH of the formamide sequences in the PVFA-co-PVAm and molecular mass had important influences on the amount that was adsorbed onto the wood samples. The higher the DH and molecular mass, then the more hydrophobic the coated surfaces were.
- The EDX measurements showed the homogenous distribution of nitrogen on the wood veneer surfaces, and therefore the homogeneous distribution of the adsorbed PVFA-co-PVAm. The homogeneity was not affected by the adsorption parameters.
- Neither the weight concentration of PVFA-co-PVAm in the aqueous solutions nor the adsorption times were important for obtaining hydrophobic surfaces.
ACKNOWLEDGMENTS
This work was performed within the Federal Cluster of Excellence EXC 1075 “MERGE Technologies for Multifunctional Lightweight Structures” and supported by the German Research Foundation (DFG). The financial support is gratefully acknowledged. BASF SE is gratefully acknowledged for providing the PVFA-co-PVAm solutions. The authors would like to thank Prof. M. Hietschold and D. Dentel (TU Chemnitz) for providing the opportunity to take electron micrographs. Additionally, the authors would like to thank Prof. W. A. Goedel and Dr. S. Ebert for providing the contact angle measuring instrument. Furthermore, the authors acknowledge A. Richter for her experimental preliminary work, and J. Schell for proofreading.
REFERENCES CITED
Achari, A. E., Coqueret, X., Lablache-Combier, A., and Loucheux, C. (1993). “Preparation of polyvinylamine from polyacrylamide: A reinvestigation of the hofmann reaction,” Macromol. Chem. Physic. 194(7), 1879-1891. DOI: 10.1002/macp.1993.021940703
Ashori, A. (2008). “Wood–plastic composites as promising green-composites for automotive industries!” Bioresource Technology, 99, 4661-4667. DOI: 10.1016/j.biortech.2007.09.043
Badesso, R. J., Pinschmidt Jr., R. K., and Sagl, D. J. (1993). “Synthesis of amine functional homopolymers with N-ethenylformamid,” Proc. Am. Chem. Soc. Div. Polym. Mat. Sci. Eng. 69, 251-252.
BASF SE (2004). Polyvinylamin, Ludwigshafen, Germany.
Buchelt, B., Siegel, C., Wagenführ, A., Nendel, W. (2015). “Veneer prepreg – biobased prepregs for thermoplastic processing methods,” Zeitschrift Kunststofftechnik / Journal of Plastics Technology 11(6), 355-374.
Carus, M., Müssig, J., and Gahle, C. (2008). Naturfaserverstärkte Kunststoffe. Pflanzen – Rohstoffe, Produkte, Fachagentur Nachwachsende Rohstoffe e. V., Güzlow, Germany.
Colom, X., Carrasco, F., Pagès, P., and Cañavate, J. (2003). “Effects of different treatments on the interface of HDPE/lignocellulosic fiber composites,” Compos. Sci. Technol. 63(2), 161-169. DOI: 10.1016/S0266-3538(02)00248-8
Dalväg, H., Klason, C., and Strömvall, H.-E. (1985). “The efficiency of cellulosic fillers in common thermoplastics. Part II. Filling with processing aids and coupling agents,” International Journal of Polymeric Materials and Polymeric Biomaterials 11(1), 9-38. DOI: 10.1080/00914038508078651
DIN 52188 (1979). “Bestimmung der Zugfestigkeit parallel zur Faser,” Deutsches Institut für Normung e.V., Berlin, Germany.
DIN EN ISO 527-4 (1997). “Bestimmung der Zugeigenschaften – Teil 4: Prüfbedingungen für isotrop und anisotrop faserverstärkte Kunststoffverbundwerkstoffe,” Deutsches Institut für Normung e.V., Berlin, Germany.
Felix, J. M., and Gatenholm, P. (1991). “The nature of adhesion in composites of modified cellulose fibers and polypropylene,” J. Appl. Polym. Sci. 42(3), 609-620. DOI: 10.1002/app.1991.070420307
Fengel, D., and Grosser, D. (1975). “Chemische Zusammensetzung von Nadel- und Laubhölzern,” Holz Roh. Werkst. 33(1), 32-34. DOI: 10.1007/BF02612913
Fischer, T., and Heitz, W. (1994). “Synthesis of polyvinylamine and polymer analogous reactions,” Macromol. Chem. Physic. 195(2), 679-687. DOI: 10.1002/macp.1994.021950225
Fowler, P. A., Hughes, J. M., and Elias, R. M. (2006). “Biocomposites: Technology, environmental credentials and market forces,” J. Sci. Food Agr. 86(12), 1781-1789. DOI: 10.1002/jsfa.2558
Geffroy, C., Labeau, M. P., Wong, K., Cabane, B., and Cohen Stuart, M. A. (2000). “Kinetics of adsorption of polyvinylamine onto cellulose,” Colloid. Surface. A 172(1–3), 47-56. DOI: 10.1016/S0927-7757(00)00499-4
Gindl, M., and Tschegg, S. (2002). “Significance of the acidity of wood to the surface free energy components of different wood species,” Langmuir 18(8), 3209-3212. DOI: 10.1021/la011696s
Gu, L., Zhu, S., and Hrymak, A. N. (2002). “Acidic and basic hydrolysis of poly(N-vinylformamide),” J. Appl. Polym. Sci. 86(13), 3412-3419. DOI: 10.1002/app.11364
Hanselka, H. (1998). “Faserverbundwerkstoffe aus nachwachsenden Rohstoffen für den ökologischen Leichtbau,” Materialwiss. Werkst. 29(6), 300-311. DOI: 10.1002/mawe.19980290610
Herrmann, A. S., Nickel, J., and Riedel, U. (1998). “Construction materials based upon biologically renewable resources—From components to finished parts,” Polym. Degrad. Stabil. 59(1–3), 251-261. DOI: 10.1016/S0141-3910(97)00169-9
Jung, B., and Roffael, E. (2002). “Über die Acidität einheimischer Holzarten,” Holz Roh. Werkst. 60(2), 154-154. DOI: 10.1007/s00107-001-0278-5
Kazayawoko, M., Balatinecz, J. J., and Matuana, L. M. (1999). “Surface modification and adhesion mechanisms in woodfiber-polypropylene composites,” J. Mater. Sci. 34(24), 6189-6199. DOI: 10.1023/A:1004790409158
Khabbaz, F., Eriksson, P., Fare, J., and Furberg, A. (2008). “Method of producing a wood based product,” WO Patent No. 2007139501.
Kirwan, L. J., Papastavrou, G., Borkovec, M., and Behrens, S. H. (2004). “Imaging the coil-to-globule conformational transition of a weak polyelectrolyte by tuning the polyelectrolyte charge density,” Nano Letters 4(1), 149-152. DOI: 10.1021/nl034912l
Kuo, P.-L., Hou, S.-S., Teng, C.-K., and Liang, W.-J. (2001). “Function and performance of silicone copolymer (VI). Synthesis and novel solution behavior of water-soluble polysiloxanes with different hydrophiles,” Colloid Polym. Sci. 279(3), 286-291. DOI: 10.1007/s003960000448
Lai, S.-M., Yeh, F.-C., Wang, Y., Chan, H.-C., and Shen, H.-F. (2003). “Comparative study of maleated polyolefins as compatibilizers for polyethylene/wood flour composites,” J. Appl. Polym. Sci. 87(3), 487-496. DOI: 10.1002/app.11419
Miao, C., Chen, X., and Pelton, R. (2007). “Adhesion of poly(vinylamine) microgels to wet cellulose,” Ind. Eng. Chem. Res. 46(20), 6486-6493. DOI: 10.1021/ie0705608
Mullier, M., and Smets, G. (1957). “Polymers and group interaction. IV. Hofmann reaction on polyvinylamides,” J. Polym. Sci. 23(104), 915-930. DOI: 10.1002/pol.1957.1202310435
Pelton, R. (2014). “Polyvinylamine: A Tool for engineering interfaces,” Langmuir 30(51), 15373-15382. DOI: 10.1021/la5017214
Piasta, D., Bellmann, C., Spange, S., and Simon, F. (2009). “Endowing carbon black pigment particles with primary amino groups,” Langmuir 25(16), 9071-9077. DOI: 10.1021/la900407n
Pinschmidt, Jr., R. K., Renz, W. L., Carroll, W. E., Yacoub, K., Drescher, J., Nordquist, A. F., and Chen, N. (1997). “N-vinylformamide – Building block for novel polymer structures,” J. Macromol. Sci. Pure 34(10), 1885-1905. DOI: 10.1080/10601329708010315
Poptoshev, E., Rutland, M. W., and Claesson, P. M. (1999). “Surface forces in aqueous polyvinylamine solutions. I. Glass surfaces,” Langmuir 15(22), 7789-7794. DOI: 10.1021/la990322k
Poptoshev, E., Rutland, M. W., and Claesson, P. M. (2000). “Surface forces in aqueous polyvinylamine solutions. 2. Interactions between glass and cellulose,” Langmuir 16(4), 1987-1992. DOI: 10.1021/la990961v
Raj, R. G., Kokta, B. V., and Daneault, C. (1989). “Polypropylene-wood fiber composites: Effect of fiber treatment on mechanical properties,” International Journal of Polymeric Materials and Polymeric Biomaterials 12(3), 239-250. DOI: 10.1080/00914038908031503
Sain, M., and Panthapulakkal, S. (2004). “Chapter 9: Green fibre thermoplastic composites,” in: Green Composites, C. Baillie (ed.), Woodhead Publishing, Cambridge, UK, pp. 181-206.
Sain, M., and Panthapulakkal, S. (2006). “Bioprocess preparation of wheat straw fibers and their characterization,” Ind. Crop. Prod. 23(1), 1-8. DOI: 10.1016/j.indcrop.2005.01.006
Sandermann, W., and Rothkamm, M. (1959). “Über die Bestimmung der pH-Werte von Handelshölzern und deren Bedeutung für die Praxis,” Holz Roh. Werkst. 17(11), 433-440. DOI: 10.1007/BF02605386
Seifert, S., Höhne, S., Simon, F., Hanzelmann, C., Winkler, R., Schmidt, T., Frenzel, R., Uhlmann, P., and Spange, S. (2012). “Adsorption of poly(vinylformamide-co-vinylamine) polymers (PVFA-co-PVAm) on copper,” Langmuir 28(42), 14935-14943. DOI: 10.1021/la302855f
Seifert, S., Simon, F., Baumann, G., Hietschold, M., Seifert, A., and Spange, S. (2011). “Adsorption of poly(vinyl formamide-co-vinyl amine) (PVFA-co-PVAm) polymers on zinc, zinc oxide, iron, and iron oxide surfaces,” Langmuir 27(23), 14279-14289. DOI: 10.1021/la203479n
Shirley, D. A. (1972). “High-resolution X-Ray photoemission spectrum of the valence bands of gold,” Phys. Rev. B 5(12), 4709-4714. DOI: 10.1103/PhysRevB.5.4709
Shulga, A., Widmaier, J., Pefferkorn, E., Champ, S., and Auweter, H. (2003a). “Kinetics of adsorption of polyvinylamine on cellulose fibers: I. Adsorption from salt-free solutions,” J. Colloid Interf. Sci. 258(2), 219-227. DOI: 10.1016/S0021-9797(02)00153-4
Shulga, A., Widmaier, J., Pefferkorn, E., Champ, S., and Auweter, H. (2003b). “Kinetics of adsorption of polyvinylamine on cellulose fibers: II. Adsorption from electrolyte solutions,” J. Colloid Interf. Sci. 258(2), 228-234. DOI: 10.1016/S0021-9797(02)00154-6
Spange, S., Meyer, T., Voigt, I., Eschner, M., Estel, K., Pleul, D., and Simon, F. (2004). “Poly(vinylformamide-co-vinylamine)/inorganic oxide hybrid materials,” in: Polyelectrolytes with Defined Molecular Architecture I, M. Schmidt (ed.), Springer Berlin Heidelberg, Berlin, Germany, pp. 43-78.
Torres, F. G., and Cubillas, M. L. (2005). “Study of the interfacial properties of natural fibre reinforced polyethylene,” Polym. Test. 24(6), 694-698. DOI: 10.1016/j.polymertesting.2005.05.004
Voigt, I., Simon, F., Estel, K., and Spange, S. (2001). “Structure and surface polarity of poly(vinylformamide-co-vinylamine) (PVFA-co-PVAm)/silica hybrid materials,” Langmuir 17(10), 3080-3086. DOI: 10.1021/la001366s
Voigt, I., Simon, F., Komber, H., Jacobasch, H.-J., and Spange, S. (2000). “Controlled synthesis of stable poly(vinyl formamide-co-vinyl amine)/silica hybrid particles by interfacial post-cross-linking reactions,” Colloid Polym. Sci. 278(1), 48-56. DOI: 10.1007/s003960050007
Wagenführ, A. (2008). ”Die strukturelle Anisotropie von Holz als Chance für technische Innovationen,” in Sitzungsbericht der Sächsischen Akademie der Wissenschaften zu Leipzig, S. Hirzel Verlag, Stuttgart, Germany, pp. 1-39.
Woodhams, R. T., Thomas, G., and Rodgers, D. K. (1984). “Wood fibers as reinforcing fillers for polyolefins,” Polym. Eng. Sci. 24(15), 1166-1171. DOI: 10.1002/pen.760241504
Article submitted: 2017-05-05; Peer review completed: July 1, 2017; Revised version received and accepted: September 11, 2017; Published: September 15, 2017.
DOI: 10.15376/biores.12.4.8134-8159
APPENDIX
Additional Information
DIN EN ISO 527-4 (1997) and DIN 52188 (1979)
The number of test specimens is at least five. When testing the ultimate tensile stress parallel to the fiber direction, the tensile forces should be homogeneous distributed on the test specimen and the maximum forces should be reached after 90 s. Therefore, the testing speed of 2 mm/s was chosen.
Poly(N-Vinylformamide-co-Vinylamine)
Table S1. Stock Concentration of the Used Aqueous PVFA-co-PVAm Solutions
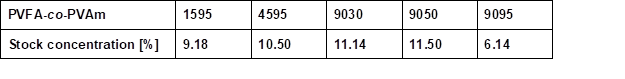
Table S2. Advancing Contact Angle Measurement (θadv) of Wood Veneer, Coated with 0.5 wt.% Aqueous Solution of PVFA-co-PVAm 9030, 9050, and 9095, as Function of pH Value*
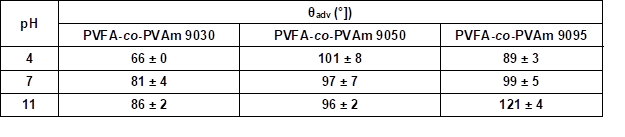
* The adsorption time was 30 s.
Table S3. Contact Angle Hysteresis Measurement (Δθ) of Wood Veneer, Coated with 0.5 wt.% Aqueous Solution of PVFA-co-PVAm 9030, 9050, and 9095, as Function of pH value *

* The adsorption time was 30 s.
Table S4. Advancing Contact Angle Measurement (θadv) and Contact Angle Hysteresis (Δθ) Measurement of Wood Veneer, Coated with 0.5 wt.% Aqueous PVFA-co-PVAm 9095 Solution at pH 11, as Function of the Adsorption Time
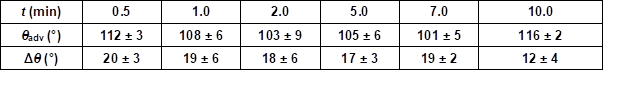
Table S5. Advancing Contact Angle Measurement (θadv) and Contact Angle Hysteresis Measurement (Δθ) of Wood Veneer, Coated with Aqueous PVFA-co-PVAm 9095 Solution at pH 11 for 30 s, as Function of the Mass Concentration

Table S6. Advancing Contact Angle Measurement (θadv) of Wood Veneer, Coated with 0.5 wt.% Aqueous Solution of PVFA-co-PVAm 1595, 4595, and 9095, as Function of pH Value*
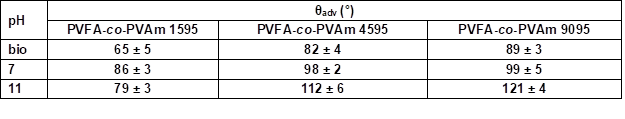
* The adsorption time was 30 s.
Table S7. Contact Angle Hysteresis Measurement (Δθ) of Wood Veneer, Coated with 0.5 wt.% Aqueous Solution of PVFA-co-PVAm 1595, 4595, and 9095, as Function of pH Value*
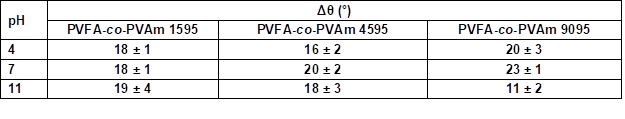
* The adsorption time was 30 s.
Table S8. Quantitative Determination of the [N]:[C] Ratio by EDX Measurements at 1000-fold Magnification*
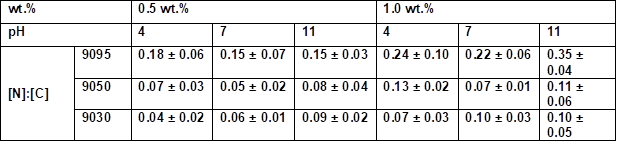
* Samples were coated 30 s with an aqueous PVFA-co-PVAm solution 9095, 9050, and 9030 at different pH values
Table S9. Quantitative Determination of the [N]:[C] ratio by EDX Measurements at 1000-fold Magnification*
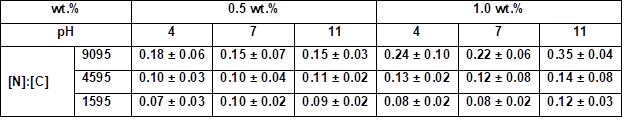
* Samples were coated 30 s with an aqueous PVFA-co-PVAm solution 9095, 4595, and 1595 at different pH values
Additional Reference Information
BASF SE. (2004). “Polyvinylamin,” information material, Ludwigshafen, Germany.
DIN EN ISO 527-4 (1997). “Bestimmung der Zugeigenschaften – Teil 4: Prüfbedingungen für isotrop und anisotrop faserverstärkte Kunststoffverbundwerkstoffe,” Deutsches Institut für Normung e.V., Berlin, Germany.
DIN 52188 (1979). “Bestimmung der Zugfestigkeit parallel zur Faser,” Deutsches Institut für Normung e.V., Berlin, Germany.
