Abstract
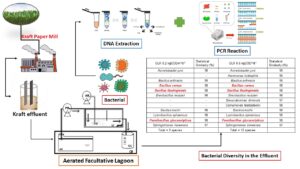
The microbiological diversity was evaluated in an aerated facultative lagoon system with organic loading rates of 0.2 and 0.6 kgCODm-3d-1 treating effluent from the kraft pulp industry for bioaugmentation purposes. Samples were taken from a laboratory-scale lagoon at steady state treating kraft pulp effluent and operated with two different rates for 120 days. The analysis was performed by 16S rDNA sequencing. The autochthonous bacteria were identified through statistical similarity obtained from the NCBI-BLAST database. The lagoon performance was assessed based on the removal efficiency of biochemical oxygen demand (94% and 80%), chemical oxygen demand (51% and 44%), total organic carbon (49% and 41%), lignin-derived compounds (13% and 27%), turbidity (94% and 87%), for the low and high rates used respectively. Color and TPC were not significantly removed during the biological treatment of the industrial matrix. In the biomass samples collected at a steady state, 9 and 12 species of bacteria were identified at the applied rates. The species Bacillus cereus, Bacillus thuringiensis, and Paenibacillus glucanolyticus found in this matrix presented significant removal of the parameters in the kraft effluent. The three referred species show great promise in the removal of specific parameters in an AFL biological treatment system using bioaugmentation.
Download PDF
Full Article
Bacterial Diversity in Aerated Facultative Lagoon Treating Kraft Cellulose Effluent with Bioaugmentation
Jackeline Valendolf Nunes,a,* Mac Wendell Barbosa da Silva,a Gustavo Henrique Couto,a Izadora Cervelin Flôr,b Vania Aparecida Vicente,b José Daniel de Almeida,c Fernanda Celinski,c and Claudia Regina Xavier a
The microbiological diversity was evaluated in an aerated facultative lagoon system with organic loading rates of 0.2 and 0.6 kgCODm-3d-1 treating effluent from the kraft pulp industry for bioaugmentation purposes. Samples were taken from a laboratory-scale lagoon at steady state treating kraft pulp effluent and operated with two different rates for 120 days. The analysis was performed by 16S rDNA sequencing. The autochthonous bacteria were identified through statistical similarity obtained from the NCBI-BLAST database. The lagoon performance was assessed based on the removal efficiency of biochemical oxygen demand (94% and 80%), chemical oxygen demand (51% and 44%), total organic carbon (49% and 41%), lignin-derived compounds (13% and 27%), turbidity (94% and 87%), for the low and high rates used respectively. Color and TPC were not significantly removed during the biological treatment of the industrial matrix. In the biomass samples collected at a steady state, 9 and 12 species of bacteria were identified at the applied rates. The species Bacillus cereus, Bacillus thuringiensis, and Paenibacillus glucanolyticus found in this matrix presented significant removal of the parameters in the kraft effluent. The three referred species show great promise in the removal of specific parameters in an AFL biological treatment system using bioaugmentation.
DOI: 10.15376/biores.17.4.6556-6568
Keywords: Bacterial; Bioaugmentation; Kraft effluent; Microbiological diversity
Contact information: a: Academic Department of Chemistry and Biology, Federal Technological University of Paraná, Dep. Heitor Alencar Furtado Street, 5000 – Cidade Industrial de Curitiba, Curitiba – Paraná, Brazil; b: Department of Basic Pathology, Federal University of Paraná, Av. Cel. Francisco H. dos Santos, 100 – Jardim das Américas, Curitiba – Paraná, Brazil; c: Cocelpa CIA. of Cellulose and Paper of Paraná, Rodovia do Xisto, S/N – Km 14,5 – Jardim Alvorada, Araucária – Paraná, Brazil;
* Corresponding author: jacke.valendolf@hotmail.com
GRAPHICAL ABSTRACT

INTRODUCTION
Biological treatments comprise bioremediation processes, which use living organisms to reduce or eliminate environmental risks arising from the accumulation of toxic chemicals and other hazardous waste (Nagda et al. 2021). Bioremediation processes are classified as natural attenuation, biostimulation, or bioaugmentation. Natural attenuation transforms pollutants into substances that are less harmful to the environment through biodegradation carried out by microorganisms (Liu et al. 2019). Biostimulation improves the conditions of the environment for microorganisms through the addition of nutrients, oxygen, temperature adequacy, pH, aeration, or reduction potential; the process enhances the performance of microorganisms in the treatment (Abena et al. 2019). Bioaugmentation introduces allochthonous bacteria, which are not native to the environment, or autochthonous bacteria, which are native to the environment, isolated or in the consortium. This process is based on the spontaneous and controlled action of microorganisms to increase their quantity, allowing them to degrade pollutants in the soil, water bodies, and in industrial and domestic effluents (Abena et al. 2019). The microorganisms can act in synergy with the local native species, without interfering with natural biogeochemical processes (Braga 2018).
According to Nagda et al. (2021), the application of biotechnological processes involving microorganisms in consortium or individually has been on the increase. There is increasing demand for versatile microorganisms capable of efficiently degrading many pollutants at a low operating cost (Singh and Singh 2019).
Aerated facultative lagoons (AFL) are biological systems used in the treatment of effluent from the pulp and paper industry (Kamali and Khodaparast 2015; Bailón-Salas et al. 2017; Lewis et al. 2018). In Brazil, such systems are widely used due to climatic conditions, their simple maintenance, low cost, and greater stability against shock loads in comparison to other systems, such as activated sludge (Subashini 2015; Von Sperling 2016).
Before 2007, the bioaugmentation technique was not used in Brazil since it depended on the agreement and authorization of government agencies and environmental inspection agencies, such as the Companhia de Tecnologia de Saneamento Ambiental (CETESB). However, on June 22, 2007, CETESB, through Board Decision No. 103/2007/C/E, authorized the use of allochthonous microorganisms in bioaugmentation (Cetesb 2007). Thus, bioaugmentation started to be allowed in Brazil, more specifically in the State of São Paulo, following the specific guidelines presented in the referred document. Among the standards to be followed, CETESB’s technical standard No. L1.022 stands out for referring to the use of biotechnological products, which consist of microorganisms intended for the treatment of liquid effluents, solid waste, and soil and water remediation (Cetesb 2007).
Bioaugmentation can also be used with genetically modified organisms to improve the treatment system. However, because these are allochthonous microorganisms, there is Brazilian legislation that regulates their use: Biosafety Law No. 11.105/05, in effect since March 24, 2005 (Brasil 2005).
The autochthonous bacterial species, commonly present in biological treatments of cellulose effluents, can live in extreme environments of temperature, pH, BOD5, COD, and low oxygen concentration (Ghribi et al. 2016). Due to such characteristics, they are of significant importance to the treatment of kraft effluent (Hooda et al. 2018).
The objective of this paper is to analyze the microbiological diversity in an AFL system treating effluent from the kraft pulp industry with organic loading rates (OLR) of 0.2 and 0.6 kgCODm-3d-1 for bioaugmentation purpose.
EXPERIMENTAL
Aerated Facultative Lagoon Treatment System
The industrial effluent used for continuous treatment in the AFL system was kindly provided by an unbleached kraft pulp mill based in the metropolitan region of Curitiba, in the state of Paraná, Brazil. The aforementioned mill treats its effluents through biological systems with aerated facultative lagoons and tertiary treatment to reach discharge criteria.
The continuous biological treatment used in this research was carried out in a bench-scale AFL reactor, as shown in Fig. 1, in transparent acrylic material with 1 L of useful volume and a sedimentation zone, to which the OLRs of 0.2 and 0.6 kgCODm-3d-1 was applied for a period of 120 days and separated into the two lagoons. Aeration was promoted by air pumps with a flow rate of 35 L/h, and the dissolved oxygen was measured in parallel with the temperature inside the reactor, in the aeration zone, and in the sedimentation zone (Ordaz-Diaz et al. 2016).
Fig. 1.: AP – Aeration pump; AR – Affluent reservoir; CB – Cool box; D – Air diffuser; PP – Peristaltic pump; SZ – Sedimentation zone; TEC – Treated effluent collector; 🡪 – Flow direction. Source: Adapted Machado et al. (2018).
For the composition of the AFL system biomass, sludge was collected from the bottom of the aerated lagoon. The biomass was inoculated to a final concentration of 70 mgVSSL-1, an intermediate value among those used in biological systems (Von Sperling 2014).
The efficiency of the treatment system was assessed as a function of the reduction in the following parameters: biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total organic carbon (TOC), lignin-derived compounds, total phenolic compounds (TPC), aromatic compounds, color, and turbidity (Çeçen 2003; Chamorro et al. 2009; Apha 2017), whose analyses were performed at the Multiuser Laboratory of Chemical Analysis of the Federal Technological University of Paraná (LAMAQ-UTFPR).
Microbiological Analysis
Isolation, cultivation, and Gram staining
The microbiological analyses were carried out at the Microbiology Laboratory of the Federal University of Paraná (UFPR). The isolated and analyzed samples came from the final stabilization period of each OLR in the lagoons.
To separate the cultivated bacteria, colonies were isolated on solid culture media in Petri dishes (Diniz 2018). The culture media used were nutrient agar and Luria-Bertani medium. After the seeding, the dishes were incubated at 37 °C, where they remained for 24 h in the absence of light and CO2.
The isolated bacteria were classified as Gram-negative and Gram-positive (Stinghen et al. 2002). Gram stain slides were analyzed under an optical microscope at 400x magnification to classify bacteria into Gram-negative and Gram-positive.
DNA extraction and amplification
16S rDNA strand was extracted by adapting the method of Vicente et al. (2008), which consists of three phases: membrane lysis, cleaning of contaminants (proteins and other macromolecules), and DNA precipitation. Polymerase chain reaction (PCR) was performed following the technique adapted from Vicente et al. (2008). The products were analyzed by gel electrophoresis in 1% agarose at 108 V for 1 h. The bands were evaluated through the PhotoDoc-It™ Imaging System.
Sequence analysis
The DNA fragments corresponding to the 16s DNA strand were purified and sequenced in the Laboratory of Biochemistry of the Department of Biological Sciences – UFPR. The sequence data were analyzed with the help of the database of the National Center for Biotechnology Information (NCBI) to obtain statistical similarity with NCBI-BLAST. The statistical similarity result with a value above 97% reveals a specific species of bacteria (Yarza et al. 2014).
The alignments of the nitrogenous bases, which were previously obtained in the genetic sequencing step, were done through the bootstrap method using the MEGA software to provide greater reliability to the result according to the genetic evolution of the species, which were later displayed in a phylogenetic tree. When the number of replicas is 100, the species whose replica is closest to 100 is the one identified in the sample, originating from a common ancestor (Hall 2013).
RESULTS AND DISCUSSION
Performance of Treatment System
Figure 2 presents the organic matter removal data in terms of BOD5 and COD in the two lagoons. The average BOD5 removal values were greater than 90%, with maximum removal of 94% in the two systems, which is in line with the literature that suggests that aerated lagoon systems can vary between 50 to 95% in the removal of BOD5 in effluent from the pulp and paper industry (Machado et al. 2018; Peitz and Xavier 2020).
Regarding COD, at both loading rates, there was removal ranging between 40 and 60% during the 120 days of operation. The removal level was similar to that obtained by Machado et al. (2018) using the same volumetric organic loading rate employed in this research for an aerated facultative lagoon.
The TOC removal analysis had an average of 49% for the AFL with an OLR of 0.2 kgCODm-3d-1 and 41% removal for the one with an OLR of 0.6 kgCODm-3d-1. The results obtained at these loading rates were similar to the result obtained by Lewis et al. (2018) in an aerated facultative lagoon.
Fig. 2. Removal of organic compounds as BOD5 and COD. a) OLR 0.2 kgCODm-3d-1. b) OLR 0.6 kgCODm-3d-1. BOD5 – biochemical oxygen demand. COD – chemical oxygen demand
Figure 3 shows data for the removal of specific compounds, namely: total phenolic compounds, lignin compounds, aromatic compounds, and lignosulphonic compounds, in addition to the parameters of color and turbidity.
Figure 3 (a) shows that the TPC had increased during the AFL treatment with an average of 26%, and in Fig. 3 (b), there was a TPC removal of approximately 11%. Kraft effluent shows an increase in total phenolic compounds in aerated biological systems (Chamorro et al. 2009; Duarte et al. 2018; Machado et al. 2018; Melchiors 2019; Peitz and Xavier 2019).
About the other specific compounds of the kraft pulp effluent, the removal of lignin compounds was approximately 13% and 27% at the OLRs of 0.2 and 0.6 kgCODm-3d-1, respectively. For the aromatic compounds, the removal average was 16% and 18% at the OLRs of 0.2 and 0.6 kgCODm-3d-1, respectively. The lignosulphonic compounds had an average removal of 8% at both OLRs.
Possible increments of specific compounds derived from lignin in aerated lagoons have been observed by Machado et al. (2018) and Peitz and Xavier (2019). The compounds are related to the biotransformation of high-molecular-weight molecules in these systems.
Fig. 3. Evaluation of the removal of specific compounds, color, and turbidity. a) OLR equals 0.2 kgCODm-3d-1. b) OLR equals 0.6 kgCODm-3d-1. AC – aromatic compounds. TPC – total phenolic compounds. LC – ligninic compounds. LSC – lignosulfonic compounds
As shown in Figs. 3 (a) and (b), there was no expressive color removal, reaching 4% and 10% at the OLRs of 0.2 and 0.6 kgCODm-3d-1, respectively. The increase in color may be related to the process of biotransformation of chromophore units and the condensation of color-forming compounds without mineralization of the effluent (Lewis et al. 2018; Peitz and Xavier 2019). Low color removal occurs during treatment through aerated lagoons (Kamali and Khodaparast 2015; Peitz and Xavier 2019).
Regarding turbidity removal, the system showed an average removal of 94% and 87% at the OLRs of 0.2 and 0.6 kgCODm-3d-1. The AFL system, in both phases, showed removal in this parameter, indicating a potential for clarification of the effluent in the AFL sedimentation zone.
Microbiological analysis
The genetic sequencing of the bacteria was performed through a comparison of information from the NCBI database and statistical similarity analysis, naming the bacteria in the sample by their high similarity with the microorganisms in the database.
Table 1 shows the bacteria identified at the steady state of the two applied OLRs.
Table 1. Cultivable Bacteria Identified in AFLs
The total number of microorganisms identified was 9 species of bacteria at the OLR of 0.2 kgCODm-3d-1 and 12 species at the OLR of 0.6 kgCODm-3d-1.
In the literature, two aspects were observed regarding the study of biological diversity. The first for those observed in biological treatment systems that are related to reactor performance, especially regarding specific parameters such as phenolic compounds, and color; and the second is those from studies in which there was the isolation of bacteria with subsequent bioaugmentation treatment utilizing these selected groups to improve the removal of specific parameters from cellulose effluents.
In the investigation conducted by Saleem et al. (2014), the removal of parameters such as BOD5, COD, and color was 66%, 61%, and 90%, respectively, using Bacillus cereus. Chandra et al. (2012) also used Bacillus cereus associated with Serratia marcescens and Serratia liquefaciens and obtained removal of color (65%), TPC (63%), COD (63%), and BOD5 (64%).
Sonkar et al. (2019) used Bacillus thuringiensis in a sequential batch process and obtained the removal of BOD5 (93%), COD (89%), TOC (82%), and color (73%). By using Bacillus thuringiensis, Arous et al. (2018) verified a removal in terms of COD and TPC of 61% and 64%, respectively.
Raj et al. (2014) obtained removal of color (68%), lignin compounds (54%), total phenol (86%), BOD5 (83%), and COD (78%) by using Paenibacillus sp. Mathews et al. (2016) used Paenibacillus glucanolyticus and verified the potential to degrade cellulose, hemicellulose, and lignin. The three identified species (Bacillus cereus, Bacillus thuringiensis, and Paenibacillus glucanolyticus) show great promise in removing specific parameters in an AFL biological treatment system using bioaugmentation.
Phylogenetic tree
Figures 4 and 5 display the phylogenetic tree of the species Bacillus cereus, Bacillus thuringiensis, and Paenibacillus glucanolyticus, identified at the two OLRs employed in the AFL, whose data were obtained using the MEGA software. The results from the application of the Bootstrap method closest to 100 indicate the species of bacteria present in the sample. The accession number of bacteria in NCBI database for Bacillus cereus is NR_114582, Bacillus thuringiensis is NR_114581, and Paenibacillus glucanolyticus is NR_115597.
Fig. 4. a) Phylogenetic tree of the species Bacillus cereus. The phylogenetic tree was obtained using the union of neighbors with Bacillus megaterium as an outer group, obtaining a replica of 97 for the Bacillus cereus. b) Phylogenetic tree of the species Bacillus thuringiensis. The phylogenetic tree was obtained using the union of neighbors with Bacillus megaterium as an outer group, obtaining a replica of 98 for the Bacillus thuringiensis.
Fig. 5. Phylogenetic tree of the species Paenibacillus glucanolyticus. The phylogenetic tree was obtained using the union of neighbors with Bacillus subtilis as an outer group, obtaining a replica of 100 for the Paenibacillus glucanolyticus.
The microorganisms identified in the kraft effluent were found in other studies (Chandra et al. 2012; Raj et al. 2014; Saleem et al. 2014; Bailón-Salas et al. 2017; Sonkar et al. 2019). The species with the greatest potential for removing the specific parameters of pulp and paper effluents is Bacillus cereus, especially regarding color removal in cellulose effluent treatment, as analyzed by Nunes et al. (2021) and Salem et al. (2014).
CONCLUSIONS
- The microbiological diversity was analyzed in an aerated facultative lagoon system treating kraft pulp industry effluent at organic load rates of 0.2 and 0.6 kgCODm-3d-1 for bioaugmentation purposes. In the treatment, it was possible to observe that the removal of organic matter in terms of biological oxygen demand (BOD5), chemical oxygen demand (COD), and total organic carbon (TOC) was efficient at the low organic loading rates (OLR), reaching a total of 94%, 51%, and 49%. Color and TPC were not significantly removed during the biological treatment. Turbidity had 94%, and lignin-derived compounds had an average removal of 13%.
- For the high OLR, the removal percentages of BOD5, COD, and TOC were 94%, 51%, and 49%. Color and total phenolic compounds (TPC) were not significantly removed during the biological treatment. Turbidity had 87%, and lignin-derived compounds had an average removal of 27%.
- The bacterial species identified from the samples collected in the lagoons were named through BLAST, which showed high statistical similarity for Acinetobacter junii (98%), Aeromonas hydrophila (96%), Bacillus anthracis (99%), Bacillus cereus (98 %), Bacillus kochii (98%), Bacillus thuringiensis (98%), Brevibacillus reuszeri (98%), Brevundimonas diminuta (97%), Comamonas testosteroni (98%), Bacillus sphaericus (98%), Paenibacillus glucanolyticus (98%), and Sphingomonas koreensis (94%).
- The identified species, namely Bacillus cereus, Bacillus thuringiensis, and Paenibacillus glucanolyticus, show great promise in the removal of specific parameters in an AFL biological treatment system using bioaugmentation.
ACKNOWLEDGMENTS
The authors are grateful to the Cocelpa cellulose and paper supplying the effluent, to the Federal Technological University of Paraná, the Department of Chemistry and Biology, the Wastewater Treatment Laboratory, UTFPR Microbiology Laboratory, Ecotoxicology Laboratory, UFPR Microbiology, and Parasitology Laboratory, and the Effluent Treatment Research Group (GTEF) for the support and opportunity to develop this work.
REFERENCES CITED
Abena, M. T. B., Li, T., Shah, M. N., Zhong, W. (2019). “Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation,” Chemosphere 234, 864-874. DOI: 10.1016/j.chemosphere.2019.06.111
American Public Health Association (APHA) (2017). Standard Methods for the Examination of Water and Wastewater (23rd Ed.), E. W. Rice and R. B. Baird, A. D. Eaton (eds.), American Public Health Association, Washington, D.C.
Arous, F., Hamdi, C., Kmiha, S., Khammassi, N., Ayari, A., Neifar, M., Mechichi, T., and Jaouani, A. (2018). “Treatment of olive mill wastewater through employing sequencing batch reactor: Performance and microbial diversity assessment,” 3 Biotech 8(481), 1-14. DOI: 10.1007/s13205-018-1486-6
Bailón-Salas, A. M., Medrano-Roldán, H., Valle-Cervantes, S., Ordaz-Díaz, L. A., Urtiz-Estrada, N., and Rojas-Contreras, J. A. (2017). “Review of molecular techniques for the identification of bacterial communities in biological effluent treatment facilities at pulp and paper mills,” BioResources 12(2), 4384-4409. DOI: 10.15376/biores.12.2. 4384-4409
Braga, L. G. C. (2018). “Estudo de biorremediação aplicada em áreas contaminadas por hidrocarbonetos derivados de petróleo,” 46 f. Monografia (Graduação em Engenharia Ambiental e Sanitária) – Departamento de Ciência e Tecnologia Ambiental, Centro Federal de Educação Tecnológica de Minas Gerais, Belo Horizonte.
Brasil. (2005). “Lei nº 11.105, de 24 de março de 2005,” Regulamenta os incisos II, IV e V do § 1º do art. 225 da Constituição Federal, estabelece normas de segurança e mecanismos de fiscalização de atividades que envolvam organismos geneticamente modificados – OGM e seus derivados, cria o Conselho Nacional de Biossegurança – CNBS, reestrutura a Comissão Técnica Nacional de Biossegurança – CTNBio, dispõe sobre a Política Nacional de Biossegurança – PNB, revoga a Lei nº 8.974, de 5 de janeiro de 1995, e a Medida Provisória nº 2.191-9, de 23 de agosto de 2001, e os arts. 5º, 6º, 7º, 8º, 9º, 10 e 16 da Lei nº 10.814, de 15 de dezembro de 2003, e dá outras providências. Diário oficial. 2005.
Çeçen, F. (2003). “The use of UV-VIS measurements in the determination of biological treatability of pulp bleaching effluents,” in: Conf. Proc. – 7th International Water Association Symposium on Forest Industry Wastewaters, Seattle, WA, pp. 135-142.
Cetesb. (2007). “Avaliação do Uso de Produtos Biotecnológicos para Tratamento de Efluentes Líquidos, Resíduos Sólidos e Remediação de Solos e Águas – L1.022,” Norma Técnica L1.022/2007. 21 f. Disponível em: https://cetesb.sp.gov.br/wp-content/uploads/2021/01/L1.022_Avaliacao-do-uso-de-produtos-biotecnologicos-para-tratamento-de-efluentes-liquidos-residu-Revogada.pdf.
Chamorro, S., Pozo, Z., Jarpa, M., Hernandes, V., Becerra, J., and Vidal, G. (2009). “Monitoring endocrine activity in kraft mill effluents treated by an aerobic moving bed bioreactor system,” Water Science and Technology 62(1), 154-161. DOI: 10.2166/wst.2010.297
Chandra, R., Singh, R., and Yadav, S. (2012). “Effect of bacterial inoculum ratio in mixed culture for decolorization and detoxification of pulp paper mill effluent,” Journal of Chemical Technology & Biotechnology 87(3), 436-444. DOI: 10.1002/jctb.2758.
Diniz, C. G. (2018). “Roteiro de aulas práticas,” Disciplina: Bacteriologia Curso: Farmácia. Departamento de Parasitologia, Microbiologia e Imunologia – Setor de Microbiologia. Universidade Federal de Juiz de Fora.
Duarte, J. C., Peitz, P., and Xavier, C. R. (2018). “Avaliação do tratamento de efluente kraft com meio de suporte esponjoso em reator sequencial em batelada (RSB) [Evaluation of the treatment of kraft effluent with spongy support medium in sequential batch reactor],” in: Anais XIV Simpósio Ítalo-Brasileiro de Engenharia Sanitária Ambiental – SIBESA, Foz do Iguaçu, Brazil.
Ghribi, M., Meddeb-Mouelhi, F., and Beauregard, M. (2016). “Microbial diversity in various types of paper mill sludge: Identification of enzyme activities with potential industrial applications,” SpringerPlus 5, 1492. DOI: 10.1186/s40064-016-3147-8
Hall, B. G. (2013). “Building phylogenetic trees from molecular data with MEGA,” Molecular Biology and Evolution 30(5), 1229-1235. DOI: 10.1093/molbev/mst012
Hooda, R., Bhardwaj, N. K., and Singh, P. (2018). “Brevibacillus parabrevis MTCC 12105: A potential bacterium for pulp and paper effluent degradation,” World Journal of Microbiology and Biotechnology 34(2), 31-41. DOI: 10.1007/s11274-018-2414-y
Kamali, M., and Khodaparast, Z. (2015). “Review on recent developments on pulp and paper mill wastewater treatment,” Ecotoxicology Environmental Safety 114, 326-342. DOI: 10.1016/j.ecoenv.2014.05.005
Lewis, R., Cohen, J., Awad, J., Burger, H., Marzouk, J., Burch, G., Lewis, D. M., and Van Leeuwen, J. A. (2018). “Study of the impacts of process changes of a pulp and paper mill on aerated stabilization basin (ASB) performance,” Chemosphere 211, 767-774. DOI: 10.1016/j.chemosphere.2018.07.178
Liu, J., Yao, J., Wang, F., Min, N., Gu, J., Li, Z., Sunahara, G., Duran, R., Solevic-Knudsen, T., Hudson-Edwards, K. A., Alakangas, L. (2019). “Bacterial diversity in typical abandoned multi-contaminated nonferrous metal(loid) tailings during natural attenuation,” Environmental Pollution 247, 98-107. DOI: 10.1016/j.envpol.2018.12.045
Machado, E. P., Xavier, C. R., and Couto, G. H. (2018). “Tratamento de efluente Kraft em lagoa aerada facultativa empregando enzimas lignolíticas [Kraft effluent treatment in an optional aerated lagoon using lignolytic enzymes],” Interciencia 43(8), 590-596.
Mathews, S. L., Grunden, A. M., and Pawlak, J. (2016). “Degradation of lignocellulose and lignin by Paenibacillus glucanolyticus,” International Biodeterioration & Biodegradation 110, 79-86. DOI: 10.1016/j.ibiod.2016.02.012
Melchiors, E. (2019). Avaliação do Desenvolvimento de Biofilme em Meio Suporte Esponjoso em Reator Biológico de Leito Móvel (MBBR) no Tratamento de Efluente de Indústria de Celulose [Evaluation of the development of biofilm in spongy support medium in biological reactor of mobile bed (MBBR) in the treatment of effluent from the cellulose industry], Master’s Thesis, Universidade Tecnológica Federal do Paraná – Curitiba, Paraná, Brazil.
Nagda, A., Meena, M., Shah, M. P. (2021). “Bioremediation of industrial effluents: A synergistic approach”, Journal of Basic Microbiology 62, 395-414. DOI: 10.1002/jobm.202100225
Nunes, J. V., Silva, M. W. B., Couto, G. H., Bordin, E. R., Ramsdorf, W. A., Flôr, I. C., Vicente, V. P., Almeida, J. D., Celinski, F., and Xavier, C. R. (2021). “Microbiological diversity in an aerated lagoon treating kraft effluent,” BioResources 16(3), 5203-5219. DOI: 10.15376/biores.16.3.5203-5219
Ordaz-Diaz, L. A., Valle-Cervantes, S., Rojas-Contreras, J. A., Rodriguez-Flores, F. J., and Bailón-Salas, A. M. (2016). “Optimization of a microbial formulation acclimated for pilot scale biodegradation of paper mill effluent,” BioResources 11(1), 1071-1079. DOI: 10.15376/biores.11.1.1071-1079
Peitz, C., and Xavier, C. R. (2020). “Moving bed biofilm reactor for treatment of kraft pulp effluent with high organic load rate,” Revista Ambiente & Água 15(4), 1-10. DOI: 10.4136/ambi-agua.2512
Peitz, C., and Xavier, C. R. (2019). “Evaluation of aerated lagoon modified with spongy support medium treating kraft pulp mill effluent,” Revista Facultad de Ingeniería Universidad de Antioquia 92, 70-79. DOI: 10.17533/udea.redin.20190725
Raj, A., Kumar, S., Haq, I., and Singh, S. K. (2014). “Bioremediation and toxicity reduction in pulp and paper mill effluent by newly isolated ligninolytic Paenibacillus sp.,” Ecological Engineering 71, 355-362. DOI: 10.1016/j.ecoleng.2014.07.002
Saleem, M., Ahmad, S., and Ahmad, M. (2014). “Potential of Bacillus cereus for bioremediation of pulp and paper industrial waste,” Annals of Microbiology 64(2), 823-829. DOI: 10.1007/s13213-013-0721-y
Singh, R. L., Singh, R. P. (2019). Advances in Biological Treatment of Industrial Wastewater and Their Recycling for a Sustainable Future, Singapore: Springer, p. 225.
Sonkar, M., Kumar, M., Dutt, D., and Kumar, V. (2019). “Treatment of pulp and paper mill effluent by a novel bacterium Bacillus sp. IITRDVM-5 through a sequential batch process,” Biocatalysis and Agricultural Biotechnology 20, 101232.
Stinghen, A. E. M., Albini, C. A., and Souza, H. A. P. H. M. (2002). “Coloração de Gram: Como fazer, interpretar e padronizar,” 70 p. Curitiba: Microscience.
Subashini, A. M. (2015). “Review on biological treatment processes of pulp and paper industry wastewater,” International Journal of Innovative Research in Science, Engineering and Technology 4(5), 3721-3725. DOI: 10.15680/IJIRSET.2015.0405195
Vicente, V. A., Attili-Angelis, D., Pie, M. R., Queiroz-Telles, F., Cruz, L. M., Najafzadeh, M. J., Hoog, G. S., Zhao J. S., and Pizziranikleiner, A. (2008). “Environmental isolation of black yeast-like fungi involved in human infection,” Studies in Mycology 61, 137-144.
Von Sperling, M. (2016). Urban Wastewater Treatment in Brazil (IDB-TN-970), Department of Sanitary and Environmental Engineering, Federal University of Minas Gerais, Brazil.
Von Sperling, M. (2014). “Introdução à qualidade das águas e ao tratamento de esgotos [Introduction to water quality and sewage treatment],” in: Princípios do tratamento biológico de águas residuárias [Principles of biological wastewater treatment], Belo Horizonte, Minas Gerais, Brazil.
Yarza, P., Yilmaz, P., Pruesse, E., Glokner, F. O., Ludwig, W., Schleifer, K. H., Whitman, W. B., Euzéby, J., Amann, R., and Rosselló-Móra, R. (2014). “Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences,” Nature Reviews Microbiology 12, 635-645. DOI: 10.1038/nrmicro3330
Article submitted: August 22, 2022; Peer review completed: September 11, 2022; Revised version received and accepted: September 23, 2022; Published: October 6, 2022.
DOI: 10.15376/biores.17.4.6556-6568
