Abstract
A rapid and green preparation of lignin nanoparticles was demonstrated starting from bio-refinery lignin containing grafted carbohydrates. The particles were prepared by recovering a fraction of the lignin, which contained 24% carbohydrate (by weight) as the insoluble fraction in 0.5 M NaOH. The carbohydrate content of this fraction was verified with a wet chemistry analytical technique, nuclear magnetic resonance, and X-ray diffraction. This fraction was then dissolved in a NaOH/urea/water system and added dropwise to water under a high shear, which rapidly formed precipitated particles in a size range of approximately 100 nm. This carbohydrate-containing fraction of the lignin was soluble in a green solvent system that was not suited for lignin alone. The generated particles were stable in different organic solvents and water. Overall, the dissolution of the bio-refinery lignin in the NaOH/urea/water system, followed by precipitation in water can be regarded as a green and rapid method to produce stable nanoparticles. The generated nanoparticles, containing both carbohydrates and lignin, are expected to have unique applications because of their bi-component nature. Furthermore, this is the first publication to show how materials with high levels of lignin can be solubilized in solvents that are conventionally used for cellulose.
Download PDF
Full Article
Bi-component Carbohydrate and Lignin Nanoparticle Production from Bio-refinery Lignin: A Rapid and Green Method
Hasan Sadeghifar,a,b Richard A. Venditti,a,* Joel J. Pawlak,a and Jesse Jur c
A rapid and green preparation of lignin nanoparticles was demonstrated starting from bio-refinery lignin containing grafted carbohydrates. The particles were prepared by recovering a fraction of the lignin, which contained 24% carbohydrate (by weight) as the insoluble fraction in 0.5 M NaOH. The carbohydrate content of this fraction was verified with a wet chemistry analytical technique, nuclear magnetic resonance, and X-ray diffraction. This fraction was then dissolved in a NaOH/urea/water system and added dropwise to water under a high shear, which rapidly formed precipitated particles in a size range of approximately 100 nm. This carbohydrate-containing fraction of the lignin was soluble in a green solvent system that was not suited for lignin alone. The generated particles were stable in different organic solvents and water. Overall, the dissolution of the bio-refinery lignin in the NaOH/urea/water system, followed by precipitation in water can be regarded as a green and rapid method to produce stable nanoparticles. The generated nanoparticles, containing both carbohydrates and lignin, are expected to have unique applications because of their bi-component nature. Furthermore, this is the first publication to show how materials with high levels of lignin can be solubilized in solvents that are conventionally used for cellulose.
Keywords: Carbohydrate-lignin; Lignin nanoparticle; Bio-refinery lignin; Green processing; Sodium hydroxide; Urea; Bi-component
Contact information: a: Departments of Chemistry and Forest Biomaterials, Organic Chemistry of Wood Components Laboratory, North Carolina State University, Raleigh, NC 27695 USA; b: Department of Wood and Paper Science, Islamic Azad University, Sari Branch, Iran, P.O. Box 48161-19318; c: Department of Textile Engineering, Chemistry and Science, North Carolina State University, Raleigh, NC 27695 USA; *Corresponding author: richard_venditti@ncsu.edu
INTRODUCTION
As the second most abundant renewable natural polymer after cellulose, lignin has always attracted the interest of researchers. Lignin is available as a by-product from wood pulping to produce paper and the bio-refinery of lignocellulosic biomass. Technical lignin is a complex, multifunctional, and heterogeneous biopolymer (Lu and Ralph 1998; Crestini et al. 2011). Through bio-refinery processes, biomass is expected to be one of the main sources of future energy, biofuel, and chemical production. Among the different types of biomass sources, lignocellulosic feedstock (straw, reeds, grass, wood, paper waste, etc.), which contains various levels of cellulose, hemicellulose, and lignin, has shown good potential for bio-refinery processing (Galbe and Zacchi 2002; FitzPatrick et al. 2010). All bio-processing methods for the conversion of polysaccharides to bio-fuel leave residual lignin, along with unconverted sugars as a by-product (25% to 35% of total biomass) (Wyman 2007).
Massive amounts of lignin will be generated in the future from lignocellulosic ethanol and chemical production industries. However, bio-refinery industries mostly focus on fuel and chemical production from cellulose and hemicellulose, while lignin is usually considered a low-value residual product and used as an energy source for power generation (Yuan et al. 2013). The conversion of this low-value by-product into high-value co-products will help to offset the cost of bio-ethanol production. To do this, new applications for the biorefinery lignin must be developed. Biorefinery lignin contains a significant amount of carbohydrate bonded to the lignin. Because of the high amounts of carbohydrates associated with bio-refinery lignin, it is insoluble in regular lignin solvents and is challenging to use in the production of lignin-based materials.
Natural nanoparticles have shown good potential for applications in the food, polymer, cosmetic, drug, and other fields. After a few decades of research on nanocellulose production as a main source of natural nanoparticles, nanoparticles and microparticles based on other natural resources, such as lignin, have generated considerable research interest in recent years. The main reported methods for lignin nanoparticle preparation are anti-solvent precipitation (Stewart et al. 2014), sonication and ultrasound (Tortora et al. 2014; Gilca et al. 2015), simultaneous enzymatic saccharification and physical treatment of lignin (Shikinaka et al. 2010), reaction with silica (Cui et al. 2015), high-shear homogenization (Nair et al. 2014), self-assembly using acetylated lignin (Qian et al. 2014), and spray drying (Ago et al. 2016). A recent comprehensive review (Beisl et al. 2017) of lignin nanoparticle production did not contain any reports about the production of lignin nanoparticles from bio-refinery lignin.
In this study, bio-refinery lignin was characterized, and nanoparticles and microparticles were produced. The synthesis of bio-refinery lignin particles was performed by dissolving the lignin in NaOH/urea/water, followed by precipitation in water. This method is fast, uses green solvents, and yields final lignin particles that are stable in different solvents and water.
EXPERIMENTAL
Bio-refinery lignin produced from the solid residue from a dilute acid pretreatment and enzyme hydrolysis process from hardwood was collected from an industrial bio-refinery process and was used in an air-dry state. The lignin was first suspended in an HCl solution (pH = 2) and then washed with deionized water to remove minerals and water-soluble materials. After drying at room temperature, the prepared lignin was mixed with dimethyl sulfoxide (DMSO), dimethylformamide (DMF), 0.5 M NaOH, or acetone (1 g/20 mL) separately to investigate the solubility in these solvents. The soluble fraction was separated from the insoluble fraction using filtration. The insoluble fraction using 0.5 M NaOH was used to produce particles in this study. The nanoparticle production from the insoluble fraction of bio-refinery lignin is illustrated in Fig. 1.
A solution of NaOH/urea/water with a ratio of 7:12:81 by weight was prepared and cooled to ‑12 °C (Luo et al. 2009). The insoluble fraction (0.5 M NaOH) of the bio-refinery lignin (1 g/20 mL) was immediately dispersed into the solvent system under vigorous stirring for 10 min. Then, the mixture was cooled again to -12 °C and mixed for an additional 10 min with shaking. The insoluble part was separated using a centrifuge at 5000 rpm for 5 min and discarded. The soluble part of the bio-refinery lignin in the NaOH/urea/water system was added dropwise into 500 mL of water in a lab-scale blender at high speed. The materials in the water were directly transferred into a cellulose dialysis bag and dialyzed for 3 d against deionized water to remove all of the soluble materials and impurities. After purification, the suspension was centrifuged at 5000 rpm for 5 min, and the settled particles were separated from the non-settled ones. The non-settled fraction was used for this study as nanoparticles.
Fig. 1. Schematic illustration of lignin nanoparticle production from bio-refinery lignin
Particle size and shape evaluations were performed using transmission electron microscopy (TEM) (JEM-1400, JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV. Particle surface analysis was performed by scanning electron microscopy (SEM) using a Phenom G2 Pro system (Thermo Fisher Scientific, Waltham MA, USA) at different magnifications. Zeta potential and dynamic light scattering (DLS) measurements were performed on the particle suspension at 25 °C using a Nano ZS particle sizer (Malvern Instruments, Malvern, UK) with a detection angle of 173° and a 3-mW He-Ne laser operating at a wavelength of 633 nm. The hydrodynamic size (dhyd) DLS values were the Z-average diameters (mean hydrodynamic diameters based on the intensity of the scattered light). The X-ray diffraction (XRD) patterns of the original bio-refinery lignin and its particles were determined using a Rigaku Smartlab X-ray diffractometer (Tokyo, Japan). The diffractometer was equipped with Be-filtered Cu Kα radiation with a wavelength of 1.54 Å generated at 35 kV and 25 mA. The hydroxyl groups of the bio-refinery lignin were analyzed by quantitative 31P nuclear magnetic resonance (NMR) using published procedures (Sadeghifar et al. 2014). The carbohydrate content in the insoluble lignin fraction was measured using an acid hydrolysis method (Klason lignin) (TAPPI T222 OM 002).
RESULTS AND DISCUSSION
The bio-refinery lignin used in this research contained approximately 20% carbohydrates and was mostly insoluble in the usual organic and alkaline solvents for lignin. Mixing the bio-refinery lignin in typical lignin solvents (DMF, DMSO, 0.5 M NaOH, and acetone) indicated that approximately 25% of the material was soluble in these solvents. The soluble fraction can be understood as being the part of the lignin that has not bonded with carbohydrate. The bio-refinery lignin was fractionated using the 0.5 M NaOH solution into soluble and insoluble fractions, and the insoluble fraction was used for particle production. This process (fractionation of lignin with a NaOH solution and solubilization with NaOH/urea/water) creates a carbohydrate-rich lignin that is a unique material and utilizes a green solvent system for lignin. Furthermore, the generated particles, with both carbohydrates and lignin, may have unique applications because of their bi-component nature. These might include lignin properties, such as ultraviolet light blocking or radical scavenging, and carbohydrate properties, such as compatibility with other carbohydrates in composites or enzymatic susceptibility.
The carbohydrate content in the insoluble fraction was measured using an acid hydrolysis method (Klason lignin) (TAPPI T222 OM 002) and was 24%. The total phenolic and aliphatic hydroxyl group contents of the insoluble fraction were measured by 31P NMR (Fig. 2). The total aliphatic OH, phenolic OH, and acid contents of the insoluble fraction were 1.96 mmol/g, 4.58 mmol/g, and 0.2 mmol/g, respectively. Due to the presence of carbohydrate in the insoluble fraction, the 31P NMR spectra of the insoluble fraction were divided into two separate signals related to lignin and carbohydrates, with partially overlapping peaks. The phenolic part of the spectra indicates the biomass source is hardwood (Sadeghifar et al. 2014).
Fig. 2. 31P NMR spectra of the insoluble fractions of the bio-refinery lignin
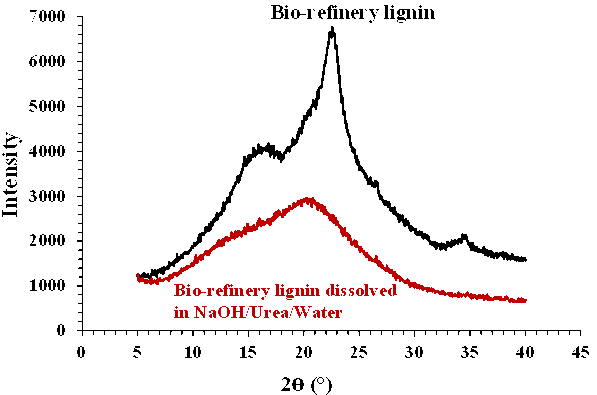
Fig. 3. XRD spectra of bio-refinery lignin and dissolved sample in the NaOH/urea/water system
Figure 3 shows the XRD spectra of the bio-refinery lignin before and after dissolution in the NaOH/urea/water system. The bio-refinery lignin showed an order similar to the cellulose patterns with signals at the 2θ values 22.5° and 14° to 16°. After dissolving the sample in the NaOH/urea/water system, the results showed an amorphous structure.
Figure 4 shows the shape and size of the bio-refinery lignin nanoparticles using transmission electron spectroscopy. The images showed spherical particles that were less than 100 nm in size. The SEM images (Fig. 5) show the morphology of the freeze-dried sample. The dried samples tended to self-assemble into ultrafine powders, which exhibited a broad size distribution of micro-sized particles. These results show the possibility of micro-sized particle preparation in the dry state.
Fig. 4. TEM images of bio-refinery lignin particles from mass to single particle (A to C)
Fig. 5. SEM images of the freeze-dried bio-refinery lignin particles in smaller (A) length scale of 20 microns, and (B) length scale of 10 microns are indicated on the figures
The zeta potential is commonly used to measure the electrostatic potential of particles at the electrical double layer. The surface properties of the bio-refinery lignin particles suspended in water were analyzed using zeta potential measurements at a neutral pH. The results showed a negative surface charge of ζ equal to -36 mV ± 0.5 mV.
Dynamic light scattering was used to measure the sizes of the colloidal bio-refinery lignin particles without any drying. Because of the spherical structure of the particles, as is shown in the TEM results, the DLS results could be used to estimate the particle sizes. The DLS results showed a wide distribution of sizes, though with many particles in the range of 1 nm to 1000 nm (Fig. 6). Agglomeration of the particles may have been the source of the micro-sized lignin particles that are shown in Fig. 6.
The particles were dispersed in several organic liquids (DMF, DMSO, methanol, and acetone) and alkaline water, and were determined to be non-soluble in these liquids.
Fig. 6. DLS size distrubution of the bio-refinery lignin particles
CONCLUSIONS
- A facile procedure was developed to show that readily available lignin-based materials with high levels of lignin can be solubilized in solvents that are conventionally used for cellulose.
- The dissolution of bio-refinery lignin in the NaOH/urea/water system, followed by precipitation in water, can be regarded as a greener method than typical cellulose solvents such as heavy metal complexes and ionic liquids.
- Compared to regular cellulose solvent processes that need multiple stage processes to generate regenerated cellulose/cellulosic compounds, this method is faster and more facile due to the one-pot process to produce stable nanoparticles that contain lignin covalently bonded to carbohydrates.
- The bi-component nanoparticles generated will have unique applications and properties because of their covalently bonded lignin and cellulose composition.
REFERENCES CITED
Ago, M., Huan, S., Borghei, M., Raula, J., Kauppinen, E. I., and Rojas, O. J. (2016). “High-throughput synthesis of lignin particles (~30 nm to ~2 μm) via aerosol flow reactor: Size fractionation and utilization in Pickering emulsions,” ACS Appl. Mater. Inter. 8(35), 23302-23310. DOI: 10.1021/acsami.6b07900
Beisl, S., Miltner, A., and Friedl, A. (2017). “Lignin from micro- to nanosize: Production methods,” Int. J. Mol. Sci. 18(6), 1244-1274. DOI: 10.3390/ijms18061244
Crestini, C., Melone, F., Sette, M., and Saladino, R. (2011). “Milled wood lignin: A linear oligomer,” Biomacromolecules 12(11), 3928-3935. DOI: 10.1021/bm200948r
Cui, J., Sun, H., Wang, X., Sun, J., Niu, M., and Wen, Z. (2015). “Preparation of siliceous lignin microparticles from wheat husks with a facile method,” Ind. Crop. Prod. 74, 689-696. DOI: 10.1016/j.indcrop.2015.05.061
FitzPatrick, M., Champagne, P., Cunningham, M. F., and Whitney, R. A. (2010). “A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products,” Bioresour. Technol. 101(23), 8915-8922. DOI: 10.1016/j.biortech.2010.06.125
Galbe, M., and Zacchi, G. (2002). “A review of the production of ethanol from softwood,” Appl. Microbiol. Biot. 59(6), 618-628. DOI: 10.1007/s00253-002-1058-9
Gilca, I. A., Popa, V. I., and Crestini, C. (2015). “Obtaining lignin nanoparticles by sonication,” Ultrason. Sonochem. 23, 369-375. DOI: 10.1016/j.ultsonch.2014.08.021
Lu, F., and Ralph, J. (1998). “The DFRC method for lignin analysis. Part 3. NMR studies,” J. Wood Chem. Technol. 18(2), 219-233. DOI: 10.1080/02773819809349578
Luo, X., Liu, S., Zhou, J., and Zhang, L. (2009). “In situ synthesis of Fe3O4/cellulose microspheres with magnetic-induced protein delivery,” J. Mater. Chem. 19(21), 3538-3545. DOI: 10.1039/B900103D
Nair, S. S., Sharma, S., Pu, Y., Sun, Q., Pan, S., Zhu, J. Y., Deng, Y., and Ragauskas, A. J. (2014). “High shear homogenization of lignin to nanolignin and thermal stability of nanolignin-polyvinyl alcohol blends,” ChemSusChem 7(12), 3513-3520. DOI: 10.1002/cssc.201402314
Qian, Y., Deng, Y., Qiu, X., Li, H., and Yang, D. (2014). “Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor,” Green Chem.16(4), 2156-2163. DOI: 10.1039/C3GC42131G
Sadeghifar, H., Dickerson, J. P., and Argyropoulos, D. S. (2014). “Quantitative 31P NMR analysis of solid wood offers an insight into the acetylation of its components,” Carbohyd. Polym.113, 552-560. DOI: 10.1016/j.carbpol.2014.07.046
Shikinaka, K., Fujii, N., Egashira, S., Murakami, Y., Nakamura, M., Otsuka, Y., Ohara, S., and Shigehara, K. (2010). “Polyfunctional nanometric particles obtained from lignin, a woody biomass resource,” Green Chem. 12(11), 1914-1916. DOI: 10.1039/C0GC00140F
Stewart, H., Golding, M., Matia-Merino, L., Archer, R., and Davies, C. (2014). “Manufacture of lignin microparticles by anti-solvent precipitation: Effect of preparation temperature and presence of sodium dodecyl sulfate,” Food Res. Int. 66, 93-99. DOI: 10.1016/j.foodres.2014.08.046
TAPPI T222 om-02. (2002). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
Tortora, M., Cavalieri, F., Mosesso, P., Ciaffardini, F., Melone, F., and Crestini, C. (2014). “Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules,” Biomacromolecules 15(5), 1634-1643. DOI: 10.1021/bm500015j
Wyman, C. E. (2007). “What is (and is not) vital to advancing cellulosic ethanol,” Trends Biotechnol. 25(4), 153-157. DOI: 10.1016/j.tibtech.2007.02.009
Yuan, T.-Q., Xu, F., and Sun, R.-C. (2013). “Role of lignin in a biorefinery: Separation characterization and valorization,” J. Chem. Technol. Biot. 88(3), 346-352. DOI: 10.1002/jctb.3996
Article submitted: January 31, 2019; Peer review completed: April 8, 2019; Revised version received and accepted: June 14, 2019; Published: June 19, 2019.
DOI: 10.15376/biores.14.3.6179-6185
