Abstract
This paper describes the preparation of poly(vinyl alcohol)/kenaf fiber (PVOH/KF) composites with entrapped urea. The major FTIR peaks of these composites could be identified. These composites are intended for agricultural applications as biodegradable mulches and could be potential carrier materials for fertilizer. The water solubility, release behavior, chemical properties, and thermal stability of the composites were evaluated. The composites lost 25% of their weight after 7 days in water. In a wet environment, urea was released from the composites through its dissolution in water, and around 57% of the urea was released from the composites in 24 h; Thermagravimetric analysis showed that these composites were stable up 150 °C. These composites would be able to withstand rain and protect seedlings from the sun when applied in the field as mulches. For around three to four weeks, these biobased mulches could slowly disintegrate as the PVOH binder was gradually dissolved by moisture, releasing the kenaf fibers to serve as soil fertilizer without leaving any undegradable waste for disposal. Hence, they would not pose any risks to the land or biological systems.
Download PDF
Full Article
Biodegradable Mulches Based on Poly(vinyl Alcohol), Kenaf Fiber, and Urea
Boon Khoon Tan,a Yern Chee Ching,a,* Seng Neon Gan,b and Shaifulazuar Rozali a
This paper describes the preparation of poly(vinyl alcohol)/kenaf fiber (PVOH/KF) composites with entrapped urea. The major FTIR peaks of these composites could be identified. These composites are intended for agricultural applications as biodegradable mulches and could be potential carrier materials for fertilizer. The water solubility, release behavior, chemical properties, and thermal stability of the composites were evaluated. The composites lost 25% of their weight after 7 days in water. In a wet environment, urea was released from the composites through its dissolution in water, and around 57% of the urea was released from the composites in 24 h; Thermagravimetric analysis showed that these composites were stable up 150 C. These composites would be able to withstand rain and protect seedlings from the sun when applied in the field as mulches. For around three to four weeks, these biobased mulches could slowly disintegrate as the PVOH binder was gradually dissolved by moisture, releasing the kenaf fibers to serve as soil fertilizer without leaving any undegradable waste for disposal. Hence, they would not pose any risks to the land or biological systems.
Keywords: Kenaf fiber; PVOH; Urea; Biodegradable; Mulches
Contact information: a: Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia; b: Chemistry Department, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia; *Corresponding author: chingyc@um.edu.my
INTRODUCTION
There have been increasing concerns in recent years regarding environmental pollution consisting of non-biodegradable polyethylene mulch films. Previous literature has reported the uses of polyethylene films as mulches in reducing soil erosion and maintaining soil moisture for better crop growth (Adegbidi et al.2003). Petroleum-based films are widely used due to their properties such as low density, good mechanical properties, excellent water resistance, and low cost (Nurfatimah et al. 2015). However, this synthetic plastic has generated a number of disposal difficulties because it requires huge labor forces for manual disposal. Land application is not a suitable disposal method for non-degradable plastic mulch due to its interference in the root development of subsequent crops (Kasirajan and Ngouajio 2012). An alternative disposal method for agricultural plastic wastes is through biodegradation processes.
Many studies have demonstrated the use of biodegradable materials as alternatives to conventional polyethylene mulch films (Moreno and Moreno 2008). Some of the biodegradable materials used were composites developed from a combination of cellulose and starch (Moreno and Moreno 2008). These biodegradable films have not gained widespread use, possibly due to their high production cost and the unpredictable nature of their biodegradation behavior (Kasirajan and Ngouajio 2012).
Poly(vinyl alcohol) (PVOH) is a non-toxic material that presents no adverse effects to biological systems, and has become highly attractive for various applications (DeMerlis and Schoneker 2003; Yong et al. 2015). In the agriculture sector, PVOH could be a promising material for biodegradable mulches due to its water solubility (Guohua et al. 2006). This material can be biodegraded and absorbed into the soil instead of requiring removal from the field. The use of biodegradable mulch films can solve the waste disposal difficulties and high labor cost issues associated with PE films.
Kenaf fiber is an abundant, sustainable high-yield fiber crop (Monti and Alexopoulou 2013; Yong et al. 2015). Due to the film-forming characteristics of PVOH, it can serve as a binder for kenaf fibers to form biodegradable mulches. Moreover, the kenaf fiber could serve as fertilizer for the seedling after the degradation of PVOH in soil.
In agriculture, urea is the fertilizer with the highest nitrogen content (46%). In its field application, it has not been fully absorbed by the plants, but a greater part is lost to the environment (40 to 70%), particularly due to rapid leaching and surface runoff into the soil, resulting in low crop production and environment pollution (Chen et al. 2008; Ni et al. 2013; Thanh et al. 2015). For this reason, controlled-release fertilizers made from a variety of matrix materials have been developed, such as starch, polyacrylic acid, chitosan, polylactic acid, ethyl cellulose, and poly(vinyl alcohol) (Chen et al.2008; Wu and Liu 2008; Ni et al. 2009; Calabria et al. 2012; Yip et al. 2013; Zhong et al. 2013).
Nevertheless, the implementation of controlled-release fertilizers is limited by their relatively high cost, in spite of their potential benefits (Trenkel 1997). Tao et al. (2011) have reported a triple polymeric layers for slow-release of urea, with water-retaining characteristics by using a combination of polyethylene, poly(acrylic acid-co-acrylamide), and poly(butyl methacrylate).
Realizing these complications, Castro-Enríquez et al. (2012) developed homogeneous, thin porous membranes, containing oriented fiber obtained from wheat gluten via an electrospinning technique, with the aim of building a release system at lower cost. However, the product formed released 98% of the total urea in 300 minutes, which is incompatible with the established standards for agricultural use (Castro-Enríquez et al. 2012). Wheat gluten is regarded as a suitable material for such applications because it is renewable, biodegradable, inexpensive, and widely available (Castro-Enríquez et al. 2012). In another case, Yip et al. (2013) also encapsulated urea in starch-based composites to reduce the cost. Although the price of starch based coating is low, its nutrient release lifespan is lower than the polymer coating formulations. Furthermore, some of the starch coating formulations are occasionally incompatible with crop metabolic needs (Azeem et al. 2014).
In the present research, poly(vinyl alcohol)/kenaf fiber composites with entrapped urea were prepared via a solution casting method. Urea was added as a slow-release fertilizer. The functionality, water solubility, and thermal stability of the prepared composites were evaluated. The release behavior of urea through the composites and their feasibility as potential fertilizer carriers were examined. Moreover, this report further investigated the time scale of urea release after water penetration. This material was designed to provide a two-fold advantage with respect to environmental management: the urea is released to the plants in a controlled manner, and the biodegradable mulches can disintegrate after a number of weeks without leaving any unnecessary residue behind.
EXPERIMENTAL
Materials
Polyvinyl alcohol (PVA-220S), with a degree of hydrolysis between 87 to 89 mole percent, was purchased from Kuraray Asia Pacific Pte. Ltd (Singapore) and used without further purification. The urea was purchased from Systerm ChemAR (Malaysia). The kenaf fibers were supplied by National Kenaf & Tobacco Board (Malaysia). The fibers were cleaned, ground, and sieved to an average size of 50 µm, then dried in an oven to less than 10% moisture prior to use.
Methods
Preparation of PVOH/KF composites
The composites were prepared according to a procedure mentioned in previous literature (Yip et al. 2013), with some minor modifications. A predetermined amount of PVOH powder was first dissolved in cold water to produce a PVOH solution with 10% solids content. The solution was homogenized under constant stirring at 80 °C for 1 h. A 100-g portion of the PVOH solution was transferred into a 250-mL beaker, and kenaf fiber was added until it represented 10 wt% of the PVOH solution. Various levels of urea loading (3, 5, 7, and 10 wt%) were added, and the mixture was stirred continuously until it became homogeneous. Finally, the mixture was cast on a 20-cm × 20-cm non-stick mold and left to dry overnight at ambient temperature (27 to 28 °C). The sample was then hot pressed at 150 °C for 8 min to obtain a sheet with a uniform thickness of around 1 mm. The produced samples were stored in a drying cabinet for 7 d at 25 ±0.5 °C and 35% relative humidity (RH) before characterization. The morphological images of the composites were examined by a Dino-Lite AM3113T digital microscope (Dino-Lite, Taiwan). The composites had a good appearance and smooth surfaces without any bubbles or surface cracks as show in Fig. 1.
Fig. 1. Morphology of biodegradable mulches
Characterization
For the water solubility test, a total of 5 samples for each formulation were cut into 2-cm × 2-cm squares of 1 mm thickness, and dried overnight in an oven until a constant weight was achieved. The dry weight of the samples was measured using an analytical balance. The samples were then immersed in distilled water at room temperature. After the specified time, the specimens were taken out of the water and wiped with a soft tissue. The specimens were dried in a vacuum oven at a temperature of 50 °C until a constant weight was achieved. The final weight was measured and recorded, and the water solubility was calculated as the ratio of lost weight to the original weight. All readings were measured in triplicate.
The urea content in the water was determined by using the Kjeldahl method, with minor modifications (AOAC 1995). The water used in the solubility test, described in the previous paragraph, was mixed with 25 mL of a 50 wt% sodium hydroxide solution in a conical flask fitted with a rubber stopper that carried a delivery tube. The mixture was heated to boiling point, and the released ammonia gas was directed through the delivery tube into a known volume of 0.1-M hydrochloric acid solution. The acid/ammonia solution was then back-titrated using a 0.1-M sodium hydroxide solution. The number of moles of ammonia was calculated by subtracting the moles of base added from the moles of acid at the end point of the titration. Finally, the urea content released in the water was determined based on number of moles of ammonia. For comparison with the experimental results, another standard solution was prepared by mixing urea in distilled water at concentrations of 3, 5, 7, and 10 wt%. This experiment was carried out in triplicate.
The functional groups of the composites were characterized by the attenuated total reflection (ATR) technique using a Perkin Elmer Spectrum 400 spectrometer (Perkin Elmer, USA). The sample was placed in direct contact with the crystal surface. The probe was then gently pressed down into the sample. A series of FTIR spectra were recorded, consisting of 16 scans in the wave number range of 4000 to 400 cm-1. The thermogravimetric measurements were performed by using a TGA 851/LF instrument from Mettler-Toledo (Switzerland). The samples (approximately 6 to 8 mg) were placed in a clean platinum pan and heated from 30 °C to 600 °C at a scanning rate of 10 °C/min under a nitrogen atmosphere.
RESULTS AND DISCUSSION
Water Solubility
Figure 2 presents the results of water solubility test the composites after 1 week of water immersion. It was found that the water solubility of the composites was not affected by the loading of small amounts of urea; the entrapped urea in the PVOH/KF composites could dissolve much more slowly than urea in the water. All of the samples presented similar patterns, and the water solubility percentage increased with increasing time. The 10-wt% PVOH/KF/U composite achieved the highest water solubility percentage after 7 d of water immersion. The composite reached 12% solubility in 24 h, increasing slowly to 14% in 3 d. The composite achieved 25% solubility after 7 d in the water, implying that the composite could last for a reasonable length of service time in static aquatic conditions.
When the composite was immersed in water, the water molecules diffused into the composite, and presumably the hydroxyl groups in PVOH and kenaf fiber could take part in intermolecular hydrogen bonding with water, causing the lower molecular weight fractions to diffuse out and dissolve (Nosbi et al. 2010). The water-solubility of the composite gradually increased over 24 h (Fig. 2). This effect was enhanced by the cellulose content of kenaf fiber, which contributed to more water penetration into the composites (Tan et al. 2014). Mazuki et al. (2010) also reported the same observation when pultruded kenaf fiber reinforced composites were immersed in water. They claimed that this phenomenon was attributable to the penetrability of water and capillary action.
Azwa et al. (2013) also reported that moisture is one of the factors that influences the degradation of natural fiber/polymer composites. The leaching of water-soluble substances from PVOH/KF composites would cause the debonding of kenaf fibers from the composite. Thus, the dissolution of a composite in water would ultimately lead to the degradation of the composite (Azwa et al. 2013). Similar findings have been reported by Dhakal et al. (2007), who investigated the water absorption behaviour of hemp fiber-reinforced unsaturated polyester composites.
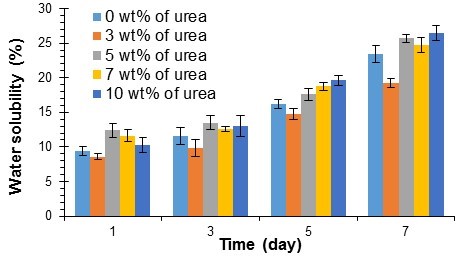
Fig. 2. Water solubility of PVOH/KF composites with different urea loading values
Content of Urea Released in Water
The controlled release property of the composites was investigated by measuring the amount of urea released into the water. The nutrients released through the polymer carrier were mainly dependent on the temperature and moisture permeability of the polymer carrier. Therefore, it is possible to estimate the nutrient release for a given time (Trenkel 1997). In Figure 3, all of the samples displayed a similar pattern, and the content of urea released from the composites increased with increasing time and urea loading. The 10-wt% PVOH/KF/U composite released the highest amount of urea after 24 h; 25% of the urea content was released in the first hour. The urea content in the water increased to 36% after 10 h, while a total of 57% of the urea was released into the water after 24 h. This finding indicates that the urea was released in a controlled and delayed manner after being entrapped in the composite.
Figure 4 compares the leached urea content with the standard urea solution. Apparently, the leached urea content was reduced after the urea was entrapped in the composites. This result indicates that the poly(vinyl alcohol)/kenaf fiber composites had a controlled release property. Niu and Li (2012) also found that the rate of urea release had been evidently slowed down by starch-g-poly(vinyl acetate) matrices because the pure urea usually dissolved very quickly in water in several seconds. The 3-wt% PVOH/KF/U composite released around 48% of the urea content, whereas the 5-wt% composite released up to 55% in 24 h. The leached urea content continued to increase with increasing urea loading in the composites. The 7- and 10-wt% PVOH/KF/U composites released approximately 56% and 57% of the urea content, respectively. Niu and Li (2012) reported that urea was released slowly in the first (about 10% of urea) and third (about 15% of urea) stages. The release of urea from starch-g-poly(vinyl acetate) membrane depends on the graft efficiency of poly(vinyl acetate). The data in Fig. 4 are shown in units of g/g in order to compare the amount of urea released between the composites and the standard urea solution.
According to the nutrient release rate requirements published by the International Fertilizer Industry Association, the desired nutrient release rate should not exceed 15% in 24 h (Trenkel 1997). Despite the observed increase in the nutrient release rate, these composites still retained the necessary characteristics for a mulching film and potential carrier for fertilizer. However, depending on the polymer coating material used, the incorporation of a cross-linking agent could reduce the moisture permeability of the polymers, which would subsequently reduce the nutrient release rate (Hussain et al. 2012).
Fig. 3. Urea content released from the composites in 24 h at different urea loading values
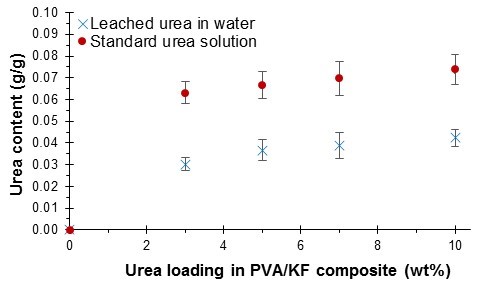
Fig. 4. Urea content in the water after 24 h for PVOH/KF composites at different urea loading values
FTIR-ATR Analysis
FTIR was used to identify that the characteristics peaks of the various components in the composites. Figure 5 shows the IR spectra of PVOH, KF, PVOH/KF, and the 10-wt% PVOH/KF/U composite. The region between 833 cm-1 and 837 cm-1 indicated the presence of a PVOH skeletal backbone . The PVOH and kenaf fiber shared the same band, at 3299 cm-1 and 3334 cm-1, respectively, which was assigned to the –OH groups (Ali et al. 2014). The stretching of –OH groups from the intermolecular and intramolecular hydrogen bonds among PVOH chains was ascribed to the high hydrophilic forces (Reis et al. 2006; Yong et al. 2015). However, the existence of this band in kenaf fiber was due to the stretching of hydroxyl groups, which are present in cellulose, hemicellulose, and lignin. The signal at 3297 cm-1 was related to the stretching of –NH groups in urea. Similarly, the stretching of –CH alkyl groups was also seen at 2928 cm-1 and 2921 cm-1 for PVOH and KF, respectively.
Figure 6 displays the IR spectrum of the 10-wt% PVOH/KF/U composite before and after the water solubility test. Both curves show a similar transmittance, even though the upper curve represents a system following 24 h of water solubility testing The band at 1647 cm-1 was associated with the bending of –NH groups, while the band at 1662 cm-1 corresponded to the stretching of C=O bonds in urea molecules. This observation supported the controlled release property of PVOH/KF and its potential as a carrier for fertilizer. The main IR bands are summarized in Table 1.
Table 1. Main FTIR Bands of PVOH, Kenaf Fiber, PVOH/KF and 10-wt% PVOH/KF/U
Fig. 5. FTIR of (a) PVOH, (b) raw KF, (c) PVOH/KF, and (d) 10-wt% PVOH/KF/U
Fig. 6. FTIR of 10-wt% PVOH/KF/U before and after soaking in water
Thermal Properties
In the application as agricultural mulches, the materials are not subjected to high temperatures. Nevertheless thermogravimetric analysis was carried out to check if the weight losses at different temperature ranges could be related to the wt% of the components. With reference to Fig. 7, both samples (a) and (b) exhibit a small weight loss (arount 10%) in the range 50 to 150 C due to volatile component (water).
a)
b)
Fig. 7. Comparison of TG and DTG curves for a) PVOH/KF composites and b) 10-wt% PVOH/KF/U composites
The next major weight loss (about 60%) occurred from 250 to 350 C due to thermal degradations of lower molecular weight fraction of PVA and the finer fibers. The higher molecular weight fraction of PVA and larger fibers were broken down at temperatures higher than 350 C. In curve (b), the 10% urea were seen to break down at around 400 C, as indicated by dW/dT with a sharper peak. The maximum decomposition temperature, Tmax, for each sample is listed in Table 2.
Table 2. TGA Characterization of Virgin PVOH, PVOH/KF, and 10-wt% PVOH/KF/U in the Temperature Range of 150 to 450 °C
The result is in good agreement with the findings of Azwa et al.(2013), who found that approximately 60% of the thermal decomposition of most natural fibres occurred within a temperature range between 215 and 310 °C.
Table 2 shows that the incorporation of fibers into the composites had a profound effect on the thermal stability of the PVOH matrix (Ng et al. 2014). The composites showed a lower degradation temperature (by about 50 °C) than pure PVOH. A similar observation was reported using poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) composites with bamboo fiber (Lee and Wang 2006). Julkapli and Akil (2010) also reported that addition of kenaf dust in chitosan film reduced the thermal stability of chitosan film and that the reduction was proportional to the amount of kenaf dust added. The incorporation of urea into the composites did not influence the thermal stabilization of the composites, even at a loading of 10 wt%. Imam et al. (2005) reported a decrease in the thermal stability of PVOH/starch/orange peel composites, particularly because the addition of a high amount of low-molecular-weight components, such as urea and glycerol, resulted in early decomposition of the composites. Therefore, the low decomposition temperature of urea must be considered during the preparation of composites for high temperature applications (Imam et al. 2005).
CONCLUSIONS
- Both the PVOH/KF and urea-entrapped PVOH/KF composites were prepared by a solution casting technique.
- The composites exhibited good water solubility and controlled release property and were readily degradable in the environment, suggesting their suitability for several practical applications, such as carriers for urea and as mulches for agriculture.
- The release behavior of urea was examined, and it was found that the entrapped urea could diffuse slowly out of the polymer matrix into the soil through the water medium.
- The composites were degradable in water, so no undesirable accumulation of plastic residues would occur in the soil. The leached kenaf fiber would serve as a natural fertilizer for the seeding of plants.
- Composites were successfully made at a fixed ratio of PVOH/KF. The fiber is relatively cheaper than PVOH. The chosen ratio is very close to the highest possible fiber per unit weight of PVOH used for pressing into the rectangular mould. Other compositions would result in different rates of the release of urea.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support of the High Impact Research MoE Grant UM.C/625/1/HIR/MoE/52 and FP030-2013A from the Ministry of Education of Malaysia; research grants University of Malaya – RU022A-2014, PV117-2012A, and PG079-2012B for the success of this project.
REFERENCES CITED
Adegbidi, H. G., Briggs, R. D., Volk, T. A., White, E. H., and Abrahamson, L. P. (2003). “Effect of organic amendments and slow-release nitrogen fertilizer on willow biomass production and soil chemical characteristics,” Biomass Bioenerg. 25(4), 389-398. DOI: 10.1016/S0961-9534(03)00038-2
Ali, M. E., Yong, C. K., Ching, Y. C., Chuah, C. H., and Liou, N. S. (2014). “Effect of single and double stage chemically treated kenaf fibers on mechanical properties of polyvinyl alcohol film,” BioResources 10(1), 822-838. DOI: 10.15376/biores.10.1.822-838
AOAC. (1995). Official Methods of Analysis, 16th Ed., Association of Analytical Communities International, Gaithersburg, MD.
Azeem, B., KuShaari, K., Man, Z. B., Basit, A., and Thanh, T. T. (2014). “Review on materials & methods to produce controlled release coated urea fertilizer,” J. Control Release 181, 11-21. DOI: 10.1016/j.jconrel.2014.02.020
Azwa, Z. N., Yousif, B. F., Manalo, A. C., and Karunasena, W. (2013). “A review on the degradability of polymeric composites based on natural fibres”, Mater. Design 47, 424-442. DOI: 10.1016/j.matdes.2012.11.025
Calabria, L., Vieceli, N., Bianchi, O., Oliveira, R.V. B., Filho, I. D. N., and Schmidt, V. (2012). “Soy protein isolate/poly(lactic acid) injection-molded biodegradable blends for slow release of fertilizers,” Ind. Crop. Prod. 36(1), 41-46. DOI: 10.1016/j.indcrop.2011.08.003
Castro-Enríquez, D. D., Rodríguez-Félix, F., Ramírez-Wong, B., Torres-Chávez, P. I., Castillo-Ortega, M. M., Rodríguez-Félix, D. E., Armenta-Villegas, L., and Ledesma-Osuna, A. I. (2012). “Preparation, characterization and release of urea from wheat gluten electrospun membranes,” Materials 5(12), 2903-2916. DOI: 10.3390/ma5122903
Chen, L., Xie, Z., Zhuang, X., Chen, X., and Jing, X. (2008). “Controlled release of urea encapsulated by starch-g-poly(L-lactide),” Carbohyd. Polym. 72(2), 342-348. DOI: 10.1016/j.carbpol.2007.09.003
DeMerlis, C., and Schoneker, D. (2003). “Review of the oral toxicity of polyvinyl alcohol (PVOH),” Food Chem. Toxicol. 41(3), 319-326. DOI: 10.1016/S0278-6915(02)00258-2
Dhakal, H. N., Zhang, Z. Y., and Richardson, M. O. W. (2007). “Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites,” Compos. Sci. Technol. 67(7-8), 1674-1683. DOI: 10.1016/j.compscitech.2006.06.019
Guohua, Z., Ya, L., Cuilan, F., Min, Z., Caiqiong, Z., Zongdao, C. (2006). “Water resistance, mechanical properties and biodegradability of methylated-corn starch/poly(vinyl alcohol) blend film,” Polym. Degrad. Stab. 91(4), 703-711. DOI: 10.1016/j.polymdegradstab.2005.06.008
Hussain, M. R., Devi, R. R., and Maji, T. K. (2012). “Controlled release of urea from chitosan microspheres prepared by emulsification and cross-linking method,” Iran. Polym. J. 21(8), 473-479. DOI: 10.1007/s13726-012-0051-0
Imam, S. H., Cinelli, P., Gordon, S. H., and Chiellini, E. (2005). “Characterization of biodegradable composite films prepared from blends of poly(vinyl alcohol), corn starch, and lignocellulosic fiber,” J. Polym. Environ. 13(1), 47-55. DOI: 10.1007/s10924-004-1215-6
Julkapli, N. M., and Akil, H. M. (2010). “Thermal properties of kenaf-filled chitosan biocomposites,” Polym. Plast. Technol. Eng.49(2), 147-153. DOI:
10.1080/03602550903284206
Kasirajan, S., and Ngouajio, M. (2012). “Polyethylene and biodegradable mulches for agricultural applications: a review,” Agron. Sustain. Dev. 32(2), 501-529. DOI: 10.1007/s13593-011-0068-3
Lee, S. H., and Wang, S. (2006). “Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent,” Compos. Pt. A-Appl. Sci. Manuf. 37(1), 80-91. DOI: 10.1016/j.compositesa.2005.04.015
Mazuki, A. A. M., Akil, H. M., Safiee, S. Ishak, Z. A. M., and Bakar A. A. (2010). “Degradation of dynamic mechanical properties of pultruded kenaf fiber reinforced composites after immersion in various solutions,” Compos. Pt. B-Eng. 42(1), 71-76. DOI: 10.1016/j.compositesb.2010.08.004
Monti, A., and Alexopoulou, E. (2013). Kenaf: a multi-purpose crop for several industrial applications: new insights from the biokenaf project. Springer-Verlag, London.
Moreno, M. M., and Moreno, A. (2008). “Effect of different biodegradable and polyethylene mulches on soil properties and production in a tomato crop,” Sci. Hortic. 116(3), 256-263. DOI: 10.1016/j.scienta.2008.01.007
Ng, T. S., Ching, Y. C., Awanis, N., Ishenny, N., Rahman, M. R.(2014). “Effect of bleaching condition on thermal properties and UV-transmittance of PVOH/cellulose biocomposites,” Mater. Res. Innov. 18(S6), 400-404.
Ni, B., Liu, M., and Lü, S. (2009). “Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations,” Chem. Eng. J. 155(3), 892-898. DOI: 10.1016/j.cej.2009.08.025
Ni, X., Wu, Y., Wu, Z., Wu, L., Qiu, G., and Yu, L. (2013). “A novel slow-release urea fertiliser: Physical and chemical analysis of its structure and study of its release mechanism,” Biosyst. Eng. 115(3), 274-282. DOI: 10.1016/j.biosystemseng.2013.04.001
Niu, Y., and Li, H. (2012). “Controlled release of urea encapsulated by starch-g-poly(vinyl acetate),” Ind. Eng. Chem. Res. 51(38), 12173-12177. DOI: 10.1021/ie301684p
Nosbi, N., Akil, H. M., Mohd Ishak, Z. A., and Abu Bakar A. (2010). “Degradation of compressive properties of pultruded kenaf fiber reinforced composites after immersion in various solutions,” Mater. Des. 31(10), 4960-4964. DOI: 10.1016/j.matdes.2010.04.037
Nurfatimah, A. B., Ching, Y. C., Luqman, C. A., Chantara, T. R., and Nor, A. I. (2015). “Thermal and dynamic mechanical properties of grafted kenaf filled poly(vinyl chloride)/ethylene vinyl acetate composites,” Mater. Design. 65 (2015) 204-211.
Reis, E. F. D., Campos, F. S., Lage, A. P., Leite, R. C., Heneine, L. G., Vasconcelos, W. L., Lobato, Z. I. P., and Mansur, H. S. (2006). “Synthesis and characterization of poly(vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption,” Mater. Res. Bull. 9(2), 185-191. DOI: 10.1590/S1516-14392006000200014
Tan, B. K., Ching, Y. C., Gan, S. N, Ramesh, S., and Rahmmah, R. V. (2014). “Water absorption properties of kenaf fibre- PVA composites,” Mater. Res. Innov. 18, 144-146.
Tao, S., Liu, J., Jin, K., Qiu, X., Zhang, Y., Ren, X., and Hu, S. (2011). “Preparation and characterization of triple polymer‐coated controlled release urea with water‐retention property and enhanced durability,” J. Appl. Polym. Sci. 120(4), 2103-2111. DOI: 10.1002/app.33366
Thanh, T. H., Kushaari, K., Shuib, A. S., Ismail, L., and Azeem, B. (2015). “Modelling the release of nitrogen from controlled release fertiliser: Constant and decay release,” Biosyst. Eng. 130, 34-42. DOI: 10.1016/j.biosystemseng.2014.12.004
Trenkel, M. E. (1997). Controlled-Release and Stabilized Fertilizers in Agriculture, International Fertilizer Industry Association, Paris, France.
Wu, L., and Liu, M. (2008). “Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention,” Carbohyd. Polym. 72(2), 240-247. DOI: 10.1016/j.carbpol.2007.08.020
Yip, H. L., Shaaban, A., Mitan, N. M. M., Dimin, M. F., Mohamad, N., Hamid, N., and Sian, M. S. (2013). “Characterization of urea encapsulated by biodegradable starch-PVOH-glycerol,” J. Polym. Environ. 21(4), 1083-1087. DOI: 10.1007/s10924-012-0552-0
Yong, C. K, Ching, Y. C., Chuah, C. H., and Liou, N. S. (2015). “Effect of fiber orientation on mechanical properties of kenaf-reinforced polymer composite,” BioResources 10(2), 2597-2608. DOI: 10.15376/biores.10.2.2597-2608.
Yong, C. K., Ching Y. C., Mohamad, A., Lim, Z. K., and Chong, K. E. (2015). “Mechanical and thermal properties of chemical treated oil palm empty fruit bunches fiber reinforced polyvinyl alcohol composite,” J. Biobased Mater. Bioenergy. 9, 231-235.
Zhong, K., Lin, Z. T., Zheng, X. L., Jiang, G. B., Fang, Y. S., Mao, X. Y., and Liao, Z.W. (2013). “Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers,” Carbohyd. Polym. 92(2), 1367-1376. DOI: 10.1016/j.carbpol.2012.10.030
Article submitted: April 13, 2015; Peer review completed: June 20, 2015; Revised version received and accepted: July 6, 2015; Published: July 20, 2015.
DOI: 10.15376/biores.10.3.5532-5543
