Abstract
Bio-ethanol is considered as an important renewable fuel to partly replace fossil-derived fuels. In this study, bioethanol production, which includes cellulase production, saccharification of the cellulose content of sesame seed residue, and ethanol production, was investigated. Out of the hundreds of cellulase-producing bacterial strains isolated from sesame seed residue during this study, the B isolate was found to have the highest cellulase enzyme production. This isolate was identified as Bacillus cereus by 16S rRNA sequencing. The effects of different growth parameters, including inoculum concentration, incubation time, temperature, pH, and carbon and nitrogen sources, were investigated to optimize the growth conditions of the bacterium. The maximum cellulase activity was achieved with an inoculum concentration of 3% after 48 h in a basal medium at a pH of 7 and an incubation temperature of 35 °C. The best nitrogen and carbon sources were yeast extract and sesame seed residue, respectively. The results showed the liberation of 2.3 g/L of reducing sugar by the dinitrosalicylic acid method. This total reducing sugar produced 15 g/L of ethanol after 48 h when Saccharomyces cerevisiae was used as a fermentation agent. Hence, bioethanol was successfully produced from the cellulose of sesame seed residue using the cellulase enzyme from B. cereus.
Download PDF
Full Article
Bioethanol Production with Cellulase Enzyme from Bacillus cereus Isolated from Sesame Seed Residue from the Jazan Region
Emad A. Abada,a,b,* Yahya S. Masrahi,a Mohamed A. Al-Abboud,aHassien M. Alnashiri,a and Khaled E. El-Gayar a,c
Bio-ethanol is considered as an important renewable fuel to partly replace fossil-derived fuels. In this study, bioethanol production, which includes cellulase production, saccharification of the cellulose content of sesame seed residue, and ethanol production, was investigated. Out of the hundreds of cellulase-producing bacterial strains isolated from sesame seed residue during this study, the B isolate was found to have the highest cellulase enzyme production. This isolate was identified as Bacillus cereus by 16S rRNA sequencing. The effects of different growth parameters, including inoculum concentration, incubation time, temperature, pH, and carbon and nitrogen sources, were investigated to optimize the growth conditions of the bacterifum. The maximum cellulase activity was achieved with an inoculum concentration of 3% after 48 h in a basal medium at a pH of 7 and an incubation temperature of 35 °C. The best nitrogen and carbon sources were yeast extract and sesame seed residue, respectively. The results showed the liberation of 2.3 g/L of reducing sugar by the dinitrosalicylic acid method. This total reducing sugar produced 15 g/L of ethanol after 48 h when Saccharomyces cerevisiae was used as a fermentation agent. Hence, bioethanol was successfully produced from the cellulose of sesame seed residue using the cellulase enzyme from B. cereus.
Keywords: Sesame cake; Cellulase; Biofuel; Bacillus cereus
Contact information: a: Biology Department, Faculty of Science, Jazan University, Jazan, Saudi Arabia; b: Botany and Microbiology Dep., Faculty of Science, Helwan University, Cairo, Egypt; c: The Holding Company for Biological Products & Vaccines (VACSERA), Cairo, Egypt;
* Corresponding author: emadm_abada@yahoo.co.uk; eabada@jazanu.edu.sa
INTRODUCTION
Bioethanol is a renewable fuel and it is important to solve the problem of bioethanol production to partially replace fossil-derived fuels. The global production of bioethanol rose from 50 million m3 in 2007 to more than 100 million m3 in 2012 (Kang et al. 2014). In developing economies, these food-related raw materials can preferably be replaced by the non-food part of sweet corn or cassava raw materials (a woody shrub native to South America). Biofuel is a promising alternative for maintaining a better quality of both human health and the environment through the reduction of harmful emissions that result from biofuel-run diesel engines (Mofijur et al.2016).
Bioethanol production can be produced by the use of plant residues that are rich in lignocellulose and cellulose. Industrial ethanol is mainly produced petrochemically through the acid-catalyzed hydration of ethylene. Ethanol for use in alcoholic beverages, and the vast majority of ethanol for use as biofuel, is produced by fermentation, in which certain species of yeast (e.g., Saccharomyces cerevisiae) or bacteria (e.g., Zymomonas mobilis) metabolize sugars in oxygen-lean conditions to produce ethanol and carbon dioxide.
When sugar-based raw materials are used in fermentation, the product is referred to as “first-generation” bioethanol, and when lignocellulosic raw materials (sustainable feedstock that cannot be used directly for food production) are used, it is called “second-generation” bioethanol. Algal bioethanol, which is called “third-generation” bioethanol, is still under investigation. Although the enzymes that are used to hydrolyze cellulose are expensive, the production of second-generation bioethanol uses cellulose-released sugars. To develop this generation of bioethanol, a number of cellulose-containing agricultural byproducts, such as wood trimmings, husks, straw, bamboo, rapeseed oil, and sawdust, are used. One of the most important sources for bioethanol production is residues that remain after production processes, such as rice husks, bagasse, sesame hulls, and straw. Other important agroindustrial biomass residues are the byproducts of agriculture or its related industries, including wheat, rice straw, cotton stalk, maize cobs, coconut shells, and rice husks (Demirbas et al. 2009).
Saccharomyces cerevisiae, the yeast commonly used for first-generation bioethanol production, cannot metabolize xylose. Other yeasts and bacteria are under investigation for the fermentation of xylose and other pentose sugars into ethanol (Kang et al. 2014). Because of the higher water content in the broth for bacteria and yeast, additional distillation efforts are required.
When using enzymatic hydrolysis, separate hydrolysis and fermentation steps are suggested. During separate hydrolysis and fermentation, the liberated cellulose is treated in a different reactor for hydrolysis and subsequent fermentation than the hydrolyzed hemicellulose and lignin. Despite the simplification of the optimization of each separate reactor and the selection of sugar-appropriate microorganisms to ferment the different sugars, the higher investment cost for two separate reactors and the inhibition of the fermenting organisms with a high glucose concentration are major disadvantages (Aden and Foust 2009; Kazi et al. 2010).
Sesame (Sesamum indicum) is cultivated in several countries, such as India, Sudan, China, and Burma, which are considered to be the major producers and are responsible for 60% of the total global production (Abou-Gharbia et al. 1997). Not only are its seeds used for the production of oil, but it is also used in the manufacturing of tahini paste and in food formulations, such as Halaweh (sweetened tehineh), java beans, and salads (Namiki 1995). Sesame seeds are composed of oil (44% to 58%), protein (18% to 25%), carbohydrates (13.5%), and ash (5%) (Kahyaoglu and Kaya 2006). Additionally, the lignin content of the deep, brown colored sesame husk is high, at almost 40%. This separated lignin can likewise be recovered and used, which would make the bioethanol process more economical (Purkait et al. 2011; Ji 2015).
The Jazan region is famous for its production of high-quality agricultural crops, such as mangos, bananas, sorghum, and sesame. According to a report published in 2009 (unpublished report) by the Saudi ministry of agriculture about the production of sesame in King Saudi Arabia (KSA), the Jazan region produces approximately 67.5% of the total sesame production of the KSA. Algeljlan, also known as asulait oil, is extracted by crushing sesame seeds in juicers. The seed residue is used as animal food, while the remainder could cause serious environmental pollution through the deterioration of the residues.
The work presented herein concentrated on the isolation of cellulose-degrading bacteria from sesame seed residue (seed cake) and the evaluation of their cellulolytic activity on the cellulose content of sesame residues. The successive culturing of cellulose-degrading bacteria followed by yeast was done for the saccharification and fermentation of the cellulose from the sesame residue for the production of bioethanol. To the best knowledge of the authors, this is the first study about the production of bioethanol from sesame seed residue from the Jazan region.
EXPERIMENTAL
Sesame Seed Residues Collection
The sesame seed residues were collected from the Jazan juicers shops around the Jazan region. Normally the sesame seeds are crushed to obtain sesame oil. The remaining material from the seeds is called sesame seed residues. The sesame residue samples were transferred to the laboratory inside sterile plastic bags.
Isolation of Cellulose Degrading Bacteria
The sesame seed residues of the collected samples were inoculated in a basal salt medium (KH2PO4 5 g, KHPO4 10 g, (NH4)2SO4 0.5 g, NaCl 0.2 g, MgSO4 0.1 g, yeast extract 10 g, Carboxy-Methyl Cellulose (CMC) 10 g in a liter) for the isolation of cellulolytic bacteria. The cultures were incubated for 2 days in a shaker incubator at 30 °C and 120 rpm. In order to isolate the bacterial colonies capable of utilizing cellulose as sole source of carbon, the colonies were grown on cellulose agar media composed of (KH2PO4 5 g, KHPO4 10 g, (NH4)2SO4 0.5 g, NaCl 0.2 g, MgSO4 0.1 g, yeast extract 10 g, CMC 10 g, agar 20 g in a liter) with a pH 7. To confirm the cellulose-degrading ability of bacterial isolates, the isolates were streaked on the cellulose Congo red agar medium. Congo red is regarded as an indicator for cellulose degradation in an agar medium; colonies showing discoloration of Congo red were taken as positive cellulose-degrading bacterial colonies (Gupta et al. 2012; Hendricks et al. 1995).
Identification of Potential Cellulase Producing Isolates by 16S rRNA
Genomic DNA was isolated from the celluase producing strains using the protocol of the Gene Jet genomic DNA purification kit (Thermo). Amplification of the 16S rRNA gene was carried out by means of 16S rRNA pair of primer named as 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and 1492R (5′-TACGGYTACCTTGTTACGA-CTT-3′). The PCR reaction was performed in a 50 µL reaction mixture containing 5 µL Template DNA, 25 µL 2X Maxima hot Start PCR master Mix, 1 µL of primer F and R (20 µM), and 18 µL water, nuclease-free. The amplification program was done according to the following program: Initial denaturation (10 min at 94oC), followed by 35 cycles of denaturation at 94 oC for 30 sec., annealing at 65 oC for 1 min, extension at 72 oC for 1.5 min and a final extension at 72 oC for 10 min (Abada, 2014a). The PCR product was electrophorized at 1 % low melting agarose, and purified by using the GeneJETTM PCR purification Kit (Thermo). Macrogen Company (Macrogen Company, South Korea) sequenced the purified PCR product by the use of ABI 3730 xl DNA sequencer using 785F (5′-GGATTAGATACCCTGGTA-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) as sequencing primers. The gene homology and related sequences were carried out by public databases BLAST at the NCBI server (http://www.ncbi.nlm.nih.gov/ blast/).
Cellulase Production
The cellulose degrading bacteria were cultured on production media composed of (MgSO4 0.25 g, KH2PO4 0.5 g, CMC 2.0 g, gelatin 2 g, agar 15 g and 1 L distilled water). The culture were grown at optimum temp., pH, and 120 rpm. After 24 h of incubation period, the cultures were centrifuged for 10 min at 8000 rpm and at 4 oC. The supernatant were collected and stored as crude enzyme at -20 oC for further investigations (Tailliez et al. 1989).
Cellulase Enzyme Activity Assay
The cellulose activity was determined by measuring the amount of reducing sugar formed from sesame seed residues. The enzyme activity was determined according to the methods recommended by the International Union of Pure and Applied Chemistry (IUPAC) commission on biotechnology (Ghose et al. 1987). 0.5 mL of supernatant was added to 0.5 mL of 2% CMC in 0.05 mL sodium acetate buffer (pH 4.8). The mixture was incubated at optimum temperature for 1 h. The reaction was terminated by adding 3 mL of 3,5-dinitrosalicylic acid (DNS) reagent to 1 mL of reaction mixture (Miller 1959). The mixture were boiled in water bath then cooled by ice. The colour intensity was measured at OD 540 nm. The reducing sugars were estimated spectrophotometrically with 3,5-dinitrosalicylic acid using glucose standard curve previously prepared. The enzymatic activity of cellulase defined in international units (IU). One unit of enzymatic activity is defined as the amount of enzyme that releases 1 μmol reducing sugars (measured as glucose) per mL per minute.
Optimization of fermentation parameters
In these experiments, the conditions for cellulase production by cellulose producing bacteria were optimized. Three replicates were used in each determination, and values were recorded as the mean of the three replicate ± standard deviation. Simply, the optimization parameters in this category were initial inoculums concentration, incubation time, temperature, pH, carbon and nitrogen sources (Abada 2014a,b).
Production and estimation of bioethanol
To initiate the saccharification process, the cellulose degrading bacteria were grown in basal medium at optimum fermentation conditions with shaking at 120 rpm for 48 h. The culture was centrifuged; the supernatant was resterilized by 0.2 µm filter for the growth of Saccharomyces cerevisiae at 32 oC for 48 h. The total reducing sugar was estimated before and after the fermentation process by DNS method. The fermentation broth samples were centrifuged at 8000 rpm at 4 °C for 3 min to separate suspended particles, and the clear liquid was analysed for the presence of fermentation products. Then, the clarified samples were filtered through a 0.45-mm Whatman nylon filter to remove insoluble materials. Ethanol concentration was measured according to Ire et al.(2016) using Gas Chromatography (Shimadzu-2014, Shimadzu Co. Ltd., Tokyo, Japan) with a packed column (Gaskuropack5460/80;GC-2014Glass ID:3:2φ X 2:1 m, GL Science Co. Ltd., Tokyo, Japan), with the following operational conditions: temperature of column and detector were 110 and 250 °C, respectively, nitrogen gas flow rate 60 mL/min and the injected sample volume 2 μL.
Results and discussion
Isolation, Screening, and Identification of the Cellulase-producing Bacteria
The sesame seed residues were collected in sterile plastic bags and numbered. The residues were serially diluted using sterile distilled water and cultured on basal medium agar plates containing carboxymethylcellulose (CMC) as a substrate for cellulase production. Hundreds of colonies were grown on the CMC plates, and they were screened for their ability to produce cellulase using Congo red dye as an indicator for cellulose hydrolysis (Hendricks et al. 1995; Gupta et al. 2012). Ten isolates showed the highest clear zone of Congo red dye (Fig. 1).
Fig. 1. Cellulase activity of the Bacillus cereus grown on the basal medium containing Congo red dye; the clear zone is an indication of cellulose degradation by B. cereus
The ten isolates were subjected to quantitative estimation for cellulase activity by growing them on a basal medium containing CMC, as was mentioned previously. The cellulase activity was measured using the dinitrosalicylic acid (DNS) method (Miller 1959; Ghose et al. 1987). One isolate showing the highest cellulase activity, temporarily named “B isolate”, was chosen for further studies. Many studies have used a similar procedure to select bacterial isolates with a high cellulase activity (Ahmad et al. 2013; Gautam and Sharma 2014; Rasul et al. 2015). Gram staining showed that the B isolate was a gram-positive bacterium with a rod shape. The genomic DNA of B isolate was separated, purified, and identified by 16S rRNA gene analysis. The gene sequence of the B isolate was studied for similar sequences in the databases using a BLAST search of the NCBI server. The gene sequence alignment of the B isolate indicated that the DNA sequence was 100% homologous with the 16S rRNA gene sequences of Bacillus cereus (accession number: NR-074540.1). The B isolate was identified as B. cereus, as indicated in the phylogenetic tree shown in (Fig. 2).
Bacillus cereus is a gram-positive, rod-shaped, aerobic, motile, and beta-hemolytic bacterium commonly found in soil and food. Some strains are harmful to humans and cause food borne illnesses, while others have beneficial applications, such as probiotics for animals. There are reports on the production of cellulase by B. cereus strains and on their potential for the production of bioethanol (Yan et al.2011; Behera et al. 2014).
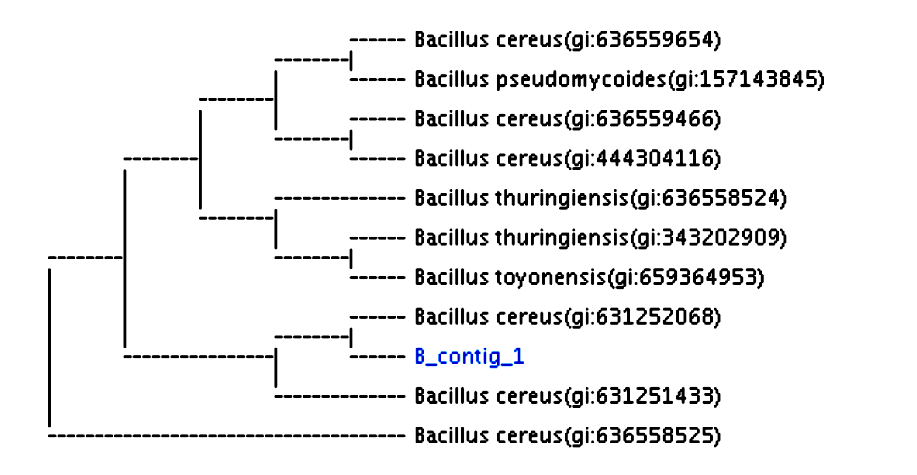
Fig. 2. Phylogenetic tree of the 16S rRNA sequence results of B. cereus
Optimization of the Culture Conditions for High-Cellulase Activity
Effect of the inoculum concentration
The effect of different initial inoculum concentrations, including 1%, 2%, 3%, 4%, and 5% (v/v), of the B. cereus cultures on the cellulase production was studied. The inoculum concentration that resulted in the highest cellulase activity was 3%, which had an activity of 19 IU/mL (Fig. 3).
Fig. 3. Effect of the inoculum concentration on the cellulase production from B. cereus
The growth of the bacteria is affected by the growth phase at which it grows. During the decline phase the number of bacteria decrease due to the consuming or depletion of nutrients materials; this in turn affects the enzyme production. This can explain the observed decrease in cellulase activity with increasing inoculum concentration. The present results agreed with those for B. amyloliquefaciens and B. alcalophilus, which also exhibit a maximum cellulase activity at a 3% inoculum concentration (Abou-Taleb et al. 2009).
Effect of the incubation time
The cellulase produced from B. cereus showed a higher activity after 48 h of incubation time, and had an activity of 27 IU/mL (Fig. 4).
Fig. 4. Effect of the incubation time on the cellulase production from B. cereus
It has been shown that isolates from the Persian Gulf have a higher cellulase activity after 48 h (Samira et al. 2011). However, the optimum incubation time may vary from strain to strain according to their characteristics and culturing conditions (Bajaj et al. 2009). This effect is due to the depletion of nutrients or accumulation of other byproducts in the fermentation media, which leads to a decrease in cellulase activity.
Effect of the temperature
Temperature plays a large role in the physiology, growth, and enzymatic activity of microorganisms.
Fig. 5. Effect of the temperature on the cellulase production from B. cereus
Bacillus cereus was grown at different temperatures, including 20 °C, 30 °C, 35 °C, 40 °C, 45 °C, and 50 °C. The maximum cellulase activity was achieved at 35 °C, and was 30 IU/mL (Fig. 5). The increase in temperature, above the optimum values, results in a loss of enzyme activity due to thermal denaturation of enzymes; hence low enzyme activity was observed above 35 °C.
The enzyme activity decreased as the temperature increased because of the thermal denaturation of the enzyme. It has been reported that the cellulase enzyme from B. pumilis showed a higher activity at 35 °C (Kanmani et al. 2011).
Effect of the pH
The cellulase activity of the B. cereus isolated from sesame seed residue at different pH ranges (2.0 to 12.0) was studied. The maximum cellulase activity was achieved at a pH of 7.0 and was 27 IU/mL (Fig. 6). An optimum pH is required to maintain the three-dimensional shape of the active site of enzyme and the change in pH results in loss of functional shape of enzyme due to alteration in the ionic bonding of enzyme. The obtained bacterial isolate had an optimum pH at 7 above or below this pH, the bacterial growth is affected and in turn reduce cellulase activity.
Fig. 6. Effects of the initial pH of the media on the cellulase production from B. cereus
Nasr et al. (2011) reported that the maximum cellulase activity was achieved at a pH of 7.0 for Bacillus sp. 17 and Bacillus sp. 8. Moreover, the highest cellulase activity of the Bacillus sp. was reached at a pH of 7.0, which was reported by Padilha et al. (2015).
Effect of different carbon sources
Different carbon sources, including glucose, maltose, sucrose, CMC, sesame seed residue, and lactose, at a 1% concentration (w/v) were used to study their effects on the cellulase activity (Fig. 7). Our results indicated that the cellulase production is improved when CMC or sesame cake were used as a carbon source rather than the glucose, maltose, sucrose, and lactose.
Fig. 7. Effect of different carbon sources (1%, w/v) on the cellulase production from B. cereus
The cellulase from B. cereus had a maximum activity when the sesame seed residues were used as a carbon source, and had an activity of 22 IU/mL. Sesame seed cake, beside it being a source of cellulose, may favor the growth of the bacteria as a natural supplement and hence increase the enzyme production. It has been stated by Yang et al. (2014) that the carbon source type is a very important factor that affects the cellulase production for a diverse array of microorganisms. Also, it has been reported that the use of CMC resulted in the maximum cellulase activity of the different Bacillus sp. (Deka et al. 2011; Singh et al. 2014). Herein, it has been reported for the first time that sesame cake is the best carbon source to use for the production of cellulase from B. cereus.
Effect of the nitrogen source
Different nitrogen sources, such as yeast extract, peptone, NH4Cl, (NH4)2SO4, and NaNO3, at a concentration of 1% (w/v) were used to study their effect on the cellulase activity of B. cereus. The maximum cellulase activity was achieved when yeast extract was used as a nitrogen source, and was 10 IU/mL (Fig. 8).
Fig. 8. Effect of different nitrogen sources (1%, w/v) on the cellulase production from B. cereus
The presence of an external nitrogen source is essential for the fermentation media during extracellular enzyme production for the effective utilization of soluble carbohydrates (Ray et al. 2007). The use of organic nitrogen sources, when compared with inorganic sources, was found to be more suitable for maximum cellulase production (Lugani et al. 2015).
Bioethanol production from the saccharification and fermentation of the sesame seed residues
The reducing sugar content was 0.26 g/L for the fermentation medium before the saccharification process. After the growth of the Bacillus cereus for 48 h under optimum fermentation conditions, the reducing sugar content was elevated to 8.8 fold (2.3 g/L) of reducing sugars, as measured by the DNS method (data not shown). The culture was centrifuged and sterilized for the growth of of S. cerevisiae under at 32 oC. The analysis of the fermentation medium by GC-MS showed an ethanol concentration of 15.0 g/L (Fig. 9).
Fig. 9. Chromatogram of the ethanol produced by the successive culture of B. cereus and S. cerevisiae
These results showed that there was a higher concentration of ethanol when B. cereus was used to degrade the sesame seed residues, which suggested there was a higher conversion of the substrate to reducing sugars. Banerjee et al. (2010) explained that enzymatic hydrolysis is done by cellulase enzymes that are highly substrate specific. The obtained yield can be compared with the yield obtained from other wild-type bacteria. Svetlitchnyi et al. (2013) reported a maximum ethanol yield of 3.5 g/L from the wild-type bacterium Caldicellulosiruptor DIB 004C. Sato et al. (1993) reported a bioethanol production of 4 g/L from the wild-type Clostridium thermocellum strain I-1-B, and an improved 23.6-g/L ethanol yield by the same strain when it was grown on an optimized medium.
The bioethanol yield obtained in this study was higher than the yield (7.5 g/L) obtained from the fermentation of sugarcane bagasse hydrolysate using Pichia stipitis DSM 3651, as reported by Ira et al.(2016). However, the yield from the co-culture was lower than the yield from banana pseudo stem (17 g/L) reported by Ingale et al.(2014).
Conclusions
- To enhance the cellulose enzyme production, the bacterial growth conditions were optimized. The results indicated that the cellulase activity of B. cereus was improved by adjusting the different growth parameters.
- Additionally, the maximum ethanol concentration produced in the fermentation medium was 15.0 g/L, which was estimated by GC-MS analysis after 48h growth of the S. cerevisiae on the saccharification process achieved by B. cereus.
- This study is the first to demonstrate the efficient production of bioethanol by applying a successive culture strategy through saccharification and fermentation of sesame seed residue from the Jazan region.
Acknowledgement
The authors would like to thank the Deanship of Scientific Research, Jazan University, KSA for the financial support of this work (Project number 37/7/0033; Production of bioethanol by cellulase enzyme from sesame residue of Jazan region).
REFERENCES CITED
Abada, E. (2014a). “Production and purification of lipase from Pseudomonas sp. AB2 with potential application in biodiesel production,” J. Pure Appl. Microbio. 8, 133-142.
Abada, E. (2014b). “Production optimization of extracellular amidase enzyme by newly isolated Pseudomonas putida AP-2 from agricultural soil,” Rend. Lincei-Sci. Fis. 25(4), 523-530. DOI: 10.1007/s12210-014-0347-4
Abou-Gharbia, H. A., Shahidi, F., Adel, A., Shehata, Y., and Youssef, M. M. (1997). “Effect of processing on oxidative stability of sesame oil extracted from intact and dehulled seed,” J. Am. Oil Chem. Soc.74(3), 215-221. DOI: 10.1007/s11746-997-0126-9
Abou-Taleb, K. A. A., Mashhoor, W. A., Nasr, S. A., Sharaf, M. S., and Abdel-Azeem, H. H. M. (2009). “Nutritional and environmental factors affecting cellulase production by two strains of cellulolytic Bacilli,” Australian Journal of Basic and Applied Sciences 3(3), 2429-2436.
Ahmad, B., Nigar, S., Shah, S. S. A., Bashir, S. J., Ali, J., Yousaf, S., and Bangash, J. A. (2013). “Isolation and identification of cellulose degrading bacteria from municipal waste and their screening for potential antimicrobial activity,” World Applied Science Journal27(11), 1420-1426. DOI: 10.5829/idosi.wasj.2013.27.11.81162
Bajaj, B. K., Pangotra, H., Wani, M. A., Sharma, P., and Sharma, A. (2009). “Partial purification and characterization of a highly thermostable and pH stable endoglucanase from a newly isolated Bacillus strain M-9,” Indian J. Chem. Techn. 16(5), 382-387.
Banerjee, S., Mudliar, S., Sen, R., Giri, B., Satpute, D., Chakrabarti, T., and Pandey, R. A. (2010). “Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies,” Biofuel. Bioprod. Bior. 4(1), 77-93. DOI: 10.1002/bbb.188
Behera, B. C., Parida, S., Dutta, S. K., and Thatoi, H. N. (2014). “Isolation and identification of cellulose degrading bacteria from mangrove soil of Mahanadi River Delta and their cellulase production ability,” American Journal of Microbiological Research 2(1), 41-46. DOI: 10.12691/ajmr-2-1-6
Deka, D., Bhargavi, P., Sharma, A., Goyal, D., Jawed, M., and Goyal, A. (2011). “Enhancement of cellulase activity from a new strain of Bacillus subtilis by medium optimization and analysis with various cellulosic substrates,” Enzyme Research 2011, 1-8. DOI: 10.4061/2011/151656
Demirbas, M. F., Balat, M., and Balat, H. (2009). “Potential contribution of biomass to the sustainable energy development,” Energ. Convers. Manage. 50(7), 1746-1760. DOI: 10.1016/j.enconman.2009.03.013
Ghose, T. K. (1987). “Measurement of cellulase activity,” Pure and Applied Chemistry 59, 257-268.
Gupta, P., Samant, K., and Sahu, A. (2012). “Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential,” International Journal of Microbiology. DOI: 10.1155/2012/578925
Hendricks, C. W., Doyle, J. D., and Hugley, B. (1995). “A new solid medium for enum-erating cellulose-utilizing bacteria in soil,” Appl. Environ. Microb. 61(5), 2016-2019.
Ingale, S., Joshi, S. J., and Gupte, A. (2014). “Production of bioethanol using agricultural waste: Banana pseudo stem,” Braz. J. Microbiol. 45(3), 885-892.
Ira, F. S., Ezebuiro, V., and Ogugbue, C. J. (2016). “Production of bioethanol by bacterial co-culture from agro-waste-impacted soil through simultaneous saccharification and co-fermentation of steam-exploded bagasse,” Bioresources and Bioprocessing 3(26), 1-12. DOI: 10.1186/s40643-016-0104-x
Ji, L.-Q. (2015). “An assessment of agricultural residue resources for liquid biofuel production in China,” Renew. Sust. Energ. Rev. 44, 561-575. DOI: 10.1016/j.rser.2015.01.011
Kahyaoglu, T., and Kaya, S. (2006). “Modelling of moisture, color and texture changes in sesame seeds during the conventional roasting,” J. Food Eng. 75(2), 167-177. DOI: 10.1016/j.jfoodeng.2005.04.011
Kang, Q., Appels, L., Tan, T., and Dewil, R. (2014). “Bioethanol from lignocellulosic biomass: Current findings determine research priorities,” Sci. World J. 2014, 1-13. DOI: 10.1155/2014/298153
Kanmani, R., Vijayabaskar, P., and Jayalakshmi, S. (2011). “Saccharification of banana-agro waste and clarification of apple juice by cellulase enzyme produced from Bacillus pumilis,” World Applied Sciences Journal 12(11), 2120–2128.
Kazi, F. K., Fortman, J. A., Anex, R. P., Hsu, D. D., Aden, A., Dutta, A., and Kothandaraman, G. (2010). “Techno-economic comparison of process technologies for biochemical ethanol production from corn stover,” Fuel 89(1), 20-28. DOI: 10.1016/j.fuel.2010.01.001
Lugani, Y., Singla, R., and Sooch, B. S. (2015). “Optimization of cellulase production from newly isolated Bacillus sp. Y3,” Journal of Bioprocessing and Biotechniques 5(11), 1-6. DOI: 10.4172/2155-9821.1000264
Miller, G. L. (1959). “Use of dinitrosalicylic acid reagent for determination of reducing sugar,” Anal. Chem. 31(3), 426-428. DOI: 10.1021/ac60147a030
Mofijur, M., Rasul, M. G., Hyde, J., Azad, A. K., Mamat, R., and Bhuiya, M. M. K. (2016). “Role of biofuel and their binary (diesel–biodiesel) and ternary (ethanol–biodiesel–diesel) blends on internal combustion engines emission reduction,” Renew. Sust. Energ. Rev.53, 265-278. DOI: 10.1016/j.rser.2015.08.046
Namiki, M. (1995). “The chemistry and physiological functions of sesame,” Food Rev. Int. 11(2), 281-329. DOI: 10.1080/87559129509541043
Nasr, S. A., Abozaid, A. A., Hussein, Y. A., and Al-Salemi, F. A. (2011). “Cellulase production by local bacteria isolated from taif in Saudi Arabia,” Journal of Agriculture Science 19(1), 163-170.
Padilha, I. Q. M., Carvalho, L. C. T., Dias, P. V. S., Grisi, T. C. S. L., da Silva, F. L. H., Santos, S. F. M., and Araújo, D. A. M. (2015). “Production and characterization of thermophilic carboxymethyl cellulase synthesized by Bacillus sp. growing on sugarcane bagasse in submerged fermentation,” Braz. J. Chem. Eng. 32(1), 35-42. DOI: 10.1590/0104-6632.20150321s00003298
Purkait, B. S., Ray, D., Sengupta, S., Kar, T., Mohanty, A., and Misra, M. (2011). “Isolation of cellulose nanoparticles from sesame husk,” Ind. Eng. Chem. Res. 50(2), 871–876. DOI: 10.1021/ie101797d
Rasul, F., Afroz, A., Rashid, U., Mehmood, S., Sughra, K., and Zeeshan, N. (2015). “Screening and characterization of cellulase producing bacteria from soil and waste (molasses) of sugar industry,” International Journal of Bioscience 6(3), 230-238. DOI: 10.12692/ijb/6.3.230-238
Samira, M., Mohammad, R., and Gholamreza, G. (2011). “Carboxymethyl-cellulose and filter-paperase activity of new strains isolated from Persian Gulf,” Microbiology Journal 1, 8-16. DOI: 10.3923/mj.2011.8.16
Sato, K., Tomita, M., Yonemura, S., Goto, S., Sekine, K., Okuma, E., Takagi, Y., Hon-Nami, K., and Saikit, T. (1993). “Characterization of and ethanol hyper-production by Clostridium thermocellum I-1-B,” Biosci. Biotech. Bioch. 57(12), 2116-2121. DOI: 10.1271/bbb.57.2116
Singh, S., Moholkar, V. S., and Goyal, A. (2014). “Optimization of carboxymethyl-cellulase production from Bacillus amyloliquefaciensSS35,” 3 Biotech 4(4), 411-424. DOI: 10.1007/s13205-013-0169-6
Svetlitchnyi, V., Kensch, O., Falkenhan, D. A., Korseska, S. G., Lippert, N., Prinz, M., Sassi, J., Schickor, A., and Curvers, S. (2013). “Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria,” Biotechnol. Biofuels 6, 31-46. DOI: 10.1186/1754-6834-6-31
Tailliez, P., Girard, H., Millet, J., and Beguin, P. (1989). “Enhanced cellulose fermentation by an asprogenous and ethanol tolerant mutant of Clostridium thermocellum,” Applied Environmental Microbiology55, 207-211.
Yan, H., Dai, Y., Zhang, Y., Yan, L., and Liu, D. (2011). “Purification and characterization of an endo-1,4-β-glucanase from Bacillus cereus,” Afr. J. Biotechnol. 10(72), 16277-16285. DOI: 10.5897/AJB11.155
Yang, W., Meng, F., Peng, J., Han, P., Fang, F., Ma, L., and Cao, B. (2014). “Isolation and identification of a cellulolytic bacterium from the Tibetan pig’s intestine and investigation of its cellulase production,” Electron. J. Biotechn. 17(6), 262-267. DOI: 10.1016/j.ejbt.2014.08.002
Article submitted: February 6, 2018; Peer review completed: March 18, 2018; Revised version received: March 25, 2018; Accepted: March 28, 2018; Published: April 16, 2018.
DOI: 10.15376/biores.13.2.3832-3845
