Abstract
Brewer’s spent grain (BSG) is the main solid by-product of the brewing sector. High moisture and nutrient-rich content render BSG easily perishable, leading to waste generation and environmental impacts. BSG has narrow applications in both feed and food sectors due to its composition including high fiber and low protein. Therefore, a processing strategy leading to the nutritional valorization of BSG could widen its applications. In this study, submerged cultivation of edible filamentous fungi (Aspergillus oryzae, Neurospora intermedia, and Rhizopus delemar) was introduced as a strategy to enhance the protein content of BSG. The growth of all strains in BSG increased the protein content of the fermented BSG. The highest increase of protein content (from 22.6% to 34.6%), was obtained by cultivation using A. oryzae and medium supplementation. The protein content increase was followed by a decrease in the content of polysaccharides (up to ca. 50%), namely starch, glucan, xylan, and arabinan. The addition of cellulase resulted in enhanced ethanol production from BSG but led to lower concentration of recovered solids. In conclusion, simple processing of BSG using edible filamentous fungi can lead to quality improvement of BSG, providing potential economic and environmental benefits to the brewing sector.
Download PDF
Full Article
Brewing Process Development by Integration of Edible Filamentous Fungi to Upgrade the Quality of Brewer’s Spent Grain (BSG)
Mohsen Parchami,* Jorge A. Ferreira, and Mohammad J. Taherzadeh
Brewer’s spent grain (BSG) is the main solid by-product of the brewing sector. High moisture and nutrient-rich content render BSG easily perishable, leading to waste generation and environmental impacts. BSG has narrow applications in both feed and food sectors due to its composition including high fiber and low protein. Therefore, a processing strategy leading to the nutritional valorization of BSG could widen its applications. In this study, submerged cultivation of edible filamentous fungi (Aspergillus oryzae, Neurospora intermedia, and Rhizopus delemar) was introduced as a strategy to enhance the protein content of BSG. The growth of all strains in BSG increased the protein content of the fermented BSG. The highest increase of protein content (from 22.6% to 34.6%), was obtained by cultivation using A. oryzae and medium supplementation. The protein content increase was followed by a decrease in the content of polysaccharides (up to ca. 50%), namely starch, glucan, xylan, and arabinan. The addition of cellulase resulted in enhanced ethanol production from BSG but led to lower concentration of recovered solids. In conclusion, simple processing of BSG using edible filamentous fungi can lead to quality improvement of BSG, providing potential economic and environmental benefits to the brewing sector.
Keywords: Edible filamentous fungi; Brewer’s spent grain; Protein recovery; Submerged cultivation
Contact information: Swedish Centre for Resource Recovery, University of Borås, 50190 Borås, Sweden;
* Corresponding author: mohsen.parchami@hb.se
GRAPHICAL ABSTRACT
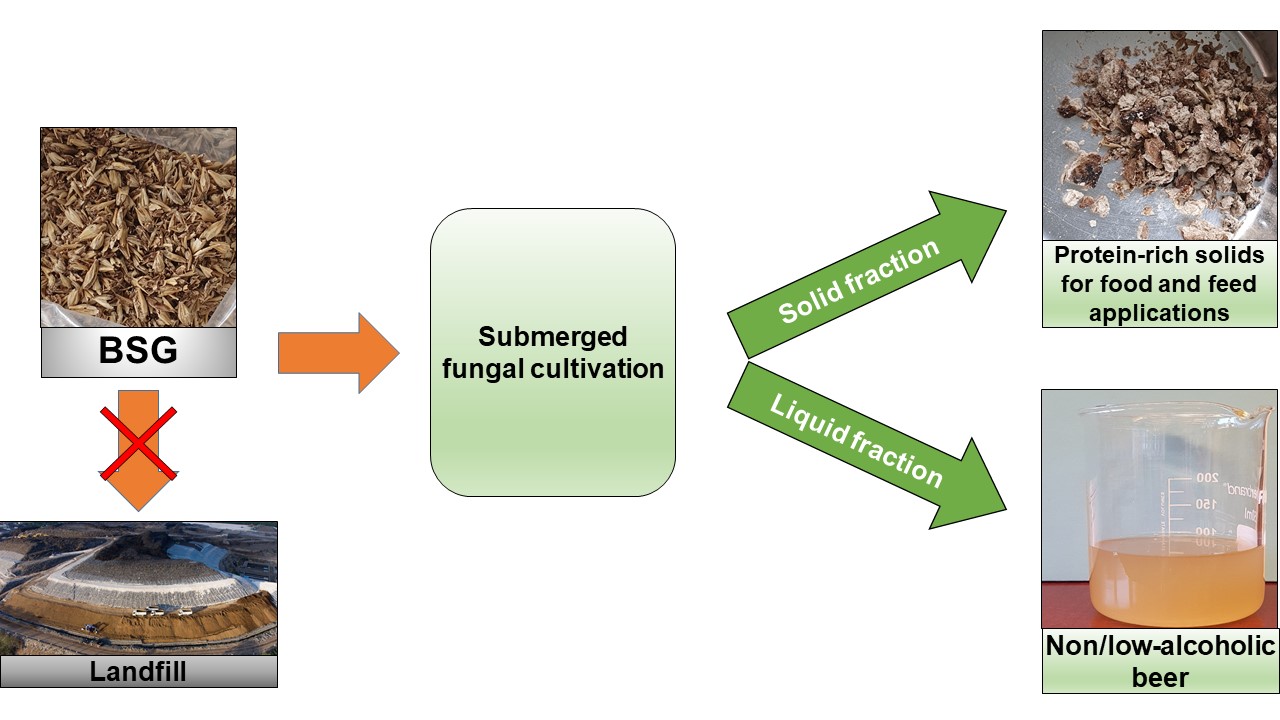
INTRODUCTION
One sector in the food industry that retains huge market value is the brewing industry. In 2018, 188 billion liters of beer (made from malt and excluding beer containing ≤ 0.5% v/v alcohol) were manufactured worldwide with the revenue of 504 billion Euro. China was the leading producer, accounting for approximately 20% of the global production; the USA followed with 11% of the global production (Barth-Haas Group 2019).
The main raw material for beer production is barley, which, before brewing, undergoes malting steps for enzyme activation. The malt is then milled, transferred to the mash tun, and mixed with water and adjuncts. During mashing, enzymatic hydrolysis originates a sweet liquid called wort. Brewer’s spent grain (BSG) is the solid fraction collected after the filtration of wort (Fig. 1) (Hardwick 1995; Mussatto et al. 2006). BSG is mainly barley grain husk mixed with (testa) seed coat and pericarp layers, and depending on the adjuncts, parts of other grains can be found. The main components of BSG are proteins, cellulosic and non-cellulosic polysaccharides, lignin, lipids, vitamins, and minerals (Mussatto 2014; Lynch et al. 2016). The brewing process originates other by-products such as spent hops and spent yeast, but BSGs is the most abundant by-product of brewing, accounting for 85% of total by-products and 30% of the initial malt weight (Townsley 1979; Mussatto et al. 2006). Considering the average generation of 20 kg wet BSG (70 to 80% moisture) for every 100 liters of beer (Steiner et al. 2015), the global amount of BSG generated in 2018 was approximately 37.6 million metric tons. Due to high moisture and nutrient-rich content, BSG is highly susceptible to biological deterioration, leading to waste generation and environmental impacts (El‐Shafey et al. 2004).
Fig. 1. An overview of the beer production process
Currently, BSG encounters limited applications in animal feed, due to low digestibility, rendering landfilling the most common disposal route. Recently proposed valorization routes have included the incorporation of BSG in food products with potential health benefits (Mussatto 2014). There are a number of studies on the direct application of BSG as a cheap enrichment source for the nutritional fortification of food products such as bread, cookies, cakes (Townsley 1979; Waters et al. 2012; Fărcaş et al. 2014), and egg pasta (Cappa and Alamprese 2017). Nonetheless, the use of BSG is also limited in food systems (about 5 to 10%) due to undesired changes in the final product properties such as taste, texture, and color (Mussatto et al. 2006). Production of phenolic compounds (Moreira et al. 2012; Spinelli et al. 2016; Guido and Moreira 2017), arabinoxylans (Coelho et al. 2014; Vieira et al. 2014), absorbents (Chiang et al. 1992), and paper (Ishiwaki et al. 2000), or energy recovery through combustion and pyrolysis (Anal 2018), are other approaches proposed for valorization of BSG.
Upgrading the nutritional profile of BSG could lead to higher incorporation in both feed and food systems, and the use of edible filamentous fungi for conversion of cellulosic and non-cellulosic polysaccharides into nutritious biomass and protein could achieve this goal. Increasing population and changing diets support the need of alternative protein sources for animal feed and meat replacers, respectively.
Filamentous fungi share low substrate specificity and wide range of potential value-added products (Ferreira et al. 2016). Different filamentous fungal species, such as Aspergillus spp., Rhizopus spp., and Neurospora spp., are used for the production of native East Asian foods and beverages (e.g., tempe, oncom, miso, syoyu, and sake) (Karimi et al. 2019). Consequently, various species are categorized as generally regarded as safe (GRAS) microorganisms, indicating their suitability for the valorization of wastes into food and feed products. Their high protein content with essential and non-essential amino acids, low fat, and cholesterol-free content make filamentous fungi a nutritive source of food and feed. Moreover, filamentous fungi secrete a large number of different enzymes and secondary metabolites, with great importance in different food and feed processing industries, making them advantageous for food and feed applications (Ghorai et al. 2009). Nutritional valorization of BSG has been investigated on low-moisture solid-state fermentation, which requires a difficult scale-up (Cooray and Chen 2018; Ibarruri et al. 2019; Tan et al. 2019; Gmoser et al. 2020). Another strategy is needed to cope with the cumbersome amounts of BSG available.
This study upgraded BSG using submerged cultivation by edible filamentous fungi, a strategy currently applied at the industrial scale (Ferreira et al. 2016). The effects of fungal strain, medium supplementation, and cellulase addition on the amounts of recovered solids and protein content were investigated.
EXPERIMENTAL
Substrate
The BSG was obtained from Göteborgs Nya Bryggeri AB (Gothenburg, Sweden). The collected wet BSG was air-dried at room temperature for three days, followed by storage in zip-sealed bags at room temperature.
Filamentous Fungi
Three filamentous fungi, namely Aspergillus oryzae var. oryzae CBS 819.72 (Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands), Neurospora intermedia CBS 131.92, and Rhizopus delemar CBS 145940, isolated originally from tané koji used for sake making (CBS strain database), fermented peanuts for making Oncom (CBS strain database), and leaves of Tectona grandis used to produce tempe (CBS strain database), respectively, were used in this study. The strains were kept on potato dextrose agar (PDA) plates containing 20 g/L glucose, 15 g/L agar, and 4 g/L potato infusion powder. New PDA plates were prepared by flooding pre-grown plates with 20 mL of sterile distilled water, and a disposable plastic L-shape spreader was used to bring the spores into solution. New plates were inoculated with 100 μL of spore solution, incubated at 30 °C for four days, and stored at 4 °C.
Cultivation in Shake-Flasks
Submerged fungal cultivations were performed in batch mode using 250 mL cotton-plugged Erlenmeyer flasks containing 100 mL of medium. The composition of the medium for each cultivation condition can be found in Table 1. The salt and BSG/yeast extract solutions were autoclaved at 121 °C for 20 min separately before cultivation. For all conditions, the pH was adjusted to 5.5 ± 0.1 using 6.0 M NaOH except for the cultivation without the addition of nutrients (initial pH = 5.2). For the cultivation with addition of cellulase cocktail, 10 FPU Cellic Ctec3/g glucan (Novozymes, Copenhagen, Denmark) was added to the flask prior to inoculation with spore solution. The enzyme activity was 234 FPU/mL. The flasks were inoculated with 2 mL of spore solution, as presented in Table 1. The cultivations were carried out in a water bath with shaking at 125 rpm and at 35 °C for three days. During the cultivation period, samples of 1 mL were taken every 12 h and centrifuged for 10 min at 14,000 rpm. The supernatant was filtered through a syringe filter of 0.2 μm pore size and stored at −20 °C until high-performance liquid chromatography (HPLC) analysis.
Analytical Methods
After cultivation, the solids (fungal biomass entangled with undigested BSG components) were recovered by sieving using a fine mesh (1 mm2 pore size), washed with distilled water until a clear effluent was obtained, and oven-dried at 70 °C until they reached a constant weight. The total solids, structural carbohydrates, and total lignin of both initial and cultivation-derived samples were measured according to the National Renewable Energy Laboratory (NREL) method for the determination of structural carbohydrates and lignin in biomass (Sluiter et al. 2008).
The crude protein content of BSG and of recovered solids from the cultivations was determined according to the Kjeldahl method using an InKjel P digestor and a behrotest® S1 distillation unit (Behr Labor‐Technik, Düsseldorf, Germany). In the first step, 20 mL of 98% H2SO4, antifoam, and KT1 tablets (Thompson & Capper Ltd, Runcorn, UK) were added to 0.5 ± 0.0 g material and digested for 100 min at 100% power (where 10 min were needed for heating the digestion block). Afterwards, the digested solutions were neutralized with 32% NaOH solution and distilled for 5 min; the distillation vapor was collected in 50 mL of 4% H3BO4. Finally, the condensates were titrated with 0.1 M HCl until the pH reached 4.6. For conversion of nitrogen to protein, a factor of 6.25 was used (Magomya et al. 2014), whereas the percentage of protein content increment of BSG following fungal cultivation was calculated according to Eq. 1,
where X is the increase in protein content of the BSG, Xbiomass is the protein content of the biomass and XBSG is the protein content of the BSG.
The liquid samples from cultivation and structural carbohydrate and lignin analysis were analyzed using a HPLC system (Waters 2695, Waters Corporation, Milford, MA, USA). A hydrogen-ion based ion exchange column (Aminex HPX87-H, BioRad Laboratories, München, Germany) at 60 °C and with 0.6 mL/min 5 mM H2SO4 as eluent was used for the analysis of glucose, sugars other than glucose, and ethanol.
Table 1. Medium Recipes and Spore Concentrations Used at the Various Conditions Studied for Cultivation of Three Edible Filamentous Fungi
In addition, a lead (II)-based column (Aminex HPX-87P, Bio-Rad) at 85 °C and 0.6 mL/min ultrapure water was used for the analysis of xylose, galactose, arabinose, and mannose. An ultraviolet (UV) absorbance detector (Waters 2487), operating at 210 nm wavelength, was used in series with a refractive index (RI) detector (Waters 2414).
Statistical Analysis
All experiments and analyses were performed in duplicate. The statistical analysis of the data was carried out using MINITAB® 17 (Minitab Ltd., Coventry, UK). The error bars and intervals reported in the text, tables, and graphs represent two times the standard deviation. The one-way ANOVA (analysis of variance) with a confidence interval of 95% was used for analyzing the results and pairwise comparisons were carried out according to Tukey’s test.
RESULTS AND DISCUSSION
Biological quality upgrading of BSG for feed application is a promising method for reducing the negative environmental impact of the brewing industry. Although BSG is a food-grade byproduct rich in nutrients with great potential for production of food products, most of the research attention has been directed to the production of chemicals. Hence, this study investigated the nutritional valorization of BSG through submerged cultivation of edible filamentous fungi. The industrial applicability of the process was considered during the process design. The submerged cultivation was chosen as the cultivation mode because it is a similar to the technology used in the beer industry and is easier to scale up than solid-state fermentation. Moreover, the proposed process was kept very simple, at a minimum number of stages, providing a higher chance of integration into an already established brewery. The submerged cultivation of three edible filamentous fungi, namely A. oryzae, N. intermedia, and R. delemar, are reported in this study. In addition to fungal strain, the effect of the addition of nutrients and cellulase on the concentration of recovered solids and respective protein content were evaluated. Finally, a discussion is provided on the implications of the proposed process through proposal of scenarios for process integration and future research studies.
Brewer’s Spent Grain as a Substrate for Fungal Cultivation
The main components of BSG were polysaccharides (64.9 ± 4.34%), proteins (22.65 ± 0.10%), and lignin (15.68 ± 0.20%). The main polysaccharides were glucan, accounting to 17.52 ± 1.43%, starch, accounting to 20.88 ± 0.10%, and xylan, accounting to16.30 ± 0.17% (Table 2).
The batch cultivation of BSG without the addition of nutrients resulted in recovery of solids ranging from 15.62 to 20.61 g/L. The concentrations of recovered solids and respective protein contents following cultivation of A. oryzae and R. delemar were not significantly different, while cultivation with N. intermedia led to the lowest concentration of recovered solids and respective protein content (p-value = 0.003 and 0.008, respectively) (Table 3). The fungal cultivation with each of the different filamentous fungal strains resulted in an increase in protein content in comparison with the initial protein content of BSG (Fig. 2). The cultivation with A. oryzae resulted in the highest protein content increment (19.43 ± 0.16%; p-value = 0.008) in comparison with the other two fungal strains.
Table 2. Chemical Composition (% w/w) of BSG and of the Recovered Solids from Cultivations with Three Fungal Strains and Addition of Supplementary Nutrients
AIL – acid-insoluble lignin; ASL – acid-soluble lignin
Fig. 2. Increase percentage in protein content of BSG through comparison with the recovered solids following cultivation with the three filamentous fungal strains
Table 3. Obtained Concentration of Recovered Solids and Protein Content from Cultivation with Different Fungal Strains and Conditions
The addition of nutrients boosted both the concentration of recovered solids and protein content in all three fungal cultivations (Table 3), which directly improved the efficiency of protein recovery through fungal cultivation of BSG. The increase in the concentration of recovered solids and protein content was statistically significant for the three fungi (p-value ≤ 0.05) in comparison to that obtained from cultivation without the addition of nutrients. This result implies that either BSG organic and inorganic nutrients are not enough for efficient fungal growth or that the filamentous fungal strains did not manage to access the needed nutrients, rendering addition of nutrients necessary. The highest increase in concentration of recovered solids and protein content was observed from the cultivation with N. intermedia, while the lowest increase was obtained from the cultivation with A. oryzae. The cultivation with N. intermedia led to an increase in concentration of recovered solids and protein content from 15.62 ± 0.01 g/L and 24.99 ± 0.10% (from cultivation without addition of nutrients) to 22.32 ± 0.05 g/L and 31.70 ± 1.97%, respectively. Unlike the cultivations without addition of nutrients, not significantly different concentration of recovered solids (~24 g/L) and protein content (~31%) were obtained from the cultivation with different fungal strains with addition of nutrients. The increase in protein content compared to that of initial BSG was more than 40%. These results are more than the double of those obtained by Ibarruri et al. (2019), where there was less than 20% increase in protein content after three days of solid-state fermentation with genetically modified Rhizopus sp. However, the authors obtained 50% increase in protein content after eight days of fermentation. In a long solid-state fermentation (35 days) of BSG with A. oryzae, Ogunjobi et al. (2011) achieved a 55% increase in the protein content.
A compositional comparison between the recovered solids after fungal cultivation with addition of supplementary nutrients and that of BSG showed approximately 50% decrease in polysaccharides content in form of starch, glucan, xylan, and arabinan following the cultivation with N. intermedia and R. delemar, while the polysaccharides content decrease was ca. 42% for A. oryzae cultivation. This decrease in polysaccharides was followed by an increase in lignin on a percentage weight basis.
The ethanol yields (Table 4) show that BSG composition in the current state (without addition of nutrients and enzyme) was favorable for production of fungal biomass rather than ethanol production. The highest ethanol yield of 16.84 ± 1.54% (percentage of the theoretical yield) was achieved from the cultivation with N. intermedia. This filamentous fungus is a potential ethanol producer compared with other ascomycetes and zygomycetes filamentous fungi, during cultivation in sidestreams from industrial ethanol production (Ferreira et al. 2014).
Table 4. Ethanol Yields Obtained from Cultivations with Addition of Supplementary Nutrients and Different Fungal Strains
Effect of Cellulase Addition on Biomass Production
The BSG is a lignocellulosic material and of recalcitrant nature, which limits the access of filamentous fungi to polysaccharides and consequently their growth. To overcome the recalcitrance, the lignocellulosic substrate needs to undergo either energy and chemical-intensive physical-chemical pretreatment or time consuming biological pretreatment step (Kumari and Singh 2018). Hence, no pretreatment was applied in this work, and cellulase enzyme was added to the cultivation medium to complement the enzymes possibly produced by filamentous fungi and boost the conversion of BSG. However, the compositional analysis of the recovered solids (Table 2) showed no considerable improvement in sugar consumption. The glucan level of the recovered solids from the cultivation of all three fungal strains, without the addition of enzyme, was similar to the respective values obtained from the cultivation with the addition of cellulase. This result indicates limited access of the enzyme to cellulose due to the recalcitrant structure of BSG, normally related to the presence of lignin (Mussatto et al. 2008). The fungal strains used in this work are not traditional producers of lignin-modifying enzymes, which might explain the results obtained and the need of other pretreatment strategies for proper access of polymers in BSG. However, these pretreatment methods would increase the complexity of BSG processing and therefore were not considered in this research work, where the ultimate goal was to achieve nutritional upgrading with simple processing. However, the addition of cellulase resulted in faster release of the accessible glucose; as observed in Fig. 3. The initial glucose concentration in cultivations with the addition of cellulase was more than 10 times the initial values in cultivations without the addition of cellulase. During cultivation with the addition of cellulase, the growth of all three fungal strains led to higher ethanol production yields (Table 4). The addition of the cellulase resulted in more than 75% increase in ethanol production yield. Bátori et al. (2015) reported a similar trend for the cultivation of N. intermedia and A. oryzae in whole stillage. They obtained an up to 85% increase in ethanol production after using the cellulase cocktail at 1 FPU/g SS (suspended solids).
Fig. 3. Profiles of ethanol, glucose, sugars other than glucose during cultivation with addition of supplementary nutrients and edible filamentous fungi in BSG. (a) A. oryzae and without addition of cellulase cocktail; (b) A. oryzae and addition of cellulase cocktail; (c) N. intermedia and without addition of cellulase cocktail; (d) N. intermedia and with addition of cellulase cocktail; (e) R. delemar and without addition of cellulase cocktail; (f) R. delemar and with addition of cellulase cocktail
The concentration of recovered solids for each fungal strain with the respective value obtained at cultivation with the addition of enzyme was significantly decreased (p-value ≤ 0.05), while the protein content percentage was similar. A decrease in concentration of recovered solids ranging from 24% to 32% was observed. For instance, the concentration of recovered solids from cultivation with N. intermedia with the addition of cellulase was 16.28 ± 1.30 g/L, representing a 32% reduction compared to that obtained from the cultivation without enzyme addition (Table 3).
Process Implications
The brewery sector is one of the main sectors in the food industry, and it generates huge amounts of solid by-products, mainly BSG, throughout the year. Handling the waste streams negatively affects process economics; the problem becomes more acute as more strict laws on waste management are put in place. Currently, a low amount of BSG is used in animal feeding practices, while most ends up in landfills. However, upgrading the nutritional composition of BSG can increase their incorporation in both feed and food systems.
Fig. 4. The proposed scenarios for the valorization of BSG
This study showed that a simple cultivation of edible filamentous fungi in BSG resulted in up to 47% weight reduction and 40% increase in protein content, which can have significant implications. For instance, the BSG generated in 2018 contained around 8 million tons of protein, which can be increased to 11.2 million tones by the simple fungal cultivation proposed in this study; the amount of BSG can be reduced from 37.6 million tons to 19.9 million tons. These results make BSG a suitable substrate for the production of protein-rich products with simple processing. Considering the source of BSG and experimental results, two different scenarios (Fig. 4) were considered for valorization.
In the first scenario, the fungal protein production is integrated into an already established brewery. The proposed submerged fungal cultivation of BSG is a very simple process with minimum complexity and number of steps, which share the same technology with the brewery; therefore, it has a high potential for integration. In addition to protein recovery from BSG through fungal cultivation, the remaining liquid following recovery of solids can be used as a new type of non-alcoholic or low-alcohol beer. The production of additional products from the same substrate is being increasingly motivated in view of potential economic benefits, establishment of biorefineries, positive environmental impacts, and contributing to circular bioeconomy. Additionally, by converting the BSG to new value-added products, the previous waste management costs for disposal of BSG are eliminated, which contributes further to overall process economy. Although the conversion of BSG into a nutritionally superior product seems a promising valorization method, further studies devoted to process optimization, full analysis of the product (e.g., amino acid profile, digestibility in feed systems), techno-economic analysis, and life-cycle assessment are essential to corroborate the potential of the proposed process. Furthermore, the integration of the fungal cultivation of BSG into other established industrial processes, depending on the desired product, could be studied. For instance, if the desired product is ethanol, the low ethanol liquid stream from the fungal cultivation needs to go through a costly distillation process. However, this low ethanol stream is valuable for an already established bioethanol plant; thus integrating the described approach into a running bioethanol plant could be an interesting option (Ferreira et al. 2018).
In the second scenario, the BSG is a substrate for the production of multiple products such as ethanol, lignin, and protein, in a biorefinery system. A pretreatment step is needed to deconstruct the lignocellulosic material into lignin-, cellulose-, and hemicellulosic compounds-rich streams, where organosolv pretreatment has been receiving a lot of research interest because it allows easy lignin recovery (Ferreira and Taherzadeh 2020). As the price of substrate and products directly affect the economic feasibility of an industrial process, the fact that BSG is a cheap substrate and readily available can have positive economic implications. Moreover, there is the possibility of utilizing other waste streams from the brewery, e.g., spent yeast stream and wastewater. For every liter of beer produced between 3 to 10 liters of wastewater, rich in nutrients, is generated. This wastewater can be used as a suitable medium for cultivation of filamentous fungi (Hultberg and Bodin 2017). By using the wastewater, the costly water reclamation will be reduced, while potentially offsetting the need of medium supplementation. In the second scenario, additional process steps (e.g., pretreatment, transportation) are involved. Thus, in addition to techno-economic analysis of the process, the LCA analysis of the process is crucial to process development. Moreover, other issues such as process optimization and product development should be addressed.
CONCLUSIONS
- In this study, the protein recovery from brewer’s spent grains (BSG) through submerged fungal cultivation was proposed as a novel strategy for valorization. The submerged fungal cultivation of BSG successfully increased the protein content of the final product, but medium supplementation was necessary for higher process output.
- The addition of nutrients resulted in similar concentration of recovered solids (~24 g/L; p-value = 0.108) and protein content (~31%; p-value = 0.652) from the cultivation with all fungal strains investigated.
- The addition of cellulase cocktail reduced the concentration of recovered solids by 24 to 32%.
- Two different scenarios were proposed for valorization of BSG that can represent a basis for extensive future research studies.
ACKNOWLEDGMENTS
This work was supported by the Swedish Agency for Economic and Regional Growth (Tillväxtverket) through a European Regional Development Fund. We would like to thank Göteborgs Nya Bryggeri AB for kindly providing the brewer’s spent grain used throughout this work.
REFERENCES CITED
Anal, A. K. (ed.) (2018). Food Processing By-Products and their Utilization, Wiley, Hoboken, NJ, USA.
Barth-Haas Group (2019). “Leading 10 countries in worldwide beer production in 2018,” (https://www.statista.com/statistics/270269/leading-10-countries-in-worldwide-beer-production/), Accessed 28 June 2020.
Bátori, V., Ferreira, J. A., Taherzadeh, M. J., and Lennartsson, P. R. (2015). “Ethanol and protein from ethanol plant by-products using edible fungi Neurospora intermedia and Aspergillus oryzae,” BioMed Research International, 2015, 1-10. DOI: 10.1155/2015/176371
Cappa, C., and Alamprese, C. (2017). “Brewer’s spent grain valorization in fiber-enriched fresh egg pasta production: Modelling and optimization study,” LWT – Food Science and Technology 82, 464-470. DOI: 10.1016/j.lwt.2017.04.068
CBS strain database (2020). “CBS 131.92,” (http://www.wi.knaw.nl/collections/BioloMICS.aspx?Fields=All&ExactMatch=T&Table=CBS+strain+database&Name=CBS+131.92), Accessed 5 July 2020.
CBS strain database (2020). “CBS 819.72,” (http://www.wi.knaw.nl/collections/BioloMICS.aspx?Fields=All&ExactMatch=T&Table=CBS+strain+database&Name=CBS+819.72), Accessed 5 July 2020.
CBS strain database (2020). “CBS 145940,” (http://www.wi.knaw.nl/collections/BioloMICS.aspx?Fields=All&ExactMatch=T&Table=CBS+strain+database&Name=CBS+145940), Accessed 5 July 2020.
Chiang, P.-C., Chang, P., and You, J.-H. (1992). “Innovative technology for controlling VOC emissions,” Journal of Hazardous Materials 31(1), 19-28. DOI: 10.1016/0304-3894(92)87036-f
Coelho, E., Rocha, M. A. M., Saraiva, J. A., and Coimbra, M. A. (2014). “Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides,” Carbohydrate Polymers 99, 415-422. DOI: 10.1016/j.carbpol.2013.09.003
Cooray, S. T., and Chen, W. N. (2018). “Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value,” Journal of Functional Foods 42, 85-94. DOI: 10.1016/j.jff.2017.12.027
El‐Shafey, E. I., Gameiro, M. L. F., Correia, P. F. M., and De Carvalho, J. M. R. (2004). “Dewatering of brewer’s spent grain using a membrane filter press: A pilot plant study,” Separation Science and Technology 39(14), 3237-3261. DOI: 10.1081/SS-200028775
Fărcaş, A., Tofană, M., Socaci, S., Mudura, E., Scrob, S., Salanţă, L., and Mureşan, V. (2014). “Brewers’ spent grain–A new potential ingredient for functional foods,” Journal of Agroalimentary Processes and Technology 20(2), 137-141.
Ferreira, J. A., Brancoli, P., Agnihotri, S., Bolton, K., and Taherzadeh, M. J. (2018). “A review of integration strategies of lignocelluloses and other wastes in 1st generation bioethanol processes,” Process Biochemistry 75, 173-186. DOI: 10.1016/j.procbio.2018.09.006
Ferreira, J. A., Lennartsson, P. R., and Taherzadeh, M. J. (2014). “Production of ethanol and biomass from thin stillage using food-grade zygomycetes and ascomycetes filamentous fungi,” Energies 7(6), 3872-3885. DOI: 10.3390/en7063872
Ferreira, J. A., Mahboubi, A., Lennartsson, P. R., and Taherzadeh, M. J. (2016). “Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects,” Bioresource Technology 215, 334-345. DOI: 10.1016/j.biortech.2016.03.018
Ferreira, J. A., and Taherzadeh, M. J. (2020). “Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment,” Bioresource Technology 299, 122695. DOI: 10.1016/j.biortech.2019.122695
Ghorai, S., Banik, S. P., Verma, D., Chowdhury, S., Mukherjee, S., and Khowala, S. (2009). “Fungal biotechnology in food and feed processing,” Food Research International 42(5), 577-587. DOI: 10.1016/j.foodres.2009.02.019
Gmoser, R., Fristedt, R., Larsson, K., Undeland, I., Taherzadeh, M. J., and Lennartsson, P. R. (2020). “From stale bread and brewers spent grain to a new food source using edible filamentous fungi,” Bioengineered 11(01), 582-598. DOI: 10.1080/21655979.2020.1768694
Guido, L. F., and Moreira, M. M. (2017). “Techniques for extraction of brewer’s spent grain polyphenols: A review,” Food and Bioprocess Technology, 10(7), 1192-1209. DOI: 10.1007/s11947-017-1913-4
Hardwick, W. A. (1995). “An overview of beer making,” Handbook of Brewing (Food Science and Technology), W. A. Hardwick (ed.), CRC Press, Boca Raton, FL, USA, pp. 87-95.
Hultberg, M., and Bodin, H. (2017). “Fungi-based treatment of brewery wastewater—biomass production and nutrient reduction,” Applied Microbiology and Biotechnology 101(11), 4791-4798. DOI: 10.1007/s00253-017-8185-9
Ibarruri, J., Cebrián, M., and Hernández, I. (2019). “Solid state fermentation of brewer’s spent grain using Rhizopus sp. to enhance nutritional value,” Waste and Biomass Valorization 10(12), 3687-3700. DOI: 10.1007/s12649-019-00654-5
Ishiwaki, N., Murayama, H., Awayama, H., Kanauchi, O., and Sato, T. (2000). “Development of high value uses of spent grain by fractionation technology,” Technical quarterly-Master Brewers Association of the Americas 37(2), 261-265.
Karimi, S., Mahboobi Soofiani, N., Lundh, T., Mahboubi, A., Kiessling, A., and Taherzadeh, M. J. (2019). “Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: Protein, lipid, and mineral composition,” Fermentation 5(4), 99. DOI: 10.3390/fermentation5040099
Kumari, D., and Singh, R. (2018). “Pretreatment of lignocellulosic wastes for biofuel production: A critical review,” Renewable and Sustainable Energy Reviews 90, 877-891. DOI: 10.1016/j.rser.2018.03.111
Lynch, K. M., Steffen, E. J., and Arendt, E. K. (2016). “Brewers’ spent grain: A review with an emphasis on food and health,” Journal of the Institute of Brewing 122(4), 553-568. DOI: 10.1002/jib.363
Magomya, A. M., Kubmarawa, D., Ndahi, J. A., and Yebpella, G. G. (2014). “Determination of plant proteins via the Kjeldahl method and amino acid analysis: A comparative study,” International Journal of Scientific & Technology Research 3(4), 68-72.
Moreira, M. M., Morais, S., Barros, A. A., Delerue-Matos, C., and Guido, L. F. (2012). “A novel application of microwave-assisted extraction of polyphenols from brewer’s spent grain with HPLC-DAD-MS analysis,” Analytical and Bioanalytical Chemistry 403(4), 1019-1029. DOI: 10.1007/s00216-011-5703-y
Mussatto, S. I. (2014). “Brewer’s spent grain: a valuable feedstock for industrial applications,” Journal of the Science of Food and Agriculture 94(7), 1264-1275. DOI: 10.1002/jsfa.6486
Mussatto, S. I., Dragone, G., and Roberto, I. C. (2006). “Brewers’ spent grain: generation, characteristics and potential applications,” Journal of Cereal Science 43(1), 1-14. DOI: 10.1016/j.jcs.2005.06.001
Mussatto, S. I., Fernandes, M., Milagres, A. M. F., and Roberto, I. C. (2008). “Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain,” Enzyme and Microbial Technology 43(2), 124-129. DOI: 10.1016/j.enzmictec.2007.11.006
Ogunjobi, A. A., Mejeha, O. K., and Fagade, O. E. (2011). “Protein enrichment of brewery spent grains using Aspergillus oryzae,” AU Journal of Technology 15(1), 53-56.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2008). Determination of Structural Carbohydrates and Lignin in Biomass (NREL/TP-510-42618), National Renewable Energy Laboratory, Golden, CO, USA.
Spinelli, S., Conte, A., and Del Nobile, M. A. (2016). “Microencapsulation of extracted bioactive compounds from brewer’s spent grain to enrich fish-burgers,” Food and Bioproducts Processing 100, 450-456. DOI: 10.1016/j.fbp.2016.09.005
Statista (2020). “Beer – worldwide,” (https://www.statista.com/outlook/10010000/100/beer/worldwide), Accessed 26 June 2020.
Steiner, J., Procopio, S., and Becker, T. (2015). “Brewer’s spent grain: Source of value-added polysaccharides for the food industry in reference to the health claims,” European Food Research and Technology 241(3), 303-315. DOI: 10.1007/s00217-015-2461-7
Tan, Y. X., Mok, W. K., Lee, J., Kim, J., and Chen, W. N. (2019). “Solid state fermentation of brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17,” Fermentation 5(3), 52. DOI: 10.3390/fermentation5030052
Townsley, P. M. (1979). “Preparation of commercial products from brewer’s waste grain and trub,” Technical Quarterly Master Brewers Association of America 16, 130-134.
Vieira, E., Rocha, M. A. M., Coelho, E., Pinho, O., Saraiva, J. A., Ferreira, I. M. P. L. V. O., and Coimbra, M. A. (2014). “Valuation of brewer’s spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans,” Industrial Crops and Products, 52, 136-143. DOI: 10.1016/j.indcrop.2013.10.012
Waters, D. M., Jacob, F., Titze, J., Arendt, E. K., and Zannini, E. (2012). “Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment,” European Food Research and Technology 235(5), 767-778. DOI: 10.1007/s00217-012-1805-9
Article submitted: November 4, 2020; Peer review completed: January 9, 2021; Revised version received and accepted: January 18, 2021; Published: January 21, 2021.
DOI: 10.15376/biores.16.1.1686-1701
