Abstract
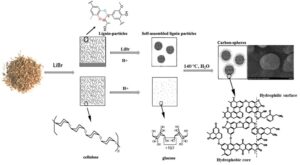
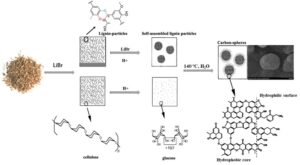
Exploring an efficient technique for carbon sphere preparation has attracted extensive attention. Herein, acidic lithium bromide hydrate (ALBH) was used in the hydrothermal carbonization (HTC) process to overcome the recalcitrance of lignocellulose, such that nano-carbon spheres were prepared at mild condition: 140 °C for 150 min with 0.8 M of HCl from 20-40 meshed corn stover. That carbon spheres showed decent morphology properties and abundant functional groups, which was better than that from pine and poplar wood. The corn stover derived carbon nanospheres could efficiently adsorb both heavy ions and methyl orange in wastewater. Meanwhile, the ALBH after reaction could be recovered and reused. Specifically, the morphologies and adsorption capability of the prepared carbon nanospheres using recovered ALBH were negligibly affected even after 5 cycles. These results verified the practical production of carbon nanospheres from lignocellulose at mild conditions, which provided more potential for the synthesis of novel biomass-based materials for comprehensive applications.
Download PDF
Full Article
Carbon Nanospheres Derived from Crop Residues by Acidic Lithium Bromide Hydrate Treatment
Jian Chen,a,b Xianqin Lu,a,c,d,* Zhiqiang Pang,a Bingbing Li,e and Cuihua Dong a,b,*
Exploring an efficient technique for carbon sphere preparation has attracted extensive attention. Herein, acidic lithium bromide hydrate (ALBH) was used in the hydrothermal carbonization (HTC) process to overcome the recalcitrance of lignocellulose, such that nano-carbon spheres were prepared at mild condition: 140 °C for 150 min with 0.8 M of HCl from 20-40 meshed corn stover. That carbon spheres showed decent morphology properties and abundant functional groups, which was better than that from pine and poplar wood. The corn stover derived carbon nanospheres could efficiently adsorb both heavy ions and methyl orange in wastewater. Meanwhile, the ALBH after reaction could be recovered and reused. Specifically, the morphologies and adsorption capability of the prepared carbon nanospheres using recovered ALBH were negligibly affected even after 5 cycles. These results verified the practical production of carbon nanospheres from lignocellulose at mild conditions, which provided more potential for the synthesis of novel biomass-based materials for comprehensive applications.
Keywords: Corn stover; Carbon spheres; Acidic LiBr; Cr(VI); Methyl orange
Contact information: a: State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Jinan, 250353, Shandong Province, PR China; b: School of Light Industry and Engineering, Qilu University of Technology (Shandong Academy of Sciences) Jinan, 250353, China; c: School of Bioengineering, Qilu University of Technology, Jinan, 250353, Shandong Province, PR China; d: Advanced Research Institute for Multidisciplinary Science, Qilu University of Technology, Jinan, 250353, Shandong Province, PR China; e: Shandong Shunfeng Biological Technology Co., Ltd., Jinan, 250353, Shandong Province, PR China;
*Corresponding authors: lxq_sdu@163.com; Xiaodong771111@163.com
GRAPHICAL ABSTRACT
INTRODUCTION
Biochar is a carbon-rich material formed by heating biomass under certain conditions (Ahmad et al. 2014). As an environmentally friendly adsorbent, biochar shows excellent capability for metal ion and aromatic compound adsorption, such as hexavalent chromium, iron ions, methyl violet, polycyclic aromatic hydrocarbons, and chlorobenzene (Qiu et al. 2009; Vithanage et al. 2017; Zhang et al. 2018, 2019a).
The fabrication of carbon spheres has attracted extensive attention in the field of materials science. Due to its unique properties, including abundant surface functional groups, high surface area, and controllable particle size, carbon spheres have broad application prospects in catalysis, electrochemical conversion, energy storage, and environmental purifications (Qiu et al. 2009; Vithanage et al. 2017; Zhang et al. 2018, 2019a). The properties of carbon spheres heavily rely on the synthesis method and conditions. Over the past decades, many researchers have made great advances in the synthesis methods of carbon spheres, such as the template methods (hard template and soft template), the hydrothermal carbonization method, and the microemulsion polymerization method (Mi et al. 2008; Fechler et al. 2013; Mao et al. 2018). Template methods involved multi-steps: a) the synthesis of the template, b) the preparation of the carbon precursors, c) the pyrolysis carbonization process, and d) template removal at harsh conditions (Claesson and Philipse 2005). The complex coating process and the harsh post-treatment condition of hard template method restricted its wide applications (Tian et al. 2019). The soft template methods omitted the harsh post-treatment process, but they raised high demands on the templates. Many soft template precursors were employed with emulsion droplets, micelles, or surfactant as templates, which could be decomposed during the carbonization process (Fechler et al. 2013). In soft template methods, the template should tolerate the temperature before the carbonization and decompose during the carbonization process (Liang et al. 2008). In addition, the high tendency of carbon precursors to crosslinking resulted in monodispersed carbon spheres (Liu et al. 2015). Compared with template methods, the hydrothermal carbonization method (HTC) has many advantages, such as environmental friendliness and mild treatment conditions. The hydrothermal carbonization methods were commonly performed at temperatures from 200 to 250 °C for about 12 h to 48 h (Sevilla and Fuertes 2009; Simsir et al. 2017). Many biomass-derived carbon precursors, such as glucose, fructose, and sucrose, could be transformed into spherical carbon through the HTC methods (Mi et al. 2008; Nizamuddin et al. 2017; Mao et al. 2018; Tian et al. 2019). However, the raw lignocellulose was resistant to hydrothermal treatment and hardly produced the carbon spheres during HTC process (Simsir et al. 2017; Zhang et al. 2019b).
The acidic concentrated lithium bromide hydrolysis (ALBH) process was reported as a green and recyclable method to overcome the resistance of biomass and cleave the linkages of cellulose into glucose under mild conditions without pretreatment (80 °C to 110 ℃) (Pan and Shuai 2015; Yang et al. 2016; Lu et al. 2020). Previous research suggested that the ALBH process could be used to synthesize biochar from corn stover (Lu et al. 2020). This reaction was carried out under mild conditions to directly transform lignocellulose into biochar without any pretreatment (Pan and Shuai 2015; Yang et al. 2016). The carbon nano-spheres were formed by the ALBH method via one mild treatment (140 °C for 150 min) with raw crop residue as the carbon precursors (Pan and Shuai 2015). Thus, the potential of ALBH in carbon nano-spheres production is worthy of further development. The biochar synthesis depended on the synthesis of biomass types and conditions (Li et al. 2017). Raw lignocellulose, such as agricultural residues and forest waste, is abundant in nature and is the most common feedstock for biochar synthesis. The properties of lignocellulose, such as the compositions of carbon precursor, the inorganic compounds, the degree of crystalline etc., were reported to affect the properties of biochar (Zhang et al. 2015). In addition, many studies have shown that the parameters of synthesis conditions, such as temperature, rate of heating, and residence time, also affected the properties of biochar (Zhang et al. 2015; Nizamuddin et al. 2017). On this basis, this work further investigated the impact of the factors including the chemical composition and the origin of carbon precursor, the substrate size, and the reaction conditions on the spherical morphology of biochar and considered the general process of biochar balling under ALBH conditions. Moreover, the recovery of ALBH solution was explored and excellent results were obtained.
Chromium, one of the most common heavy metals pollution in wastewater, has a wide range of sources including leather tanning, landfill leachate, printing industry, electroplating, and textile industries. Cr(VI) is a swallowable and inhalation poison that is easily absorbed by the body. Skin contact with Cr(VI) can cause sensitive and genetic defects and even lead to cancer by invading the respiratory tract (Liu et al. 2016). Methyl orange (MO) is an acid anion azo dyes representative. It is widely used in the dye industry, accounting for a large share of the global dye market. Due to the toxicity, mutagenicity and carcinogenicity of MO, it will also cause safety hazards to people’s lives (Liu et al. 2021). With the rapid development of industry, a large amount of industrial wastewater is discharged into the ocean every year, which greatly aggravates the pollution to the environment. Therefore, how to remove the MO and Cr ions present in sewage has become a hot spot of concern. Adsorption is an important way to remove heavy metals and dyes in sewage, and it is urgent for people to find an efficient adsorbent.
The biochar synthesis depends on the biomass type and conditions (Li et al. 2017). The raw lignocellulose, such as agricultural residues and forest waste, is abundant in nature and was the most common feedstock for biochar preparation (Zhang et al. 2015). Thus, this study was performed to prepare biochar using different biomass as feedstock by the ALBH method. In addition, the optimum conditions for carbon nano-spheres production were determined, and the adsorption capacity of biochar on heavy metal ions (Cr(VI)) and polyvalent aromatic hydrocarbons (MO) were also evaluated.
EXPERIMENTAL
Materials
Poplar and pine chips were provided by Shandong Sun Paper United Co., Ltd. (Jining, China) and corn stover were obtained from farmland in Jinan, Shandong Province, and the compositions of the feedstocks are shown in Table 1. Lithium bromide was purchased from Macklin Reagent Co., Ltd. (Shanghai, China). Holocellulose was prepared from corn stover as reported by Guo et al. (2014). Lignin was provided by Shandong Longlive Bio-Technology Co., Ltd. (Jinan, China).
Table 1. The Composition of Different Raw Materials
Preparation of Biochar
The preparation of biochar was carried out in a pressure-tight high temperature reactor as described before (Lu et al. 2019, 2020). Briefly, the feedstocks were reacted with 60 wt% lithium bromide and 0.5 M HCl at 140 °C for 150 min. After the reaction, the carbon residue and the filtrate were separated by suction filtration. The filtrate was collected and stored at 4 °C. The solid residue was washed with tap water to neutrality, then dried in an oven at 40 °C for 24 h. The control experiments were conducted under the same conditions. The yields of biochar prepared by ALBH method were calculated using the Eq. 1,
(1)
where Y is biochar yield (%), is mass of carbon residual after ALBH treatment (g), and is initial mass of the feedstock (g).
Characterization of Biochar
The Fourier transform infrared spectroscopy (FTIR) analysis of biochar was performed using an infrared spectrometer (Alpha, Bruker, Karlsruhe, Germany) to analyze its functional groups. The surface element compositions were analyzed by X-ray photoelectron spectroscopy (XPS) (ESCALAB Xi+; Thermo Fisher Scientific, Waltham, MA, USA). The Brunauer-Emmett-Teller (BET) surface of biochar was measured using an automatic gas adsorption analyzer ASAP 2460 (Micromeritics, Norcross, GA, USA). The morphologies of biochar were observed by a Hitachi Regulus 8220 (Tokyo, Japan) scanning electron microscope (SEM).
Adsorption Experiments
Cr(VI) solution (265 mg/L) was prepared by dissolving K2Cr2O7 into distilled water. The pH of the solution was adjusted by 30% HCl. A total of 0.05 g biochar and 25 mL Cr (VI) solution were incubated at 150 rpm, 37 °C for 24 h in an automatic thermostatic oscillation incubator. After incubation, the residue and supernatant were separated by filtration. The concentration of Cr(VI) in the supernatant was measured by a UV-visible spectrophotometer (UV-4800; Unico, Shanghai, China) at 540 nm as reported before (Lu et al. 2019).
The concentrated methyl orange solution (500 mg/L) was prepared by dissolution of methyl orange in distilled water, and the pH of the solution was adjusted to 2 with HCl. Then, 500, 255, 206, 175, 110, and 50 mg/L orange solution gradient was prepared by dilution of the concentrated solution with HCl aqueous (pH 2). The adsorption was performed by shaking the mixture (0.1 g biochar and 10 mL methyl orange solution) in an automatic thermostatic oscillator at 150 rpm, 37 °C for 24 h. After that, the residue and solution were filtered, and the concentration of methyl orange was detected by UV-visible spectrophotometer at 470 nm as described before (Siyasukh et al. 2018).
RESULTS AND DISCUSSION
Effect of Composition of Carbon Precursor on Morphology of Biochar
The composition of carbon precursor was an essential factor influencing the production of biochar (Vaughn et al. 2013; Simsir et al. 2017). Cellulose, hemicellulose, and lignin are the main components of lignocellulose; during the pyrolysis process, the hemicellulose was broken down at temperatures of 220 to 315 °C, and cellulose could endure relatively high temperature with decomposition temperature from 315 to 400 °C (van Zandvoort et al. 2013; Zhang et al. 2015). Lignin, aromatic polymer in nature, was reported to be decomposed over a relatively wide range of temperature from 160 to 900 °C (van Zandvoort et al. 2013; Ragauskas et al. 2014). Thus, as carbon precursor, varying lignocellulosic compositions may affect the morphologies of biochar. To verify the formation mechanism of carbon nanospheres during ALBH treatment, holocellulose (cellulose and hemicellulose), lignin, and homocellulose-lignin mixture were respectively used as the precursor to produce biochar under the same treatment conditions (Fig. 1). Carbon nanospheres derived from holocellulose presented irregular shape and size varied from 50 nm to 100 nm. In respect of lignin as substrate, some sphere and micropores were observed on the surface of lignin. During ALBH treatment, part of linkages among lignin were cleaved, and the hydrophobic lignin units was self-assembled and colloid spheres were formed (approximately 100 nm diameter) in the aqueous phase. Subsequently, the colloid spheres were released from the surface of lignin, and the porous structure of lignin formed. For the holocellulose-lignin mixture system, charcoal morphology changed with the proportion of lignin and holocellulose. The results showed that carbon favorably formed at a suitable holocellulose-lignin ratio, where the carbon-particles at 100 to 200 nm diameter were produced.
Fig. 1. The morphology of biochar with varying holocellulose and lignin ratio: (a) holocellulose: lignin = 0:3; (b) holocellulose: lignin = 1:2; (c) holocellulose: lignin = 1.5:1.5; (d) holocellulose: lignin = 2:1; (e) holocellulose: lignin = 2.5:0.5; (f) holocellulose: lignin = 3:0
The ALBH has powerful ability for dissolution and hydrolysis of lignocellulose (Mosier et al. 2005; Deng et al. 2015; Pan and Shuai 2015; Li et al. 2016). For carbon pellets preparation, most cellulose and hemicellulose were first hydrolyzed into monosaccharides, whereas lignin negligibly changed. Subsequently, soluble polymers from glucose were formed by the intermolecular hydration and condensation reaction. Meanwhile, the polymers interacted with lignin and the aromatic nuclei formed. The various species in the solution diffused to the surface of the aromatic nuclei and linked to the nuclei by the oxygen-containing functional groups. As a result of the growth process, the carbon spheres were formed and a high concentration of reactive oxygen groups were distributed on the surface of the carbon spheres. Therefore, the aromatic nuclei were essential for the spheres forming.
Fig. 2. Schematic diagram of lignocellulose biochar formation
The self-assembling lignin particles in the solution could provide more hydrophobic cores for the sphere growth. In addition, more active hydroxyl groups were formed on the surface due to the cleavage of β-O-4 linkages, providing additional linkages on the core. Thus, the cleavage ability of ALBH on cellulose and lignin favored the formation of carbon spheres. The formation of carbon spheres using ALBH system is schematically showed in Fig. 2.
The Effect of Feedstock Species on the Biochar Characterization
Based on the above analysis, poplar, pine, and corn stover were selected as carbon precursors to produce biochar using ALBH treatment. Table 2 shows the elemental composition of biochar produced using different lignocellulose. The carbon content of biochar prepared from pine wood, poplar, and corn stover was 64.49 wt%, 63.46 wt%, and 61.81 wt%, respectively. These findings suggested that carbonization of lignocellulose occurred during the ALBH treatment and biochar was formed. Moreover, the yield of biochar was affected by the lignocellulose species, and corn stover resulted in the lowest yield (40.44 wt%) among the selected three raw materials. A higher content of lignin in lignocellulose resulted in a higher yield of the final biochar product. The linkages of hemicellulose were easily cleaved under thermal treatment, whereas cellulose and lignin were relatively more stable (van Zandvoort et al. 2013; Zhang et al. 2015). For the superior thermal stability of lignin, high lignin content of pine (27.30%) and poplar (24.20%) accounts for high yield of pine biochar (46.71 wt%) and polar biochar (47.63 wt%).
Table 2. Elemental Analysis of Biochar Produced From Corn Stover, Pine, and Poplar (%)
Fig. 3. The morphology of biochar produced from different raw materials
Figure 3 shows the morphology of biochar. Typical carbon sphere shape is displayed, especially for corn stover as feedstock. Biochar from corn stover presented uniform sphere and was evenly dispersed. However, only few carbon spheres were observed for the biochar prepared from wood pine and poplar, and most carbon spheres were compactly piled. The irregular morphology of biochar from pine and poplar was primarily due to the incomplete carbonization of lignocellulose.
The BET analysis on biochars were conducted (Fig. 4). The biochar prepared from corn stover had 83.9 m2/g surface area, which was higher than that of the biochar-pine (42.8 m2/g) and biochar-poplar (45.8 m2/g). The BET results show that compared with wood raw materials, the surface of biochar prepared from corn straws had a rich pore structure. During adsorption process, the rich pore structure was beneficial to the adsorption capacity of biochar. The functional groups on the surface of biochar were analyzed using FTIR spectroscopy (Fig. 5). Specifically, wide peaks located at 2900 and 3400 cm-1 belonged to O-H vibration, and the peaks of 1000 to 1600 cm-1 indicated the existence of -C-O and -C=O vibration, and the peaks of approximately 700 cm-1 was ascribed to the vibration of -C-H (Lu et al. 2020). The FTIR analysis suggested that a large number of hydroxyl and carboxyl oxygen-containing groups were present on the surface of ALBH biochar. Those oxygen functional groups on the biochar could provide the interaction sites for the metal ions (Wu et al. 2019).
Fig. 4. BET and adsorption pore radius analysis of different biochar
Fig. 5. The functional groups presented on the surface of biochar by FTIR
The Production and Characterization of Biochar From Corn Stover by ALBH Treatment
The above results showed that corn stover as feedstock for biochar preparation can obtain higher specific surface area and exceptional morphology. Moreover, the effect of substrate size on the carbon spheres formation was analyzed. The feedstock was ground and sieved into portions of > 60-mesh (< 250 μm), 20- to 40-mesh (380 to 830 μm), and 40- to 60-mesh (250 to 380 μm), and carbon spheres could be formed in all sizes of corn stover (Fig. 3). Through reducing the feedstock size, rupture of carbon sphere appeared, and aggregation of the small sphere occurred. Large spheres formed for the biochar prepared from corn stover with bigger size (60-mesh). Meanwhile, feedstock at too small size was also unfavorable for the forming of biochar sphere. The biochar produced from the corn stover with size bigger than 60-mesh (< 250 μm) possessing poor surface area, which further confirmed the above statement (Fig. 4). Comparatively, the biochar from corn stalks sized with 20- to 40-mesh (380 to 830 μm) was selected for further study.
The effect of reaction temperature and acid dosage on the morphology of biochar were also discussed (Table 3). The morphology of biochar prepared under T1 through T6 conditions was observed by SEM (Fig. 6). The carbon spheres distributed more uniformly under the conditions of T1 (140 °C), and the particle size of the carbon spheres had poor uniformity, with a broad distribution, for the samples of T2 (160 °C) and T3 (180 °C). The effect of various acid concentration (Entries T4, T5, and T6) on the ALBH reaction was further explored at 140 °C. The acid concentration had a slight effect on the morphology of the biochar (Fig. 6B). In comparison, the carbon spheres for HCl concentration of 0.8 M (T5) were more uniformly distributed. Summarily, lower temperature and acid concentration will lead to poor pelletizing performance of biochar, while too high temperature and acid concentration will destroy the complete biochar sphere. The carbon spheres with good properties using 20- to 40-meshed corn stover as feedstock could be prepared at 140 °C for 150 min with 0.8 M HCl.
Table 3. The Different Reaction Conditions for ALBH Biochar Preparation From Corn Stover (T1 Through T6)
Fig. 6. The morphology of biochar at different treatment conditions: (A) Effect of temperature, biochar prepared at 140 °C (T1), 160 °C (T2), 180 °C (T3); (B) Effect of HCl concentration, biochar prepared at 0.6 M HCl (T4), 0.8 M HCl (T5), 1.0 M HCl (T6)
The Recyclability of LiBr Solution
After reaction, the biochar was separated from the reaction system by filtering, and the solution was analyzed using HPLC. Taking T5 treatment (140 °C, 150 min, and 0.8 M HCl) as an example, no sugar compounds were detected in the filtrate form. The dehydration production of sugars in this experiment, such as 5-hydroxymethylfurfural (5-HMF), furfural, and levulinic acid (LA) were detected. Only slight LA was detected in the filtrate, and almost no 5-HMF and furfural were detected. Thus, the filtrate after treatment can be directly reused.
Figure 7 shows the morphology of the biochar prepared using the LiBr after the fifth run recycling, where carbon spheres with uniform size distribution was still observed. The biochar using the recovered filtrate could be obtained with yields of 36% to 40% (40% for run 1, 38% for run 2, 38% for run 3, 38% for run 4, and 36% for run 5), and the biochar had abundant functional groups after the five cycles (Fig. 8). The results verified the recyclability of the treated solution and confirmed green production of biochar using ALBH treatment.
Fig. 7. The morphology of biochar prepared from LiBr solution after the fifth run recycling
Fig. 8. FTIR spectrum of the biochar using LiBr solution with different cycles
The Removal Ability of Biochar to Heavy Ions and Methyl Orange
The adsorption capability of biochar on Cr(VI) at the initial concentration of 265 mg/L was analyzed (Table 4). The biochar (the T5 sample) obtained an adsorption capability of 111.0 mg/g Cr(VI), and the corn stover-ALBH biochar had a higher value than the elephant grass-ALBH biochar. Additionally, the recycle of biochar showed a slight effect on the adsorption capability on heavy ions, and adsorption of 94.71 mg/g Cr(VI) was obtained even after five recycling runs. As an adsorbent, the surface area and the functional groups played important roles relative to the interaction between the ions and the biochar. In this study, the morphology spheres provided more binding sites for the ions. Meanwhile, the abundant functional groups also improved the adsorption of biochar on heavy ions.
Table 4. Adsorption Capacity of Biochar on Cr(VI) During Five Runs of Continual Recycling of LiBr Solution
The removal ratio of biochar on methyl orange is shown in Fig. 9. Methyl orange as a water-soluble azo dye is widely used in the industrial sector, and the adsorption was found to be an effective technique for methyl orange removal. Herein, the corn stover-ALBH biochar had high potential in the removal methyl orange than other reported carbon spheres (Siyasukh et al. 2018). A good linear correlation was detected between the concentration of methyl orange and the adsorption capability of biochar, and the adsorption capability of biochar on methyl orange increased with the initial dosage of methyl orange. With the further increase of the concentration of methyl orange in the solution to 2000 mg methyl orange /g biochar, the removal ratio of methyl orange reached to 84.8%.
Fig. 9. The removal capability of biochar on methyl orange (a linear correlation with R2 = 0.9998 between the concentration of methyl orange and the adsorption capability of biochar); Qe means equilibrium adsorption capacity; Ce means equilibrium concentration after adsorption.
CONCLUSIONS
- The carbon spheres from corn stover exhibited excellent morphology properties and abundant functional groups, which was better than that from pine and poplar wood.
- Through optimization of the reaction conditions, regular spherical structure with even size distribution could be obtained, and the product showed strong removal capacity on both heavy metal ions and methyl orange.
- The LiBr solution could be readily recycled and reused for many runs. The technique opened a new pathway for biomass utilization and functional carbon sphere preparation.
- As a green and solvent-recyclable technique, one-pot preparation of carbon spheres from corn stover was achieved using acidic lithium bromide hydrate (ALBH) treatment.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Foundation of State Key Laboratory of Biobased Material and Green Papermaking (No. 2419010205, No. 23190444, and No. KF201823), Foundation of the Jiangsu Provincial Key Laboratory of Pulp and Paper Science and Technology (No. KL201906), Natural Science Foundation of Shandong Province (ZR2019BC022), and the National Natural Science Foundation of China (Grant No. 31901269).
REFERENCES CITED
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., Vithanage, M., Lee, S. S., and Ok, Y. S. (2014). “Biochar as a sorbent for contaminant management in soil and water: A review,” Chemosphere 99, 19-33. DOI: 10.1016/j.chemosphere.2013.10.071
Claesson, E. M., and Philipse, A. P. (2005). “Monodisperse magnetizable composite silica spheres with tunable dipolar interactions,” Langmuir 21(21), 9412-9419. DOI: 10.1021/la051127a
Deng, W., Kennedy, J. R., Tsilomelekis, G., Zheng, W., and Nikolakis, V. (2015). “Cellulose hydrolysis in acidified LiBr molten salt hydrate media,” Industrial & Engineering Chemistry Research 54(19), 5226-5236. DOI: 10.1021/acs.iecr.5b00757
Fechler, N., Fellinger, T. P., and Antonietti, M. (2013). “Salt templating: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids,” Advanced Materials 25(1), 75-79. DOI: 10.1002/adma.201203422
Guo, F., Shi, W., Sun, W., Li, X., Wang, F., Zhao, J., and Qu, Y. (2014). “Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism,” Biotechnology for Biofuels 7, article no. 38. DOI: 10.1186/1754-6834-7-38
Li, H., Dong, X., Silva, E. B., Oliveira, L. M., Chen, Y., and Ma, L. Q. (2017). “Mechanisms of metal sorption by biochars: Biochar characteristics and modifications,” Chemosphere 178, 466-478. DOI: 10.1016/j.chemosphere.2017.03.072
Li, N., Pan, X., and Alexander, J. (2016). “A facile and fast method for quantitating lignin in lignocellulosic biomass using acidic lithium bromide trihydrate (ALBTH),” Green Chemistry 18(19), 5367-5376. DOI: 10.1039/c6gc01090c
Liang, C., Li, Z., and Dai, S. (2008). “Mesoporous carbon materials: Synthesis and modification,” Angewandte Chemie International Edition 47(20), 3696-3717. DOI: 10.1002/anie.200702046
Liu, J., Wickramaratne, N. P., Qiao, S. Z., and Jaroniec, M. (2015). “Molecular-based design and emerging applications of nanoporous carbon spheres,” Nature Materials 14, 763-774. DOI: 10.1038/nmat4317
Liu, Q., Liu, Q., Ma, W., Liu, W., Cai, X., and Yao, J. (2016). “Comparisons of two chelating adsorbents prepared by different ways for chromium(VI) adsorption from aqueous solution,” Colloids and Surfaces A: Physicochemical and Engineering Aspects 511, 8-16. DOI: 10.1016/j.colsurfa.2016.09.022
Liu, X., Zhang, W., Mao, L., Yin, Y., and Hu, L. (2021). “Synthesis of feoclmos2 with excellent adsorption performance for methyl orange,” Journal of Materials Science 56, 6704-6718. DOI: 10.1007/s10853-020-05715-y
Lu, X., Liu, X., Zhang, W., Wang, X., Wang, S., and Xia, T. (2019). “The residue from the acidic concentrated lithium bromide treated crop residue as biochar to remove Cr (VI),” Bioresource Technology 296, article ID 122348. DOI: 10.1016/j.biortech.2019.122348
Lu, X., Chen, J., Lu, J., Wang, S., and Xia, T. (2020). “Monosaccharides and carbon nanosphere obtained by acidic concentrated LiBr treatment of raw crop residues via optimizing the synthesis process,” Bioresource Technology 310, article ID 123522. DOI: 10.1016/j.biortech.2020.123522
Mao, H., Chen, X., Huang, R., Chen, M., Yang, R., Lan, P., Zhou, M., Zhang, F., Yang, Y., and Zhou, X. (2018), “Fast preparation of carbon spheres from enzymatic hydrolysis lignin: Effects of hydrothermal carbonization conditions,” Scientific Reports 8, article no. 9501. DOI: 10.1038/s41598-018-27777-4
Mi, Y., Hu, W., Dan, Y., and Liu, Y. (2008). “Synthesis of carbon micro-spheres by a glucose hydrothermal method,” Materials Letters 62(8-9), 1194-1196. DOI: 10.1016/j.matlet.2007.08.011
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., and Ladisch, M. (2005). “Features of promising technologies for pretreatment of lignocellulosic biomass,” Bioresource Technology 96, 673-686. DOI: 10.1016/j.biortech.2004.06.025
Nizamuddin, S., Baloch, H. A., Griffin, G. J., Mubarak, N. M., Bhutto, A.W., Abro, R., Mazari, S. A., and Ali, B. S. (2017). “An overview of effect of process parameters on hydrothermal carbonization of biomass,” Renewable and Sustainable Energy Reviews 73, 1289-1299. DOI: 10.1016/j.rser.2016.12.122
Pan, X., and Shuai, L. (2015). “Saccharification of lignocellulosic biomass,” U. S. Patent No. 9187790B2.
Qiu, Y., Zheng, Z., Zhou, Z., and Sheng, G. D. (2009). “Effectiveness and mechanisms of dye adsorption on a straw-based biochar,” Bioresource Technology 100(21), 5348-5351. DOI: 10.1016/j.biortech.2009.05.054
Sevilla, M., and Fuertes, A. B. (2009). “The production of carbon materials by hydrothermal carbonization of cellulose,” Carbon 47(9), 2281-2289. DOI: 10.1016/j.carbon.2009.04.026
Simsir, H., Eltugral, N., and Karagoz, S. (2017). “Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization,” Bioresource Technology 246, 82-87. DOI: 10.1016/j.biortech.2017.07.018
Siyasukh, A., Chimupala, Y., and Tonanon, N. (2018). “Preparation of magnetic hierarchical porous carbon spheres with graphitic features for high methyl orange adsorption capacity,” Carbon 134, 207-221. DOI: 10.1016/j.carbon.2018.03.093
Tian, H., Liang, J., and Liu, J. (2019). “Nanoengineering carbon spheres as nanoreactors for sustainable energy applications,” Advanced Materials 31(50), a rticle ID 1903886. DOI: 10.1002/adma.201903886
van Zandvoort, I., Wang, Y., Rasrendra, C. B., van Eck, E. R., Bruijnincx, P. C., Heeres, H. J., and Weckhuysen, B. M. (2013). “Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions,” ChemSusChem 6(9), 1745-1758. DOI: 10.1002/cssc.201300332
Vaughn, S. F., Kenar, J. A., Thompson, A. R., and Peterson, S. C. (2013). “Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates,” Industrial Crops and Products 51, 437-443. DOI: 10.1016/j.indcrop.2013.10.010
Vithanage, M., Herath, I., Joseph, S., Bundschuh, J., Bolan, N., Ok, Y. S., Kirkham, M. B., and Rinklebe, J. (2017). “Interaction of arsenic with biochar in soil and water: A critical review,” Carbon 113, 219-230. DOI: 10.1016/j.carbon.2016.11.032
Wu, J., Wang, T., Zhang, Y., and Pan, W. P. (2019). “The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: Considering the increased oxygen functional groups by HCl treatment,” Bioresource Technology 291, article ID 121859. DOI: 10.1016/j.biortech.2019.121859
Yang, X., Li, N., Lin, X., Pan, X., and Zhou, Y. (2016). “Selective cleavage of the aryl ether bonds in lignin for depolymerization by acidic lithium bromide molten salt hydrate under mild conditions,” Journal of Agricultural and Food Chemistry 64(44), 8379-8387. DOI: 10.1021/acs.jafc.6b03807
Zhang, X., Hewetson, B. B., and Mosier, N. S. (2015). “Kinetics of maleic acid and aluminum chloride catalyzed dehydration and degradation of glucose,” Energy & Fuels 29(4), 2387-2393. DOI: 10.1021/ef502461s
Zhang, K., Sun, P., Faye, M., and Zhang, Y. (2018). “Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation,” Carbon 130, 730-740. DOI: 10.1016/j.carbon.2018.01.036
Zhang, J., Shao, J., Jin, Q., Li, Z., and Chen, H. (2019a). “Sludge-based biochar activation to enhance Pb(II) adsorption,” Fuel 252, 101-108. DOI: 10.1016/j.fuel.2019.04.096
Zhang, X., Zhang, P., Yuan, X., Li, Y., and Han, L. (2019b). “Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar,” Bioresource Technology 296, article ID 122318. DOI: 10.1016/j.biortech.2019.122318
Article submitted: June 28, 2021; Peer review completed: August 21, 2021; Revised version received: Sept. 4, 2021; Accepted: Sept. 5, 2021; Published: September 10, 2021.
DOI: 10.15376/biores.16.4.7220-7233
