Abstract
Biomass catalytic fast pyrolysis is one of the most promising technologies for the production of renewable aromatics and olefins directly from solid biomass. In this study, catalytic pyrolysis experiments were carried out on biomass in a fluidized bed reactor using typical metal-loaded (Mg, K, Fe, Ga, and Ni) ZSM-5 zeolites as catalysts. The effects of catalysts on the product distribution and bio-oil components were investigated to determine the cheapest and most efficient metal-loaded catalyst. The results showed that bio-oil yields with metal-loaded catalysts (40 to 43.4 wt.%) were a little lower than that of pure ZSM-5 (46.4 wt.%). Metal-loaded catalysts produced more CO2 and CO than did pure ZSM-5. Fe/ZSM-5 produced the highest yield of CO2 (13.8 wt.%), as well as the highest yield of olefins (2.7 wt.%). Fe/ZSM-5 showed the same catalytic characteristics as Ga/ZSM-5 (aromatic hydrocarbon proportion in bio-oils of more than 80%), but it is much cheaper than Ga/ZSM-5.
Download PDF
Full Article
Catalytic Pyrolysis of Willow Wood with Me/ZSM-5 (Me = Mg, K, Fe, Ga, Ni) to Produce Aromatics and Olefins
Huiyan Zhang, Jian Zheng, and Rui Xiao*
Biomass catalytic fast pyrolysis is one of the most promising technologies for the production of renewable aromatics and olefins directly from solid biomass. In this study, catalytic pyrolysis experiments were carried out on biomass in a fluidized bed reactor using typical metal-loaded (Mg, K, Fe, Ga, and Ni) ZSM-5 zeolites as catalysts. The effects of catalysts on the product distribution and bio-oil components were investigated to determine the cheapest and most efficient metal-loaded catalyst. The results showed that bio-oil yields with metal-loaded catalysts (40 to 43.4 wt.%) were a little lower than that of pure ZSM-5 (46.4 wt.%). Metal-loaded catalysts produced more CO2 and CO than did pure ZSM-5. Fe/ZSM-5 produced the highest yield of CO2 (13.8 wt.%), as well as the highest yield of olefins (2.7 wt.%). Fe/ZSM-5 showed the same catalytic characteristics as Ga/ZSM-5 (aromatic hydrocarbon proportion in bio-oils of more than 80%), but it is much cheaper than Ga/ZSM-5.
Keywords: Biomass; Metal-loaded ZSM-5; Catalytic pyrolysis; Fluidized bed
Contact information: Ministry of Education of Key Laboratory of Energy Thermal Conversion and Control, School of Energy and Environment, Southeast University, Nanjing 210096, P.R. China; *Corresponding author: ruixiao@seu.edu.cn
INTRODUCTION
Biomass is the most abundant and inexpensive sustainable source of carbon that can be used to produce renewable fuels and chemicals (Huber et al. 2006). The development of energy-efficient processes for biomass conversion is of great importance in light of future petroleum shortages and the associated environmental issues worldwide. Many studies have focused on biomass fast pyrolysis with pyrolysis oil (bio-oil) as the product (Bridgwater and Peacocke 2000; Dobele et al. 2007; Liu et al. 2013). As a pre-treatment step to increase the energy density of biomass, pyrolysis could reduce transport costs up to 87% (Luque et al. 2008). However, the produced bio-oil has many disadvantages, such as high oxygen content, high viscosity, corrosivity, and thermal instability. Therefore, improvement of bio-oil is necessary to extend its applications.
Catalytic fast pyrolysis (CFP) is one of the most promising technologies for producing renewable aromatic and olefin compounds directly from solid biomass. In the CFP process, biomass first thermally decomposes to pyrolysis vapors. These vapors then enter the pores of zeolite catalysts and are converted into aromatic and olefin compounds, along with CO, CO2, H2O, char, and coke. Biomass pyrolysis and catalytic upgrading can be conducted in one reactor, which eliminates the costly condensation/re-evaporation processes required for bio-oil upgrading.
The catalyst used in the CFP process is the key factor for producing a high yield of hydrocarbons. Many catalysts, including microporous (e.g., ZSM-5, USY) and mesoporous (e.g., MCM-41, MSU, SBA-15) materials, have been used for CFP of biomass. Among these catalysts, ZSM-5 is one of the most suitable for producing hydrocarbons due to its special pore structure and activity (Cheng and Huber 2012; Zhang et al. 2012; Zhao et al. 2012). However, the rapid deactivation of ZSM-5 catalyst and low hydrocarbon yield are the two main barriers for CFP of biomass. This can be attributed to the strong acidity of ZSM-5, which leads to a decrease in the organic fraction of bio-oil via further cracking to hydrocarbons, gases, and more coke. The acid sites contribute to the deoxygenation of pyrolysis vapors and deactivation of the catalyst (Mortensen et al. 2011). Tuning the acid sites is important in designing the catalyst, as it not only affects the selectivity of products, but also determines the extent of coke formation.
It has been suggested that the presence of transition metals affects the mode of oxygen rejection by producing more carbon oxides and less water, which makes more hydrogen available to form hydrocarbons. Therefore, transition metal-modified zeolite catalysts (Ce-ZSM-5, Co-ZSM-5, Ga-ZSM-5, and Ni-ZSM-5) were used in biomass pyrolysis to verify whether these materials can produce higher yields of hydrocarbons and less coke (Cheng et al. 2012; French and Czernik 2010; Neumann and Hicks 2012). Recently, the highest yield of aromatics (23.2%) was achieved using gallium-substituted ZSM-5. This new catalyst boosted the hydrocarbon yield by 40% compared to pure ZSM-5 (Cheng et al. 2012). However, gallium-substituted ZSM-5 is still very expensive. More efforts should be focused on designing efficient and cheap metal-loaded catalysts for CFP of biomass.
In this work, some cheap metal-loaded (Mg, K, Fe, and Ni) ZSM-5 catalysts were synthesized for CFP of biomass. Ga-loaded ZSM-5 was synthesized for comparison. CFP of willow wood was conducted in a fluidized bed reactor using these catalysts. The catalytic characteristics of all metal-loaded catalysts on the product yields and selectivities were studied and compared with that of pure ZSM-5 catalyst. Fe/ZSM-5, which is more efficient and cheaper than Ga/ZSM-5, was utilized in this study.
EXPERIMENTAL
Materials
Willow wood was used as the biomass feedstock. The elemental composition of the willow wood (air-dry basis) was 44.05 wt.% carbon, 4.97 wt.% hydrogen, 0.73 wt.% nitrogen, and 46.77 wt.% oxygen (by difference). The willow wood’s lower heating value was 16.19 MJ/kg. Its proximate analysis (air-dry basis) was 3.56 wt.% moisture, 73.8 wt.% volatiles, 19.16 wt.% fixed carbon, and 3.48 wt.% ash. Prior to all experiments, the feedstock was cut and sieved to the range of 1.5 to 2.5 mm in diameter and 4.0 to 6.0 mm in length. The particles were then dried at 105 °C until a constant weight was achieved.
A mixture of quartz sand and catalyst was used in all the experiments. The particle size of quartz sand was from 0.05 to 0.075 mm, while that of the catalyst was from 0.075 to 0.10 mm because of its lower density. The mass ratio of sand to catalyst was 1. The total amount of the heat transfer material (sand and catalyst) in the fluidized bed was 20 g. The catalyst material (provided by Sinopec Yangzi Petrochemical Company Ltd.) was an equilibrium, commercially diluted ZSM-5 catalyst. This zeolitic catalyst was further modified with typical metals (Fe, Ni, Ga, Mg, and K) via a traditional wet impregnation method at 2 wt.% metal loading using aqueous solutions of corresponding nitrate salts. All of the metal-modified catalysts were calcined under an air atmosphere at 600 °C for 6 h. The characteristics of the diluted ZSM-5 and its metal-loaded (Mg, K, Ga, Fe and Ni) catalysts are listed in Table 1.
Table 1. Characteristics of Diluted ZSM-5 and Its Metal-loaded Catalysts
Methods
Experimental setup
A schematic diagram of the pyrolysis system used in this study is shown in Fig. 1. This reactor was refitted from the one used in previous studies (Zhang et al. 2009, 2011). It consisted of a flow control unit, temperature control unit, fluidized bed reactor, feed unit, condenser, gas purifier, accumulative flowmeter, and gas-collecting bag. The inside diameter and height of the fluidized bed reactor were 32 mm and 450 mm, respectively. A porous plate and two pieces of wire netting (200-mesh size) at the bottom of the reactor were used to support the bed materials and provide uniform distribution of the fluidizing gas.
Fig. 1. Schematic diagram of a fluidized bed system for biomass catalytic pyrolysis (1. Nitrogen; 2. Mass flowmeter; 3. Mass flow controller; 4. Gas pre-heater; 5. Temperature controller; 6. K-type thermocouple; 7. Electrical furnace; 8. Fluidized bed reactor; 9. Feed hopper; 10. Temperature controller; 11. Ceramic filter; 12. Condenser; 13. Cotton wool filter; 14. Silica gel filter; 15. Accumulative flowmeter; 16. Gas-sampling bag)
The reactor used quartz sand with a particle size of 0.6 to 0.9 mm or its mixture with catalyst as the bed material and pure nitrogen (99.999%) as the fluidizing gas. A mass flow controller was used to control the flow rate of the carrier gas. Before entering the reactor, the carrier gas was heated to 400 °C by a pre-heater. A cylindrical furnace was used to supply the heat needed in the pyrolysis reactions. The connecting pipe between reactor and condensers was maintained at about 400 °C to prevent tar condensation by the strip heater. A ceramic filter was located after the reactor to remove fine particles. Following the ceramic filter, the product vapors were passed through a condenser to collect liquid products. A cotton filter and silica gel filter were used to ensure that all of the condensable vapors were captured. The non-condensable gas was collected with a bag for analysis.
Procedure and product analysis
At the beginning of each test, the bed materials were placed in the reactor and 2 g of willow was placed in the feed hopper, which was purged with 450 mL/min N2 to guarantee a reduction atmosphere during the experiment. Next, the furnace started to heat the reactor. After the desired temperature (600 °C) in the reactor was reached, the feedstock was fed into the bed. Liquid products condensed in the condenser, and non-condensable gases were collected using a gas-collecting bag. The experiment was carried out for approximately 10 min to ensure the complete pyrolysis of willow wood. After the experiment, the furnace was turned off, and the N2 was maintained until the reactor reached room temperature to avoid the oxidation of char. The char was separated from bed materials and weighed. The condenser was cleaned using ethanol, and the washings were heated at 60 °C for ethanol evaporation. The total liquid products included the liquids collected by the condenser, the weight increases of the cotton wool and silica gel filters, and the weight of the washing evaporation residues. The liquid products were identified by gas chromatography-mass spectrometry (GC-MS; GC, 7890A, Agilent; MS, 5975C, Agilent). The bed materials were dried at 120 °C until a constant weight was reached and then combusted with air in a muffle furnace at 600 °C for 2 h. The coke output was determined by the difference in weight of the bed materials before and after combustion. The gas output was calculated by the total collected gas volume measured by the accumulative flowmeter, as well as the components and their percentages determined by GC-FID/TCD (Shimadzu 2014 GC system). The product yields were calculated by the weight of the products divided by the feedstock weight (air-dry basis). The liquid yields were obtained by difference. Each experiment was repeated three times under the same conditions to ensure its repeatability.
RESULTS AND DISCUSSION
The bio-oil, non-condensable gas, char, and coke deposits on the catalyst obtained by the fast pyrolysis with different metal-loaded catalysts are shown in Fig. 2. Compared to pure ZSM-5, all of the metal-loaded catalysts increased the gaseous products, the biomass residue (char), and the coke deposits on the catalyst at the expense of the total liquid yield. It is obvious that each catalyst seemed to affect the product yields to a different extent. The highest liquid yield (46.4 wt.%) was achieved with pure ZSM-5 catalyst. Ga/ZSM-5 produced the highest coke yield, while K/ZSM-5 produced the highest yield of bio-oil. Depending on the material, the liquid yield ranged from 40 wt.% to 43.4 wt.%. The char yield decreased, ranging from 25.4 wt.% to 33.9 wt.% while the coke increased, ranging from 3.7 wt.% for the control case to 5.8 wt.%. Gaseous products increased in the presence of all metal-loaded catalysts, ranging from 29.1 wt.% to 31.3 wt.%, compared to 24.5 wt.% with pure ZSM-5. The liquid yields and elemental analysis of organic fraction are listed in Table 2.
Fig. 2. Product yields of willow wood catalytic pyrolysis with different metal-loaded catalysts
Table 2. Yields of Liquids and Elemental Analysis of Organic Fraction (wt.%)
a by difference.
The compositions of bio-oil obtained from catalytic pyrolysis experiments with diluted ZSM-5 catalysts are shown in Fig. 3. The most representative organic compounds of the thermal bio-oil were classified into 4 major functional groups: aromatic hydrocarbons, aliphatic hydrocarbons, acetals, and others, the latter of which included phenols, furans, acids, esters, alcohols, ethers, aldehydes, ketones, nitrogen compounds, and heavier compounds. Among these, aromatic hydrocarbons and aliphatic hydro-carbons are considered to be the most desirable products for biofuel production. As can be seen from Fig. 3, Fe/ZSM-5, Ni/ZSM-5, and Ga/ZSM-5 showed good characteristics in the CFP process. Aromatic hydrocarbons proportion was more than 80% in the bio-oil with these catalysts, which is much higher than other catalysts. More detailed chemical compositions of the bio-oils are listed in Table 3. Fe/ZSM-5 and Ni/ZSM-5 showed the same catalytic characteristics as Ga/ZSM-5, but they are much cheaper than Ga/ZSM-5. CFP of biomass with K/ZSM-5 produced more acetals and fewer aromatics. Some studies have obtained similar results with catalytic pyrolysis of lignin with HZSM-5 and K/ZSM-5 (Jackson et al. 2009).
Figure 4 shows the relative content of aromatic hydrocarbons in the bio-oils of catalytic pyrolysis with different metal-loaded catalysts, principally benzene, toluene, xylenes, and naphthalene. The reported proportions of aromatic hydrocarbons are based on chromatogram peak areas. Toluene made up the largest part of the liquid products from willow wood fast pyrolysis with every ZSM-5 catalyst. Ga/ZSM-5 showed the highest toluene relative content (37.4%), while Ni/ZSM-5 showed the highest relative content of xylenes (27.3%). Fe/ZSM-5 produced the highest relative content of benzene (17.9%). The naphthalene content was lower than benzene, toluene, and xylenes. The relative contents of naphthalene were less than 5% with every catalyst.
| Fig. 3. Area percentages of different chemical groups of the bio-oils obtained from catalytic pyrolysis of willow wood with different metal-loaded catalysts | Fig. 4. Relative content of aromatic hydrocarbons in bio-oils obtained from catalytic pyrolysis of willow wood with different metal-loaded catalysts |
Table 3. Chemical Composition of Bio-oils
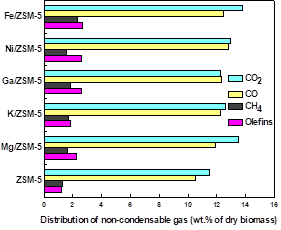
Figure 5 shows the non-condensable gas obtained by CFP of willow wood with different catalysts.
 |
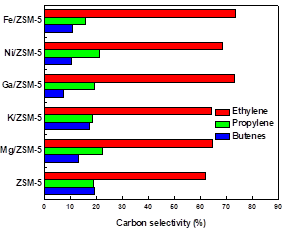 |
| Fig. 5. Yields of the main gas product components in non-condensable gas from catalytic pyrolysis of willow wood with different metal-loaded catalysts | Fig. 6. The carbon selectivities of ethylene, propylene, and butanes in olefins obtained from catalytic pyrolysis of willow wood with different metal-loaded catalysts |
The main gaseous products were CO2, CO, CH4, and C2-C4 olefins. The use of metal-loaded catalysts led to an increase in all of the gas components, especially olefins. The main aim of using catalysts is deoxygenating the bio-oil. Oxygen can be removed from the pyrolysis vapors in the form of CO2, CO, and H2O. The most effective way of deoxygenating is via CO2; the high-value hydrogen would be reserved, and one carbon could release two oxygen atoms. Fe/ZSM-5 gave the highest CO2 yield (13.8%) among the catalysts.
Olefins are desired compounds that can be used as petrochemicals. Fe/ZSM-5 also produced the highest yield of olefins (2.7%). The obtained olefins contained ethylene, propylene, and butylenes. The carbon selectivities of these products in olefins are shown in Fig. 6. Both Fe/ZSM-5 and Ga/ZSM-5 gave higher ethylene selectivity (about 73%). Fe/ZSM-5 and Ga/ZSM-5 gave the lowest selectivities of propylene and butanes, respectively.
The color of all the catalysts became darker after experiments. Besides, the metal-loaded catalysts led to the significant increase of coke yield according previous product analysis. It seems that the metal-loaded catalysts favored the deposition of polymerizing compounds on the surface of the bed materials. All of the catalysts showed similar surface appearance before and after reactions, so Fe/ZSM-5 was chosen as the example for SEM analysis.
As can be seen from Fig. 7, the fresh catalyst had surface irregularities, which are beneficial to catalytic reactions. However, the surface of the used catalyst was smoother than that of the fresh one due to coke formation. Abrasion is another important factor for the smoothening of the catalyst surface.
Fig. 7. SEM analysis of the Fe/ZSM-5 catalyst before and after the reaction
CONCLUSIONS
- Catalytic fast pyrolysis of willow wood with different typical metal-loaded ZSM-5 catalysts (Mg/ZSM-5, K/ZSM-5, Ga/ZSM-5, Fe/ZSM-5, and Ni/ZSM-5) was conducted in a fluidized bed reactor. Fe/ZSM-5 showed the same catalytic characteristics as Ga/ZSM-5 (aromatic hydrocarbon proportion in bio-oils more than 80%), but it is much cheaper than Ga/ZSM-5.
- The bio-oil yields of metal-loaded catalysts were a little lower than that of pure ZSM-5, but all of them were between 40 and 47 wt.%. CO2 is the best method of bio-oil deoxygenation. Metal-loaded catalysts produced more CO2 and CO than pure ZSM-5, especially the Fe/ZSM-5 catalyst. Fe/ZSM-5 also produced the highest yield of olefins (2.7 wt.%).
- The synthesized catalysts loaded with Ga, Fe, or Ni improved the percentages of benzene, toluene, and xylenes in bio-oils. Ga/ZSM-5 showed the highest relative content of toluene (37.4%), while Ni/ZSM-5 showed the highest relative content of xylenes (27.3%). Fe/ZSM-5 produced the highest relative content of benzene (17.9%).
ACKNOWLEDGMENTS
The authors acknowledge the financial support of the National Natural Science Foundation of China (Grant No. 51076031, 51306036), National Basic Research Program of China (973 Program) (Grant No. 2010CB732206), the Jiangsu Natural Science Foundation (Grant No. BK20130615), and the China Postdoctoral Science Foundation (Grant No. 2012M520978).
REFERENCES CITED
Bridgwater, A. V., and Peacocke, G. V. C. (2000). “Fast pyrolysis processes for biomass,” Renew. Sust. Energ. Rev. 4(1), 1-73.
Cheng, Y. T., and Huber, G. W. (2012). “Production of targeted aromatics by using Diels-Alder classes of reactions with furans and olefins over ZSM-5,” Green Chem. 14(11), 3114-3125.
Cheng, Y. T., Jae, J., Shi, J., Fan, W., and Huber, G. W. (2012). “Production of renewable aromatic compounds by catalytic fast pyrolysis of lignocellulosic biomass with bifunctional Ga/ZSM-5 catalysts,” Angew. Chem. Int. Ed. 51(6), 1387-1390.
Dobele, G., Urbanovich, I., Volpert, A., Kampars, V., and Samulis, E. (2007). “Fast pyrolysis – Effect of wood drying on the yield and properties of bio-oil,” BioResources 2(4), 699-706.
French, R., and Czernik, S. (2010). “Catalytic pyrolysis of biomass for biofuels production,” Fuel Process. Technol. 91(1), 25-32.
Huber, G. W., Iborra, S., and Corma, A. (2006). “Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering,” Chem. Rev. 106(9), 4044-4098.
Jackson, M. A., Compton, D. L., and Boateng, A. A. (2009). “Screening heterogeneous catalysts for the pyrolysis of lignin,” J. Anal. Appl. Pyrol. 85(1), 226-230.
Liu, B., Li, Y. M., Wu, S. B., Li, Y. H., Deng, S. S., and Xia, Z. L. (2013). “Pyrolysis characteristic of tobacco stem studied by Py-GC/MS, TG-FTIR, and TG-MS,” BioResources 8(1), 220-230.
Luque, R., Herrero-Davila, L., Campelo, J. M., Clark, J. H., Hidalgo, J. M., Luna, D., Marinas, J. M., and Romero, A. A. (2008). “Biofuels: A technological perspective,” Energ. Environ. Sci. 1(5), 542-564.
Mortensen, P. M., Grunwaldt, J. D., Jensen, P. A., Knudsen, K. G., and Jensen, A. D. (2011). “A review of catalytic upgrading of bio-oil to engine fuels,” Appl. Catal. A-Gen. 407(1-2), 1-19.
Neumann, G. T., and Hicks, J. C. (2012). “Effects of cerium and aluminum in cerium-containing hierarchical HZSM-5 catalysts for biomass upgrading,” Top. Catal. 55(3-4), 196-208.
Zhang, H. Y., Carlson, T. R., Xiao, R., and Huber, G. W. (2012). “Catalytic fast pyrolysis of wood and alcohol mixtures in a fluidized bed reactor,” Green Chem. 14(1), 98-110.
Zhang, H. Y., Xiao, R., Huang, H., and Xiao, G. (2009). “Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor,” Bioresour. Technol. 100(3), 1428-1434.
Zhang, H. Y., Xiao, R., Wang, D. H., He, G. Y., Shao, S. S., Zhang, J. B., and Zhong, Z. P. (2011). “Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2atmospheres,” Bioresour. Technol. 102(5), 4258-4264.
Zhao, Y., Fu, Y., and Guo, Q. X. (2012). “Production of aromatic hydrocarbons through catalytic pyrolysis of gamma-valerolactone from biomass,” Bioresour. Technol. 114, 740-744.
Article submitted: June 30, 2013; Peer review completed: August 27, 2013; Revised version received: Sept. 13, 2013; Accepted: Sept. 14, 2013; Published: September 20, 2013.
