Abstract
Pressing beech veneers at high temperatures has been shown to be a reliable method for manufacturing laminated boards without adhesives. The reasons behind the self-bonding phenomenon as well as the causes of the waterproof character gained by the boards being pressed at 250 °C were investigated. Water leachates from the dried and the hot-pressed veneers were analysed by UV-spectroscopy, high-performance liquid chromatography (HPLC), and solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance (CP/MAS 13C NMR). Press-plate temperatures during hot pressing were 200, 225, and 250 °C. After pressing, an increased content of 5-(hydroxymethyl)furfural (not at 250 °C) and conjugated phenols was observed in the bonding lines (interfaces) compared to the inner part of veneers of the self-bonded boards. Furfural contents were low and relatively similar, but 5-(hydroxymethyl)furfural (HMF) showed an abrupt decrease in the bonding line when the temperature increased from 200 °C to 225 °C and especially to 250 °C. The contribution of caramelization to browning and bonding is suggested. In studies with CP/MAS 13C NMR, a higher content of phenolic units in beech lignin was observed during hot pressing at 225 °C. Homolytical cleavage of b-O-4 structures in lignin as well as the condensation reactions involved are discussed.
Download PDF
Full Article
Changes in Content of Furfurals and Phenols in Self-Bonded Laminated Boards
Carmen Cristescu,a and Olov Karlsson b,*
Pressing beech veneers at high temperatures has been shown to be a reliable method for manufacturing laminated boards without adhesives. The reasons behind the self-bonding phenomenon as well as the causes of the waterproof character gained by the boards being pressed at 250 oC were investigated. Water leachates from the dried and the hot-pressed veneers were analysed by UV-spectroscopy, high-performance liquid chromatography (HPLC), and solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance (CP/MAS 13C NMR). Press-plate temperatures during hot pressing were 200, 225, and 250 ⁰C. After pressing, an increased content of 5-(hydroxymethyl)furfural (not at 250 ⁰C) and conjugated phenols was observed in the bonding lines (interfaces) compared to the inner part of veneers of the self-bonded boards. Furfural contents were low and relatively similar, but 5-(hydroxymethyl)furfural (HMF) showed an abrupt decrease in the bonding line when the temperature increased from 200 ⁰C to 225 ⁰C and especially to 250 ⁰C. The contribution of caramelization to browning and bonding is suggested. In studies with CP/MAS 13C NMR, a higher content of phenolic units in beech lignin was observed during hot pressing at 225 ⁰C. Homolytical cleavage of -O-4 structures in lignin as well as the condensation reactions involved are discussed.
Keywords: Self-bonding; Heat treatment; Beech; Veneers; Furfurals; Phenols; HPLC; UV-spectroscopy; CP/MAS 13C NMR; Binderless
Contact information: a: Luleå University of Technology, TVM, Div. of Wood Science and Engineering, Wood Technology, LTU Skellefteå,SE-93187, Sweden; b: Luleå University of Technology, TVM, Div. of Wood Science and Engineering, Wood Physics, LTU Skellefteå, SE-93187, Sweden;
Corresponding authors: carmen@ltu.se; olov.karlsson@ltu.se
INTRODUCTION
Self-bonding in wooden products has become of great interest ever since Masonite started to produce panel boards (Mason 1928). The reason suggested by Mason for self-adhesion was that the coating of lignins on the wood fibres had softened, and, when cooled, had effectively welded the wood fibres together into a solid mass (Moore 1961). Self-bonding of beech veneers was mentioned by Runkel and Jost (1948). They used a gastight press, since they believed that the volatile products (condensable and permanent gases) formed during the first stage of the gastight pressing would further hydrolyse the wood constituents, resulting in the decomposition of the lignin-carbohydrate compound. The more recent welding of solid wood can also be considered to be a self-bonding process, where friction has a decisive role (Suthoff et al. 1996). Similar to the Masonite hypothesis, the melting of middle lamella lignin is considered to be one of the main causes of the bonding (Pizzi et al. 2003). The influence of the oxidative activation of wood particles and fibres before hot pressing into boards without the addition of adhesive has been studied (Stofko 1974; Westermark and Karlsson 2002; Widsten 2002). Here, the formation of water-soluble compounds during oxidative treatment and reaction with wood components during hot pressing was suggested to be a reason for the low swelling of the boards in water (Westermark and Karlsson 2003).
Lately, self-bonding of lignocellulosic material has gained increased attention from researchers. The materials studied have varied from kenaf (Okuda et al. 2006) to oil palm biomass (Hashim et al. 2011) and bagasse (Nonaka et al. 2013). Ando and Sato (2009) used kenaf core powder to bond plywood that had a shear strength in dry conditions less than 1 MPa.
The possibility of producing laminated boards without adhesives or an intermediate lignocellulosic material from rotary-cut, dry beech veneers in a heated open press was recently discovered (Cristescu 2006). Not only were the boards compact and self-bonded without using any additives or binders, but they had a dry shear strength above 3 MPa for boards pressed at 225 °C and they were also water-resistant when the press-plate temperature was 250 oC (Cristescu 2008). At this press-plate temperature, the boards were darker, harder, and more brittle than at lower pressing temperatures. Furthermore, unpublished results indicated that the higher the temperature used when manufacturing them, the more resistant to fungal attack the boards became.
Apart from durability, other physical properties of our boards, such as colouring, hardness, and water resistance, similar to those of thermally treated wood, had been noticed during the experiments. The characteristics especially resemble torrefied wood (Bourgois et al. 1988). The explanations rest on the fact that the process conditions show similarities: the temperature is in the same range, above 220 °C, and the moisture content at the beginning of the process is similar (12% for torrefied wood, 9% for the boards).
According to observations during the experiment, when hot-pressing the beech veneers they developed a darker bond-line (Fig. 1 bottom). It is assumed that drying the veneers at 140 °C after cutting them from steamed logs would lead to the migration and accumulation of sugars at veneer surfaces (Theander et al. 1993). Hot-pressing veneers with deposits of soluble sugars could cause not only the formation of volatile compounds but also the formation of browning and bonding products through caramelization (Sehlstedt-Persson 1995) among other possible chemical reactions.
It was of interest to study whether the high water-resistance of boards obtained at 250 °C could be related to chemical changes during hot pressing. In this paper we will present results on the presence of water-soluble furans, water-soluble unconjugated and conjugated phenolic compounds, as well as lignin-like compounds, during hot pressing of beech veneers (Fagus sylvatica) at various temperatures. For that purpose, high performance liquid chromatography (HPLC), ultraviolet-spectroscopy (UV), and nuclear magnetic resonance (CP/MAS 13C-NMR) were used. Special interest was directed to studying the structure of the areas that are darker in colour (bond-line) than the inner part of the hot-pressed veneer.
EXPERIMENTAL
Board Manufacturing
Rotary-cut veneer sheets of beech (Fagus sylvatica), 2.2 mm thick were provided from Brasov, Romania. Prior to cutting, the logs were plasticized by heating with steam at 80 oC, then rotary-cut at 40 to 50 oC, followed by drying at 140 to 170 °C to reach a moisture content (MC) of between 7 and 10%.
Five layers of veneer with dimensions of 140 mm x 140 mm x 2 mm and 9% MC were overlaid in parallel grain direction and placed in a Fjällman laboratory press. Three pressings in total were run at three different temperature levels: 200, 225, and 250 °C. The pressure used was 5 MPa, and the pressing time was 300 s for all three pressings. The pressing time was counted precisely from the moment when the press plates were used to apply the chosen pressure to the veneer pack.
Veneers before and after pressing at 250 °C, 5 MPa, and 300 s are presented as raw material (top) and board (bottom) in Fig. 1:
Fig. 1. Beech veneers before pressing (top) and after pressing (bottom) for 300 s at 250 ⁰C, 5 MPa
The boards were allowed to cool at ambient parameters of temperature and air humidity and left in our laboratory room until further analysis.
Filtration of Wood Particles
Furfurals and monosaccharides in boards
The surfaces of the veneer that was not pressed (used as a reference) were shaved by hand with a very sharp blade into small particles. The veneers within the hot-pressed board were delaminated with a sharp knife. Pieces taken from the inner part of the veneers in the core of hot-pressed boards and from their bond-lines (see Fig. 2) were shaved by hand into small particles and placed in a sealed container to avoid the release of volatile compounds.
The bond-line was shown to be denser (Cristescu 2006) and darker (Cristescu 2008), as shown in Fig. 2. However, it must be pointed out that a complete separation of the bond-line and inner part of veneer could not be achieved in this way, as the bond-line was too thin and entangled with the inner veneer. This means that chemical differences between the bond-line and inner part of veneer could be even larger than indicated in our paper.
According to the temperatures recorded by thermocouples within veneers, all four bonding lines from each samples reached the press plate temperature before 270 s. According to our previous unpublished studies, the core bonding-line was the weakest when soaked in water and when tested to shear strength. Therefore we decided to focus on bonding of core layers only in this study. Temperature curves of each layer will be the subject to another article. A quantity of 0.50 g of particles from each sample was mixed in 30 mL of water, treated in an ultrasonic bath for one hour, and then left under magnetic stirring overnight in a closed vessel. The mixture was filtered and the mother liquor collected.
Descriptions of the provenance of the particles (veneer or bond-line) and the pressing temperature used when manufacturing the board are given in Table 1 together with the abbreviations.
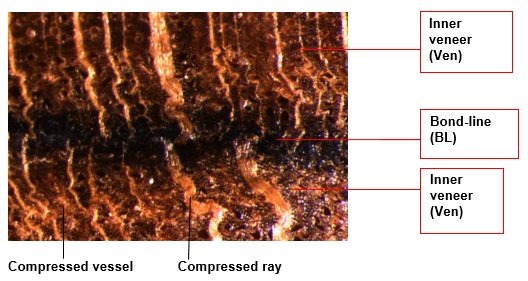
Fig. 2. Image of a bond-line in a laminated board pressed at 250 ⁰C, 5 MPa for 300 s. Light microscopy, 30X magnification
Table 1. Numbering and Abbreviations of the Samples
Monosaccharides in veneers before pressing
An isolation of veneer surface and veneer bulk before pressing was performed according to an analogue procedure. From a veneer sheet, first the darker surface part of the veneer was shaved off with a sharp knife into small particles and then the brighter inner veneer was shaved into small particles
HPLC (High-Performance Liquid Chromatography) Analysis
The filtered water solution (50 μm) was analysed with an HPLC device equipped with a Water Hi-plex Pb-column (8 μm and 250 x 7.7 mm) at 60 °C using water as the eluent at a flow of 0.3 mL/min.
Furfural and 5-hydroxymethylfurfural HMF were detected with a UV-detector operating at 280 nm and a refractive index-detector. A Varian Prostar autosampler (model 410), equipped with a 10 μL loop, was used, and quantitative estimations were made by comparison with calibration curves of the individual compounds.
A separate HPLC analysis of monosaccharides content in surface of veneers and bulk of veneers before pressing was performed in the same conditions.
UV Analysis
The UV-absorption of the water leachates were recorded with a Hitachi UV/VIS U-1500 spectrophotometer. Water-soluble phenols were analysed using the Δε method (Aulin-Erdtman 1953; Wexler 1964). Samples diluted in water were analysed in a 1 cm wide cuvette at a range of 200 to 600 nm. The pH of the samples was adjusted to pH 12 with a strong alkaline solution, and samples were analysed in the same way. The differ-rences between the absorptions in water extracts and the alkali-treated samples were calculated.
CP/MAS 13C NMR
The NMR tests were performed at Umeå University, Sweden. Three groups of samples were ground prior to high resolution spectroscopic analysis. Fine particles were shaved with a sharp blade by hand from a raw material (veneer), inner veneer, and the bond-line of boards was pressed at 250 °C and 5 MPa for 300s. The NMR procedure was adapted following Wikberg (2004) and Wikberg and Maunu (2004).
CP/MAS 13C NMR measurements were taken with a Varian/Chemagnetics 400 MHz spectrometer operating at 100.6 MHz for carbon. For all the wood samples, the spinning speed was 8000 Hz, contact time 2 ms, acquisition time 5.12 ms, and delay between pulses 1.5 s.
In the dipolar dephasing (DD) measurements, the high-powered decoupler was turned off for 80 μs before data acquisition.
RESULTS AND DISCUSSION
Sample Preparation
Four darker horizontal lines corresponding to the bonding lines, as shown in Fig. 1, were clearly visible in the hot-pressed board. A light microscopy image of the bond-line together with the adjacent ‘inner veneer’ of a board pressed at 250 °C is shown in Fig. 2. Although it was a compact board that came out of the press, each veneer can be considered as a board itself subjected to hot-pressing. The images of the wood particles after filtration (Fig. 3) and of the filtered solution (Fig. 4) show that the darkening in the wood particle was not directly correlated to the colour of the filtered solution. This observation will be discussed below.
Fig. 3. Wood particles from veneers and bond-lines after leaching with water. The numbering corresponds to that in Table 1.
Fig. 4. Filtered solutions from leaching of veneers and bond-lines with water. The numbering corresponds to that in Table 1.
HPLC (High Pressure Liquid Chromatography) Analysis
Water leachates from hot-pressed veneers, bond-lines, and veneers that were not pressed were analysed with HPLC. The content of 5-(hydroxymethyl)furfural (HMF) and furfural in veneers and bond lines were calculated, the results are presented in Table 2, and the corresponding chart is presented in Fig. 5.
Table 2. Content of 5-(Hydroxymethyl)furfural (HMF), Furfural, Glucose, and Fructose from Water Leaching of Hot-Pressed Veneers, Bond-lines, and Veneers that Were Not Pressed
Fig. 5. Content of HMF and furfural in dry wood (%) in water extracts from veneers and bond lines according to HPLC measurement
As can be seen in Fig. 5 and Table 2, the HMF content was found to be greater than that of furfural up to the press-plate temperature of 225 °C. The largest amount of HMF was found in the bond-line in a board that had been pressed at 200 °C, and the amount decreased with increasing press temperatures. A decrease is also indicated in the inner parts of the pressed veneer, but the amount of HMF found was considerably smaller than from the bond-line, except at high pressing temperatures (Fig. 5).
HMF is mainly formed from carbohydrates of the hexose type. A relatively high content of glucose and fructose was found in material from the surfaces of veneers that had not been hot pressed, but these monosaccharides were absent in the boards after hot pressing (Table 2). Therefore, it is suggested that the main sources of formation of HMF are low-molecular-weight sugars.
The higher content of HMF in bond-line compared to inner veneer could be explained by the migration of the monosaccharides to the surface of veneer during drying (as a previous stage in the preparation of the raw material). That is why it was decided to separately check the content of monosaccharides of the veneer before pressing at the surface and in the bulk of the veneer as well.
The results of the HPLC test, which compared the content of monosugars from veneer surface and veneer bulk, showed that there was approximately 3 times more glucose and fructose at the veneer surface (compared to bulk) already before pressing. A similar result was reported by Hiltunen et al. (2008) when comparing the sugar content of light-coloured inner part to the discoloured (darkened) surface of European white birch after vacuum drying: sugars (mainly glucose and fructose) were 2.7 more concentrated at the surface layer. It is interesting to notice that the ratio between HMF at BL200C and Ven200C was 2.77, a value close to 3. The HMF ratio reached 2.33 at 225 °C and fell to less than 1 at 250 °C press temperature (Table 2, Fig. 5). As the ratio between HMF content in bonding-line and veneer bulk decreased with heating temperature and as the quantity of HMF decreased with temperature above 200 °C, it is likely that the formed HMF reacted further or evaporated at the higher press temperatures. Although colour measurements were not performed during this study, it is obvious that the colour intensity of the filtered solution (Fig. 4) relates to the content of HMF (Fig. 5) while the colour of wooden particles does not. The wood gets darker while the HMF content in the wood decreases (see Fig. 3 and Fig. 5), and this observation supports the hypothesis that HMF might participate into forming new brown bonding polymers.
Caramelization, browning, and HMF formation are of high interest in food research, for which HMF is actually used as a colour indicator (Kroh 1994; Ramirez- Jimenez et al. 2000). Relevant results about HMF levels were obtained by Ait Ameur et al.(2006) when baking cookies above 200 °C; some conditions were similar to ours: low water content, high temperature, and the presence of sugars, while other conditions were different: an absence of pentoses, lignins, and extractives. Ait Ameur et al. (2008) noticed significantly more HMF than furfural when baking below 250 °C followed by a decrease in HMF in the matrix and a simultaneous increase of HMF in the baking vapours at 300 °C. Those results indicated that part of the HMF formed could be involved in the formation of brown polymers in the cookie matrix, with furfural as one possible degradation product of HMF (Ait Ameur et al. 2008).
In our study furfural content was considerably lower than HMF for 200 °C and 225 °C (Fig. 5). The presence of furfural was similar or increased somewhat with increased treatment temperature. Furfural could be formed from hexoses or from HMF, but it is more likely that it originates from pentoses, such as xylan, as a similar decrease observed in HMF content was not found for furfural (Fig. 5). Since hardwoods (especially beech) have a high content of xylan (Beyer et al. 2006) it is intriguing to find such low levels of furfural in the veneers and bond-lines. Our results are supported in the literature. For instance, Okuda et al. (2006) reported that little furfural was generated from hemicellulose during pressing of kenaf boards without steam-explosion pretreat-ment. It is difficult to estimate how much furfural participates in the colour formation, as it evaporates and oxidises very fast.
UV-spectrographic Analysis
UV-absorption at 280 nm
Water leachates analysed by UV-spectroscopy are presented in Table 3. It can be seen that the absorption at 280 nm of water leachates from inner veneers at 200, 225, and 250 °C (Ven200, Ven225, Ven250) were fairly similar and somewhat higher than in the untreated material and in the bonding line at 250 °C (BL250).
A larger UV-absorption in extracted material from the bond-line at 225 °C and an even larger UV-absorption from the bond-line at 200 °C were observed (Table 3). Those data correspond well to the amount of HMF determined by HPLC (Fig. 5). It can be seen in Table 3 that considerable parts of UV absorption were related to HMF but that also UV-absorptions from other types of compounds contributed, such as phenolic substances (see below). It is especially interesting to note that a lower absorption at 280 nm could be found in the bond-lines at higher press-plate temperatures (Table 3). As it was discussed above, it is unlikely that the initial formation of furfurals was lower at the higher temperature. However, with higher temperature, evaporation as well as further reactions with furfurals, such as degradation or condensation, might occur. In Fig. 5 the amount of furfurals at BL250 seems to be the lowest among the treated wood, but in Table 3, BL250 is shown as having a higher absorbance compared to all inner veneers. This difference between the two methods might be caused by other compounds than furfurals, for example, phenols, as indicated by the difference in UV-spectroscopy (see below). It could also be caused by new products of degradation and condensation. Condensation between furans and phenols has been suggested to occur during pressing kenaf into binderless boards using steam injection (Widyorini et al. 2005).
Table 3. UV-Absorption at 280 nm, Different UV-Absorption at 250, and Content of Phenols (Δε at 250 nm) in Water Extracts from Pressed and Not-pressed Veneers as well as from Corresponding Bond-lines
- Extinction coefficient of 210 L*g-1*cm-1 (Singh et al. 1948).
- Extinction coefficient of 15 L*g-1*cm-1 (Wexler 1964).
Content of water-soluble phenols (Δε-curve at 250 nm)
In Fig. 6, the difference UV-spectra (Δε-curve) are presented, wherein the 250 nm peak corresponds to the total amount of phenolic compounds (Wexler 1964). The curves are presented at similar dilution level, or they could not be compared in the figure. An absorption coefficient of 15 mL/(mg*cm) (Wexler 1964) corresponds to a content of water-soluble phenols in the reference veneer of ca. 2.3 % (Table 3). The content of water soluble phenols in beechwood depends on the position in the tree, and phenolic contents from 2 to 12 mmol/100 g wood has been reported when using the Folin-Ciocalteu (FC) method (Albert et al. 2003; Vek and Oven 2010). All three samples from the bond-line had higher phenolic content than the corresponding veneers (Fig. 6). The concentration of water-soluble phenols in the hot-pressed veneers was somewhat lower than in the reference veneer which suggests that migration of phenols during drying was rather slow. Material from the bond-line at 200 oC seemed to have the highest content of water-soluble phenols. This indicates that phenols react further, especially at higher press temperatures.
Fig. 6. Δε-curve (difference between UV-absorption in neutral and alkaline solutions) of water-soluble compounds in veneers and bond-lines (similar dilution level)
Differences in UV-absorptions at higher wavelengths could be related to the structure of the phenols. However, even though the absorption coefficient is unknown for such compounds, we believe that useful information could still be gained by comparison of such absorptions. Absorption around 300 nm corresponds to unconjugated phenols (Lin 1992), such as those found in tannins (Kubel et al. 1988; Mämmelä 2001; Zule and Može 2003), lignans, or simpler phenols, such as syringol. In green silver birch, lignans can be found but at a low content (Hiltunen et al. 2006). Significant amounts of lignans: syringaresinol, less medioresinol, and pinoresinol, have been found in extracts from thermally modified hardwoods (Peters et al. 2008). However, the present results showed a trend of decreasing absorptions at 300 nm with increased temperature within the veneer as well as for the bond-line, indicating that water-soluble unconjugated phenols decreased during heat treatment (Fig. 6). Thus, this type of structure seems to react further when the treatment temperature rises during hot pressing of the boards. Furthermore, our data indicated that the unconjugated phenols were more abundant in the bond-line than in the inner veneer under all three temperature conditions, probably due to migration during drying of veneers.
For the untreated veneer as well as for material from the bonding line, formed during hot pressing at 200 °C, absorption at 350 nm was also obtained (Fig. 6). Absorp-tions around 350 nm in the Δε-curve can be due to the presence of phenols conjugated with an -carbonyl group, such as syringaldehyde and vanillin (Aulin-Erdtman 1953).
Absorptions at higher wavelengths than 350 nm were obtained at higher treatment temperatures (Fig. 6). These peaks describe phenols that are members of more extended conjugated systems, as found at 421 nm in sinapyl aldehyde and at 414 nm in conifer-aldehyde (Goldschmid 1971; Wexler 1964). In vacuum-dried European white birch, even at the surface layer, the content of sinapyl aldehyde, coniferaldehyde, and syringaldehyde is very low (Hiltunen et al. 2008). Since UV-absorptions at wavelengths higher than 350 nm were not found in the untreated material but in material hot-pressed at 225 °C or higher, it is likely that conjugated phenols such as sinapyl aldehyde and coniferaldehyde were formed and they became more prominent at higher temperatures (Fig. 6). It could be added that coniferaldehyde and sinapyl aldehyde were found in water extracts from the thermal modification of hardwoods, including beech (Peters et al. 2008).
Absorptions when approaching visible parts of the spectrum in Fig. 6 can be seen in bond-line at 225 °C and to a greater extent at 250 °C. This indicates that conjugated phenols are also involved in the formation of colour of compounds from the water leachates.
Taher and Cates (1974) concluded that the yellow colour that accompanies the degradation of sugars (D-glucose) into furans is due to the formation of unsaturated dicarbonyl compounds through oxidation.
CP/MAS 13C-NMR
Studies of chemical changes in wood during hot pressing at 225 °C were performed with CP/MAS 13C NMR using similar conditions as described by Wikberg and Maunu (2004). The NMR spectra of the samples are presented in Fig. 7 and those based on the use of DD in Fig. 8. As can be seen, the DD resulted in the removal of signals from the carbohydrates (in the range from 60 ppm to 105 ppm), and thus the spectra in Fig. 8 show mainly changes related to lignins.
Fig. 7. CP/MAS 13C NMR spectra of beech: BL225 in black, Ven225 in red, and Ref in green
Fig. 8. CP/MAS 13C NMR, DD=80 dipolar dephasing: BL225 in black, Ven225 in red, and Ref in green
The peak at 56 ppm corresponds to the aryl methoxyl groups in lignin (Lin 1992); Wikberg and Maunu 2004). The signal is larger for the bonding line in both spectra than for the treated veneer (Figs. 7 and 8). The methoxyl content per phenyl-propane unit has been reported to be 1.7 for beech, which indicates that the ratio of syringyl and guaiacyl propane units is close to 1 (Akiyama et al. 2005). The methoxylation of lignin is unlikely to have taken place, and a possible explanation could be that lignin degrades under heat and pressure and its byproducts migrate to the bond-line. In that case, syringyl propane units are a more likely candidate than guaiacyl propane units, as the former have been reported to be more reactive than the latter (Rousset et al. 2009).
In Fig. 9, annotations of carbons in phenylpropane units of lignin are shown.
Fig. 9. The monomeric units used in formation of lignin: a) trans-p-coumaryl alcohol, b) trans-coniferyl alcohol, representing guaiacyl (G) unit, and c) trans-sinapyl alcohol, representing syringyl (S) unit (following Wikberg 2004)
According to studies by Wikberg and Maunu (2004), the signal at 148 ppm in Figs. 7 and 8 is assigned to aromatic carbons C-3 and C-5 of phenolic S units as shown in Fig. 9c. Furthermore, carbons C-3 and C-4 in phenolic G units (Fig. 9b), as well as in etherified G-units also contribute to the absorption at 148 ppm. As it can be seen in Figs. 7 and 8, the signal at 148 ppm was low in untreated veneers but increased during hot pressing of veneers at 225 °C to a fairly considerable extent. From these data we suggest that an increase in phenolic syringyl propane units in lignin takes place during hot pressing.
One possibility for the formation of phenols is via homolytic cleavage of phenolic -ethers in arylglycerol -aryl ethers in lignin. It could be seen in Fig. 6 that water-soluble phenols with an extended conjugated system, such as that found in sinapyl aldehyde and coniferaldehyde, increased with increased treatment temperatures. Sinapyl aldehyde and coniferaldehyde have been found in studies on heat treatment of model compounds of -ether type, and a radical exchange reaction was proposed to explain their formation (Li et al. 2000). The signal at 153 ppm in Figs. 7 and 8 is assigned to C-3 and C-5 of S units that are etherified at C-4 (Wikberg and Maunu 2004). The signal at 136 ppm is assigned to C-1 and C-4 of S and G units that are etherified at C-4 (Wikberg 2004).
Only small changes could be seen for the signals at 153 ppm and 136 ppm in Fig. 8, and cleavage of -ether bonds seems not to be extensive at hot pressing at 225 °C. However, it should be noted that the relaxation time and thus intensity of absorptions in the NMR spectrum can differ for lignin fragments; the increase in the relaxation times of decayed relative to non-decayed wood samples was attributed to an increase in the mobility of the molecular components of the cell wall and of the water in the cell walls (Wikberg and Maunu 2004)
Furthermore, a larger signal at 153 ppm was observed for material from the bonding line than in hot-pressed veneer (Fig. 8). This is in agreement with the assumption made above that lignin fragments are enriched in the bond-line during the hot pressing treatment. In such a case, the fragments seem to also contain etherified lignin units.
Moreover, Li et al. (2000) found that not only radical exchange took place, but also recombination of radicals and formation of carbon-carbon bonds of , -5, and -1 during the heat treatment of lignin model compounds of -ether type. It was observed in the present work that water stability of the bond-line (measured as the delamination rate when samples were soaked in room temperature water for at least 48 h) increased with the pressing temperature. Formation of stable chemical bonds of the mentioned type could be an explanation for this behaviour.
The peak at 128 ppm has been proposed to be related to condensation products, such as biphenyl (5–5) and diphenylmethane structures, caused by thermal modification (Wikberg 2004). This peak seemed to be more intense for the bonding line in Fig. 8 than for the veneer. However, to answer if this is related to higher lignin content or a higher extent of condensed lignin units, further study is needed.
Finally, the signals at 21 ppm and 173 ppm in Fig. 7 are considered to correspond to methyl (CH3) and carboxylic carbons (C=0), respectively, in acetyl groups (COCH3) attached to xylan in beech wood (Wikberg and Maunu 2004). According to Gilardi et al. (1995) some of the intensity at 174 ppm could also be due to uronic acid groups in xylan and pectins. Figure 7 shows the differences in intensity of signals at 21 ppm and 173 to 174 ppm in untreated and hot-pressed veneer were relatively weak, reflecting low activity of the acetyl groups of hemicellulose during hot pressing under dry conditions. This is supported by the observation that the pH of water leachates from the hot-pressed boards was close to neutral conditions.
CONCLUSIONS
- Data showed differences in chemical composition of the bond-line and the inner part of veneers in a self-bonded laminated board. The temperature of the pressing plate was also found to have a large influence on the chemical composition of the boards.
- From the relatively high concentration of glucose and fructose found in the surface of dried veneers, we concluded and confirmed that monosaccharides had migrated towards veneer surfaces during veneer drying. The monosaccharides in the untreated veneer were transformed into dehydration products, such as furfurals, when heated. The content of HMF was higher in the bond-line than in the veneer (200 and 225 °C).
- HMF content peaked at the highest value in both inner veneers and bonding lines of boards pressed at 200 °C, but it gradually decreased in boards pressed at 225 and 250 °C. This means that the presence of HMF in the material is related to a complex process that might include caramelization. HMF might contribute to newly formed products involved in browning.
- From studies with CP/MAS 13C-NMR it was found that the content of lignin containing β-ether structures and methoxyl groups was higher in the bond-line than in the pressed veneer. Migration of the degraded lignin towards the bonding line, where a condensation reaction might occur especially at 250 °C, is thus likely, and such a process will increase the water-resistance property acquired by boards pressed at this temperature due to the higher hydrophobicity of lignin than wood.
- Conjugated water-soluble phenols were formed, particularly at high temperatures, in both the bonding line and the inner veneer. Formation of such compounds indicates the occurrence of homolytical cleavage of phenolic β-ether structures and thereby also condensation reactions that might contribute to the bonding process, making a developing bond-line more water resistant. Formation of such structures could not be confirmed in this paper and needs further studies.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the ‘SkeWood’ programme at Luleå University of Technology. Greger Orädd is gratefully acknowledged for running the NMR analysis.
REFERENCES CITED
Ait Ameur, L., Trystram, G., and Birlouez-Aragon, I. (2006). “Accumulation of 5-hydroxymethyl-2-furfural in cookies during the baking process: Validation of an extraction method,” Food Chem 98, 790–796.
Ait Ameur, L., Rega, B., Giampaoli, P., Trystram, G., and Birlouez-Aragon, I. (2008). “The fate of furfurals and other volatile markers during the baking process of a model cookie,” Food Chem. 111, 758-763.
Akiyama, T., Goto, H., Nawawi, D. S., Syafii, W., Matsumoto, Y., and Meshitsuka, G. (2005). “Erythro/threo ratio of β-O-4-5 structures as an important structural characteristic of lignin. Part 4: Variation in the erythro/threo ratio in softwood and hardwood lignins and its relation to syringyl/guaiacyl ratio,” Holzforschung 59(3), 276-281.
Albert, L., Hofmann, T., Németh, Zs.l., Rétfalvi, T., Koloszár, J., Varga, Sz., and Csepregi, I. (2003). “Radial variation of total phenol content in beech (Fagus sylvatica L.) wood with and without red heartwood,” Holz Roh Werkstoff 61, 227-230.
Ando, M., and Sato, M. (2009). “Manufacture of plywood bonded with kenaf core powder,” J. Wood Sci. 55, 283-288.
Aulin-Erdtman, G. (1953). “Spectrographic contributions to lignin chemistry. III. Investigations on model compounds,” Svensk Papperstidn. 56(3), 91-101.
Beyer, M., Koch, H, and Fischer, K. (2006). “Role of hemicelluloses in the formation of chromophores during heat treatment of bleached chemical pulps,” Macromol Symp. 232, 98-106
Bourgois, J., and Guyonnet, R. (1988). “Characterisation and analysis of torrefied wood,” Wood Sci. Technol. 22, 143-155.
Cristescu, C. (2006). “Bonding laminated veneers with heat and pressure only,” In: Proceedings of the 2nd International Conference on Environmentally-Compatible Forest Products “Ecowood”, Porto, Portugal, pp. 339-348.
Cristescu, C. (2008). “Bonding veneers using only heat and pressure. Focus on bending and shear strength,” Licentiate thesis, Luleå University of Technology, 2008: 43.
Gilardi, G., Abis, L., and Cass, A. E. G. (1995). “Carbon-13 CP/MAS solid-state NMR and FT-IR spectroscopy of wood cell wall biodegradation,” Enzyme Microb. Technol. 17, 267.
Goldschmid, O. (1971). “Ultraviolet spectra,” In: Lignins. Occurrence, Formation, Structure, and Reactions; Sarkanen, K. V., and Ludwig, C. H. (eds.), Wiley-Interscience, New York, London, Sydney, Toronto, 241-266.
Hashim, R., Nadhari, W.N.A.W., Sulaiman, O., Kawamura, F., Hiziroglu, S., Sato, M., Sugimoto, T., Seng, T. G., and Tanaka, R. (2011). “Characterization of raw materials and manufactured binderless particleboard from oil palm biomass,” Mater. Design 32(1), 246-254.
Hiltunen, E., Pakkanen, T. T., and Alvila, L. (2006). “Phenolic compounds in silver birch (Betula pendula Roth) wood,” Holzforschung 60, 519-527.
Hiltunen, E., Mononen, K., Alvila, L., and Pakkanen, T. T. (2008). “Discolouration of birch wood: Analysis of extractives from discoloured surface of vacuum-dried European white birch (Betula pubescens) board,” Wood Sci. Technol. 42, 103-115.
Kroh, L. W. (1994). “Caramelisation in food and beverages,” Food Chem. 4, 373-379.
Kubel, H., Weissmann, G., and Lange, W. (1988). “Untersuchungen zur Cancerogenität von Holzstaub. Die Extraktstoffe von Buche und Fichte,” Holz Roh Werkstoff 46, 215-220.
Li, S., Lundquist, K., and Westermark, U. (2000). “Cleavage of arylglycerol -aryl ethers under neutral and acid conditions,” Nord. Pulp Paper Res. 4, 292-299.
Lin, S. Y. (1992). “Ultraviolet spectroscopy,” In: Methods in Lignin Chemistry; Lin, S. Y., and Dence, C. V. (eds.), Springer-Verlag, 217-232.
Mason, W. (1928). “Hard grainless fiber products and process of making same,” Patent US1663505.
Moore, J. H. (1961). “Exploded wood,” J. South. Hist. 27(2), 169.
Mämmelä, P. (2001). “Phenolics in selected European hardwood species by liquid chromatography–electrospray ionization mass spectrometry,” Analyst 126, 1535-1538.
Nonaka, S., Umemura, K., and Kawai, S. (2013). “Characterization of bagasse binderless particleboard manufactured in high-temperature range,” J. Wood Sci.59, 50-56.
Okuda, N., Hori, K., and Sato, M. (2006). “Chemical changes of kenaf core binderless boards during hot pressing (I): Influence of the pressing temperature condition,” J. Wood Sci. 52, 244-248.
Peters, J., Fischer, K., and Fischer, S. (2008). “Characterization of emissions of thermally modified wood and their reduction by chemical treatment,” BioResources 3(2), 491-502.
Pizzi, A., Properzi, M., Leban, J. M., Zanetti, M., and Pichelin, F. (2003). “Mechanically-induced wood welding,” Maderas. Cienca y Tecnologia 5(2), 101-106.
Ramírez-Jiménez, A., Guerra-Hernández, E., and García-Villanova, B. (2000). “Browning indicators in bread,” J. Agric. Food Chem. 48(9), 4176-4181.
Rousset, P., Lapierre, C., Pollet, B., Quirino, W., and Perre, P. (2009). “Effect of severe thermal treatment on spruce and beech wood lignins,” Ann. For. Sci. 66(110), 1-8.
Runkel, R., and Jost, J. (1948). “Verfahren und Vorrichtung zur Herstellung von Formungen aus Holz, Holzabfällen oder verholzten Pflanzenteilen unter Druck, bei höheren Temperaturen. (Method and apparatus for the production of mouldings from wood, wood waste, or vegetable origin under fibers under pressure at elevated temperatures),” Patent DE841055.
Sehlstedt-Persson, M. (1995). “High temperature drying of Scots pine. A comparison between HT- and LT-drying,” Holz Roh Werkstoff 53, 55-59.
Taher, A. M., and Cates, D. M. (1974). “A spectrophotometric investigation of the yellow color that accompanies the formation of furan derivatives in degraded-sugar solutions,” Carbohydr. Res. 34, 249-291.
Theander, O., Bjurman, J., and Boutelje, J. B. (1993). “Increase in the content of low-molecular carbohydrates at lumber surfaces during drying and correlations with nitrogen content, yellowing, and mould growth,” Wood Sci. Technol. 27, 381-389.
Stofko, J. (1974). “The autoadhesion of wood,” PhD thesis, Univ. of California.
Suthoff, B., Schaaf, A., Hentschel, H., and Franz, U. (1996). “Verfahren zum reibschweiβartigen Fügen von Holz. (Method for joining wood),” Patent DE 196 20 273 A 1.
Vek,V., and Oven, P. (2010) “Variability in content of total phenols in beech stem,” First Serbian Forestry Congress, 11-13 November 2010, Belgrade, Republic of Serbia, p. 279.
Westermark, U., and Karlsson, O. (2002). “Resin-free particleboard by oxidation of wood,” In: 6th Pacific Rim Bio-Based Composites Symposium & Workshop on the Chemical Modification of Cellulosics, Nov 2002, Portland, Oregon.
Westermark, U., and Karlsson, O. (2003). “Auto-adhesive bonding by oxidative treatment of wood,” In: 12th International Symposium for Wood, Fibre and Pulping Chemistry, Madison, WI, pp. 365-368.
Wexler, A. S. (1964). “Characterization of lignosulfonates by ultraviolet spectrometry, direct and difference spectrograms,” Anal. Chem. 36(1), 213-222.
Widyorini, R., Xu, J., Watanabe, T., and Kawai, S. (2005). “Chemical changes in steam-pressed kenaf core binderless particleboard,” J. of Wood Sci. 51(1), 26-32.
Widsten, P. (2002). “Oxidative activation of wood fibres for the manufacture of medium-density fibreboard,” Doctoral thesis, Helsinki University.
Wikberg, H. (2004). “Advanced solid state NMR spectroscopic techniques in the study of thermally modified wood,” PhD dissertation, Helsinki.
Wikberg, H., and Maunu, S. L. (2004). “Characterisation of thermally modified hard- and softwoods by 13C CPMAS NMR,” Carbohydr. Polym. 58, 461-466.
Zule, J., and Može, A. (2003). “GC analysis of extractive compounds in beech wood,” J. Sep. Sci. 26, 1292-1294.
Article submitted: November 22, 2012; Peer review completed: January 4, 2013; Revised version received and accepted: June 6, 2013; Published: June 11, 2013.
