Abstract
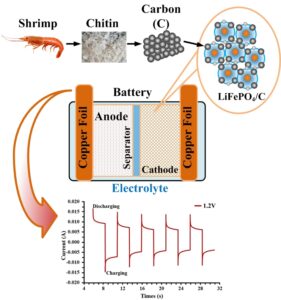
A LiFePO4 battery is the best device for energy storage. Batteries are currently being developed for higher capacity using novel materials. Carbon is one material that can be used to improve the properties of LiFePO4 batteries. The chitin produced from shrimp shell is a viable material that can be transformed into organic carbon. The chitin is revealed to be an element of 36.6 wt% carbon (C). Carbon is formed of small crystallites comprising electrode composite with a uniform carbon coating that can improve the electrochemical activation for LiFePO4/C composites. When the electrochemical reaction was operated at 1.2 V, the flow rate was increased 80%. The average charge-discharge capacities were 100 and -100 mAh/g, respectively, while the average energy density over a period of 20 cycles was 336 Wh/kg (maximum ~350 Wh/kg). Therefore, organic carbon can be used to remarkably improve the properties of LiFePO4 batteries with low-cost materials.
Download PDF
Full Article
Characteristics of Carbon from Chitin-coated LiFePO4 and its Performance for Lithium Ion Battery
Ekawat Ratchai,a Montri Luengchavanon,b,* Kua-anan Techato,a Warakorn Limbuta,c Aujchariya Chotikhun,d and Nyuk Yoong Voo e
A LiFePO4 battery is the best device for energy storage. Batteries are currently being developed for higher capacity using novel materials. Carbon is one material that can be used to improve the properties of LiFePO4 batteries. The chitin produced from shrimp shell is a viable material that can be transformed into organic carbon. The chitin is revealed to be an element of 36.6 wt% carbon (C). Carbon is formed of small crystallites comprising electrode composite with a uniform carbon coating that can improve the electrochemical activation for LiFePO4/C composites. When the electrochemical reaction was operated at 1.2 V, the flow rate was increased 80%. The average charge-discharge capacities were 100 and -100 mAh/g, respectively, while the average energy density over a period of 20 cycles was 336 Wh/kg (maximum ~350 Wh/kg). Therefore, organic carbon can be used to remarkably improve the properties of LiFePO4 batteries with low-cost materials.
DOI: 10.15376/biores.18.3.4399-4412
Keywords: Carbon; Chitin; Shrimp shell; LiFePO4
Contact information: a: Faculty of Environmental Management, Prince of Songkla University, Hatyai, Songkhla, 90110, Thailand; b: Sustainable Energy Management Program, Wind Energy and Energy Storage Systems Centre (WEESYC), Faculty of Environmental Management, Center of Excellence in Metal and Materials Engineering (CEMME), PSU-Electric Vehicle Development Center, Engineering Faculty, Prince of Songkla University, Hatyai, Songkhla, 90110, Thailand; c: Division of Health and Applied Sciences, Thailand Center of Excellence for Innovation in Chemistry, Center of Excellence for Trace Analysis and Biosensor, Faculty of Science, Prince of Songkla University, Hatyai, Songkhla, 90110, Thailand; d: Faculty of Science and Industrial Technology, Prince of Songkla University, Surat Thani Campus, Mueang, Surat Thani 84000, Thailand; e: Applied Physics, Faculty of Science, Universiti Brunei Darussalam;
* Corresponding author: montri.su@psu.ac.th, ID ORCID: 0000-0001-8586-194X
GRAPHICAL ABSTRACT

INTRODUCTION
Presently, the best kind of battery is made of lithium iron phosphate (LiFePO4). Such batteries can be applied to many technologies for the most sustainable energy storage due to their properties and functions. There are many factors, such as preparation, materials, and methodologies that need to be developed for higher performance lithium ion batteries. Generally, the structure of a lithium ion battery consists of a cathode, electrolyte, and anode, which researchers can develop separately. The specific preparation methodologies, as well as the materials used for the development of cathode, anode, and electrolyte, greatly influence the properties. Today, technologies should be considered using environmentally friendly biomaterials to reduce waste in the future. An example is an additive of free gelatin based on the sol-gel procedure, which has been developed to synthesize LiFePO4/C powders. Gelatin is a biomaterial that is commercially available, cheap, and environmentally friendly, obtained from the hydrolysis of collagen (Gao et al. 2014), while activated carbon from coconut shell is fabricated for electrodes in electric double-layer supercapacitors (Chopngam et al. 2021).
The cathode materials used for LiFePO4 can perform at high voltage (~3.4 V vs. Li/Li+) (Liu et al. 2018) and possess high theoretical capacity (~170 mAh/g). Other desirable properties include avoidance of environmental pollution, low cost, thermal energy stability, and a long-life cycle (Aravindan et al. 2015; Kim et al. 2019). Moreover, LiFePO4 comprises Fe element that is of low cost and readily available in the market, though it has disadvantages, including low conductivity (∼10-9 to 10-10 S/cm), low Li+ ion diffusivity (∼10-14 to 10-16 S/cm2) (Lu et al. 2008; Wang and Sun 2012; Zaghib et al. 2013), and low tap density (Wang and Sun 2012; Zaghib et al. 2013). Many researchers are currently attempting to improve the conductivity of LiFePO4 cathodes. For example, such efforts include increasing conductivity and accelerating the diffusion rate of Li+ ions through porous carbon and carbon coating layers. Such developments have used the coating of conducted materials on the surface of LiFePO4 material (Zaghib et al. 2013; Zhang et al. 2015; Yao et al. 2017; Wu 2018), doping transition metals ions (Wang et al. 2010; Chekannikov et al. 2018), and reducing the size of particles (Wang et al. 2014; Yang et al. 2014). The use of LiFePO4 material has successfully improved the energy storage of lithium-ion batteries, which increased power production by adding an external carbon source (Arias-Thode et al. 2013). Chitin, an organic material derived from raw shrimp shell, goes through a typical reaction without the use of a catalyst or oxidant. In a typical reaction, 100 mg of chitin (4 mmol of carbon), 5 mL of 2 M NaOH, and a stirrer bar were loaded into the autoclave (Gao et al. 2016). The most available source of chitin and a major source is crustaceans, which are processed into chitin and chitosan. Chitosan after blending with a solid polymer electrolyte, plasticized with various weight percentages of polyethylene glycol (PEG) and LClO2 have been successfully prepared by a solution-casting method. This preparation can be used effectively as a promising material for the production of solid-state lithium ion batteries (Leo Edward et al. 2021).
This study assessed the modification of carbon produced from chitin (obtained from shrimp shells) for the carbon composite electrode. The carbon comprises small crystallites of electrode material with a uniform carbon coating that can improve the electrochemical activation of LiFePO4 batteries. The study focuses on comparing the physical and electrochemical performances of polymer batteries using pure LiFePO4 based on chitin-derived carbon coated on LiFePO4, which comprised of a modified electrochemical activation process.
EXPERIMENTAL
Preparation of LiFePO4 Material
The first step was the preparation of the chitin produced from the shrimp shells (Suratthani Province, Thailand), which were washed and cleaned before being processed into chitin. The samples were then sun-dried for 2 to 3 days before being completely dried in an oven at around 60 °C and being ground. Following that, 10 g of shrimp shell power was mixed with 20 mL of acetone (Anantanara Co., Ltd., Thailand). Afterwards, 200 mL of hydrochloric acid (37% HCl; RCL Labscan, Thailand) was gradually added, and stirred in a flask that was kept in a tank of ice until the mixture was completely dissolved, which was then kept at 4 °C for 24 h. After 24 h, the substrate mixture was filtered through 2 to 3 layers of cheesecloth and added to 600 mL of a 50% ethanol solution (RCL Labscan, Thailand) with continuous stirring until precipitation, after which the top layer of the water was drained. Deionized water was then added in the same amount as the drained water, after which the pH balance was measured at 7.0. The substrate mixture was then centrifuged at 5,000 rpm for 10 min, steamed in an autoclave at 120 °C for 15 min, and stored at 10 °C.
The second step was to prepare the FePO4·2H2O precursor, which was done in a flask with iron (III) oxide (Fe2O3; Sigma Aldrich, Japan) powder and phosphoric acid (H3PO4; Sigma Aldrich, Japan) solution (85%), mixed with deionized water (DW), with the Fe/P/DW ratio being 2 g: 1.8 mL: 2.5 mL (Jiang et al. 2020). The mixtures were then transferred to a horizontal ball mill for 9 h, heated at 90 °C for 5 h to form a suspension, and then cooled to room temperature in the current study. In the final step, the FePO4·2H2O precursor was mixed with lithium carbonate (Li2CO3; Sigma Aldrich, Tokyo, Japan), chitin, and DW in the following proportions: 1 g: 0.485 g: 0.1 g: 32 mL (Ying et al. 2006) at room temperature. After that, the mixture was placed in an ultrasonic bath for 30 min. The mixed slurries were then oven-dried for 24 h at 85 °C. The mixture was then sintered at 650 °C in a tube furnace for 10 h with an argon flow of 3 °C/min to obtain the LiFePO4 material shown in Fig. 1.
Fig. 1. Schematic illustration showing the preparation of LiFePO4 material
Characterization of LiFePO4 material
The phase identification of the LiFePO4 material was carried out using X-ray diffraction (XRD; PANalytical Empyrean, Netherlands). The morphology and energy dispersive X-ray (EDX analysis) of the LiFePO4 material were observed by scanning electron microscopy (SEM) with Quanta 400 (Thermo Fisher Scientific Quanta, Czech Republic). The specific surface areas were estimated using the multipoint BET (Brunauer−Emmett−Teller, BET) model (Micromeritics equipment ASAP2460, USA), determining 11 closely spaced points in the relative pressure (P/P0) range from 0.05 to 0.35. Prior to testing, samples (based on 50 to 200 mg in the form of powder) were degassed for 1 h at 30 °C under vacuum conditions to remove weakly adsorbed species.
Electrochemical measurements
For preparing the cathode, the LiFePO4 material, carbon from chitin, and polyvinylidene fluoride (PVDF; Sigma Aldrich, Japan) were mixed at a ratio of 80:10:10 by weight. This mixture was mixed in a viscous slurry using N-methyl-2-pyrrolidone (NMP; Sigma Aldrich, Japan). The mixture was covered with aluminum foil and dried in a vacuum at 100 °C for 5 h. The composition was dried and formed into a film, which was loaded at a level of approximately 4.8 mg/cm2 and used as the cathode. Meanwhile, copper plates were used as the current collector, reference electrode, and LiFePO4 cathode material as the cathode. The electrolyte consisted of a solution of 1 mol/L LiPF6 in ethylene carbon (EC)/dimethyl carbonate (DMC) (1:1 w/w) (Sigma Aldrich, Japan). The nano-power carbon (Sigma Aldrich, Japan) coated copper foil was used for the anode side.
The measurements of the battery cells were taken using an Autolab electrochemical impedance spectroscopy (Metrohm, PGSTAT302N, USA) as potentiostatic charge and discharge between 1.2 to 3.3 V with a scan rate of 0.1 to 0.5 V s-1 at room temperature. Cyclic voltammetry (CV) was also tested. An electrochemical impedance spectrometer (EIS) was used to characterize samples using Autolab electrochemical impedance spectroscopy by adjusting the amplitude signal at 5 mV and a frequency range of 0.01 Hz to 100 kHz.
RESULTS AND DISCUSSION
Figure 2 shows the XRD patterns of LiFePO4 synthesized with carbon produced from chitin. The XRD patterns of the sample revealed a highly crystalline stoichiometric LiFePO4 phase in which 90% of major peaks were matched by the LiFePO4 standard.
Fig. 2. XRD spectra of LiFePO4 coated carbon (LiFePO4/C) produced from chitin
The major phase of synthesized composite was confirmed with the Inorganic Crystal Structure Database (ICSD) file (No. 01-078-7911, Crystal system: Orthorhombic, Pnma (62), a = 10.3254 Å, b = 6.0035 Å, c = 4.6879 Å) (Gao et al. 2014). The XRD patterns of LiFePO4 indicated a chitin peak of carbon contains calcium, presumably in the form of CaCO3, sodium, and sulphur (joined together in Na2S and its derivatives Na2S4 or Na2S5) (Conder et al. 2019). This LiFePO4 coated carbon composite was assessed for carbon element content with the EDX technique shown in Fig. 4.
Fig. 3. A SEM micrograph of LiFePO4 with carbon from chitin at (a) scale bar: 2 µm, (b) scale bar: 1 µm, and (c) scale bar: 500 nm
Fig. 4. EDX map of LiFePO4/C composite; homogenous distribution of P, Fe, and C related to the presence of LiFePO4 composite
Figure 3(a) shows SEM images of the LiFePO4/C composite with C from chitin, indicating the high magnification of the coating. The LiFePO4/C composite showed uniform particle size distribution as well as small grain sizes of approximately 0.5 to 1.0 μm. This result was attributed to the carbon coating on the surface. The C-coating was homogeneously deposited on LiFePO4 composite. Conductive carbon can be co-deposited with LiFePO4, which enhances the electrical conductivity of olivine-type LiFePO4 thin films (Lu et al. 2008). Figure 3(b) shows a high magnification SEM image demonstrating both LiFePO4 particles and carbon particles. The carbon particles can be found harmoniously mixed in the LiFePO4/C composite. The particle aggregation was indicated by the particle size distribution measurement. Figure 3(c) shows a high magnification image based on the nano-size, indicating carbon particles with nano-particles. The sintering was confirmed to occur during the calcination process on the surface of the plates (Lyczko et al. 2012). The particle aggregation based on elements of LiFePO4/C can be revealed by colour, as shown in Fig. 4.
Figure 4 shows an EDX map of LiFePO4 composite with carbon particles from chitin. The elemental contents of 5.20 wt% phosphorus (P) and 10.6 wt% iron (Fe) were mainly obtained from the LiFePO4 composite. The content of 36.6 wt% carbon (C) was revealed from the carbon particles prepared from chitin, while 52.4 wt% consisted of other elements combined between Li and O. Additionally, the EDX map and SEM image (Fig. 3) certainly exhibited the highly homogenous composite of LiFePO4/C. The LiFePO4/C composite with co-deposited carbon showed homogeneous distribution throughout the LiFePO4/C films. It was found that 2 mol% carbon was the optimized amount for the highest capacity (Lu et al. 2008). The mechanical activation operation is reported to be particularly efficient for synthesizing small particles with homogenous morphology (Kim et al. 2019).
Table 1. Surface Area and Pore Size of LiFePO4/C
The LiFePO4 composites with carbon particle (LiFePO4/C) crystals are mainly composed of smaller micro-crystals that are assembled in an orthorhombic structure, as shown in Fig. 2. Brunauer−Emmett−Teller (BET) analysis is shown in Table 1. The carbon coating of the samples (LiFePO4/C) exhibited 4.47 m2/g surface area and 157 to 169 Å pore size. (Paolella et al. (2016) revealed 4.20 m2/g surface area of the LiFePO4/C with added Ca composite for an increased surface area.
Fig. 5. Cyclic voltammetry of LiFePO4/C cell curves at different scan rates
Cyclic voltammetry (CV) studies revealed the effect of carbon from chitin on the electrochemical properties of LiFePO4 using a scanning rate of 0.1 to 0.5 V/s, as shown in Fig. 5. When the scanning rate was increased, the starting point of the cyclic voltammetry curve changed slightly, indicating that carbon can activate the stable cycling performance and that CV has no pseudo capacitance (Jiang and Xie 2019). The carbon from chitin for a cathode composite had stable electrochemical cycling properties. The CV of LiFePO4/C from chitin composites showed a highly symmetrical and sharper shape of the anodic/cathodic peaks, which indicates the better electrochemical process (Wang et al. 2010). The multi cycle from the first scan curve showed an irreversible cathodic peak at around 0.4 V and -1.0 V that can correspond to the generation of a solid electrolyte interphase (SEI) (Tao et al. 2020), and the appearance of well-indicated pair redox peaks indicates the redox activity of Fe2+/Fe3+ that is generated by lithium ion extraction/insertion in LiFePO4 during the charge and discharge processes (Wu et al. 2016).
Figure 6 shows the Nyquist plot from the electrochemical impedance spectroscopy (EIS) of the LiFePO4/carbon from chitin electrodes, which aimed to further study their electrochemical kinetics. The electrochemical activity of the LiFePO4 cell 1.2 V was compared with cell 2.6 V and cell 3.1 V using the EIS technique. The EIS measurements were taken to explore the effect of carbon coating from chitin on the electrochemical performance of the LiFePO4 cathode.
Fig. 6. Nyquist plot of electrochemical impedance spectra curves including cells at 1.2 V, 2.6 V, and 3.1 V
The charge transfer resistance represented the values for the cross point of the semicircle on the horizontal axes. The carbon from chitin was able to affect the first Nyquist plot (Z’) at around 41 Ω, and the curves were moderately increased with higher voltage, while the end of the Nyquist plot was formed in a straight line that was slowly transformed to a semicircle with higher voltage. The straight line represents the Warburg impedance, which is associated with lithium-ion diffusion on the inside of LiFePO4 particles. Additionally, charge transfer resistance involves a close relationship between the composition and conductivity of the interface (Zhang et al. 2016; Sofyan et al. 2018).
In investigations using LiFePO4 with carbon from chitin as a pumping solution, LiFePO4 also showed an increase in current. For the LiFePO4 with carbon from chitin, when operated at 1.2 V, the flow rate was increased to 80%, as shown in Fig. 7(a). This charge-discharge curve showed the high efficiency of the flow rate current. Electrochemical activation can be operated so that addition of Li+ to the pumping solution increases the ionic strength and flow rate of the pump (Baek et al. 2018). In a solution containing Li+, C may increase the high conductivity of carbon from chitin, which can result in faster charge and discharge (Wu 2018). Figure 7(b) shows the operation of the charge-discharge curves measured for LiFePO4 with carbon from chitin when changing the active voltage at 1.9 V, 2.6 V, 3.1 V, and 3.4 V. The level of current is increased following the active voltage from 1.9 V to 4.3 V. When the cation was Li+, carbon exhibited the highest efficiency with the largest flow rate for the smallest current value. The grain size and crystallinity of the composite strongly influences the capacity. In terms of the capacity of organic carbon sources, LiFePO4/C composite electrode prepared with a carbon source have a higher capacity (Chen et al. 2019).
Fig. 7. (a) Charge and discharge current profiles of 1.2 V, and (b) comparison of charge and discharge current profiles between 1.9 V, 2.6 V, 3.1 V and 3.4 V
Figure 8(a) shows that the LiFePO4/C cell has good rate capability with average charge-discharge capacities of 100 and -100 mAh/g once the voltage is operated at 3.4 V within 20 cycles. These results demonstrate the high cycling performance, capacity retention, and rate capability (Mohamed et al. 2014; Liu et al. 2015). Figure 8(b) shows the charge capacity of the LiFePO4/C electrode versus the cycle number at a cycling specific capacity of 100 mAh/g; within 20 cycles, the capacity increases during the first 4 cycles. Thereafter, the trends were slightly decreased to 20 cycles (∼100 mAh/g for cycle 20 charge capacity) with an improvement of 100% coulombic efficiency. The coulombic efficiency referred to the steady charge operation. The coulombic efficiency of these LiFePO4/C electrodes subjected to galvanostatic charge/discharge was high with added amount of carbon in LiFePO4 (Varzi et al. 2014).
Fig. 8. (a) Charge and discharge profiles of 3.4 V; (b) cycling performance combined with coulombic efficiency of 3.4 V
Figure 9(a) shows the trends compared between the specific capacity and energy density at 20 cycles. The LiFePO4/C electrode was revealed to have good rate capability with average charge capacities of 100 mAh/g, once the voltage was operated at 3.4 V, while the average energy density over a period of 20 cycles for LiFePO4/C electrodes was 336 Wh/kg (maximum ~350 Wh/kg). The trends of specific capacity and energy density were similarly conducted; the trends were higher at 1 to 4 cycles and then gently were reduced to a straight line until 20 cycles. This manner of trend for energy density can occur in LiFePO4 using carbon fibre-reinforced polymer (Moyer et al. 2020). Figure 9(b) shows the trends compared between the columbic efficiency and energy density at 20 cycles. At 1 to 4 cycles, both trends were different in that the trend for energy density was gently arched. At 4 to 20 cycles, both trends were formed in a straight line. This characteristic of the energy density trend can be formed at a high level in the first cycle and gently decrease to a straight line. This activation is generated in LiFePO4 applying reinforced carbon fibre (Moyer et al. 2020), while the coulombic efficiency of LiFePO4/C composite referred to charge/discharge is very high with an added amount of carbon (Varzi et al. 2014). The cost of LiFePO4 when compared between this investigation and the market (Alibaba.com) indicates that materials from the market are cheaper than those in this investigation, while the manufacturing production permits low cost fabrication. However, this study used materials from an organic composite (chitin), which conformed to environmental composition standards for LiFePO4/C batteries, as shown in Table 2.
Fig. 9. (a) Cycling performance combined with energy density of 3.4 V; (b) energy density combined with coulombic efficiency of 3.4 V
Table 2. Comparison of LiFePO4 Between this Investigation and the Market
CONCLUSIONS
1. This study used carbon (C) fabricated from chitin (produced from shrimp shell). The C formed small crystallites of the electrode composite with a uniform carbon coating that can improve the electrochemical activation of LiFePO4 batteries.
2. The C produced from chitin revealed a C-elemental content of 36.6 wt%, which can be homogenously mixed for LiFePO4/C composition.
3. The electrochemical reaction of charge showed an increased current when LiFePO4 used carbon from chitin; while operated at 1.2 V, the flow rate was increased 80%.
4. LiFePO4 added with C from chitin cells exhibited good rate capability with average charge-discharge capacities of 100, and -100 mAh/g once the voltage operated at 3.4 V within 20 cycles.
5. LiFePO4 added with C from chitin can generate average energy density of 336 Wh/kg (maximum ~350 Wh/kg) over a period of 20 cycles.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Graduate School Fund: 2020, Prince of Songkla University, Thailand.
REFERENCES CITED
Aravindan, V., Lee, Y. -S., Yazami, R., and Madhavi, S. (2015). “TiO2 polymorphs in ‘rocking-chair’ Li-ion batteries,” Materials Today 18(6), 345-351. DOI: 10.1016/j.mattod.2015.02.015
Arias-Thode, Y. M., Hsu, L., Wotawa-Bergen, A., and Chadwick, B. (2013). “Chitin lengthens power production in a sedimentary microbial fuel cell,” in: 2013 OCEANS-San Diego, San Diego, CA, USA, pp. 1-4. DOI: 10.23919/OCEANS.2013.6741080
Baek, J., Kim, K., and Shin, W. (2018). “Development of LiFePO4/FePO4 electrode for electro-osmotic pump using Li+ migration,” Journal of Electrochemical Science and Technology 9(2), 85-92. DOI: 10.5229/JECST.2018.9.2.85
Chekannikov, A. A., Kuz’mina, A. A., Kulova, T. L., Novikova, S. A., Skundin, A. M., Stenina, I. A., and Yaroslavtsev, A. B. (2018). “Development of lithium-ion battery of the “doped lithium iron phosphate–doped lithium titanate” system for power applications,” in: Proceedings of the Scientific-Practical Conference “Research and Development – 2016,” Springer International Publishing, Cham, Switzerland, pp. 341-350. DOI: 10.1007/978-3-319-62870-7_37
Chen, L., Feng, W., Su, W., Li, M., and Song, C. (2019). “Biosynthesis of LiFePO4/C cathode materials by a sol-gel route for use in lithium ion batteries,” International Journal of Electrochemical Science 14, 2846-2856. DOI: 10.20964/2019.03.21
Chopngam, K., Luengchavanon, M., Khangkhamano, M., Chetpattananondh, K., and Limbut, W. (2021). “Coating activated carbon from coconut shells with Co3O4/CeO2 for high-performance supercapacitor applications: An experimental study,” BioResources 16(4), 8022-8037. DOI: 10.15376/biores.16.4.8022-8037
Conder, J., Vaulot, C., Marino, C., Villevieille, C., and Ghimbeu, C. M. (2019). “Chitin and chitosan-structurally related precursors of dissimilar hard carbons for Na-ion battery,” ACS Applied Energy Materials 2(7), 4841-4852. DOI: 10.1021/acsaem.9b00545
Gao, M., Liu, N., Li, Z., Wang, W., Li, C., Zhang, H., Chen, Y., Yu, Z., and Huang, Y. (2014). “A gelatin-based sol-gel procedure to synthesize the LiFePO4/C nanocomposite for lithium ion batteries,” Solid State Ionics 258, 8-12. DOI: 10.1016/j.ssi.2014.01.041
Gao, X., Chen, X., Zhang, J., Guo, W., Jin, F., and Yan, N. (2016). “Transformation of chitin and waste shrimp shells into acetic acid and pyrrole,” ACS Sustainable Chemistry & Engineering 4(7), 3912-3920. DOI: 10.1021/acssuschemeng.6b00767
Jiang, D., Zhang, X., Zhao, T., Liu, B., Yang, R., Zhang, H., Fan, T., and Wang, F. (2020). “An improved synthesis of iron phosphate as a precursor to synthesize lithium iron phosphate,” Bulletin of Materials Science 43(1), Article Number 50. DOI: 10.1007/s12034-019-1994-y
Jiang, G., and Xie, S. (2019). “Preparation and electrochemical properties of lignin porous carbon spheres as the negative electrode of lithium ion batteries,” International Journal of Electrochemical Science 14, 5422-5434. DOI: 10.20964/2019.06.36
Kim, H. -J., Bae, G. -H., Lee, S. -M., Ahn, J. -H., and Kim, J. -K. (2019). “Properties of lithium iron phosphate prepared by biomass-derived carbon coating for flexible lithium ion batteries,” Electrochimica Acta 300, 18-25. DOI: 10.1016/j.electacta.2019.01.057
Leo Edward, M., Dharanibalaji, K. C., Kumar, K. T., Chandrabose, A. R. S., Shanmugharaj, A. M., and Jaisankar, V. (2021). “Preparation and characterisation of chitosan extracted from shrimp shell (Penaeus monodon) and chitosan-based blended solid polymer electrolyte for lithium-ion batteries,” Polymer Bulletin 79, 587-604. DOI: 10.1007/s00289-020-03472-1
Liu, M., Chen, Y., Chen, K., Zhang, N., Zhao, X., Zhao, F., Dou, Z., He, X., and Wang, L. (2015). “Biomass-derived activated carbon for rechargeable lithium-sulfur batteries,” BioResources 10(1), 155-168. DOI: 10.15376/biores.10.1.155-168
Liu, R., Chen, J., Li, Z., Ding, Q., An, X., Pan, Y., Zheng, Z., Yang, M., and Fu, D. (2018). “Preparation of LiFePO₄/C cathode materials via a green synthesis route for lithium-ion battery applications,” Materials (Basel) 11(11), Article Number 2251. DOI: 10.3390/ma11112251
Lu, Z., Lo, M. -F., and Chung, J. C. Y. (2008). “Pulse laser deposition and electrochemical characterization of LiFePO4-C composite thin films,” The Journal of Physical Chemistry C 112(17), 7069-7078. DOI: 10.1021/jp0744735
Lyczko, N., Nzihou, A., Sharrock, P., Germeau, A., and Toussaint, C. (2012). “Characterization of LiFePO4/C cathode for lithium ion batteries,” Industrial and Engineering Chemistry Research 51(1), 292-300. DOI: 10.1021/ie201799x
Mohamed, S. G., Chen, C. -J., Chen, C. K., Hu, S. -F., and Liu, R. -S. (2014). “High-performance lithium-ion battery and symmetric supercapacitors based on FeCo2O4 nanoflakes electrodes,” ACS Applied Materials & Interfaces 6(24), 22701-22708. DOI: 10.1021/am5068244
Moyer, K., Boucherbil, N. A., Zohair, M., Eaves-Rathert, J., and Pint, C. L. (2020). “Polymer reinforced carbon fiber interfaces for high energy density structural lithium-ion batteries,” Sustainable Energy & Fuels 4(6), 2661-2668. DOI: 10.1039/d0se00263a
Paolella, A., Turner, S., Bertoni, G., Hovington, P., Flacau, R., Boyer, C., Feng, Z., Colombo, M., Marras, S., Prato, M., Manna, L., Guerfi, A., Demopoulos, G. P., Armand, M., and Zaghib, K. (2016). “Accelerated removal of Fe-antisite defects while nanosizing hydrothermal LiFePO4 with Ca2+,” Nano Lett 16(4), 2692−2697. DOI: 10.1021/acs.nanolett.6b00334
Sofyan, N., Alfaruq, S., Zulfia, A., and Subhan, A. (2018). “Characteristics of vanadium doped and bamboo activated carbon coated LiFePO4 and its performance for lithium ion battery cathode,” Jurnal Kimia dan Kemasan 40(1), 9-16. DOI: 10.24817/jkk.v40i1.3767
Varzi, A., Bresser, D., Zamory, J. V., Müller, F., and Passerini, S. (2014). “ZnFe2O4‐C/LiFePO4‐CNT: A novel high‐power lithium‐ion battery with excellent cycling performance,” Advanced Energy Materials 4(10), Article ID 1400054. DOI: 10.1002/aenm.201400054
Wang, J., Shao, Z., and Ru, H. (2014). “Influence of carbon sources on LiFePO4/C composites synthesized by the high-temperature high-energy ball milling method,” Ceramics International 40(5), 6979-6985. DOI: 10.1016/j.ceramint.2013.12.025
Wang, J., and Sun, X. (2012). “Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries,” Energy & Environmental Science 5(1), 5163-5185. DOI: 10.1039/C1EE01263K
Wang, L., Wang, H., Liu, Z., Xiao, C., Dong, S., Han, P., Zhang, Z., Zhang, X., Bi, C., and Cui, G. (2010). “A facile method of preparing mixed conducting LiFePO4/graphene composites for lithium-ion batteries,” Solid State Ionics 181(37), 1685-1689. DOI: 10.1016/j.ssi.2010.09.056
Wu, G., Jiang, T., Tian, X., Zhu, Y., and Zhou, Y. (2018). “An efficient carbon coating process applied in different synthetic routes of LiFePO4 cathode materials,” International Journal of Electrochemical Science 13, 8006-8021. DOI: 10.20964/2018.08.07
Wu, J., Cai, W., and Shang, G. (2016). “In situ electrochemical-AFM study of
LiFePO4 thin film in aqueous electrolyte,” Nanoscale Research Letters 11(1), 223.
DOI: 10.1186/s11671-016-1446-1
Yang, G., Cai, F. P., Jiang, B., Wang, B., Hu, S. Q., Tan, C. H., Gao, J. H., and Chen, H. (2014). “Preparation of micro/nano-LiFePO4/C cathode material for Li-ion batteries,” Journal of New Materials for Electrochemical Systems 17(4), 231-234. DOI: 10.14447/jnmes.v17i4.396
Ying, J., Lei, M., Jiang, C., Wan, C., He, X., Li, J., Wang, L., and Ren, J. (2006). “Preparation and characterization of high-density spherical Li0.97Cr0.01FePO4/C cathode material for lithium ion batteries,” Journal of Power Sources 158(1), 543-549. DOI: 10.1016/j.jpowsour.2005.08.045
Zaghib, K., Guerfi, A., Hovington, P., Vijh, A., Trudeau, M., Mauger, A., Goodenough, J. B., and Julien, C. M. (2013). “Review and analysis of nanostructured olivine-based lithium recheargeable batteries: Status and trends,” Journal of Power Sources 232, 357-369. DOI: 10.1016/j.jpowsour.2012.12.095
Zhang, K., Lee, J. -T., Li, P., Kang, B., Kim, J. H., Yi, G. -R., and Park, J. H. (2015). “Conformal coating strategy comprising N-doped carbon and conventional graphene for achieving ultrahigh power and cyclability of LiFePO4,” Nano Letters 15(10), 6756-6763. DOI: 10.1021/acs.nanolett.5b02604
Zhang, Q., Huang, S. -Z., Jin, J., Liu, J., Li, Y., Wang, H. -E., Chen, L. -H., Wang, B. -J., and Su, B. -L. (2016). “Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability,” Scientific Reports 6(1), article 25942. DOI: 10.1038/srep25942
Article submitted: September 22, 2022; Peer review completed: February 11, 2023; Revised version received: April 24, 2023; Accepted: April 30, 2023; Published: May 3, 2023.
DOI: 10.15376/biores.18.3.4399-4412
