Abstract
Water retting is a traditional retting method that enables the low-cost production of bast fibers. This study investigated the retting of flax straws by distilled water for three different durations at room temperature in laboratory condition. The retting quality was evaluated in terms of the weight loss and degumming rate together with the fiber properties, which included color, linear density, and tensile properties. The degumming rate was defined as the percentage change in pectin content of phloem regions from the raw flax to water-retted flax. It was found that the dissolution of pectin and other contaminating materials during the beginning retting stage must have played an important role in pectin (content) and weight loss besides pectin degradation, and water retting gradually improved both the apparel properties, such as whiteness and fineness, and the mechanical properties of the fibers. Given the results, a water retting duration of six days should be sufficient to provide sound retting efficiency and reasonable fiber properties.
Download PDF
Full Article
Characterization of Flax Water Retting of Different Durations in Laboratory Condition and Evaluation of Its Fiber Properties
Peiying Ruan,a,b,c Vijaya Raghavan,c Yvan Gariepy,c and Jianmin Du a,*
Water retting is a traditional retting method that enables the low-cost production of bast fibers. This study investigated the retting of flax straws by distilled water for three different durations at room temperature in laboratory condition. The retting quality was evaluated in terms of the weight loss and degumming rate together with the fiber properties, which included color, linear density, and tensile properties. The degumming rate was defined as the percentage change in pectin content of phloem regions from the raw flax to water-retted flax. It was found that the dissolution of pectin and other contaminating materials during the beginning retting stage must have played an important role in pectin (content) and weight loss besides pectin degradation, and water retting gradually improved both the apparel properties, such as whiteness and fineness, and the mechanical properties of the fibers. Given the results, a water retting duration of six days should be sufficient to provide sound retting efficiency and reasonable fiber properties.
Keywords: Water retting; Flax straws; Retting quality; Degumming rate; Fiber properties; Pectin dissolution
Contact information: a: College of Mechanical and Electrical Engineering, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, 010018 P.R. China; b: College of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo, Shandong Province, 255049 P.R. China; c: Department of Bioresource Engineering, McGill University, Sainte Anne de Bellevue, Quebec, H9X 3V9 Canada;
* Corresponding E-mails: nndjwc202@imau.edu.cn (Jianmin Du); maryruan@126.com (Peiying Ruan)
INTRODUCTION
Flax (Linum usitatissimum L.) can be used to produce flax fibers from its phloem part. As a renewable and biodegradable natural resource, flax fiber is currently being widely used in bio-composite applications as a reinforcement material (Hrabalova et al. 2011; Sojoudiasli et al. 2014) due to its superior physical-mechanical properties. Flax fibers are obtained from the phloem part of the stem between the bark and inner core tissues and exist in the form of fiber bundles cemented and surrounded mainly by pectin, such that the fibers must be separated from the non-fiber components through a process called retting or, sometimes degumming. The retting process separates the fibers into smaller bundles and elementary fibers (Akin et al. 2000b), which facilitates further processing. As it largely determines the properties of the fibers produced, retting is vital to the processing of flax, and retting quality is among the main concerns for the industries incorporating natural fibers into production (Foulk et al. 2011).
Dew retting and water retting are two traditional retting methods. Dew retting normally requires several weeks, and the quality of fibers produced depends largely on the weather conditions. Water retting involves facilitating decomposition of the pectin by placing bast straws into water and is achieved mainly by the activity of anaerobic microorganisms. Although water retting can produce fibers of good quality, the relatively long duration of 7 to 14 days and arising odor has made it less attractive (Foulk et al. 2008; Tahir et al. 2011). Warm water retting is performed at a temperature around 35 °C, and this can shorten the retting period to 100 hours, but, due to the high water consumption and unpleasant stench generated (Konczewicz et al. 2013), it is only used in some developing countries; for example, over half of fiber production in mainland of China used this method (Daenekindt 2004). Chemical and enzymatic retting methods are used as well, and these offer more control than dew and water retting. However, chemical retting, an aggressive degumming method, incurs significant pollution problems due to the large quantity of chemicals utilized, while enzymatic retting is quite expensive, even though by requiring a shorter retting time and obtaining acceptable fiber quality it carries apparent advantages over the other retting processes (Tahir et al. 2011).
Since dew retting is widely used in western countries, many efforts have been focused on this method, e.g. Fila et al. (2001) performed artificial dew retting in vitro on flax straws using 23 fungal strains, and Martin et al. (2013) investigated flax fiber’s quality required in composite from different dew retting durations, whose retting degree were measured by various techniques.
Water-retting conducted at room temperature provides a moderate, easily-operated, and low-cost way to produce bast fibers; therefore, the present study investigated the water retting of flax straws in different stages by evaluating retting quality in terms of both retting efficiency and resultant fiber properties to find an optimal water retting duration in a laboratory condition, and based on the consideration of universality, distilled water was used in this study. This study was also aimed at providing a reference for research that would be conducted to optimize the water-retting process as well as for the applications of fibers produced from the water retting.
EXPERIMENTAL
Materials
The raw flax materials of Evelin fiber flax variety were kindly provided by National Research Council, Montreal, Canada, which were harvested from Lanaupole Fiber Research Farm in Quebec. In order to achieve uniformity across all experiments, the middle part of the each stem was chosen and cut to a length of around 100 mm, with a diameter around 2.5 mm.
Methods
Water retting
Each sample of 7 g of flax straws was rinsed with distilled water and then crushed twice with a 700 g rod to maximize retting performance by increasing the exposure of phloem regions along stems (Henriksson et al. 1997). Subsequently, the sample was placed into a 250 mL glass bottle, and distilled water was added in a ratio of 1:33. The bottles containing the samples were then left at room temperature, without lids, for water-retting durations of 2, 6, and 10 days.
After the given retting duration had passed, each sample of flax straws was weighed, its mass noted as maw, and then divided into two separate groups. Group 1 was left for further treatment; Group 2, its mass noted as mww, was rinsed with distilled water for 10 s, then dried in an oven at a temperature of 105 °C for about 18 h and stored in a sealed plastic bag at room temperature until measurements. A minimum of 10 replications were completed for each water-retting treatment.
Evaluation of retting efficiency: Weight loss
The weight loss was defined as the percentage of mass change between non-retted and water-retted flax straws, and expressed in Eqs. 1 and 2,
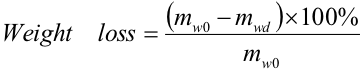 (1)
(1)
(2)
where mww and mwd represented the wet and dry masses (g), respectively, of the water-retted flax straws in Group 2; maw represented the wet mass (g) of all the water-retted flax straws; and mw0represented the dry mass (g) before water retting of flax straws in Group 2.
Evaluation of retting efficiency: Degumming rate
The degumming rate was defined as the percentage change in pectin content from non-retted to water-retted flax phloem. To measure the pectin content, the enzymatic determination method (Hansen et al.2001) was adopted, and the enzyme pectate lyase(Aspergillus niger) (Megazyme International, Ireland) was used.
To prepare the materials, the phloem of flax straws were peeled manually then cut into pieces of mesh size less than 1 mm. The pectin content determination assay was performed following the product instructions, and distilled water was used instead of deionized water.
The pectin content of phloem in the non-retted flax straws was measured in triplicate, and the pectin content of water-retted flax phloem was measured at least 10 times for each treatment. For each measurement assay, two parallel measurements were performed; for a large variation between two readings or values not in the reasonable range, the measurement assay was repeated.
Scanning electron microscopy (SEM)
To observe the fibers separation, the water-retted straws were cut into small sections and coated with gold in an ion coater Model IB5 (Eiko, Japan). The cross-sections of flax straws were observed by a scanning electron microscope S-530 (Hitachi, Japan) operating at an accelerating voltage of 25 kV.
Properties of fibers: Color
The color of flax fibers obtained was assessed through the CIE 1976 L*, a*, b* color space such that the color of the fibers was expressed by three components: L* (whiteness or brightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) (Billmeyer and Saltzman 1981).
All the fibers were obtained through manual peeling and impurity removal. The color of minimum five fiber samples for each treatment then was measured. To measure the fiber color, each fiber sample was placed on a blank paper and measured using a Tristimulus colorimeter (Minolta Co. Ltd., Japan) at 5 to 6 different points, and the averaged values was taken as the color of the fiber sample.
Linear density of fibers
The linear density of fiber was calculated using Eq. 3,
D=W/L (3)
where D is the linear density of the fiber (mg/m or tex), W is mass (mg), and L is fiber length (m) (Wang et al. 2007).
To measure the linear density, over 40 fibers were selected for each treatment and raw flax material and divided into 5 to 7 groups, such that the linear density of fibers of each treatment was the averaged value across these groups. The mass of each group was the total mass of the selected fibers, which was weighed using a balance with 0.1 mg accuracy, and the length of the group was the sum of the individual lengths.
Tensile test of single technical fibers
Over 10 single technical fibers, the fiber bundle glued by pectin, each with an even diameter and no apparent defects, were carefully selected from each treatment and raw flax fibers. Each fiber was mounted on a paper frame with gage length of 20 mm and fixed by epoxy resin at two ends. The fiber diameter was the averaged value of 4 to 6 different points within the length of the gage, which were measured using an optical microscope. Prior to the tensile test, all the fibers had been conditioned for 24 h in an environmental chamber at 20 °C and 65% RH, in accordance with ASTM D1776-04 (ASTM 2004).
For the tensile test, the paper frame was mounted onto a testing machine (Instron Model 4500, Instron Corp., USA), equipped with a 50 N capacity, 0.001 N resolution load cell. After the paper frame were cut to free the fiber, and load was applied at a constant crosshead displacement rate of 1 mm/min (Charlet et al. 2010) until the fiber fractured. All the testing data, including load, displacement, and Young’s modulus, were recorded and calculated automatically using the software Merlin IX (Instron Corp.) on an attached computer.
Statistical analysis
To analyze the data, the software programs Office Excel (2007, Microsoft, USA) and SPSS (Version 19.0 for Windows, SPSS Inc., USA) were used, in which the multiple comparisons were performed using Duncan’s test at a significance level of 0.05.
RESULTS AND DISCUSSION
Weight Loss
The weight loss represented how much of all the materials had been removed during the retting process. The results in terms of weight loss for the three different water-retting treatments, in the form of mean ± SD, are shown in Table 1.
Table 1. Weight Loss of Water Retting with Different Durations
It can be seen that extending the duration of water retting resulted in a significant increase in weight loss (P<0.05). When the duration was extended from 2 days to 6 days, the weight loss increased by 57%, from 5.54% to 8.69%; however, another increase in duration from 6 days to 10 days caused an 11% increase in weight loss. The water retting duration of 2 days produced a weight loss of 5.54%, which accounted for over 60% of the weight loss that was observed by 6 days of water retting. Therefore, it can be concluded that the beginning stage of water retting results in a large proportion of the weight loss, and that the weight loss becomes relatively stable after 6 days of water retting. It was noted that the weight loss had bigger error due to grouping and weighing wet mass.
Degumming Rate
During water retting, pectin removal is a key, so the rate of change in pectin content, i.e., the degumming rate, describes the retting efficiency in a direct way. The degumming rate results are given in Table 2.
Table 2. Degumming Rate of Water Retting with Different Durations
The data showed that extending the water retting duration increased the degumming rate significantly (P<0.01). When the duration was increased from 2 days to 6 days, the degumming rate increased by 58%, while the change in the duration from 6 days to 10 days produced an increase of only 19% in degumming rate. Moreover, 2 days of water retting resulted in a degumming rate of 6.40%, which accounted for over 60% of the degumming rate observed after 6 days of water retting. Di Candilo et al.(2010) conducted a research on well-water retting hemp straws at 35 °C for 6 different durations (from 1 to 6 days), which showed that the amount of reducing sugar in retting liquor for 2-day retting accounted for around 70% of that for 6-day retting and may thus prove the finding in terms of degumming rate in this study. In another study investigated by Liu et al. (2015), it was also found that the pectin in late-harvested hemp straws was degraded rapidly during 7 days of field retting, but the degradation slowed down at later stages.
It was apparent that the trend observed for degumming rate was consistent with the trend observed for weight loss, such that the first 2 days of water retting contributed largely to both the degumming rate and weight loss, both of which became more stable after 6 days.
Pectin, which is a generic name for the acidic polysaccharides in the plant cell wall, contains both soluble and dissoluble pectin substances (Kashyap et al. 2001). At the beginning stage of the water retting, some of the soluble components of pectin, together with contaminating inorganic salts and dust, dissolve and settle in water (Sharma and Van Sumere 1992), which contributes to the weight loss in the water retting. As the water retting progresses, the rest of the pectin components are degraded by the enzymes generated from the microorganisms and then dissolve, which contributes to the weight loss and degumming rate as well. The two-day distilled-water retting process must have resulted in weight loss and pectin (content) loss mainly due to dissolution, since the activation of enzymes should just begin after 2 days of water retting at room temperature. During the following 4 days, the enzymes were active to a large extent, and the degradation of the pectin contributed additional weight loss and increase in the degumming rate. However, after 6 days of water retting, the activity of microbes and enzymes became gradually flatter, causing the weight loss and degumming rate to become more stable.
It was noticed that the dissolution of pectin in the beginning retting stage together with the degradation of pectin played an important role in the total pectin (content) removal; therefore, to increase pectin solubility and susceptibility to the degrading enzymes, the water used in the retting process should not be high in hardness, since the calcium bridges formed inside of pectin molecules in the presence of hard water restrain these effects (Huber 1991; Rihouey et al. 1995).
SEM of Straws Cross-sections
Figure 1 presents the cross-sections of water-retted flax straws, and indicates that most of fiber bundles were relatively intact, but easy to free from the woody core after 2 days of water retting. Six days of water retting resulted in not only the separation of the fiber bundles from core, but also the formation of the smaller bundles from larger ones.
Color of Fibers Obtained
The color of the flax fibers obtained from three different water-retting treatments was expressed in terms of three color components, which are shown in Table 3.
Fig. 1. SEM micrographs of cross-sections of (a) 2-day water-retted and (b) 6-day water-retted flax straws. Scale bars equal 250 µm.
Table 3. Color of Water-Retted Flax Fibers with Different Retting Durations
Generally speaking, extending the water retting duration significantly increased the whiteness of the flax fibers (P<0.05), and there was no significant change in the redness or yellowness of the fibers. The water retting is able to improve the whiteness of fibers because colored materials and contaminating substances, such as dust, dissolve and settle in the retting water (Sharma and Van Sumere 1992) at the beginning retting stage, which darkens the water’s color but brightens the color of the flax fibers, as indicated in Fig. 2a; as the retting progressed, the color of the water became whiter, and the color change gradually stabilized due to the dissolved pectin components degraded by the enzymes (shown in Fig. 2a and 2b), which still contributed to a slight color change of the fibers. Based on the research conducted by Akin et al. (2000a), the flax fibers collected in this study after 6 days of water retting had a color that would have been reasonably expected.
a
b
Fig. 2. A comparison of retting water color from different days of the process: (a) 0.5 days, 3.5 days, and 4.5 days; and (b) 7 days and 9 days.
Linear Density of Fibers
The linear densities of the retted and the non-retted flax fiber were measured, and the results are shown in Table 4.
Table 4. Linear Density of Non-Retted and Water-Retted Flax Fibers
The linear density is the index of the fibers fineness (Montalvo and Von Hoven 2004), and the residual non-cellulose content of the extracted fibers is correlated with fineness (Beltran et al. 2002). It is shown in Table 4 that extending the duration of water retting significantly reduced the linear density, thereby improving the fineness of the fibers (P<0.05); it was observed that 10 days of water retting yielded the finest fibers, an improvement of 38% in comparison to that of raw flax fibers. Compared to the fineness of flax fibers produced from warm water-retting at 32 °C for 72 h (Nair et al. 2013), the retted fibers in this study had larger linear density, which is possibly because the obtained and selected fibers were coarser due to hand-extraction, especially the 2-day retted fibers.
It should be mentioned that the results concerning the linear density of flax fibers were consistent with the results for weight loss and degumming rate presented previously, suggesting that the reduction in non-cellulosic materials during water retting improved the fineness of flax fibers.
Tensile Properties of Fibers
The tensile parameters of the tensile strength, elongation at break, and Young’s modulus of fibers obtained from non-retted and water-retted flax are presented in Table 5.
Table 5. Tensile properties of Non-Retted and Water-Retted Fibers
The data in Table 5 indicate that the tensile strength did not differ significantly between raw flax fibers and fibers water-retted for 2 days, and between 6-day water-retted and 10-day water-retted fibers (P>0.05), perhaps because of the large coefficients of variance of these tensile strength values. However, the tensile strength of fibers tended to increase with the increase in duration of water retting. The Young’s modulus of water-retted flax fibers improved significantly (P<0.05) compared to that of non-retted fibers. A similar tendency for tensile parameters (strength, modulus and strain) of flax fibers dew-retted for 1, 9 and 19 days was found in previous research (Martin et al. 2013), and since different variety of flax used and coarser fibers tested, i.e. fiber with larger bundle/diameter, the tensile strength and Young’s modulus of fibers in this study were lower than that in this reference research, but still in the average ranges (Faruk et al. 2012).
Since higher cellulose content causes better tensile performance (Alix et al. 2009), extending the water-retting duration in this study must have resulted in lower contents of pectin and non-cellulosic substances, as demonstrated previously, thereby improving the tensile strength and Young’s modulus of the fibers.
The elongation at break of the fibers shows that the failure strain of the water-retted fibers was lower than that of raw fibers, and those values from the fibers water-retted for different durations did not differ significantly (P>0.05). Compared to the retted fibers, the raw fibers were generally found in larger fiber bundles, whereas the retted fibers took the form of smaller bundles, split from larger ones due to the retting process. When subjected to tensile testing, the larger fiber bundle containing more elementary fibers glued by pectin demonstrated a larger elongation at break, a similar phenomenon found by Sankari (2000). In addition, the treatment of pectin at 65% RH prior to tensile testing may result in improved elasticity of fibers.
CONCLUSIONS
- The degumming rate and weight loss increased significantly as the water retting duration was extended; however, the first 2 days of water retting contributed to the large percentage of degumming rate and overall weight loss in these for 6 and 10 days of water retting, and these values had become stable after 6 days of water retting.
- The dissolution of pectin and non-cellulosic substances and the degradation of pectin both contributed to the weight loss and degumming rate; therefore, the water used to ret bast fiber plants should be chosen carefully.
- The whiteness and fineness of fibers were improved significantly by increasing the water-retting duration, which also increased the tensile performance of the fibers but decreased the failure elongation in a mild way.
- From the point view of retting quality, a retting duration of 6 days should be suitable for retting flax straws by distilled water at room temperature, a period after which good retting efficiency and fibers of reasonable color, fineness, and tensile properties were obtained.
ACKNOWLEDGMENTS
The first author is thankful to the Chinese Scholarship Council for financial support during her joint Ph.D. study at University of McGill, Canada. Many thanks also go to Dr. Darwin Lyew for his kind help and advice, to Dr. Denis Rho and Gopu Raveendran Nair for providing the raw flax materials, to Mark Goettel for language editing, and to Bei Ma at IMAU for assistance in SEM analysis.
REFERENCES CITED
ASTM D1776-04. (2004). “Standard practice for conditioning and testing textiles,” American Society for Testing and Materials, West Conshohocken, PA, USA.
Akin, D. E., Epps, H. H., Archibald, D. D., and Sharma, H. S. (2000a). “Color measurement of flax retted by various means,” Textile Research Journal 70(10), 852-858. DOI: 10.1177/004051750007001002
Akin, D. E., Himmelsbach, D. S., and Morrison III, W. H. (2000b). “Biobased fiber production: Enzyme retting for flax/linen fibers,” Journal of Polymers and the Environment 8(3), 103-109. DOI: 10.1023/A:1014886631052
Alix, S., Goimard, J., Morvan, C., and Baley, C. (2009). “Influence of pectin structure on the mechanical properties of flax fibres: A comparison between a linseed-winter variety (Oliver) and a fibres-spring variety of flax (Hermès),” in: Pectins and Pectinases, H. A. Schols, R. G. F. Visser, and A. G. J. Voragen, (eds.), Wageningen Academic Publishers, The Netherlands, pp. 87-96. DOI: 10.3920/978-90-8686-677-9
Beltran, R., Hurren, C. J., Kaynak, A., and Wang, X. (2002). “Correlating the fineness and residual gum content of degummed hemp fibres,” Fibers and Polymers 3(4), 129-133. DOI: 10.1007/BF02912656
Billmeyer, F. W., and Saltzman, M. (1981). Principles of Color Technology, J. Wiley & sons, New York.
Charlet, K., Jernot, J. P., Eve, S., Gomina, M., and Bréard, J. (2010). “Multi-scale morphological characterisation of flax: From the stem to the fibrils,” Carbohydrate Polymers 82(1), 54-61. DOI: 10.1016/j.carbpol.2010.04.022
Daenekindt, A. (2004). “Flax, hemp and allied fibres in the world,” Euroflax Newsletter 21(1), 6-9.
Di Candilo, M., Bonatti, P., Guidetti, C., Focher, B., Grippo, C., Tamburini, E., and Mastromei, G. (2010). “Effects of selected pectinolytic bacterial strains on water-retting of hemp and fibre properties,” Journal of Applied Microbiology 108(1), 194-203. DOI: 10.1111/j.1365-2672.2009.04409.x
Faruk, O., Bledzki, A. K., Fink, H.-P., and Sain, M. (2012). “Biocomposites reinforced with natural fibers: 2000–2010,” Progress in Polymer Science 37(11), 1552-1596. DOI: 10.1016/j.progpolymsci.2012.04.003
Fila, G., Manici, L., and Caputo, F. (2001). “In vitro evaluation of dew-retting of flax by fungi from southern Europe,” Annals of Applied Biology 138(3), 343-351. DOI: 10.1111/j.1744-7348.2001.tb00119.x
Foulk, J., Akin, D., Dodd, R., and Ulven, C. (2011). “Production of flax fibers for biocomposites,” in: Cellulose Fibers: Bio- and Nano-Polymer Composites, S. Kalia, B. S. Kaith, and I. Kaur, (eds.), Springer Berlin Heidelberg, London New York, pp. 61-95. DOI: 10.1007/978-3-642-17370-7_3
Foulk, J. A., Akin, D. E., and Dodd, R. B. (2008). “Influence of pectinolytic enzymes on retting effectiveness and resultant fiber properties,” BioResources 3(1), 155-169. DOI: 10.15376/biores.3.1.155-169
Hansen, K. M., Thuesen, A. B., and Soderberg, J. R. (2001). “Enzyme assay for identification of pectin and pectin derivatives, based on recombinant pectate lyase,” Journal of AOAC International 84(6), 1851-1854.
Henriksson, G., Akin, D. E., Rigsby, L. L., Patel, N., and Eriksson, K.-E. L. (1997). “Influence of chelating agents and mechanical pretreatment on enzymatic retting of flax,” Textile Research Journal67(11), 829-836. DOI: 10.1177/004051759706701107
Hrabalova, M., Schwanninger, M., Wimmer, R., Gregorova, A., Zimmermann, T., and Mundigler, N. (2011). “Fibrillation of flax and wheat straw cellulose: Effects on thermal, morphological, and viscoelastic properties of poly (vinylalcohol)/fibre composites,” BioResources 6(2), 1631-1647. DOI: 10.15376/biores.6.2.1631-1647
Huber, D. J. (1991). “Acidified phenol alters tomato cell wall pectin solubility and calcium content,” Phytochemistry 30(8), 2523-2527. DOI: 10.1016/0031-9422(91)85093-F
Kashyap, D. R., Vohra, P. K., Chopra, S., and Tewari, R. (2001). “Applications of pectinases in the commercial sector: A review,” Bioresource Technology 77(3), 215-227. DOI: 10.1016/S0960-8524(00)00118-8
Konczewicz, W., Kryszak, N., Nowaczkiewicz, E., Kozlowski, R., Wojtysiak, J., and Podsiedlik, W. (2013). “Osmosis phenomena based degumming of bast fibrous plants as a promising method in primary processing,” Molecular Crystals and Liquid Crystals 571(1), 116-131. DOI: 10.1080/15421406.2012.703912
Liu, M., Fernando, D., Daniel, G., Madsen, B., Meyer, A. S., Ale, M. T., and Thygesen, A. (2015). “Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers,” Industrial Crops and Products 69, 29-39. DOI: 10.1016/j.indcrop.2015.02.010
Martin, N., Mouret, N., Davies, P., and Baley, C. (2013). “Influence of the degree of retting of flax fibers on the tensile properties of single fibers and short fiber/polypropylene composites,” Industrial Crops and Products 49, 755-767. DOI: 10.1016/j.indcrop.2013.06.012
Montalvo, J. G., and Von Hoven, T. (2004). “Analysis of cotton,” in: Near-Infrared Spectroscopy in Agriculture, C. A. Roberts, J. Workman Jr, and J. B. Reeves III, (eds.), American Society of Agronomy Inc., Crop Science Society of America Inc., Soil Science Society of America Inc., Madison, Wisconsin, pp. 671-728. DOI: 10.2134/agronmonogr44.c25
Nair, G. R., Singh, A., Zimniewska, M., and Raghavan, V. (2013). “Comparative evaluation of physical and structural properties of water retted and non-retted flax fibers,” Fibers 1(3), 59-69. DOI: 10.3390/fib1030059
Rihouey, C., Jauneau, A., Cabin-Flaman, A., Demarty, M., Lefebvre, F., and Morvan, C. (1995). “Calcium and acidic pectin distribution in flax cell walls: Evidence for different kinds of linkages in the cell junction and middle lamella of the cortical parenchyma of flax hypotocyl,” Plant Physiology and Biochemistry 33(4), 497-508.
Sankari, H. S. (2000). “Comparison of bast fibre yield and mechanical fibre properties of hemp ( Cannabis sativa L.) cultivars,” Industrial Crops and Products 11(1), 73-84. DOI: 10.1016/S0926-6690(99)00038-2
Sharma, H. S. S., and Van Sumere, C. F. (1992). “Enzyme treatment of flax,” Genetic Engineer and Biotechnologist (United Kingdom) 12(2), 19-23.
Sojoudiasli, H., Heuzey, M.-C., and Carreau, P. J. (2014). “Rheological, morphological and mechanical properties of flax fiber polypropylene composites: Influence of compatibilizers,” Cellulose 21(5), 3797-3812. DOI: 10.1007/s10570-014-0375-3
Tahir, P. M., Ahmed, A. B., SaifulAzry, S. O. A., and Ahmed, Z. (2011). “Retting process of some bast plant fibres and its effect on fibre quality: A review,” BioResources 6(4), 5260-5281. DOI: 10.15376/biores.6.4.5260-5281
Wang, B., Panigrahi, S., Tabil, L., and Crerar, W. (2007). “Pre-treatment of flax fibers for use in rotationally molded biocomposites,” Journal of Reinforced Plastics and Composites 26(5), 447-463. DOI: 10.1177/0731684406072526
Article submitted: December 16, 2014; Peer review completed: March 5, 2015; Revisions received and accepted: April 20, 2015; Published: April 27, 2015.
DOI: 10.15376/biores.10.2.3553-3563
