Abstract
A novel natural fiber derived from the Cymbopogan citratus plant was investigated for the first time. The characterization of the C. citratus fibers was conducted, and the chemical composition and physical, thermal, mechanical, crystallinity, and morphological characteristics were studied. The chemical composition analysis of Cymbopogan citratus fiber revealed that the suggested fiber was rich in cellulose contents (37.6%). The tensile test of C. citratus fiber demonstrated the fiber’s average tensile strength of 43.81 ± 15.27 MPa and modulus of elasticity of 1.046 ± 0.33 GPa. Further analysis with X-ray diffraction (XRD) confirmed that the crystallinity index of Cymbopogan citratus fiber was 35.2%, and the crystalline size was estimated as 4.28 nm. The Cymbopogan citratus fiber’s thermal stability was investigated via thermogravimetric analysis (TGA) and observed to be thermally stable (230 °C). A morphological investigation was employed on the fiber via a scanning electron microscope (SEM). The morphological study result exhibited that the fiber had a perforated and rough surface with the lumen in the center. Thus, the findings revealed that the Cymbopogan citratus fiber was a promising potential reinforcement for thermoplastic green composite applications.
Download PDF
Full Article
Characterization of Natural Cellulosic Fiber Isolated from Malaysian Cymbopogan citratus Leaves
Zatil Hafila Kamaruddin,a,b Ridhwan Jumaidin,c,* Ahmad Ilyas Rushdan,d,e Mohd Zulkefli Selamat,c and Roziela Hanim Alamjuri f,*
A novel natural fiber derived from the Cymbopogan citratus plant was investigated for the first time. The characterization of the C. citratus fibers was conducted, and the chemical composition and physical, thermal, mechanical, crystallinity, and morphological characteristics were studied. The chemical composition analysis of Cymbopogan citratus fiber revealed that the suggested fiber was rich in cellulose contents (37.6%). The tensile test of C. citratus fiber demonstrated the fiber’s average tensile strength of 43.81 ± 15.27 MPa and modulus of elasticity of 1.046 ± 0.33 GPa. Further analysis with X-ray diffraction (XRD) confirmed that the crystallinity index of Cymbopogan citratus fiber was 35.2%, and the crystalline size was estimated as 4.28 nm. The Cymbopogan citratus fiber’s thermal stability was investigated via thermogravimetric analysis (TGA) and observed to be thermally stable (230 °C). A morphological investigation was employed on the fiber via a scanning electron microscope (SEM). The morphological study result exhibited that the fiber had a perforated and rough surface with the lumen in the center. Thus, the findings revealed that the Cymbopogan citratus fiber was a promising potential reinforcement for thermoplastic green composite applications.
Keywords: Cymbopogan citratus; Natural fiber; Mechanical properties; Crystallinity index; Thermogravimetric analysis; Scanning electron microscopy
Contact information: a: Fakulti Kejuruteraan Mekanikal, Universiti Teknikal Malaysia Melaka, Hang Tuah Jaya, 76100 Durian Tunggal, Melaka, Malaysia; b: German-Malaysian Institute, Jalan Ilmiah Taman Universiti, 43000 Kajang, Selangor, Malaysia; c: Fakulti Teknologi Kejuruteraan Mekanikal dan Pembuatan, Universiti Teknikal Malaysia Melaka, Hang Tuah Jaya, 76100 Durian Tunggal, Melaka, Malaysia; d: School of Chemical and Energy Engineering, Faculty of Engineering, Universiti Teknologi Malaysia, 81310 UTM, Johor Bahru, Johor, Malaysia; e: Centre for Advanced Composite Materials, Universiti Teknologi Malaysia, 81310 UTM Johor Bahru, Johor, Malaysia; f: Faculty of Tropical Forestry, Universiti Malaysia Sabah, Jalan UMS,88400 Kota Kinabalu, Sabah, Malaysia;
* Corresponding author: ridhwan@utem.edu.my; rhanim@ums.edu.my
GRAPHICAL ABSTRACT
INTRODUCTION
Global warming, extreme emissions, and natural resource scarcity are among the significant risks that will be faced by the future generations. Finding a bio-based material originating from a natural renewable source as a substitute for synthetic materials is one way to minimize the depletion of the earth’s raw materials and the impact on the environment. In recent decades, natural fibers are attractive due to their nature, e.g., biodegradable, abundant availability, low cost, non-toxic, low density, eco-friendly, recyclable, and having a high strength-to-weight ratio (Ilyas et al. 2018b; Jumaidin et al. 2020; Kamaruddin et al. 2020). The massive discrepancy between the existing demand and the cultivation rate of conventional fibers has prompted the emergence of new natural fibers that are chemically comprised of cellulose, hemicellulose, lignin, wax, ash, and other soluble substances (Senthamaraikannan and Kathiresan 2018). Cellulose is classified as a linear polysaccharide polymer composed of glucose monosaccharide unit groups. In general, the high content of cellulose in natural fibers gives the fibers more strength and stability (Ishak et al. 2010). When characterizing a new natural fiber, its crystallinity index value is normally computed to determine its degree of crystalline or amorphous nature. The natural fibers’ mechanical, physical, and chemical properties vary from each other because of variations in the chemical composition, maturity, and the plant’s part of the derived fiber, location of the plant cultivation, soil micronutrients level, and environmental conditions (Saravanakumar et al. 2013). From the same work, the natural fibers’ importance was discovered from a comprehensive literature review. A previous study found that the percentages of the fiber cellulose content and the crystallinity index directly affected the effectiveness (Ilyas et al. 2018). Using natural fibers as reinforcements in composite materials development is effective, as confirmed by Hyness et al. (2018). They also reported that the percentage of cellulose and the value of the crystallinity index influence the fiber’s effectiveness. However, the crystalline cellulose and amorphic fractions of natural fibers (hemicellulose, lignin, and wax) vary relying on the place and conditions of the plant growing. The polymer composites’ characteristics depend on a few factors: resin nature, the fiber alignment, as well as the strong attachment between the fiber and the matrix (Chakravarthy et al. 2020).
Cymbopogan citratus is an aromatic member of the Poaceae family. It is versatile due to the massive available products that can be produced from this plant (Ranitha et al. 2014). Throughout Malaysia, the Cymbopogan citratus plant can be widely cultivated in home gardens in the rural areas of Johor, Kedah, Kelantan, Perak, Pahang, Selangor, and Negeri Sembilan (Wifek et al. 2016). The plant can be generally found throughout the country because this species naturally grows in various areas. The fresh consumption of Cymbopogan citratus is increasing every year. The Cymbopogan species from China have been reported to have potential as a reinforcement in composite materials (Bekele et al. 2017). In China, there are various species of Cymbopogan such as Cymbopogan goeringii, Cymbopogan tortilis, and Cymbopogon mekongensis (Huang et al. 2013). Unlike in China, Cymbopogan citratus is the main species in Malaysia. Usually, the Cymbopogan citratus leaves are disposed of as waste materials. To exclusively consume the waste in manufacturing useful products, the current study focuses on the fiber originated from Cymbopogan citratus leaves of Malaysia. Generally, there are many varieties of Cymbopogan, e.g., Cymbopogan flexuosus, Cymbopogon nardus, Cymbopogan winterianus (Jowitt), etc. (De Oliveira et al. 2011; Biosci et al. 2015).
In general appearance, the Cymbopogan citratus leaves resemble those of other grasses that grow in large tufts. Each leaf is composed of two distinct components: the split sheath and the blade, which are connected at the blade joint. Based on the previous study, the anatomy of the leaf epidermis is similar to that of grasses in general, with an alternating band pattern related to the tissues beneath it. The epidermis is composed of three distinct cell types: (1) long cells, (2) suberized short cells, and (3) silica cells. In addition, there are the bulliform cells of the upper epidermis. The long cells are rectangular in shape, with occasionally pointed or concave ends. Surface preparations stained with chlorozinciodide revealed that the walls of the long cells are bright blue in color and that the middle lamella is undulating and strongly silicified, resulting in a white wavy line across the surface. The long cells vary in sizes, measuring in the intercostal region of the blade up to 63 μm long, 17 μm wide, and 14 μm high, respectively. Meanwhile, the short cells usually occur in pairs with the long cells. The cells typically are rectangular in shape, especially when lying over the veins. However, they can occasionally be broadly circular or even triangular. The silica cells have a greater degree of uniformity. They are typically rectangular and parallel to the leaf axis; however, they can be oval, rounded, or even broader than long. They typically appear somewhat constricted in the middle when viewed from the surface. Silica cells do not exist in the blade’s lower epidermis (Balbaa and Johnson 1955; Bertea et al. 2003; Eltahir and Abu Reish 2010; Shah et al. 2012; Yeşil and Akalin 2015).
A recent study was conducted on natural fibers, including thermal, mechanical, morphological properties, as well as chemical composition (Ishak et al. 2012; Yusriah et al. 2014). Yusriah et al. (2014) discussed the impact of betel nut husk (BNH) maturation (raw, ripe, and matured) on its characteristics of mechanical, physical, thermal, and morphological properties. The same work discovered that the fiber of the ripe type has the most excellent tensile strength. The sugar palm fibers’ tensile and thermal properties were characterized, and the greatest tensile strength is obtained from green fiber due to optimum chemical composition, which includes a high cellulose content, as well as hemicellulose and lignin (Ishak et al. 2012).
Even though there are studies reporting on the characterization of lignocellulosic fibers as reinforcement in composite materials, none has been found on the characterization of natural cellulosic fiber obtained from Malaysian Cymbopogan citratus leaves. Therefore, the objective of this work was to understand the chemical, physical, mechanical, and thermal properties of the Malaysian Cymbopogan citratus fibers in comparison to other natural fibers known. The findings obtained through this study will be beneficial to understand the potential of this natural fiber for various biodegradable materials applications. Though, to this date, there have been few investigations on the utilization and the application of Cymbopogan citratus fiber and its composites. In the present work, the properties of Malaysian Cymbopogan citratus fiber were characterized via a series of testing for their properties that include density, chemical composition, mechanical properties, thermal stability, morphology, and functional groups. The findings were presented and compared with other natural fibers.
EXPERIMENTAL
Materials
The Cymbopogan citratus plant leaves used in this study were collected from trees located in Beranang, Selangor (Malaysia). The leaf lamina is linear and green in color.
Preparation of Fibers
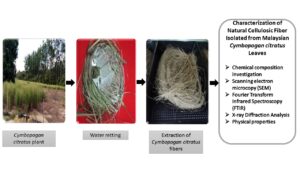
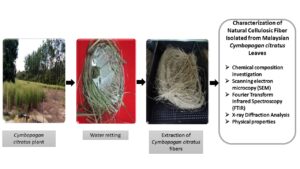
Unwanted particles were eliminated from the surface of the collected Cymbopogan citratus by washing with fresh water. Next, the cleaned leaves were retted by immersing them in normal fresh water for the microbial degradation process for 14 d in a water tank. The materials in the tank were periodically monitored to ensure that fibers were loosened to be extracted. The fibers were manually removed from the leaves after the retting process. To remove the dust particles, if any, the collected fiber was washed using distilled water and later sun-dried for 48 h before oven-dried for another 24 h at 85 °C to further eliminate the residual moisture (Huzaifah et al. 2017). Approximately 1 kg of fresh Cymbopogan citratus leaves were required to yield about 30 g of fibers. The flow processes of water retting are displayed in Fig. 1 (a) through (d).
Fig. 1. (a) Cymbopogan citratus plant, (b) water retting of Cymbopogan citratus fibers, (c) extraction of Cymbopogan citratus fibers, and (d) Cymbopogan citratus fibers
The Cymbopogan citratus fiber’s chemical composition was evaluated via neutral detergent fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL), as well as ash content analysis (Razali et al. 2015). Using Eqs. 1 and 2, the cellulose and hemicellulose percentages can be determined respectively:
Cellulose = ADF – ADL (1)
Hemicellulose = NDF – ADF (2)
Physical Properties
Cymbopogan citratus fibers diameter was evaluated under an optical microscope, Zeiss (Axiovert 200; Carl Zeiss Light Microscopy, Gottigen, Germany) that measured 15 individual fiber samples. The average diameter was randomly measured at three different positions of each image and the average value was determined (Razali et al. 2015).
Density
Cymbopogan citratus fiber density was measured using an AccuPyc 1340 TEC pycnometer (Micromeritics Instrument Corporation, Norcross, GA, USA). This pycnometer was employed to measure the volume and density in accordance with ASTM D792 (2008) of powdered fiber. This experiment utilized helium gas at a constant temperature. Three replicates of analysis were considered for this sample, and an average value was used for data analysis (Ilyas et al. 2017).
Moisture content evaluation was performed for five samples that were prepared and heated in the oven for a period of 24 h at a temperature of 105 °C. Moisture content calculation requires the initial weight prior to heating (Mi, g) as well as final weight after heating (Mf, g) (Ilyas et al. 2017). Next, the Cymbopogan citratus fiber’s moisture content was calculated using Eq. 3:
(3)
Water absorption
Five Cymbopogan citratus fiber specimens with a length range of 40 mm to 50 mm were prepared, and each sample’s average water absorption percentage was computed using Eq. 4. The specimens were weighed at room temperature before (Mi) and after (Mf) soaking in fresh water for 24 h at room temperature (Huzaifah et al. 2017), as shown in Eq. 4:
(4)
Tensile Properties
The ASTM D3379 (1998) guideline was adopted for the evaluation of the tensile properties of Cymbopogan citratus fiber by using Instron universal testing machine (5556, Noorwood, MA, USA) with 5 kN load cell capacity. Experimentation was performed with 25 mm gauge length and 1 mm/min crosshead speed. The Cymbopogan citratus fiber was carefully chosen under an optical microscope prior to the testing to guarantee that there was no damage to the fibers (Razali et al. 2015). Each fiber was fastened on the sample holder as displayed in Fig. 2, and the Cymbopogan citratus fibers with 15 replicates were used for the measurements of tensile characteristics.
Fig. 2. Sample preparation for tensile test
Thermal Characterization
Thermogravimetric analysis (TGA)
For the study of thermal stability of the Cymbopogan citratus fiber, a Thermogravimetric analyzer from TA Instruments (Mettler-Toledo AG, Schwerzenbach, Switzerland) was deployed to investigate the strength of the fibers for high-temperature applications with the thermal analysis Q series. The needed amount of Cymbopogan citratus fibers was weighed and put inside an alumina crucible and placed in a heating chamber. The study was carried out in a nitrogen environment from 25 °C to 900 °C at a 10 °C/min constant heating rate. Before the thermal study, the samples were pre-conditioned for at least 2 d at 53% (Jumaidin et al. 2017).
Scanning Electron Microscopy (SEM)
The cross-section and morphology of the Cymbopogan citratus fibers were conducted using a scanning electron microscope (SEM) instrument model Hitachi S-3400N (Kyota, Japan). The acceleration voltage used was 15 kV, and the surface of the Cymbopogan citratus fibers was pre-coated using a thin gold layer. The sample was 50 mm long and the results were acquired by enlarging at different magnifications (Arul Marcel Moshi et al. 2020).
Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) investigation of all samples was performed to determine the functional groups’ presence in the Cymbopogan citratus fiber. This analysis was performed on an IR spectrometer (Nicolet 6700 AEM, Thermo Nicolet Corporation, Madison WI, USA) experimental setup. The samples for this examination were powdered and mixed with potassium bromide. An approximate 2 mg of powdered sample was combined with potassium bromide (KBr) and later pressed into a 1-mm-thick disc. The samples’ FTIR spectra were recorded in the range of 4000 to 400 cm-1 (Jumaidin et al. 2020).
X-ray Diffraction Analysis (XRD)
The X-ray diffraction approach was chosen to evaluate the crystallinity index and crystalline size of the Cymbopogan citratus fiber using a Rigaku D/max 2500 X-ray powder diffractometer (Rigaku, Tokyo, Japan) with Cu radiation run at 40 kV and 30 mA with 0.15406 nm light wavelength. The scanning rate of 2 °min-1 in the range of diffraction angle 10° to 40° at room temperature was used to scan the samples. The crystallinity index (CI) of Cymbopogan citratus fiber was computed using subsequent Segal expression Eq. 5 (Ilyas et al. 2018a),
(5)
where I002 and Iam represent peak intensities of the crystalline and amorphous fractions, respectively. The crystallite size (CS) was determined using Scherrer’s formula shown in Eq. 6,
(6)
where k = 0.89 (Scherrer’s constant), λ = 0.1541 nm is the radiation wavelength, β is the peak’s full-width at half-maximum in radians, and θ is the corresponding Bragg angle.
RESULTS AND DISCUSSION
Chemical Composition
Natural fiber contains three main constituents, e.g., celluloses, hemicellulose, and lignin. Cellulose is known to be the key framework portion of the fiber structure. Hemicellulose and lignin compounds cover cellulose domains and give fiber strength, rigidity, and structural stability (Kabir et al. 2012). The natural fibers’ chemical composition analysis is vital in determining the fibers’ mechanical strength that is influenced by the quantity of numerous chemical components present on the natural fibers’ surface (Moshi et al. 2020). The comparison of the results from the analysis of the chemical composition of the Cymbopogan citratus fiber with different natural fibers is presented in Table 1. A total of 37.6% cellulose was found on the fibers’ surface, of the Cymbopogan citratus fibers, which was relatively higher than other natural fibers, e.g., bamboo (26 to 43%) (Kulandaivel et al. 2020), Mikania micrantha Kunth (21%) (Ganapathy et al. 2019), piassava (28.6%) (Indran and Raj 2015), coconut tree leaf sheath (27%) (Indran and Raj 2015), and less than Carica papaya (58.71%) (Arul Marcel Moshi et al. 2020), Nendran banana (73.2%) (Manimaran et al. 2020), Saharan Aloe vera (67.4%) (Balaji and Nagarajan 2017; Manimaran et al. 2020), and sugar palm (44.53%) (Huzaifah et al. 2017).
Table 1. Chemical Composition of Cymbopogan citratus Fiber with Other Natural Fibers
Researchers in this field have stated that with a higher cellulose content, the mechanical properties comprising elasticity modulus and tensile strength are improved. However, the higher content of hemicellulose in natural fiber has an adverse effect on fiber strength (Ilaiya Perumal and Sarala 2020). In this study, a hemicellulose content of 29.3% was found on the surface of Cymbopogan citratus fiber. The lignin content of Cymbopogan citratus fiber was 11.1%; it acts as a protective guard against bacterial attacks (Balaji and Nagarajan 2017). The lower amount of ash (4.28%) for Cymbopogan citratus fiber can explain its mechanical properties, such that the Cymbopogan citratus fiber has the essential properties for use as a potential reinforcement material.
Physical Properties
Density and diameter
Table 2 shows the measurements obtained for the diameter of Cymbopogan citratus fiber. The diameter measurement is important because it is essential in determining the fiber’s mechanical properties, especially tensile strength. Measurement of the Cymbopogan citratus fiber’s diameter was conducted using an optical microscope, as displayed in Fig. 3. The fiber’s average diameter was calculated as 326.67 ± 45.77 μm, which was comparable with that red banana peduncle (150 to 250 μm) (Chakravarthy et al. 2020), Agave sisalana fiber (50 to 300 μm) (Moshi et al. 2020), and banana fiber (80 to 250 μm) (Moshi et al. 2020). The source of the fiber, plant condition, plant maturity, and extraction process are the key factors affecting the physical properties (Reddy and Yang 2005).
Fig. 3. Optical microscopy image of the Cymbopogan citratus fiber
Density is an essential factor for natural fibers mass determination and influences the mass of the natural fibers composite materials that will be produced. The density of Cymbopogan citratus fiber was measured using the pycnometer setup (Micromeritics Instrument Corporation, Norcross, GA, USA) and recorded as 0.251 ± 0.002 g/cm3, which was comparatively lesser than some other natural fibers, e.g., Bambusoideae (0.600 g/cm3) (Arul Marcel Moshi et al. 2020), Prosopis juliflora (0.58 g/cm3) (Saravanakumar et al. 2013), and Furcraea foetida (0.778 g/cm3) (Manimaran et al. 2018). The comparisons of diameter and density of Cymbopogan citratus fiber with different natural fibers are presented in Table 2. The density of Cymbopogan citratus fiber revealed that it will be a great candidate for producing lightweight composite products.
Table 2. Diameter and Density of Cymbopogan citratus Fiber and Other Natural Fibers
Moisture content
One of the primary factors that must be deliberated throughout the evaluation of a novel material as a possible reinforcing agent in polymer composites is moisture content. Low moisture content fibers will have a good bond between a composite material’s fiber and polymer matrix (Jayaramudu et al. 2010). The hydrophilic behavior of natural fibers has noticeable effects on their mechanical performance, dimensional stability, and porosity of natural fiber in a composite material, resulting from high moisture absorption in wet conditions (Razali et al. 2015). Hence, low moisture containing fibers are preferable for the application. As shown in Table 3, Cymbopogan citratus fiber recorded the moisture content of 5.20 ± 2.28, which was lower compared to other natural fibers (sugar palm, roselle, banana, flax, jute, sisal, ramie, and hemp) with moisture contents of approximately 5.5 to 22% (Akil et al. 2011; Razali et al. 2015; Huzaifah et al. 2017). Therefore, increasing the cellulose content percentage in the fibers will result in an increased moisture absorption rate. This phenomenon is associated with the cellulose chemical structure itself that consists primarily of water-accessible hydroxyl groups. Moisture and hemicellulose contents of natural fibers are key factors of their moisture absorption level, thermal, and biological degradation. Therefore, natural fiber’s low moisture content is the most preferable criterion for polymer composites. Moreover, high moisture content fibers cause some negative effects on the composites, e.g., degradation, cracking, mechanical properties loss, and inviting decay fungi (Sapuan et al. 2013).
Water absorption
Natural fibers possess numerous benefits as reinforcing materials and have relatively similar characteristics to synthetic fibers. The water absorption of Cymbopogan citratus fibers was computed based on the samples’ weights before and after their immersion in water for 24 h. The main common disadvantage of using natural fiber is its high hydroxyl cellulose content, making it prone to water absorption and negatively affecting the composites’ mechanical properties. This contributes to the hydrophilicity of the fibers resulting from rising water absorption with increasing cellulose composition because of the increasing free hydroxyl groups present in the fiber (Sahari et al. 2011). The water absorption is dependent on the fiber’s temperature, content, orientation, permeability, surface area exposed, void content, as well as hydrophilicity of each component (Akil et al. 2011). All lignocellulosic materials exhibit water absorption behavior due to the water molecules that are attracted to hydrophilic groups in the fiber and interact with the hydroxyl group (-OH) of cellulose molecules, forming hydrogen bonds. The results displayed in Table 3 indicate the value of water absorption for Cymbopogan citratus fiber compared to other natural fibers. From the results, the water absorption percentages of Cymbopogan citratus fiber were lower than roselle fiber but higher than sugar palm fiber. The size of the lumen in the Cymbopogan citratus fiber has a major impact on its water uptake behavior. As more lumens exist in the unit cell of Cymbopogan citratus, this creates simple passages of water molecules entry into the cell unit and spread deeper through the fiber’s cell wall, thereby increasing the water absorption of the Cymbopogan citratus fiber.
Table 3. Physical Properties of Cymbopogan citratus Fiber with Other Natural Fibers
Table 4 compares the tensile characteristics of Cymbopogan citratus fiber samples to those of different renowned natural fibers. The average single fiber’s tensile strength, elasticity modulus, and elongation rate were 43.81 ± 15.27 MPa, 1.046 ± 0.33 GPa, and 0.84 ± 0.28%, respectively. The tensile strength of Cymbopogan citratus is dependent on plant age, source, method of fiber extraction, as well as fiber microstructure, where cracks initiated from the bigger flaw will lead to fiber failure (Bezazi et al. 2014). Hence, it is vital to test a minimum of 15 replications of each fiber sample to finalize the tensile properties. The high cellulose content and index of crystallinity are the main factors contributing towards achieving greater tensile strength in natural fibers (Saravana Kumaar et al. 2019). In the comparison with different natural fibers, although the strength of Cymbopogan citratus fiber was lower than Nendran banana (65.5 MPa) (Manimaran et al. 2020) and Sansevieria ehrenbergii (50 to 585 MPa) (Sathishkumar et al. 2013a), the tensile strength of Cymbopogan citratus showed a moderate value with coconut tree leaf sheath (46.4 MPa) (Kulandaivel et al. 2020) and oil palm fruit (49 MPa) (Arul Marcel Moshi et al. 2020). The Cymbopogan citratus fiber also revealed higher strength than the Pennisetum purpureum (13.2 MPa) (Arul Marcel Moshi et al. 2020) and aerial roots of the banyan tree (19.4 MPa) (Ganapathy et al. 2019). Natural fibers possess similar morphology; however, factors, e.g., the lumens’ internal area, lumen number, fiber cells’ number and size, secondary cell walls’ thickness, and actual cross-section of the cells, differ from each other. The cross-sectional areas of each fiber are interrelated with its tensile behavior because they are not perfectly circular and exhibit some variations. Furthermore, the results of tensile tests can be influenced not only by morphology but also by chemical composition. The most important factor in determining the fiber’s strength is the cellulose content. For example, the Nendran banana and piassava had approximately 73.2% and 28.6% cellulose content, respectively (Indran and Raj 2015; Manimaran et al. 2020). Therefore, the Nendran banana might experience greater strength that was not only because of its morphological characteristics but also its higher cellulose content. From the tensile test results, there is a possibility for the Cymbopogan citratus fiber to be applied as reinforcement for polymer composites.
Table 4. Tensile Properties of Cymbopogan citratus Fiber with Other Natural Fibers
Natural fibers have limited thermal stability at high temperatures, which is one of the drawbacks of their application as a reinforcing agent in structural composite materials. The TGA studied the Cymbopogan citratus fiber’s thermal behavior and determined the suitability of the Cymbopogan citratus for high-temperature structural applications. From the TGA curves (Fig. 4), there were three major degradation phases. The first degradation corresponded to the vaporizations of highly volatile components and moisture from the fiber. The earliest devolatilization took place at a temperature of 30 °C to approximately 110 °C due to the vaporization of water or moisture as reported by Ilyas et al. (2017). Moisture evaporation from the Cymbopogan citratus fiber occurred between 80 and 95 °C and the weight loss of the fibers was 14.2%, as shown in Table 5.
The second degradation peaks were found at 280 and 340 °C, which indicated the elimination of hemicellulose from the fiber’s surface and the mass loss of around 44.73%. The typical hemicellulose decomposition occurs at 220 °C and becomes complete at 315 °C (Yang et al. 2007). The result attained from this work was parallel with the reported hemicellulose degradation data, where the hemicellulose degradation took place from 280 °C and was completed at approximately 300 °C, as displayed in Fig. 4. The first component to degrade during thermal analysis is hemicellulose because it comprises heterogeneous polysaccharides, e.g., galactose, xylose, glucose, and mannose that exist in the amorphous state, and hence are not difficult to devolatilize at low temperatures (Yang et al. 2007).
The next thermal decomposition was cellulose as demonstrated by the clear U-shaped peak found at 338 °C from the derivative thermogravimetry (DTG) graph displayed in Fig. 4 occurred from 280 to 340 °C. The last component decomposed is lignin degradation from the fiber’s surface. Lignin and other non-cellulosic chemical components degraded as confirmed by the final clearly observable thermal degradation peak near 490 °C. The leftover material after complete lignin degradation is called the residual mass. For Cymbopogan citratus fiber, the residual mass was in the range of 15 to 20%. Overall, the thermal stability of the Cymbopogan citratus fiber was concluded to be similar to other well-known bast fibers, such as kenaf, roselle, sugar palm, and jute, as shown in Table 6.
Table 5. Thermal Degradation Analysis of Cymbopogan Citratus Fiber
Fig. 4. TG and DTG curves for Cymbopogan citratus fiber
Table 6. Temperatures of Degradation for Selected Natural Fibers
Morphological Properties
Natural fibers’ morphology is among the important factors influencing the mechanical and physical characteristics of the fibers and indicates their suitability as reinforcing material for composites. The Cymbopogan citratus fiber’s cross-section was examined under SEM, and results are shown in Fig. 5. The water absorption greatly depends on lumen size as the larger lumen and porous structures make it much easier for the fiber to absorb more water and promote good penetration when used as polymer composite’s reinforcement.
Fig. 5. SEM images of Cymbopogan citratus fiber in cross-sectional view
The Cymbopogan citratus fiber’s surface morphology was examined at different magnifications and the surface fiber’s images in the longitudinal view are displayed in Fig. 6 (a) and (b).
Fig. 6(a). SEM images of the surface structure of Cymbopogan citratus fiber (a) 200X magnification and (b) 500X magnification
Fig. 6(b). SEM images of the surface structure of Cymbopogan citratus fiber (a) 200X magnification and (b) 500X magnification
The SEM images of the surface showed irregular rough surface structure and the presence of pores of the fiber. It is predicted to promote better bondability with the polymer as well as enhancing mechanical interlinking between fiber and polymer matrix in the composite (Maheshwaran et al. 2018; Chakravarthy et al. 2020). Hence, the Cymbopogan citratus fiber was shown to be an excellent candidate for reinforcement in polymer composites. Each unit cell of fiber comprises cellulose surrounded by lignin and hemicellulose. At higher magnification, the hemicellulose was observed on the fiber’s surface indicated by the image of a white layer that might be simply eradicated with the fiber’s alkali treatment. The treatment also resulted in the improvement of the mechanical characteristics of the Cymbopogan citratus fiber by facilitating the elimination of hemicellulose as well as other undesired contaminants from the surface of the Cymbopogan citratus fiber (Moshi et al. 2020).
FTIR Analysis
The FTIR spectrum obtained from Cymbopogan citratus fiber is shown in Fig. 7, in which noticeable FTIR peak positions, the composition of the Cymbopogan citratus fiber, and chemical functional groups were revealed. From Fig. 7, a peak was detected at 3287 cm-1, indicating the presence of the O-H chemical bonds in carboxylic acid group cellulose constituents (Ilaiya Perumal and Sarala 2020; Vijay et al. 2020). The next two consecutive peaks nearby for Cymbopogan citratus fiber were noticed at 2917 cm-1 and 2850 cm-1 corresponded to the C-H stretching vibration of CH and CH2, which indicated the presence of cellulose and hemicellulose (Ilyas et al. 2017; Kulandaivel et al. 2020). A band peak at 2000 cm-1 was associated with CH2 symmetrical stretching of wax. A small observable peak at 1641 cm-1 was ascribed to the hemicellulose components (Gurukarthik Babu et al. 2019). The peak at 1517 cm-1 indicated the existence of a small number of moisture particles, and the peak at 1033 cm-1 was ascribed to lignin in the form of C-OH molecules (Manimaran et al. 2020). Table 7 provides FTIR peak locations and distributions of stretching in the Cymbopogan citratus fiber.
Table 7. FTIR Peak Positions and Chemical Stretching Allocations in the Cymbopogan citratus Fiber
Fig. 7. FTIR spectrum analyses for Cymbopogan citratus fiber
Figure 8 depicts the X-ray diffractogram of the Cymbopogan citratus fiber. Two clearly observed peaks were found in the XRD spectra of Cymbopogan citratus fiber at around 2θ = 16.39° and 22.01°. The first peak was noted at a near 16.39° diffraction angle, which indicated the existence of amorphous components in Cymbopogan citratus fiber. The next peak showed the crystalline constituents in the Cymbopogan citratus fiber. The high crystallinity index (CI value) of the fiber showed excellent mechanical properties and molecular arrangement (Indran et al. 2014). The degree of crystallinity in the fiber structure can be recognized by observing the diffraction peak’s sharpness, where the sharper the peak of diffraction, the higher the fiber’s degree of crystallinity (Alemdar and Sain 2008). The CI value of the Cymbopogan citratus fiber was computed in accordance with Segal empirical method, as shown in Eq. 5, which was 35.2% and the crystalline size was estimated as 4.28 nm. The CI value of the Cymbopogan citratus fiber was similar to other natural fibers as displayed in Table 8, which was similar to oil palm fruit (34.1%) (Jebadurai et al. 2019), Calotropis gigantea fruit bunch (36%) (Narayanasamy et al. 2020), and Tridax procumbens (34.5%) (Chakravarthy et al. 2020). The value also was lower than that of Saharan aloe vera cactus (52.6%) (Balaji and Nagarajan 2017) and tilifolia (41.7%) (Narayanasamy et al. 2020), jute (65.8%), sisal (71%), flax (80%), and hemp (88%) (Kulandaivel et al. 2020). Thus, the crystallinity value depends on the cellulose content and the types of plants. There are relationships between fiber stiffness, crystallinity degree region, as well as cellulose content. Increasing the crystallinity region will increase the fibers’ stiffness. Higher fiber crystallinity is interconnected with the fibers’ cellulose content. From the X-ray diffraction analysis, there is a possibility for the application of Cymbopogan citratus fiber as a reinforcement for polymer composites.
Table 8. Crystallinity of Cymbopogan citratus Fiber and Other Natural Fibers
Fig. 8. X-ray diffraction pattern of Cymbopogan citratus fiber
CONCLUSIONS
This study emphasizes the fibers extracted from the leaves of the Cymbopogan citratus plant in order to evaluate the possibility of using them as potential biodegradable materials and adds value to the existing knowledge on Cymbopogan citratus.
- The higher lignin content of the Cymbopogan citratus can offer relatively higher rigidity in comparison to the existing other natural fiber.
- The average tensile strength of Cymbopogan citratus from single fiber testing was 43.81 ± 15.27 MPa and the tensile modulus of Cymbopogan citratus fiber was found to be 1.046 ± 0.33 GPa, making it an ideal alternative reinforcement material to the conventional fibers.
- The density of the Cymbopogan citratus fiber was found to be lower in comparison with many other reported natural fibers and few synthetic fibers, which may enable lightweight applications.
- Thermogravimetric analysis indicated that the Cymbopogan citratus fiber was thermally stable up to 230 °C, which was estimated within the temperature range of 200 to 350 °C. Thus, the material is applicable for high-temperature applications.
- Furthermore, this study will be extended to identify the suitability of the Cymbopogan citratus fiber with biodegradable matrix derived from natural sources in order to analyze the mechanical and thermal properties of this new kind of composite, the fiber/matrix adhesion, and to evaluate the necessity of a chemical pre-treatment of the fibers. In conclusion, the findings showed that Cymbopogan citratus has potential as biodegradable materials, especially for reinforcement in bio-based composites.
ACKNOWLEDGMENTS
The authors would like to express sincere gratitude to Universiti Teknikal Malaysia Melaka and the Ministry of Higher Education Malaysia for the financial support provided through research grant RACER/2019/FTKMP-CARE/F00413. The article processing charge was funded by Universiti Malaysia Sabah and the proofreading was supported by incentive grant JURNAL/FTK/2018/Q00004. Thanks also are expressed to German Malaysian Institute for providing the scholarship award to the principal author in this project.
REFERENCES CITED
Akil, H. M., Omar, M. F., Mazuki, A. A. M., Safiee, S., Ishak, Z. A. M., and Abu Bakar, A. (2011). “Kenaf fiber reinforced composites: A review,” Materials and Design 32(8–9), 4107-4121. DOI: 10.1016/j.matdes.2011.04.008
Alemdar, A., and Sain, M. (2008). “Isolation and characterization of nanofibers from agricultural residues: Wheat straw and soy hulls,” Bioresource Technology 99(6), 1664-1671. DOI: 10.1016/j.biortech.2007.04.029
Arul Marcel Moshi, A., Ravindran, D., Sundara Bharathi, S. R., Padma, S. R., Indran, S., and Divya, D. (2020). “Characterization of natural cellulosic fiber extracted from Grewia damine flowering plant’s stem,” International Journal of Biological Macromolecules164, 1246-1255. DOI: 10.1016/j.ijbiomac.2020.07.225
ASTM D792 (2008). “Standard test methods for density and specific gravity (relative density) of plastics by displacement,” ASTM International, West Conshohocken, PA, USA
ASTM D3379 (1998). “Standard test method for tensile strength and young’s modulus for high modulus single filament materials,” ASTM International, West Conshohocken, PA, USA
Balaji, A. N., and Nagarajan, K. J. (2017). “Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves,” Carbohydrate Polymers 174, 200-208. DOI: 10.1016/j.carbpol.2017.06.065
Balbaa, S., and Johnson, C. (1955). “The microscopic structure of lemongrass leaves,” Journal of the American Pharmaceutical Association. American Pharmaceutical Association 44(2), 89-98. DOI: 10.1002/jps.3030440211
Bekele, L. D., Zhang, W., Liu, Y., Duns, G. J., Yu, C., Jin, L., Li, X., Jia, Q., and Chen, J. (2017). “Preparation and characterization of lemongrass fiber (Cymbopogon species) for reinforcing application in thermoplastic composites,” BioResources 12(3), 5664-5681. DOI: 10.15376/biores.12.3.5664-5681
Bertea, C. M., Tesio, M., D’Agostino, G., Buffa, G., Camusso, W., Bossi, S., Mucciarelli, M., Scannerini, S., and Maffei, M. (2003). “The C4biochemical pathway, and the anatomy of lemongrass (Cymbopogon citratus (DC) Stapf.) cultivated in temperate climates,” Plant Biosystems 137(2), 175-184. DOI: 10.1080/11263500312331351441
Bezazi, A., Belaadi, A., Bourchak, M., Scarpa, F., and Boba, K. (2014). “Novel extraction techniques, chemical and mechanical characterisation of Agave americana L. natural fibres,” Composites Part B: Engineering 66, 194-203. DOI: 10.1016/j.compositesb.2014.05.014
Biosci, I. J., Subramanian, P., Wan, C., Che, I., Takwa, W., and Ahmad, N. E. (2015). “Chemical composition and antibacterial activity of essential oil of Cymbopogon citratus and Cymbopogon nardus against Enterococcus faecalis,” International Journal of Biosciences (IJB) 6(9), 9-17. DOI: 10.12692/ijb/6.9.9-17
Chakravarthy, K. S., Madhu, S. M., Naga Raju, J. S., and Shariff, J. (2020). “Characterization of novel natural cellulosic fiber extracted from the stem of Cissus vitiginea plant,” International Journal of Biological Macromolecules 161, 1358-1370. DOI: 10.1016/j.ijbiomac.2020.07.230
De Oliveira, W. A., De Oliveira Periera, F., De Luna, G. C. D. G., Lima, I. O., Wanderley, P. A., De Lima, R. B., and De Oliveira Lima, E. (2011). “Antifungal activity of Cymbopogan Winterianus Jowitt ex Bor against Candida albicans,” Brazilian Journal of Microbiology 42(4), 433-441. DOI: 10.1590/S1517-83822011000200004
De Rosa, I. M., Kenny, J. M., Puglia, D., Santulli, C., and Sarasini, F. (2009). “Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites,” Composites Science and Technology 70(1), 116-122. DOI: 10.1016/j.compscitech.2009.09.013
Eltahir, A. S., and Abu Reish, B. I. (2010). “Leaf and stem anatomy of Cymbopogon citratus and Cymbopogon schoenanthus in Sudan,” Journal of Chemical and Pharmaceutical Research 3(4), 766-771. DOI: 10.13140/RG.2.2.14616.60165
Ganapathy, T., Sathiskumar, R., Senthamaraikannan, P., Saravanakumar, S. S., and Khan, A. (2019). “Characterization of raw and alkali treated new natural cellulosic fibres extracted from the aerial roots of banyan tree,” International Journal of Biological Macromolecules 138, 573-581. DOI: 10.1016/j.ijbiomac.2019.07.136
Gurukarthik Babu, B., Prince Winston, D., SenthamaraiKannan, P., Saravanakumar, S. S., and Sanjay, M. R. (2019). “Study on characterization and physicochemical properties of new natural fiber from Phaseolus vulgaris,” Journal of Natural Fibers 16(7), 1035-1042. DOI: 10.1080/15440478.2018.1448318
Huang, X. W., Feng, Y. C., Huang, Y., and Li, H. L. (2013). “Chemical composition, antioxidant and the possible use as skin-care ingredient of clove oil (Syzygium aromaticum (L.) Merr. & Perry) and citronella oil (Cymbopogon goeringii) from China,” Journal of Essential Oil Research 25(4), 315-323. DOI: 10.1080/10412905.2013.775082
Huzaifah, M. R. M., Sapuan, S. M., Leman, Z., and Ishak, M. R. (2017). “Comparative study on chemical composition, physical, tensile, and thermal properties of sugar palm fiber (Arenga pinnata) obtained from different geographical locations,” BioResources 12(4), 9366-9382. DOI: 10.15376/biores.12.4.9366-9382
Hyness, N. R. J., Vignesh, N. J., Senthamaraikannan, P., Saravanakumar, S. S., and Sanjay, M. R. (2018). “Characterization of new natural cellulosic fiber from Heteropogon Contortus plant,” Journal of Natural Fibers 15(1), 146-153. DOI: 10.1080/15440478.2017.1321516
Ilaiya Perumal, C., and Sarala, R. (2020). “Characterization of a new natural cellulosic fiber extracted from Derris scandens stem,” International Journal of Biological Macromolecules 165(Pt B), 2303-2313. DOI: 10.1016/j.ijbiomac.2020.10.086
Ilyas, R. A., Sapuan, S. M., and Ishak, M. R. (2018a). “Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata),” Carbohydrate Polymers 181, 1038-1051. DOI: 10.1016/j.carbpol.2017.11.045
Ilyas, R. A., Sapuan, S. M., Ishak, M. R., and Zainudin, E. S. (2017). “Effect of delignification on the physical, thermal, chemical, and structural properties of sugar palm fibre,” BioResources 12(4), 8734-8754. DOI: 10.15376/biores.12.4.8734-8754
Ilyas, R. A., Sapuan, S. M., Ishak, M. R., and Zainudin, E. S. (2018b). “Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties,” IOP Conference Series: Materials Science and Engineering 368(1), article ID 012006. DOI: 10.1088/1757-899X/368/1/012006
Indran, S., Edwin Raj, R., and Sreenivasan, V. S. (2014). “Characterization of new natural cellulosic fiber from Cissus quadrangularis root,” Carbohydrate Polymers 110, 423-429. DOI: 10.1016/j.carbpol.2014.04.051
Indran, S., and Raj, R. E. (2015). “Characterization of new natural cellulosic fiber from Cissus quadrangularis stem,” Carbohydrate Polymers 117, 392-399. DOI: 10.1016/j.carbpol.2014.09.072
Ishak, M. R., Leman, Z., Sapuan, S. M., Edeerozey, A. M. M., and Othman, I. S. (2010). “Mechanical properties of kenaf bast and core fibre reinforced unsaturated polyester composites,” IOP Conference Series: Materials Science and Engineering 11, article ID 012006. DOI: 10.1088/1757-899X/11/1/012006
Ishak, M. R., Sapuan, S. M., Leman, Z., Rahman, M. Z. A., and Anwar, U. M. K. (2012). “Characterization of sugar palm (Arenga pinnata) fibres tensile and thermal properties,” Journal of Thermal Analysis and Calorimetry 109(2), 981-989. DOI: 10.1007/s10973-011-1785-1
Jayaramudu, J., Guduri, B. R., and Varada Rajulu, A. (2010). “Characterization of new natural cellulosic fabric Grewia tilifolia,” Carbohydrate Polymers 79(4), 847-851. DOI: 10.1016/j.carbpol.2009.10.046
Jebadurai, S. G., Raj, R. E., Sreenivasan, V. S., and Binoj, J. S. (2019). “Comprehensive characterization of natural cellulosic fiber from Coccinia grandis stem,” Carbohydrate Polymers 207, 675-683. DOI: 10.1016/j.carbpol.2018.12.027
Jumaidin, R., Khiruddin, M. A. A., Asyul Sutan Saidi, Z., Salit, M. S., and Ilyas, R. A. (2020). “Effect of cogon grass fibre on the thermal, mechanical and biodegradation properties of thermoplastic cassava starch biocomposite,” International Journal of Biological Macromolecules 146, 746-755. DOI: 10.1016/j.ijbiomac.2019.11.011
Jumaidin, R., Sapuan, S. M., Jawaid, M., Ishak, M. R., and Sahari, J. (2017). “Effect of agar on flexural, impact, and thermogravimetric properties of thermo- plastic sugar palm starch,” Current Organic Synthesis 14(2), 200-205. DOI: 10.2174/15701794136661609211
Kabir, M. M., Wang, H., Lau, K. T., and Cardona, F. (2012). “Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview,” Composites Part B: Engineering 43(7), 2883-2892. DOI: 10.1016/j.compositesb.2012.04.053
Kamaruddin, Z. H., Sapuan, S. M., Mohamed Yusoff, M. Z., and Jumaidin, R. (2020). “Rapid detection and identification of dioscorine compounds in Dioscorea hispida tuber plants by LC-ESI-MS,” BioResources 15(3), 5999-6011. DOI: 10.15376/biores.15.3.5999-6011
Kulandaivel, N., Muralikannan, R., and KalyanaSundaram, S. (2020). “Extraction and characterization of novel natural cellulosic fibers from pigeon pea plant,” Journal of Natural Fibers 17(5), 769-779. DOI: 10.1080/15440478.2018.1534184
Li, X., Tabil, L. G., and Panigrahi, S. (2007). “Chemical treatments of natural fiber for use in natural fiber-reinforced composites: A review,” Journal of Polymers and the Environment 15(1), 25-33. DOI: 10.1007/s10924-006-0042-3
Maheshwaran, M. V., Hyness, N. R. J., Senthamaraikannan, P., Saravanakumar, S. S., and Sanjay, M. R. (2018). “Characterization of natural cellulosic fiber from Epipremnum aureum stem,” Journal of Natural Fibers 15(6), 789-798. DOI: 10.1080/15440478.2017.1364205
Manimaran, P., Pillai, G. P., Vignesh, V., and Prithiviraj, M. (2020). “Characterization of natural cellulosic fibers from Nendran banana peduncle plants,” International Journal of Biological Macromolecules 162, 1807-1815. DOI: 10.1016/j.ijbiomac.2020.08.111
Manimaran, P., Senthamaraikannan, P., Sanjay, M. R., Marichelvam, M. K., and Jawaid, M. (2018). “Study on characterization of Furcraea foetida new natural fiber as composite reinforcement for lightweight applications,” Carbohydrate Polymers 181, 650-658. DOI: 10.1016/j.carbpol.2017.11.099
Moshi, A. A. M., Ravindran, D., Bharathi, S. R. S., Indran, S., Saravanakumar, S. S., and Liu, Y. (2020). “Characterization of a new cellulosic natural fiber extracted from the root of Ficus religiosa tree,” International Journal of Biological Macromolecules 142, 212-221. DOI: 10.1016/j.ijbiomac.2019.09.094
Narayanasamy, P., Balasundar, P., Senthil, S., Sanjay, M. R., Siengchin, S., Khan, A., and Asiri, A. M. (2020). “Characterization of a novel natural cellulosic fiber from Calotropis gigantea fruit bunch for ecofriendly polymer composites,” International Journal of Biological Macromolecules 150, 793-801. DOI: 10.1016/j.ijbiomac.2020.02.134
Ranitha, M., Abdurahman, H. N., Ziad, A. S., Azhari, H. N., and Thana, R. S. (2014). “A comparative study of lemongrass (Cymbopogon citratus) essential oil extracted by microwave-assisted hydrodistillation (MAHD) and conventional hydrodistillation (HD) method,” International Journal of Chemical Engineering and Application 5(2), 104-108. DOI: 10.7763/IJCEA.2014.V5.360
Razali, N., Salit, M. S., Jawaid, M., Ishak, M. R., and Lazim, Y. (2015). “A study on chemical composition, physical, tensile, morphological, and thermal properties of roselle fibre: Effect of fibre maturity,” BioResources 10(1), 1803-1823. DOI: 10.15376/biores.10.1.1803-1824
Reddy, N., and Yang, Y. (2005). “Biofibers from agricultural byproducts for industrial applications,” Trends in Biotechnology 23(1), 22-27. DOI: 10.1016/j.tibtech.2004.11.002
Sahari, J., Sapuan, S. M., Ismarrubie, Z. N., and Rahman, M. Z. A. (2011). “Comparative study of physical properties based on different parts of sugar palm fibre reinforced unsaturated polyester composites,” Key Engineering Materials 471–472(1), 455-460. DOI: 10.4028/www.scientific.net/KEM.471-472.455
Sapuan, S. M., Pua, F. L., El-Shekeil, Y. A., and AL-Oqla, F. M. (2013). “Mechanical properties of soil buried kenaf fibre reinforced thermoplastic polyurethane composites,” Materials and Design 50, 467-470. DOI: 10.1016/j.matdes.2013.03.013
Saravana Kumaar, A., Senthilkumar, A., Sornakumar, T., Saravanakumar, S. S., and Arthanariesewaran, V. P. (2019). “Physicochemical properties of new cellulosic fiber extracted from Carica papaya bark,” Journal of Natural Fibers 16(2), 175-184. DOI: 10.1080/15440478.2017.1410514
Saravanakumar, S. S., Kumaravel, A., Nagarajan, T., Sudhakar, P., and Baskaran, R. (2013). “Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark,” Carbohydrate Polymers 92(2), 1928-1933. DOI: 10.1016/j.carbpol.2012.11.064
Sathishkumar, T. P., Navaneethakrishnan, P., Shankar, S., and Rajasekar, R. (2013a). “Characterization of new cellulose Sansevieria ehrenbergii fibers for polymer composites,” Composite Interfaces 20(8), 575-593. DOI: 10.1080/15685543.2013.816652
Sathishkumar, T. P., Navaneethakrishnan, P., Shankar, S., Rajasekar, R., and Rajini, N. (2013b). “Characterization of natural fiber and composites – A review,” Journal of Reinforced Plastics and Composites 32(19), 1457-1476. DOI: 10.1177/0731684413495322
Senthamaraikannan, P., and Kathiresan, M. (2018). “Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis L.,” Carbohydrate Polymers 186, 332-343. DOI: 10.1016/j.carbpol.2018.01.072
Shah, G., Kaur, M., Dhabiliya, F., and Shri, R. (2012). “Pharmacognostic standardization of Cymbopogon citratus (dc.) stapf leaves,” Pharmacognosy Journal 4(29), 19-25. DOI: 10.5530/pj.2012.29.3
Vijay, R., Singaravelu, D. L., Vinod, A., Paul Raj, I. D. F., Sanjay, M. R., and Siengchin, S. (2020). “Characterization of novel natural fiber from Saccharum bengalense grass (Sarkanda),” Journal of Natural Fibers 17(12), 1739-1747. DOI: 10.1080/15440478.2019.1598914
Wifek, M., Saeed, A., Rehman, R., and Nisar, S. (2016). “Lemongrass: A review on its botany, properties, applications and active components,” International Journal of Chemical and Biochemical Sciences 9, 79–84.
Yang, H., Yan, R., Chen, H., Lee, D. H., and Zheng, C. (2007). “Characteristics of hemicellulose, cellulose and lignin pyrolysis,” Fuel 86(12–13), 1781-1788. DOI: 10.1016/j.fuel.2006.12.013
Yeşil, Y., and Akalin, E. (2015). “Comparative morphological and anatomical characteristics of the species known as lemongrass (limonotu): Melissa officinalis L., Cymbopogon citratus (DC) Stapf, and Aloysia citriodora Palau,” Journal of Pharmacy of Istanbul University 45(1), 29-37. DOI: 10.16883/jfpiu.86730
Yusriah, L., Sapuan, S. M., Zainudin, E. S., and Mariatti, M. (2014). “Characterization of physical, mechanical, thermal and morphological properties of agro-waste betel nut (Areca catechu) husk fibre,” Journal of Cleaner Production 72, 174-180. DOI: 10.1016/j.jclepro.2014.02.025
Article submitted: April 27, 2021; Peer review completed: August 1, 2021; Revised version received: August 24, 2021; Accepted: August 25, 2021; Published: October 1, 2021.
DOI: 10.15376/biores.16.4.7729-7750
