Abstract
This research investigates the mechanisms behind color changes, hygroscopicity reduction, and mechanical strength loss in pine wood (Pinus kesiya var. langbianensis) and birch wood (Betula alnoides). Elemental composition changes to the surfaces of pine wood and birch wood that had undergone high-temperature heat treatment were investigated with X-ray photoelectron spectroscopy. The O/C (oxygen/carbon) ratios of the wood surfaces were reduced after the thermo-vacuum and superheated steam heat treatments, which indicated a decrease in the amount of oxygen-containing functional groups. The content of C1 (carbon atoms bonded to carbon or hydrogen atoms) increased, and that of C2 (carbon atoms bonded to one oxygen atom) decreased after the thermo-vacuum and superheated steam heat treatments. The results also indicated that the relative lignin content increased and the hydroxyl group (-OH) content in the cellulose and hemicellulose decreased. The ratio of O2 (oxygen atoms bonded to carbon atoms with a double bond) to O1 (oxygen atoms bonded to carbon atoms with a single bond) increased remarkably. Thus, the content of carbonyl groups in the lignin increased.
Download PDF
Full Article
Characterization of Wood Surface Elemental Compositions after Thermo-vacuum Treatment and Superheated-steam Heat Treatment
Yan Yang,a Chunlei Dong,a Bei Luo,a Taian Chen,a and Jianxiong Lu b,*
This research investigates the mechanisms behind color changes, hygroscopicity reduction, and mechanical strength loss in pine wood (Pinus kesiya var. langbianensis) and birch wood (Betula alnoides). Elemental composition changes to the surfaces of pine wood and birch wood that had undergone high-temperature heat treatment were investigated with X-ray photoelectron spectroscopy. The O/C (oxygen/carbon) ratios of the wood surfaces were reduced after the thermo-vacuum and superheated steam heat treatments, which indicated a decrease in the amount of oxygen-containing functional groups. The content of C1 (carbon atoms bonded to carbon or hydrogen atoms) increased, and that of C2 (carbon atoms bonded to one oxygen atom) decreased after the thermo-vacuum and superheated steam heat treatments. The results also indicated that the relative lignin content increased and the hydroxyl group (-OH) content in the cellulose and hemicellulose decreased. The ratio of O2 (oxygen atoms bonded to carbon atoms with a double bond) to O1 (oxygen atoms bonded to carbon atoms with a single bond) increased remarkably. Thus, the content of carbonyl groups in the lignin increased.
Keywords: High-temperature heat treatment (HTHT); Thermo-vacuum treatment (TVT); Superheated-steam heat treatment (SSHT); Chemical composition; Thermal degradation; X-ray photoelectron spectroscopy (XPS)
Contact information: a: Yunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming, 650224, Yunnan Province, P.R. China; b: State Key Laboratory of Tree Genetics and Breeding, Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100091, P.R. China; *Corresponding author: jianxiong@caf.ac.cn
INTRODUCTION
Wood is a renewable and naturally complex material with three main constituents, which are cellulose, hemicelluloses, and lignin (Sjöström 1981). In addition to these three constituents, wood contains extractives such as aliphatic compounds, terpenes, terpenoids, and aromatic compounds (Inari et al. 2006). High-temperature heat treatment (HTHT) (160 to 240 °C) can cause the degradation of wood components (Zaman et al. 2000). The degradation intensity of HTHT depends on several factors, such as the wood specimen (Esteves et al. 2008) and temperature and duration (Esteves et al. 2008; Korkut and Guller 2008). Moreover, degradation of the composition usually leads to changes in the wood properties. For example, reductions in the hygroscopicity and equilibrium moisture content (Kamdem et al. 2002; Pétrissans et al. 2003; Olek et al. 2013) and increase in the dimensional stability have been found after HTHT (Tjeerdsma et al. 1998; Boonstra et al. 2006; Esteves et al. 2008; Gunduz et al. 2009; José et al. 2014). Furthermore, improvement in the resistances to decay (Gosselink et al. 2004) and fungus (Kamdem et al. 2002; Weiland et al. 2003; Calonego et al. 2010) have also been observed. After HTHT, the mechanical properties of wood decrease (Surini et al. 2012; Rautkari et al. 2014). Also, darker wood tints are achieved (Aksou et al. 2011; Akgül and Korkut 2012; Allegretti et al. 2012; Srinivas and Pandey 2012; Kamperidou et al. 2013; Carvalhoet al. 2014). Different wood species have different chemical compositions; therefore, the degrees of thermal degradation vary (Kačik et al. 1992; Tjeerdsma et al. 1998; Wienhaus 1999; Kotilainen et al. 2000; Zaman et al. 2000; Weiland and Guyonnet 2003; Nuopponen et al. 2005). Ultimately, changes to the chemical composition lead to changes of the heat-treated wood properties (Sivonen et al. 2002; Weiland et al. 2003; Hakkou et al. 2005).
At present, X-ray photoelectron spectroscopy (XPS) is widely used to investigate chemical states, surface chemical composition, as well as the location of atoms on numerous lignocellulosic surface materials (Hon 1984; Sinn et al. 2001). This is because the combinations and states of carbon elements play an important role in the determination of wood component structures and properties. Inari et al. (2006) and Kocaefe et al. (2013) have successfully used the XPS method to analyze the surface elements of beech (Fagus sylvatica), jack pine (Pinus banksiana), aspen (Populus tremuloides), and birch (Betulus papyrifera). The binding energy (BE) of C1s is strongly related to the atoms or groups connected to carbon atoms. Thus, the changes to the chemical composition of wood surfaces can be inferred from the chemical shifts of the C1s peak (Dorris and Gray 1978; Barry et al. 1990; Liu et al. 1998; Kamdem et al. 2001; Nzokou and Kamdem 2005; Inari et al. 2006; Popescu et al. 2009).
Carbon atoms are bonded to other atoms or groups; thus, the C1s spectra is usually grouped into four classes (Dorris and Gray 1978; Barry et al. 1990; Liu et al. 1998; Kamdem et al. 2001; Nzokou and Kamdem 2005; Inari et al. 2006; Popescu et al. 2009). The C1 class is ascribed to carbon atoms that are bonded only to carbon or hydrogen atoms, i.e. C-H or C-C. C1atoms are mainly present in lignin, which contains phenyl propane structures and extractives, such as fatty acids, fats, waxes, and terpenoids (Shen et al. 1998). The BE of C1 is low, at approximately 284.8 eV. The C2 class contains carbon atoms that are bonded to one oxygen atom, i.e. C-O. Wood cellulose and hemicellulose molecules contain a large quantity of hydroxyl groups (-OH), which bond to carbon atoms with a single bond. Accordingly, the BE of C2 is higher, at approximately 286.5 eV. The C3 class corresponds to carbon atoms that are bonded to one carbonyl oxygen atom or two non-carbonyl oxygen atoms, i.e. C=O or O-C-O. These atoms are derived from the acetal structure (O-C-O) of cellulose and hemicellulose and the carbonyl structure (C=O) of lignin. Moreover, the wood oxidation process usually produces a new C3 structure. High oxidation states of the carbon atoms in C=O and O-C-O lead to a high BE of approximately 288 eV to 288.5 eV. The C4 class is associated with carbon atoms that are bonded to one carbonyl oxygen atom and one non-carbonyl oxygen atom, i.e. O-C=O. These are acetyl groups and glucuronic acid, which are present in hemicellulose and extractives, such as resin acids, fatty acids, and other substances. Furthermore, the BE of C4 (289 eV) is higher than that of the other carbon atom types because it has the highest oxidation state.
In accordance with the combination of oxygen and carbon atoms, the O1s spectra is usually grouped into two components (Barry et al. 1990; Nzokou and Kamdem 2005; Inari et al. 2006; Popescu et al. 2009; Kocaefe et al. 2013). The O1 peak that appears at a higher BE corresponds to oxygen atoms that are bonded to carbon atoms with a single bond. The O2 peak with a lower BE is associated with oxygen atoms that are bonded to carbon atoms with a double bond. The presence of O2 and O1 indicates that non-cellulose substances are contained in the wood.
Both pine and birch woods have a wide planting area in Yunnan Province, China, but their application is limited because of their poor dimensional stability and natural durability. With the application of HTHT, the dimensional stability and resistance to corrosion of these two species can be improved. This study aimed to characterize the changes to the elemental composition of the wood surfaces after thermo-vacuum treatment (TVT) and superheated steam heat treatment (SSHT) with XPS. These analyses aimed to identify the molecular mechanisms behind color changes, hygroscopicity reduction, and mechanical strength loss in the wood. Analyses of the O/C ratio, changes to the C1, C2, C3, and C4 contents in the C1s spectra, and changes to the O2 and O1 in the O1s spectra were conducted to evaluate the changes to the elemental composition of the wood surfaces after the TVT and SSHT processes.
EXPERIMENTAL
Materials
Pine logs were collected from Munaihe forest in Cuiyun District (The geographical coordinates are as follows: the northern latitude is 22°02′-24°50′, and the east latitude is 99°09′-102°19′), Puer City, Yunnan Province, China in April 2007. The five logs collected were approximately 35 years old with a breast height diameter (BHD) of 22 cm and height of 16 m. Logs with trunks that were 1.3 m to 5.3 m above the ground were selected and sawn into boards with a 40-mm thickness in the radial direction. Afterwards, these samples were air-dried. Six birch trees were obtained from Jinghan Town (The geographical coordinates are as follows: the northern latitude is 24°08′-24°39′, and the east latitude is 97°39′-98°17′), Longchuan County, Dehong City, Yunnan Province, China in December 2008. The average height and BHD of these trees were 10 m and 20 cm to 25 cm, respectively. Trees with trunks approximately 1.3 m to 5.3 m above the ground were selected and sawn into boards with a 40-mm thickness in the radial direction. These samples were subsequently air-dried.
The boards were cut into specimens with the dimensions 150 mm (axial direction) × 50 mm (tangential direction) × 20 mm (radial direction) from non-defective samples before HTHT. All of the samples were conditioned in a humidity box (Binder KMF720) with a constant temperature of 30 °C and relative humidity of 68%. The moisture content of all of the samples was adjusted to 10% (dry basis).
Methods
TVT process
The TVT process was conducted at 200 °C (The temperature was measured by temperature inspection instrument (XSL-A08ES2V0, Beijing, China)) under an absolute pressure of 200 hPa for 4 h with a heating rate of 15 °C/h in a vacuum treatment chamber (DZF-6210, Shanghai YiHeng, Shanghai, China). Ten replicates were processed at these treatment conditions. After the target treatment time was reached, the heating power was turned off, and the samples needed approximately 5 h to cool back to room temperature before they could be removed from the vacuum chamber.
SSHT process
The SSHT process was carried out at 200 °C (The temperature was measured by temperature inspection instrument (XSL-A08ES2V0, Beijing, China)) for 4 h under medium heated superheated steam with a heating rate of 15 °C/h. Ten replicates were treated at the treatment conditions. After the scheduled treatment time, the heating power was turned off, and the samples took approximately 5 h to cool back to room temperature.
XPS analysis
The XPS analyses were performed using a VG MKII system (MultiPak V9.3, Kanagawa-ken, Japan) with a Mg Kα X-ray source. The samples were analyzed at a 10−6 Pa pressure with a 20-eV pass energy, 8-kV operating voltage, 30-mA operating current, and 0.1-eV resolution at a temperature of 20 °C to 400 °C. The samples were thoroughly cleaned and degreased before the removal of a 2 mm × 2 mm chip with the use of a cutter blade. All of the samples were removed immediately before examination to minimize contact with bare hands. After undergoing preparation, the samples were immediately placed in a vacuum chamber. Three types of spectra were collected: a survey spectrum, i.e. a low-resolution spectrum from 0 eV to 1100 eV; a high-resolution spectrum of the C1s region from 278 eV to 298 eV; and a high-resolution spectrum of the O1s region from 523 eV to 543 eV. The O1s and C1s intensities were determined, and the O/C ratio was calculated. The C1s and O1s spectra of the wood were fitted using Origin 8.5 software (OriginLab, Northampton, MA, USA). The C1s spectra were grouped into four subpeaks, whereas the O1s spectra were grouped into only two subpeaks.
RESULTS AND DISCUSSION
Elemental Composition Analysis of the Wood Surfaces
The survey XPS spectra of the surface of the two species before and after the HTHTs are presented in Fig. 1. Table 1 gives the elemental compositions and relative content of the elements. Figure 1 and Table 1 show that in addition to C and O elements, a small amount of N, Si, and Ca elements were present on the wood surfaces. Inari et al. (2006) reported that evaporation of volatile extractives initially present in the wood or the formation of volatile by-products because of thermal degradation should increase the volatile organic compound concentration in the oven, and result in more deposition of contaminants on the wood surface. Moreover, the surfaces are rapidly contaminated even at room temperature. C1s and O1s, located at the binding energy (BE) values of 284 eV to 290 eV and 531 eV to 534 eV, respectively, were the primary wood elements. A high O/C ratio usually indicates a high relative carbohydrate content. Conversely, a low O/C ratio indicates a high relative lignin content (Kocaefe et al. 2013).
The heat treatment resulted in a noticeable decrease in the O/C ratio for both species. The O/C ratio of the pine decreased remarkably from 0.6852 before TVT to 0.5177 after TVT. Meanwhile, the O/C ratio of the birch decreased from 0.5946 to 0.4732 after TVT and to 0.5007 after SSHT. During the TVT process, the O/C ratio decreased slightly more than during the SSHT process. The O/C ratios of the wood surfaces decreased no matter what treatment process was used, which manifested as an increasing content of carbon atoms and a decreasing content of oxygen atoms. These results indicated that the predominant oxygen-containing functional groups, such as the carboxyl, acetyl, and hydroxyl groups, of the heat-treated wood surfaces were reduced. Moreover, the relative content of lignin increased and that of carbohydrates decreased (Yang et al. 2015). These findings were in good agreement with the results for Cylicodiscus spp. (Shi 2011), Pinus banksiana, Populus tremuloides, Betula papyrifera (Kocaefe et al. 2013), Fagus sylvatica (Inari et al. 2006; Gérardin et al. 2007), Pinus sylvestris(Gérardin et al. 2007; Bryne et al. 2010), Picea abies, and Pinus radiata (Bryne et al. 2010) reported in previous studies.
Table 1. Elemental Composition, Atomic Percentage, and O/C Ratio of the Untreated and Heat-treated Wood Surfaces
Sample descriptions: (a)– untreated pine wood (UN pine); (b) – thermo-vacuum-treated pine wood (TVT pine); (c) – untreated birch wood (UN birch); (d) – thermo-vacuum-treated birch wood (TVT birch); and (e)– superheated steam heat-treated birch wood (SSHT birch)
The decrease in the O/C ratio may have been because of the increased relative content of lignin and the formation of volatile by-products with the lower oxygen content, such as sugar, furfural, and acetic acid (Inari et al. 2006; Kocaefe et al. 2013). However, Kocaefe et al. (2013) found that the O/C ratio of heat-treated jack pine (Pinus banksiana) was higher than that of a group of untreated jack pine. Kamdem et al. (1991) reported that the high carbon content in wood samples is also an indication of the presence of extractives on the wood surface and a high relative content of extractives. Untreated jack pine was abundant in carbon-rich extractives, such as waxes, fats, and terpenes, and rich in lignin guaiacyl units (Gérardin et al. 2007). The increase in the O/C ratio in the jack pine was probably because of the partial removal of abundant carbon-rich extractives during the HTHT process. Wood extractions lead to higher O/C ratios, which confirms that extractives affect the carbon enrichment of the surface (Inari et al. 2011). This chain of events led to a lower O/C ratio for the untreated jack pine than for the heat-treated jack pine. The O/C ratio changes may have been related to the relative content of extractives on the wood surface and changes to the lignin and oxygen-containing functional groups of other components during the HTHT process (Kocaefe et al. 2013).
The oxygen-containing functional groups of the birch wood surface decreased slightly more after TVT than after SSHT, i.e. the increase in carbon atoms after TVT was slightly higher than after SSHT. This was attributed to the different processes that occur during the heat treatments.
Fig. 1. XPS spectra of the untreated and heat-treated samples: (a) UN pine; (b) TVT pine; (c) UN birch; (d) TVT birch; and (e) SSHT birch
In TVT, almost no oxidation reactions in wood occur because of the presence of a small amount of oxygen. Additionally, because the water boiling point is reduced under vacuum conditions, a high amount of evaporation occurs at lower temperatures, and there is almost no water within the wood during hydrolysis (Yang et al. 2015). This leads to a lower decrease in carbon atoms. During HTHT, there is always water in the wood, which is involved in hydrolysis and leads to a higher decrease in carbon atoms.
C1s Spectra Analysis of the Wood Surfaces
Figure 2 illustrates the high-resolution C1s spectra of the surfaces of both wood species before and after heat treatment. The C1s spectra of both the UN and TVT pines were grouped into four classes. The C1s spectra of both the UN and TVT birches were grouped into three classes, whereas that of the SSHT birch was grouped into four classes. The detailed BE values and content of each carbon signal group are presented in Table 2.
The above-mentioned data indicated that the C2 class carbon atoms were the most abundant before the heat treatment. The C1 class carbon was the second. The C3 class carbon atoms presented a smaller proportion than C1 and C2, and the C4 class was even less abundant. However, the content of the different types of carbons differed to a major extent between the untreated and heat-treated wood samples. This finding indicated that changes occurred to the chemical structure of the surfaces of the samples. In the present research, the content of C1 in both species obviously increased, whereas the C2 content decreased. These findings were in good agreement with previous results presented by Bryne et al. (2010), Inari et al. (2006), and Kocaefe et al. (2013).
The C1 content of the pine increased noticeably from 22.8% to 45.6% after TVT and that of the birch increased considerably from 29.3% to 42.2% after TVT. The C1 content of the birch also noticeably increased after SSHT from 29.3% to 41.7%. However, the increase in the C1 content during the TVT process was slightly more than during the SSHT process. The increase in the C1 content was observed as an increase in C-C bonds, which was attributed to the increase in the lignin and extractives contents (The contents of the benzene-ethanol extractives of the birch were increased from 1.47% to 3.99% after TVT) (Yang 2016). According to the literature, the content of lignin in birch increased from 23.7% to 32.3% (Yang et al. 2015), and that in pine increased from 28.4% to 32.9% (Yang et al. 2016) after treatment at 200 °C for 4 h. The increase in the lignin content was attributed to the increase in the C1 content. The extractive contents of the birch were also determined by the authors (Yang 2016). The results showed that the contents of the cold and hot water extractive contents of the birch were reduced from 6.26% to 2.56% and from 8.28% to 3.09%, respectively, after TVT. This was because water-soluble extractives move to the wood surface during the process of heat treatment, along with moisture. This phenomenon results in decreased cold and hot water extractive contents. The transfer of extractives to the surface was another reason for the increase in the C1 content.
The C2 content of the pine decreased from 63.5% to 42.0% after TVT and that of the birch decreased from 53.2% to 45.0% after TVT. The C2 content of the birch also remarkably decreased after SSHT from 53.2% to 46.4%. However, the decrease in the C2 content during the TVT process was slightly more than the decrease that occurred during the SSHT process. The decrease in the C2 content suggested that the content of hydroxyl groups in the cellulose and hemicellulose decreased, which led to a relative decrease in the carbohydrate content. According to the literature, the content of cellulose and hemicellulose in birch decreased from 46.8% to 44.0% and from 28.6% to 23.6%, respectively, after treatment at 200 °C for 4 h (Yang et al. 2015).
The C3 content of the pine increased slightly from 7.88% to 10.16% after TVT, which indicated the formation of new lignin carbonyl groups. In contrast, the C3 content of the birch decreased from 16.50% to 12.50% after TVT and to 7.56% after SSHT, which indicated a decrease in acetal structures in the cellulose and hemicellulose. However, the decrease in the C3content during the TVT process was slightly less than during the SSHT process.
The C4 content of the pine decreased slightly from 5.87% to 2.15% after TVT, which showed that the acetylation reactions of hemicellulose led to a decrease in the hemicellulose content. However, C4 was found on the birch surface and was 4.27% after SSHT. The occurrence of C4 is usually attributed to the emergence of carboxylate ions, which have a six-membered ring structure that is relatively stable. However, the branched chains on the six-membered ring structure were oxidized to form carboxylic acids during SSHT, or were broken down and formed low molecular weight compounds. The low molecular weight compounds can also be oxidized to form carboxylic acid. The breakdown of the branched chains resulted in the loss of C4.
This analysis of the different types of carbon indicated that the C1 content increased as the O/C ratio decreased. Overall, the wood lignin content increased, which was demonstrated by the increased C1 peak. Meanwhile, the cellulose and hemicellulose contents decreased, which was demonstrated by the decrease in the C2 peak after heat treatment.
Fig. 2. High resolution C1s XPS survey spectra of the untreated and heat-treated samples: (a) UN pine; (b) TVT pine; (c) UN birch; (d) TVT birch; and (e) SSHT birch
Table 2. High Resolution C1s Data of the Wood Surfaces
O1s Spectra Analysis of the Wood Surfaces
Figure 3 shows the changes to the high-resolution O1s spectra of the wood surfaces before and after heat treatment. The detailed BE values and content of each oxygen signal group are presented in Table 3. Oxygen atoms that bonded to carbon atoms with a single bond (C-O) at a higher BE were denoted as O1. Meanwhile, oxygen atoms that bonded to carbon atoms with a double bond (C=O) at a lower BE were denoted as O2. The existence of both O1 and O2 atoms indicated that the wood contained non-cellulosic components. Figure 3 and Table 3 show that the O2 content was much lower than the O1 content on the surface of both wood species before and after the heat treatment; therefore, it was concluded that oxygen atoms mainly bonded to carbon atoms with a single bond. The O2/O1 ratio in the pine increased considerably from 0.0147 to 0.0879 and that of the birch increased from 0.0131 to 0.0487 after TVT. Moreover, the O2/O1 ratio of the birch majorly increased from 0.0131 to 0.0523 after SSHT.
The O2 content increased, whereas the O1 content decreased after TVT and SSHT. This result was in good agreement with the data from previous studies conducted by Inari et al. (2006), Shi (2011), and Kocaefe et al. (2013). The increase in the O2/O1 ratio after TVT and SSHT showed that an increasing amount of oxygen atoms were bonded to carbon atoms with a double bond, i.e. there was an increase in carbonyl groups in the lignin and an increase in the oxidation states of the carbon atoms (Shi 2011). The slight increase in the O2 content after the heat treatment may have been because of carbonyl groups, which was an indication that oxidation and condensation reactions occurred in the lignin during HTHT. Meanwhile, the decrease in the O1 content in the wood after the heat treatment may have been the result of the dehydration reaction that occurred in the cellulose and the deacetylation reaction that occurred in the hemicellulose (Shi 2011). These reactions resulted in a decrease of oxygen-containing functional groups within the wood (Inari et al. 2006; Shi 2011).
Table 3. High Resolution O1s Data of the Wood Surfaces
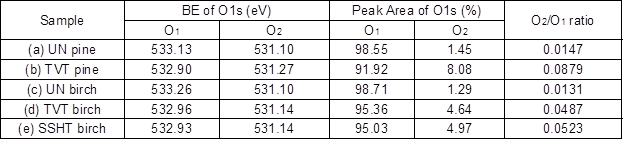
Fig. 3. High resolution O1s XPS survey spectra of the untreated and heat-treated samples: (a) UN pine; (b) TVT pine; (c) UN birch; (d) TVT birch; and (e) SSHT birch
CONCLUSIONS
- Quantitative characterizations of the elemental composition of two kinds of wood surfaces during TVT and SSHT were successfully conducted using XPS.
- The O/C ratios of the surface of both species were reduced after HTHT, which indicated there was a reduction in the amount of oxygen-containing functional groups on the wood surfaces.
- The C-H/C-C content of (C1) content of the surface of both wood species increased, whereas the C-O (C2) content decreased after heat treatment. Therefore, the relative content of non-carbohydrates increased, and the hydroxyl group content in the cellulose and hemicellulose decreased.
- The C=O/C-O (O2/O1) ratio of the surface of both species increased after heat treatment. This outcome suggested that the amount of carbonyl groups in the lignin and the oxidation states of carbon atoms increased.
- The ratios of O/C and O2/O1, and the contents of C1, C2, C3, and C4 obtained after TVT and SSHT indicated that more oxidation states of the carbon atoms occurred during the SSHT process.
ACKNOWLEDGMENTS
This work was financially supported by National key technology research and development project (No. 2017YFD0600202) , Scientific Research Start-up Project of Southwest Forestry University (No. 2017), Yunnan Provincial Education Department Projects (No. 2015Y291, 2015Y293) and National Natural Science Foundation of China (No. 31360157). The authors would like to express appreciation of Prof. Jiangmei Wu of Beijing Forestry University for the careful guidance. Chunlei Dong and Yan Yang contributed equally to this work.
REFERENCES CITED
Akgül, M., and Korkut, S. (2012). “The effect of heat treatment on some chemical properties and colour in scots pine and uludağ fir wood,” Int. J. Phys. Sci. 7(21), 2854-2859. DOI: 10.5897/IJPS12.281
Aksou, A., Deveci, M., Baysal, E., and Toker, H. (2011). “Colour and gloss changes of scots pine after heat modification,” Wood Res. 56(3), 329-336. DOI: 10.1007/s11676-013-0389-y
Allegretti, O., Brunetti, M., Cuccui, I., Ferrari, S., Nocetti, M., and Terziev, N. (2012). “Thermo-vacuum modification of spruce (Picea abies Karst.) and fir (Abies alba Mill.) wood,” BioResources 7(3), 3656-3669. DOI: 10.15376/biores.7.3.3656-3669
Barry, A. O., Koran, Z., and Kaliaguine, S. (1990). “Surface analysis by ESCA of sulfite post-treated CTMP,” J. Appl. Polym. Sci. 39(1), 31-42. DOI: 10.1002/app.1990.070390103
Boonstra, M., Pizzi, A., Ohlmeyer, M., and Paul, W. (2006). “The effects of a two stage heat treatment process on the properties of particleboard,” Holz Roh Werkst. 64(2), 157-164. DOI: 10.1007/s00107-005-0055-y
Bryne, L. E., Lausmaa, J., Ernstsson, M., Englund, F., and Wålinder, M. E. P. (2010). “Ageing of modified wood. Part 2, Determination of surface composition of acetylated, furfurylated, and thermally modified wood by XPS and ToF-SIMS,” Holzforschung 64, 305-313. DOI: 10.1515/HF.2010.062
Calonego, F. W., Severo, E. T., and Furtado, E. L. (2010). “Decay resistance of thermally modified Eucalyptus grandis wood at 140 °C, 160 °C, 180 °C, 200 °C and 220 °C,” BioresourceTechnol. 101(23), 9391-9394. DOI: 10.1016/j.biortech.2010.06.119
Carvalho, A. G., Zanuncio, A. J. V., Vital, B. R., Carneiro A. de C. O., and da Silva C. M. S. (2014). “Colorimetric and chemical changes in pre-hydrolyzed strand board particles of pine and eucalyptus,” BioResources 9(4), 7234-7242. DOI: 10.15376/biores.9.4.7234-7242
Dorris, G. M., and Gray, D. G. (1978). “The surface analysis of paper and wood fibres by ESCA. II. Surface composition of mechanical pulps,” Cell. Chem. Technol. 12, 721-734. DOI: 10.1007/BF00819664
Esteves, B. M., Domingos, I. J., and Pereira, H. M. (2008). “Pine wood modification by heat treatment in air,” BioResources 3(1), 142-154. DOI: 10.15376/biores.3.1.142-154
Gérardin, P., Petrič, M., Petrissans, M., Lambert, J., and Ehrhrardt, J. J. (2007). “Evolution of wood surface free energy after heat treatment,” Polym. Degrad. Stabil. 92(4), 653-657. DOI: 10.1016/j.polymdegradstab.2007.01.016
Gosselink, R. J. A., Krosse, A. M. A., van der Putten, J. C., van der Kolk, J. C., de Klerk-Engels, B., and van Dam, J. E. G. (2004). “Wood preservation by low-temperature carbonisation,” Ind. Crop. Prod. 19(1), 3-12. DOI: 10.1016/S0926-6690(03)00037-2
Gunduz, G., Aydemir, D., and Karakas, G. (2009). “The effects of thermal treatment on the mechanical properties of wild pear (Pyrus elaeagnifolia Pall.) wood and changes in physical properties,” Mater. Design 30(10), 4391-4395. DOI: 10.1016/j.matdes.2009.04.005
Hakkou, M., Pétrissans, M., Zoulalian, A., and Gérardin, P. (2005). “Investigation of wood wettability changes during heat treatment on the basis of chemical analysis,” Polym. Degrad. Stabil. 89(1), 1-5. DOI: 10.1016/j.polymdegradstab.2004.10.017
Hon, D. N.-S. (1984). “ESCA study of oxidized wood surfaces,” J. Appl. Polym. Sci. 29(9), 2777-2784. DOI: 10.1002/app.1984.070290908
Inari, G. N., Pétrissans, M., Dumarcay, S., Lambert, J., Ehrhardt, J. J., Šernek, M., and Gérardin, P. (2011). “Limitation of XPS for analysis of wood species containing high amounts of lipophilic extractives,” Wood Sci. Technol. 45(2), 369-382. DOI: 10.1007/s00226-010-0324-8
Inari, G. N., Pétrissans, M., Lambert, J., Ehrhardt, J. J., and Gérardin, P. (2006). “XPS characterization of wood chemical composition after heat-treatment,” Surf. Interface Anal. 38(10), 1336-1342. DOI: 10.1002/sia.2455
José, V. Z. A., Javan, P. M., Teodorico, A. da S., Elias, De S. F., and Fernando, T. P. (2014). “Physical and colorimetric changes in Eucalyptus grandis wood after heat treatment,” BioResources 9(1), 293-302.
Kačik, F., Meleer, I., and Melcerová, A. (1992). “Vergleichende Charakteristik einer hydrothermischen und thermischen Behandlung von Buchenholz,” Holz Roh Werkst. 50(1), 79-84. DOI: 10.1007/BF02684032
Kamdem, D. P., Pizzi, A., and Jermannaud, A. (2002). “Durability of heat-treated wood,” Holz Roh Werkst. 60(1), 1-6. DOI: 10.1007/s00107-001-0261-1
Kamdem, D. P., Riedl, B., Adnot, A., and Kaliaguine, S. (1991). “ESCA spectroscopy of poly(methyl methacrylate) grafted onto wood fibers,” J. Appl. Polym. Sci. 43(10), 1901-1912. DOI: 10.1002/app.1991.070431015
Kamdem, D. P., Zhang, J., and Adnot, A. (2001). “Identification of cupric and cuprous copper in copper naphthenate-treated wood by X-ray photoelectron spectroscopy,” Holzforschung55(1), 16-20. DOI: 10.1515/HF.2001.004
Kamperidou, V., Barboutis, I., and Vasileiou, V. (2013). “Response of colour and hygroscopic properties of scots pine wood to thermal treatment,” J. Forestry Res. 24(3), 571-575. DOI: 10.1007/s11676-013-0389-y
Kocaefe, D., Huang, X., Kocaefe, Y., and Boluk, Y. (2013). “Quantitative characterization of chemical degradation of heat-treated wood surfaces during artificial weathering using XPS,” Surf. Interface Anal. 45(2), 639-649. DOI: 10.1002/sia.5104
Korkut, D. S., and Guller, B. (2008). “The effects of heat treatment on physical properties and surface roughness of red-bud maple (Acer trautvetteri Medw.) wood,” Bioresource Technology 99(8), 2846-2851. DOI:10.1016/j.biortech.2007.06.043
Kotilainen, R. A., Toivanen, T.-J., and Alén, R. J. (2000). “FTIR monitoring of chemical changes in softwood during heating,” J. Wood Chem. Technol. 20(3), 307-320. DOI: 10.1080/02773810009349638
Liu, F. P. P., Rials, T. G., and Simonsen, J. (1998). “Relationship of wood surface energy to surface composition,” Langmuir 14(2), 536-541. DOI: 10.1021/la970573y
Nuopponen, M., Vuorinen, T., Jämsä, S., and Viitaniemi, P. (2005). “Thermal modifications in softwood studied by FT-IR and UV resonance Raman spectroscopies,” J. Wood Chem. Technol. 24(1), 13-26. DOI: 10.1081/WCT-120035941
Nzokou, P., and Kamdem, D. P. (2005). “X-ray photoelectron spectroscopy study of red oak- (Quercus rubra), black cherry- (Prunus serotina) and red pine- (Pinus resinosa) extracted wood surfaces,” Surf. Interface Anal. 37(8), 689-694. DOI: 10.1002/sia.2064
Olek, W., Majka, J., and Czajkowski, T. (2013). “Sorption isotherms of thermally modified wood,” Holzforschung 67(2), 183-192. DOI: 10.1515/hf-2011-0260
Pétrissans, M., Gérardin, P., El bakali, I., and Serraj, M. (2003). “Wettability of heat-treated wood,” Holzforschung 57(3), 301-307. DOI: 10.1515/HF.2003.045
Popescu, C.-M., Tibirna, C.-M., and Vasile, C. (2009). “XPS characterization of naturally aged wood,” Appl. Surf. Sci. 256(5), 1355-1360. DOI: 10.1016/j.apsusc.2009.08.087
Rautkari, L., Honkanen, J., Hill, C. A. S., Ridley-Ellis, D., and Hughes, M. (2014). “Mechanical and physical properties of thermally modified scots pine wood in high pressure reactor under saturated steam at 120, 150 and 180 °C,” Eur. J. Wood Wood Prod. 72(1), 33-41. DOI: 10.1007/s00107-013-0749-5
Shen, Q., Mikkola, P., and Rosenholm, J.B. (1998) “Quantitative characterization of the subsurface acid–base properties of wood by XPS and Fowkes theory,” Colloids and Surfaces. A: Physicochemical and Engineering Aspects 145(1), 235-241. DOI:10.1016/S0927-7757(98)00655-4
Shi, Q. (2011). The Mechanism and Effect on the Properties of Heat-treated Okan Wood, Ph.D. Dissertation, Beijing Chinese Academy of Forestry, Beijing, China.
Sinn, G., Reiterer, A., and Stanzl-Tschegg, S. E. (2001). “Surface analysis of different wood species using X-ray photoelectron spectroscopy (XPS),” J. Mater. Sci. 36(19), 4673-4680. DOI: 10.1023/A:1017954300015
Sivonen, H., Maunu, S. L., Sundholm, F., Jämsä, S., and Viitaniemi, P. (2002). “Magnetic resonance studies of thermally modified wood,” Holzforschung 56(6), 648-654. DOI: 10.1515/HF.2002.098
Sjöström, E. (1981) Wood Chemistry, Fundamentals and Applications, Academic Press, London.
Srinivas, K., and Pandey, K. K. (2012). “Effect of heat treatment on color changes, dimensional stability, and mechanical properties of wood,” J. Wood Chem. Technol. 32(4), 304-316. DOI: 10.1080/02773813.2012.674170
Surini, T., Charrier, F., Malvestio, J., Charrier, B., Moubarik, A., Castéra, P., and Grelier, S. (2012). “Physical properties and termite durability of maritime pine Pinus pinaster Ait., heat-treated under vacuum pressure,” Wood Sci. Technol. 46(1-3), 487-501. DOI: 10.1007/s00226-011-0421-3
Tjeerdsma, B. F., Boonstra, M., Pizzi, A., Tekely, P., and Militz, H. (1998). “Characterisation of thermally modified wood: Molecular reasons for wood performance improvement,” Holz Roh Werkst. 56, 149-153. DOI: 10.1007/s001070050287
Weiland, J., and Guyonnet, R. (2003). “Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy,” Holz Roh Werkst. 61(3), 216-220. DOI: 10.1007/s00107-003-0364-y
Wienhaus, O. (1999). “Modifizierung des Holzes durch eine milde Pyrolyse – Abgeleitet aus den allgemeinen Prinzipien der Thermolyse des Holzes,” Wissenschaftliche Zeitschrift der Technischen Universität Dresden 48(2), 17-22.
Yang, Y. (2016). “Studies on the mathematical model of heat and mass transfer in alder birch wood and the color control during the thermo-vacuum treatment,” Ph.D. Dissertation, Chinese Academy of Forestry, Beijing, China.
Yang, Y., Lu, J. X., Chen, T. A., Qiu, J., and Zhan, T. Y. (2016). “Influences of thermo-vacuum treatment on chemical compositions and colors in Pinus kesiya var. langbianensis,” China Forest Products Industry 43(4), 32-36.
Yang, Y., Zhan, T. Y., Lu, J. X., and Jiang, J. H. (2015). “Influences of thermo-vacuum treatment on colors and chemical compositions of alder birch wood,” BioResources 10(4), 7936-7945. DOI: 10.15376/biores.10.4.7936-7945
Zaman, A., Alén, R., and Kotilainen, R. (2000). “Thermal behaviour of scots pine (Pinus sylvestris) and silver birch (Betula pendula) at 200-230°,” Wood Fiber Sci. 32(2), 138-143.
Article submitted: September 23, 2017; Peer review completed: December 29, 2017; Revised version received and accepted: January 22, 2018; Published: January 29, 2018.
DOI: 10.15376/biores.13.1.1895-1908
