Abstract
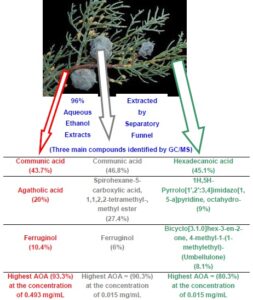
Potent antioxidant activities of solvent extracts (96% aqueous ethanol) from the fruit, leaf, and branchlet without adherent leaf of Cupressus arizonica were evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and compared with butylated hydroxytoluene (BHT) and ascorbic acid (AA). Their chemical compositions were analyzed using gas chromatography-mass spectrometry (GC/MS). Branchlet extracts (BE) were the most active as an antioxidant agent at 93.3% at the concentration of 0.493 mg/mL, which was higher than the value of vitamin C (63.3%) at the same concentration. The major components identified in the BE were communic acid (43.7%), followed by agatholic acid (20%), and ferruginol (10.4%). The extract from fruit had good antioxidant activity (90.3%) at a concentration of 0.015 mg/mL. The major compounds identified in the fruit extracts (FE) were communic acid (46.8%), spirohexane-5-carboxylic acid, 1,1,2,2-tetramethyl-, methyl ester (27.4%), and ferruginol (6%). Leaf extracts (LE) were more active as an antioxidant agent at 80.3%, which was higher than the value of BHT (75.7%) at the concentration of 0.015 mg/mL. The major components identified in the LE were hexadecanoic acid (45.1%), 1H,5H-pyrrolo[1′,2′:3,4]imidazo[1,5-a]pyridine, octahydro- (9%), bicyclo [3.1.0]hex-3-en-2-one, 4-methyl-1-(1-methylethyl)- (8.1%).
Download PDF
Full Article
Chemical Composition and Antioxidant Activity of Extracts from the Fruit, Leaf, and Branchlet of Cupressus arizonica Greene
Fatemeh Barzegari,a Seyyed Khalil Hosseinihashemi,b,* and Hadi Baseri a,*
Potent antioxidant activities of solvent extracts (96% aqueous ethanol) from the fruit, leaf, and branchlet without adherent leaf of Cupressus arizonica were evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and compared with butylated hydroxytoluene (BHT) and ascorbic acid (AA). Their chemical compositions were analyzed using gas chromatography-mass spectrometry (GC/MS). Branchlet extracts (BE) were the most active as an antioxidant agent at 93.3% at the concentration of 0.493 mg/mL, which was higher than the value of vitamin C (63.3%) at the same concentration. The major components identified in the BE were communic acid (43.7%), followed by agatholic acid (20%), and ferruginol (10.4%). The extract from fruit had good antioxidant activity (90.3%) at a concentration of 0.015 mg/mL. The major compounds identified in the fruit extracts (FE) were communic acid (46.8%), spirohexane-5-carboxylic acid, 1,1,2,2-tetramethyl-, methyl ester (27.4%), and ferruginol (6%). Leaf extracts (LE) were more active as an antioxidant agent at 80.3%, which was higher than the value of BHT (75.7%) at the concentration of 0.015 mg/mL. The major components identified in the LE were hexadecanoic acid (45.1%), 1H,5H-pyrrolo[1′,2′:3,4]imidazo[1,5-a]pyridine, octahydro- (9%), bicyclo [3.1.0]hex-3-en-2-one, 4-methyl-1-(1-methylethyl)- (8.1%).
DOI: 10.15376/biores.18.1.19-38
Keywords: Cupressus arizonica; Fruit, leaf, and branchlet extracts; Antioxidant activity; Chemical composition; GC-MS
Contact information: a: School of Chemistry, Damghan University, Damghan, Iran; b: Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran;
* Corresponding authors: hashemi@kiau.ac.ir and baseri@du.ac.ir
GRAPHICAL ABSTRACT

INTRODUCTION
Arizona cypress (Cupressus arizonica Greene) is an evergreen (coniferous) and is a medium-sized tree at 50 to 60 feet tall and 15 to 30 inches in diameter. It has blue-green foliage and scaly reddish brown bark, and is widely distributed throughout the Southwest of USA (Little and Skolmen 1989; USDA 2002; Emami et al. 2010; Swearingen and Bargeron 2016). This species is native in the United States and found in California, New Mexico, Arizona, Texas, and Mexico (Fralish and Franklin 2002). This beautiful tree was introduced into Iran in 1954 and cultivated in various parts of the country as an ornamental tree and for reforestation purposes (Sabeti 1966). Persian names of this tree are “Sarve Noqrei” (Sabeti 1976) and “Sarve Simin” (Zare 2001).
Many researchers have yet to consider the chemical composition of Cupressus spp. from different parts of the world. Some of the species that have been phytochemically analyzed include C. arizonica (Chéraif et al. 2007), Cupressus lusitanica (Kuiate et al. 2006), Cupressus cashmeriana, Cupressus chengiana, Cupressus funebris, Cupressus duclouxiana, Cupressus guadalopensis, Cupressus macnabiana, Cupressus dupreziana (Pierre-Leandri et al. 2003; Ramdani et al. 2011), and Cupressus macrocarpa (Zhang et al. 2012).
Mothana et al. (2009) demonstrated that Cupressus sempervirens L. leaves extract and essential oil have remarkable radical scavenging activity. Quercetin, rutin, caffeic acid, and p-coumaric acid have been isolated from C. sempervirens leaves (Ibrahim et al. 2007), but the bark extract of Cupressus lusitanica Mill. (Mexican white cedar) has shown high cytotoxicity on MCF-7 (estrogen receptor positive breast carcinoma) cells (Mbaveng et al. 2011).
C. arizonica Greene is an aromatic evergreen coniferous plant with great importance in urban horticulture and in the pharmaceutical and fragrance industries (Hassanpouraghdam 2011).
The results of the studies of Emami et al. (2013) showed that all methanol extracts of leaves and fruits of the six different species of Iranian common conifers: Cupressus arizonica, Pinus halepensis, Pinus nigra, Pinus brutia var. elderica, Pinus wallichiana, and Cedrus deodara, possessed antioxidant activity. Methanol extractions of Pinus spp. leaves and fruits showed the highest antioxidant activity (quite higher than α-tocopherol). Among the extracts examined, the leaves of P. halpensis methanol extract showed the lowest activity.
From the gas chromatography-mass spectrometry (GC-MS) analysis conducted by Abdulkhani et al. (2020) on the hydrophilic extractives of C. arizonica wood knots, different amounts of bioactive moieties were found, including matairesinol (MAT), curcumin, dienestrol, arctigenin (ARC), and sescoisolariciresinol (SEC). Additionally, the bioactivity of the hydrophilic extracts of C. arizonica was determined and compared with the raw hydrophilic extractives of C. sempervirens and Picea excelsa. The results of their studies revealed that P. excelsa with a total capacity of 318.8 mg.mL-1 showed the highest level of phenolics, followed by unpurified C. arizonica (257.5 mg.mL-1) and the solvent purified extract of C. arizonica (190.1 mg.mL-1). They also found that the most powerful radical scavenging activity was a raw ethanolic extract from P. excelsa wood knot with 66.67%, followed by BHA and potassium acetate purified C. arizonica with 57.96 and 56.37%, respectively.
To the best of the authors’ knowledge, the chemical composition of the fruit, leaves, and branchlet of the Arizona cypress (Cupressus arizonica) extracts have yet to be reported. However, the chemical composition of this part has been reported. Furthermore, this novel approach investigated the antioxidant activity of its extracts, as well as compared them with butylated hydroxytoluene (BHT) and ascorbic acid (AA). This paper is the first report on antioxidant activity of fruit extracts (FE), leaf extracts (LE), and branchlet extracts (BE) of C. arizonica.
EXPERIMENTAL
Materials
C. arizonica tree fruits, leaves, and branchlets were collected in April 2022 from the yard of the college of Agriculture and Natural Resources at Karaj Islamic Azad University located on the south-west of Karaj city, Iran. Voucher specimen (No. 1425) of the plant was deposited in the Herbarium of vascular plant (SOM) of the College of Agriculture and Natural Resources at Islamic Azad University, Karaj Branch, Karaj, Iran. The plant species were authenticated by Prof. Dr. Sayed Khosrow Hossein Ashrafi.
Preparation of Extracts
The innovation in this study is to consider parts of the plant that have not been tapped before for antioxidant activity. The tree parts of Arizona cypress, including fruits, leaves, and branchlets with an approximate moisture content 89.93, 94.17, and 47.06%, were separately cut into small pieces, and chopped (with 5 to 10, 5 to 15, and 5 to 20 mm particles size, respectively). In the first step, a little cotton was compressed and placed at the bottom of the 250-mL separatory funnel at the beginning of the outlet valve. In the second step, approximately 50, 50, and 40 g from pieces of fruits, leaves, and branchlets were poured into a separatory funnel, and then 200, 300, and 200 mL of 96% aqueous ethanol was added to each one. The mixture was macerated in the closed separatory funnel for 48 h. The outlet of the separatory funnel was opened, and the liquid was allowed to drip slowly, as specified in the method by Sabzikar et al. (2020). The liquid was clarified by filtration and finally concentrated to dryness, in a Petri plate at a laboratory temperature (25 ± 5 °C) under a laminar hood to avoid chemical alteration in the bioactive compounds with loss of their properties. The extracts were accumulated and dried over anhydrous sodium sulfate and then stored at 4 ºC until further analysis (Sabzikar et al. 2020). The extracts’ weights from fruit, leaf, and branchlet components were 0.20, 0.25, and 0.15 g, respectively. Each extract was prepared in the concentrations of 0.985, 0.493, 0.246, 0.123, 0.062, 0.031, and 0.015 mg/mL by diluting the extract in 70% MeOH.
Free Radical Scavenging Activity by DPPH Assay
The free radical scavenging activities of the hydroethanolic extracts of fruit, leaf, and branchlet of C. arizonica were conducted using the 1,1-dipheny-2-picrylhydrazyl (DPPH) assay as described in literature (Kim et al. 2002), because the DPPH is a stable radical, which has been used to evaluate the total antioxidant activity of plant and microbial extracts (Halliwell 1997).
Briefly, a stock solution via dissolving 3.94 mg of DPPH powder in 100 mL of 70% methanol (the stock solution of 0.1 mM DPPH with concentration of 0.0394 mg/mL) served as oxidant and was prepared just before use and stored at 4 °C in the dark to minimize degradation. The control samples were prepared with the same volume of solution, without test compounds and the referenced standards (negative control) (Pillai et al. 2019; Alam et al. 2021).
To prepare the working solution from the hydroethanolic extract, 9.85 mg (dry powder) sample of each hydroethanolic extract was dissolved in 10 mL of 70% methanol (v/v) (concentration 0.985 mg/mL), separately. Serial dilutions were made from the stock solution of 0.1 mM DPPH and the working solution of tested extract to obtain concentrations of 0.985, 0.493, 0.246, 0.123, 0.062, 0.031, and 0.015 mg/mL. The schematic of preparation process of working solution are shown in Fig. 1 (Hosseinihashemi and Aghajani 2017).
Two stock solutions of ascorbic acid (AA) and butylated hydroxytoluene (BHT) with the same concentration (0.985 mg/mL) were prepared. These served as the reference standards (positive control). Pure methanol (Sigma-Aldrich, Darmstadt, Germany) was used to make the control sample. The UV scanning spectrophotometer device (JENWAY 6320D, Standford, UK) was first calibrated and adjusted with 70% methanol.
Fig. 1. Preparation process of working solutions
The reaction mixture was mixed for 10 s and left to stand at room temperature in a dark place for 30 min. The absorbance was measured at 517 nm, using a UV scanning spectrophotometer. The experiment was performed in triplicates, and the average absorbance was recorded for both extract and concentration, separately. The DPPH free radical scavenging activity (%) was calculated using Eq. 1,
Inhibition (%) = 100 × (Ac – As)/Ac (1)
where the percentage inhibition value was calculated from the absorbance of the negative control, Ac, and of the sample, As.
The negative control contained reaction reagent except the extract or positive control substance. The values are presented as the means of triplicate analyses.
Analysis of Extracts
Gas chromatography-mass spectrometry (GC/MS) analysis of the C. arizonica fruit, leaf, and branchlet extracts was performed. Next, 100 μL of each extract was dehydrated by sodium sulfate salt and was dissolved with 100 μL of methanol, separately and run on a GC Agilent 7890A and MS Agilent 5975C mass spectrometer detector (Agilent Technologies, Palo Alto, CA, USA) equipped with a HP-5MS cross-linked capillary column (30 m long and 0.25 mm internal diameter, 0.25 μm film thickness). Helium was used as the carrier gas with a flow rate of 1 mL/min.
The GC/MS operation conditions were as follows: injector temperature of 260 °C; transfer line of 270 °C; oven temperature program of 60 °C for 4 min, 3 °C/min to 100 °C for 2 min, then 4 °C/min to 250 °C for 5 min; and carrier gas He at 1 mL/min. The intrinsic energy that hits the sample in the MS system was 70 eV. The split ratio of the sample was 50:1 with a split flow of 1 mL/min. Individual components were identified using mass spectra with data from literature, two mass spectrometric libraries (Wiley 275 L (http://www.palisade.com), 1998 and NIST-05 (http://www.nist.gov)), mass database matching, and by comparing the retention times and mass spectra of constituents with published data (Julian and König 1988; Adams 1995, 2001).
Statistical Analysis
The results are given in mean values with their standard deviations. Statistical analysis was performed using the SPSS program, version 24.0 (International Business Machines (IBM) Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) was conducted to determine the significance of differences between analytical results at p < 0.05 significance level.
RESULTS AND DISCUSSION
Antioxidant Activity (AOA)
The antioxidant activity of the 96% aqueous ethanol extracts from fruit, leaf, and branchlet of Cupressus arizonica at seven different concentrations of 0.15, 0.031, 0.062, 0.123, 0.246, 0.493, and 0.985 mg/mL were evaluated in the current study by using DPPH method and compared with BHT and vitamin C as the reference standards (Table 1 and Fig. 2). Statistically, there were highly significant differences among the treatments (fruit, leaf, branchlet extracts, BHT, vitamin C, and their concentrations (Table A1).
Table 1. Mean ± SD of Antioxidant Activity (%) as Affected by Concentration of Fruit, Leaf, and Branchlet of C. arizonica Extracts Compared with BHT and Vitamin C
As shown in Table 1 and Fig. 2, the BE exhibited high antioxidant activity overall. The lowest antioxidant activity, 70.9%, was observed in leaf extract at the concentration of 0.985 mg/mL, which was lower than for BHT (76.03%) at the same concentration. The highest activity came from branchlet extract (93.3%) at 0.985 and 0.493 mg/mL, which was also higher than the value of vitamin C (63.2 and 63.7%) at the same concentration. The same trend was observed with the reference, BHT. As the concentration of the extracts was increased, the antioxidant activity of the fruit and leaf extracts decreased, but the antioxidant activity of the branchlet extracts increased.
Additionally, among the assayed extracts, fruit and branchlet showed high antioxidant activity at 90.3 and 93.3%, respectively, when the concentration was 0.015 and 0.493 mg/mL, respectively.
On the one hand, the results of the study on the antioxidant activity and chemical composition of hydroethanolic extracts of different parts of C. arizonica indicated that C. arizonica is a rich source of phenolic compounds, with a high antioxidant capacity which may provide protection against scavenge free radicals.
On the other hand, looking at the detail of the data in Table 1 and Fig. 2, it is apparent that among the different parts of the plant, the fruit and branchlet extracts exhibited good antioxidant activity compared with the leaf extracts from C. arizonica, coinciding with high levels of terpenes and phenolic compounds in these parts.
Fig. 2. The antioxidant activity of extracts from the fruit (FE), leaf (LE), and branchlet (BE) of C. arizonica
According to the findings of Mikucka et al. (2022) and in accordance with the current research findings in Table 2, the content of total polyphenols and phenolic acids strongly correlated with antioxidant activity, indicating that these compounds provide a substantial contribution to the bioactive properties of the extracts.
Phenolic compounds are widely found in plants and play an important role in antioxidant activity and scavenging free radicals through their hydroxyl groups (Cosme et al. 2020; Ebrahimnezhad et al. 2022), where deviates depending on their molecular structure and characteristics (Qian et al. 2020; Falah et al. 2021). In addition to phenolic compounds, other structures such as terpenes could develop antioxidant capacity in plant organs both solitarily or synergistically (Álvarez-Martínez et al. 2021).
The GC/MS analysis study revealed that the tested extracts contained an abundant amount of components that can contribute to their effective antioxidant potential in DPPH assay. The chemical components identified that are classified in Table 2 confirm these statements, so that some of these components from classes such as labdane di-terpenes, abietane di-terpenes, phenolic di-terpenes, oxygenated cembrene di-terpenes, oxygenated abietane di-terpenes, phenolic acids, resin acids, monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, phenolic compounds, volatile phenolic compounds, tocopherols (Ebrahimnezhad et al. 2022), lignans (Abdulkhani et al. 2020), triterpenoids, and coumarins inhibited free radical scavenging activity in-vitro bioassay.
Table 2. Classification of the Identified Chemical Components of the C. arizonica Fruit, Leaf, and Branchlet Hydroethanolic Extracts by GC/MS Data Analysis
The antioxidant activity of hydroethanolic extracts of branchlet of C. arizonica was higher than that of fruit, leaf, BHT, and AA, which can be caused by the amount of the some mentioned antioxidant components (Table 2).
Extracts Analysis
It is clear that the extracts of the plant varied according to the plant location, variety, and position. The yields of extracts isolated from C. arizonica ranged from 0.44 to 0.79% depending on the part of the plant analyzed. The greatest yields were in branchlets and leaves (0.79 and 0.53%, respectively) and the lowest extract was in the fruits (0.44%). The extracts obtained from fresh fruit, leaf, and branchlet without adherent leaf of C. arizonica growing in Iran, were analyzed by GC/MS. A total of 55, 60, and 53 compounds were identified accounting for 98.52, 95.51, and 99.12% of the total extracts in fruit, leaf, and branchlet, respectively.
Phytochemical analysis of the fruit, leaf, and branchlet hydroethanolic extracts of C. arizonica showed the different amounts of bioactive moieties as summarized in Table 2. The preliminary phytochemical analysis of C. arizonica wood knots extract showed different amounts of bioactive moieties including matairesinol (MAT), curcumin, dienestrol, arctigenin (ARC), and sescoisolariciresinol (SEC) were found (Abdulkhani et al. 2020).
Chemical composition of C. arizonica fruit, leaf, and branchlet extracts
Figure 3 indicates the main chemical constituents in the fruit, leaf, and branchlet extracts.
Fig. 3. The main chemical constituents identified in the hydroethanolic extracts from fruit, leaf, and branchlet of C. arizonica
The chemical components identified in the hydroethanolic extracts from fruit, leaf, and branchlet of C. arizonica are presented in Tables 3, 4, and 5. The GC-MS profiling revealed the fruit, leaf, and branchlet hydroethanolic extracts of C. arizonica contained 55, 60, and 53 bioactive components, respectively. Fruit, leaf, and branchlet extracts were more active, active, and most active, respectively, and also possessed good antioxidant activity.
Other identified compounds shown in Table 3 with moderate percentages included 8-amino-2,5-dimethyl-6-methoxyquinoline (2.98%), sugiol (2.89%), totarol (1.66%), bicyclo[5.2.0]nonane, 4-methylene-2,8,8-trimethyl-2-vinyl- (1.36%), and β-retinoic acid (1.24%). A few other compounds with lower percentages were identified in the fruit extracts, including bicycloelemene, 1,10-dicyanodecane, gamma-elemene, (1R*,2R*,3S)-3-isopropenyl-2-vinylcyclohex-1-yl vinyl ether, benzenamine, 3-chloro-N-(2-pyridinylmethylene)-, di-epi-.alpha.-cedrene-(I), and mom inositol.
The other identified compounds shown in Table 4 with moderate percentages included 1-(adamantyl-1)butanol-1 (2.05%), benzeneethanol, .beta.-ethenyl- (1.75%), phyllacladan-16.alpha.-ol (1.45%), vitamin E (1.40%), pimaric acid (1.27), and totarol (1.09%). There were a few other compounds with lower percentages identified in the leaf extracts, including 1S,CIS-calamenene, aromadendrene VI, di-epi-.alpha.-cedrene-(I), phytol, catechol, 5-hydroxy-1,3,4-trimethoxy-7-methyl-6-propargynaphthalene, and .alpha.-cedrol. The other identified compounds shown in Table 5 with moderate percentages included isopimaral (2.93%), phenol, 4-ethyl-2-methoxy- (2.68%), sugiol (2.46%), 9,12-octadecadienoic acid (Z,Z)- (2.18%), (23S)-ethylcholest-5-en-3.beta.-ol (1.98%), totarol (1.44%), and 9-octadecenoic acid, (E)- (1.29%). There were a few other compounds with lower percentages identified in the branchlet extracts that included dispiro[2.1.2.1] octane, 1,1,6,6-tetramethyl-, cembrene, labda-8(17),13Z-dien-15-ol, carvacrol, hexadecanoic acid, mome inositol, and bicyclo[5.2.0]nonane, 4-methylene-2,8,8-trimethyl-2-vinyl-.
Table 3. Chemical Composition of Fruit Hydroethanolic Extracts of C. arizonica
Table 4. Chemical Composition of Leaf Hydroethanolic Extracts of C. arizonica
Table 5. Characterized Chemical Composition of Branchlet Hydroethanolic Extracts of C. arizonica
The monoterpene amounted to 0.81% in FE, 2.13% in LE, and 1.13% in BE, whereas sesquiterpenes accounted for 2.65% in FE, 6.47% in LE, and 0.58% in BE, with high amount of diterpenes 57.83% in FE and 63.22% in BE and with low amount 1.99% in LE.
In monoterpenes, monoterpene hydrocarbons were the major constituents, accounting 0.71 and 1.82%, respectively in fruit and leaf, and 0.15% in branchlet. The main monoterpene hydrocarbons were di-epi-α-cedrene-(I) 0.38% in fruits, 0.96% in leaves, and sabinene 0.04% in branchlet, respectively. In sesquiterpenes, sesquiterpene hydrocarbons were the major constituents 1.58% in fruits, 5.50% in leaves and 0.32% in branchlets.
In diterpenes, labdane diterpenoids were the major constituents, accounting 47.17 and 44.57%, respectively in fruit and branchlet extracts, but the compound was not identified in leaf extract. Furthermore, abietane-type diterpenes also were the major constituents, accounting 8.84 and 12.89%, respectively in fruit and branchlet extracts, but the compound was not found in leaf extract.
Abdulkhani et al. (2020) stated that in addition to phenolic compounds, other moieties in ethanolic extracts caused an increase in total radical scavenging activity. It has been reported that the antioxidant activity of phenolic compounds depends on the number and location of hydroxyl groups in the phenolic compounds (Sok et al. 2009). Matairesinol (MAT) was the defatted lignan in ethanolic fruit extracts of C. arizonica that have been previously reported by Abdulkhani et al. (2020) in wood knot as a predominant lignan. Dl-3,4-dehydroproline inhibits the growth of Lactobacillus arabinosus, Streptococcus lactis, Pediococcus cerevisiae, Leuconostoc dextranicum, and Escherichia coli, and the toxicities are competitively reversed by l-proline with inhibition indices of 3, 3, 3, 10, and 10, respectively (Smith et al. 1962).
Benzimidazole and some of its derivatives as 4-nitro and 5-nitro-benzimidazoles, 2-amino-, 4-amino-, and 5-aminobenzimidazoles have been tested on gastric acid secretion in Shay rats. Only 5-aminobenzimidazole decreased the gastric secretion process basally or stimulated by betazole (Trivulzio et al. 1988).
Communic acids are diterpenes with labdane skeletons found in many plants species, primarily conifers, predominating in the genus Juniperus (fam. Cupressaceae). These acids have been isolated from different parts of the plant (fruits, wood, bark, leaves, roots, etc.); they are primarily found in leaves, fruits, and bark as well as have different biological activities (antibacterial, antitumoral, hypolipidemic, relaxing smooth muscle, etc.) (Barrerol et al. 2012).
Ferruginol has antimicrobial activity (Li et al. 2008; Matsushita et al. 2006), antioxidant activity (Wang et al. 2002), gastroprotective and ulcer healing effects (Rodríguez et al. 2006), termite resistance effects (Kano et al. 2004), and growth inhibition activity against Heterosigma akashiwo (Saijo et al. 2013).
The results obtained from Wang et al. (2002) indicate that ferruginol possesses a significant inhibitory activity against the DPPH radical, followed by hinokiol, secoabietane dialdehyde, 6β-acetoxy-7α hydroxyroyleanone, and isopimarinol, with sugiol showing the least radical scavenging activity as well as ferruginol that has potential for use as a natural food preservative. The ferruginol compound value in the BE was twice that of the FE, but in the LE, this compound was not present at all. It seems that, for this reason the antioxidant activity of the BE was higher than the FE and the FE higher than the LE.
It has also been demonstrated that ferruginol is an effective antifungal compound of Taiwania cryptomerioides heartwood (Chang et al. 1999). Because both white-rot and brown-rot fungus release oxidase to cleave the cellulose or lignin of wood, it is plausible that ferruginol inhibits the growth of fungus by blocking the radical transition (Wang et al. 2002).
GC/MS analysis of Stevia rebaudiana extracts deal with in vitro antidiabetic potential has shown the major compounds of 1-hepta-triacotanol, duvatrienediol, dihydroxanthin, β-amyrin, lupenone, phytol, γ-sitosterol, agatholic acid, and fatty acids (Zaidan et al. 2019). Agatholic acid also was found in the wheat (1.46%), infected wheat by Caloglyphus berlesei (4.05%), maize (0.64%), infected maize by Caloglyphus berlesei (1.41%), and fishmeal (4.69%) extracts after three months stored (Gamal El-Din et al. 2019) as well as in the fruit benzene/acetone (10:1 v/v) extracts of Forsythia suspensa to yield of 9 mg (Kuo et al. 2014).
Diterpenoids totarol and sugiol were also detected as the major compounds in the fossil species Taxodium dubium (Otto et al. 1997). The major compound in resin extracts from T. distichum was totarol (36.75%), followed by limonene (19.24%), and α-pinene (16.06%), while resins of T. ascendens also contained higher levels of totarol (46.85%) and α-pinene (29.39%), but limonene reached only 5.42% (Špaldoňová et al. 2020).
Earlier studies of the chemical composition and biological activities of the essential oil extracted from the stem of Olea europaea sub sp. africana (Mill) showed that at the concentration of 0.15 mg/mL (149.090 μg/mL), the essential oil of Olea europaea exhibited the highest percentage of inhibition (95.03%) compared to the range given in the literature (Syamsir 2009). The crude essential oil of Olea europaea also gave strong antioxidant activities in DPPH radical scavenging test, with its IC50 values at 19.9 μg/mL and showed comparable antioxidant potential compared to ascorbic acid. Ascorbic acid showed 98.05% inhibition (IC50: 15.9 μg/mL), which serves as a standard (Syamsir 2009). Some essential stem oil of Olea europaea similarly was found in the extracts of fruit, leaf, and branchlet of C. arizonica such as spirohexane-5-carboxylic acid, 1,1,2,2-tetramethyl-, methyl ester, d-Limonene, catechol, and aromandendrene (Asfaw et al. 2022).
According to the findings of Mannai et al. (2021) regarding antifungal activities of Raphanus raphanistrum, it seems that benzeneacetonitrile, 4-fluoro as a glucosinolate product could be responsible of a part of antioxidant activity of C. arizonica leaf extracts.
According to the classification of percentage scavenging activities of DPPH radical by Syamsir (2009), the results of percentage scavenging activities of DPPH radical in the test solution at 5 mg/mL was strong when the scavenging percentage was between 71 and 100, moderate when the percentage scavenging activity was between 41 and 70, and weak when the scavenging activities were ≤ 40. Experimental findings showed that the results of percentage scavenging activities of DPPH radical in the all tested extracts was strong between 71 and 100.
Tepe et al. (2005) examined the antioxidant activity of the components of Salvia tomentosa Miller (Lamiaceae) essential oils (EOs). Of these, terpinene-4-ol, 1,8-cineole, camphor, borneol, p cymene, α-pinene, and β-pinene showed no activity. Furthermore, the main components of Achillea millefolium subsp. millefolium Afan EOs e.g., eucalyptol, camphor, β-pinene, borneol, terpinen-4-ol, and α-pinene, were all tested individually and none exhibited antioxidative activity in any of the assays employed (Candan et al. 2003). The reason that EOs showed much more activity than their constituents alone can be attributed to the high percentages of the main components, synergy among the different oil constituents, or to microcomponents acting as pro-oxidants (Viuda-Martos et al. 2010). According to the findings of Ruberto and Baratta (2000), oxygenated monoterpenes (OM), especially thymol and carvacrol, have high antioxidant activity. Although, monoterpene hydrocarbons (MH) may be considered as active antioxidants, none are stronger than oxygenated monoterpenes (OM). Sesquiterpene hydrocarbons (SH) and their oxygenated derivatives have very low antioxidant activity.
There is no data on the chemical composition and antioxidant activity of C. arizonica fruit, leaf, and branchlet extracts in the literature to be compared with the present results. However, a study on phenolic and other moieties contents in hydroethanolic extracts and antioxidant activity of its phylogenetically close taxa, C. sempervirens (CS), revealed the presence of high levels of terpenes and phenolic compounds and good antioxidant capacity in fruit, leaf, and branchlet.
It seems that diterpenes-types present in the fruit and branchlet hydroethanolic extracts of C. arizonica exhibited potent activity. The ethanolic extract of C. sempervirens fruit inhibited proliferation of human BPH-stromal cells, and the activity was localized to its chloroform-soluble, diterpene-rich fraction (Al-Snafi 2016). Diterpenes such as 6-deoxytaxodione (11-hydroxy-7, 9(11), 13-abietatrien-12-one), taxodione, ferruginol, sugiol, trans-communic acid, 15-acetoxy imbricatolic acid, and imbricatolic acid were isolated from C. sempervirens (Tumen et al. 2012).
CONCLUSIONS
The different parts of many plants and their extracts and essential oils have been used in folk medicine. In this research, the chemical composition and antioxidant capacity of hydroethanolic extracts of fruit, leaf, and branchlet of C. arizonica were studied to diversify herbal medicines. Preliminary screening of the phenolic and terpenic components and antioxidant activity of extracts from fruit, leaf, and branchlet of C. arizonica indicated the high potential of this tree for nutrition and pharmaceutical purposes. The important and general results of the current study are as follows:
1. Fruit and branchlet extracts of Cupressus arizonica exhibited a good antioxidant activity compared with leaf extracts, BHT, and vitamin C.
2. The main chemical constituents in the fruit extracts were communic acid, spirohexane-5-carboxylic acid, 1,1,2,2-tetramethyl-, methyl ester, and ferruginol; the main chemical constituents in the leaf extracts were hexadecanoic acid, 1H,5H-pyrrolo[1′,2′:3,4]imidazo[1,5-a]pyridine, octahydro-, and bicyclo[3.1.0]hex-3-en-2-one, 4-methyl-1-(1-methylethyl)-; the main chemical constituents in the branchlet extracts were communic acid, agatholic acid, and ferruginol.
3. The branchlet extracts from C. arizonica had better antioxidant activity compared with others and standard positive controls.
4. Fruit, leaf, and branchlet extracts of this species contain considerable amounts of phenols and terpenes and show good antioxidant activity, suggesting further investigation for isolation of the active components and biological characterizations and medicinal properties of the plant.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Department of Applied Chemistry, Damghan University, Damghan, Iran; and thankful for the support of the Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran.
REFERENCES CITED
Abdulkhani, A., Sedaghat, A., Alizadeh, P., and Tabil, L. G. (2020). “Extraction of bioactive moieties of Cupressus arizonica and Cupressus sempervirens wood knots,” Canadian Biosystems Engineering 62(1), 8.1-8.10. DOI: 10.7451/CBE.2020.62.8.1
Adams, R. P. (1995). Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 1st Edition, Allured Publishing Corp., Carol Stream, IL, USA.
Adams, R. P. (2001). Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 3rd Edition, Allured Publishing Corp., Carol Stream, IL, USA.
Alam, F., Ali Khan, S. H., and Bin Asad, M. H. H. (2021). “Phytochemical, antimicrobial, antioxidant and enzyme inhibitory potential of medicinal plant Dryopteris ramosa (Hope) C. Chr.,” BMC Complement. Med. Ther. 21(1), article no. 197. DOI: 10.1186/s12906-021-03370-7
Al-Snafi, A. E. (2016). “Medical importance of Cupressus sempervirens – A review,” IOSR J. Pharm. 6(6), 66-76.
Álvarez-Martínez, F. J., Barrajón-Catalán, E., Herranz-López, M., and Micol, V. (2021). “Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action,” Phytomedicine 90, article no. 153626. DOI: 10.1016/j.phymed.2021.153626
Asfaw, M. D., Mekonnen, K. N., and Asgedom, A. G. (2022). “Chemical composition and biological activities of the essential oil extracted from the stem of Olea europaea sub spp. Africana (Mill),” Nat. Prod. Chem. Res. 10(1), 1-9. DOI: 10.37533/npcr.10.1.1-9
Barrero, A. F., Mar Herrador, M., Arteaga, P., Arteaga, J. F., and Arteaga, A. F. (2012). “Communic acids: Occurrence, properties and use as chirons for the synthesis of bioactive compounds,” Molecules 17(2), 1448-1467. DOI: 10.3390/molecules17021448
Candan, F., Unlu, M., Tepe, B., Daferera, D., Polissiou, M., Sökmen, A., and Akpulat, H. A. (2003). “Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae),” J. Ethnopharmacol. 87, 215-220. DOI: 10.1016/s0378-8741(03)00149-1
Chang, S.-T., Wang, S.-Y., Wu, C.-L., Su, Y.-C., and Kuo, Y.-H. (1999). “Antifungal compounds in the ethyl acetate soluble fraction of the extractives of Taiwania (Taiwania cryptomerioides Hayata) heartwood,” Holzforschung 53, 487-490. DOI: 10.1515/HF.1999.080
Chéraif, I., Ben Jannet, H., Hammami, M., Khouja, M. L., and Mighri, Z. (2007). “Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene,” Biochem. Syst. Ecol. 35(12), 813-820. DOI: 10.1016/j.bse.2007.05.009
Cosme, P., Rodríguez, A. B., Espino, J., and Garrido, M. (2020). “Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications,” Antioxidants 9(12), 1263. DOI: 10.3390/antiox9121263
Ebrahimnezhad, Z., Dehghani, M., and Beyzaei, H. (2022). “Assessment of phenolic and flavonoid contents, antioxidant properties, and antimicrobial activities of Stocksia brahuica Benth,” Int. J. Basic Sci. Med. 7(1), 34-40. DOI: 10.34172/ijbms.2022.07.
Emami, S. A., Fakhrjafary, M., Tafaghodi, M., and Hasanzadeh, M. K. (2010). “Chemical composition and antioxidant activities of the essential oils of different parts of Cupressus arizonica Greene,” J. Essent. Oil Res. 22(3), 193-199. DOI: 10.1080/10412905.2010.9700301
Emami, S. A., Shahani, A., and Hassanzadeh Khayyat, M. (2013). “Antioxidant activity of leaves and fruits of cultivated conifers in Iran,” Jundishapur J. Nat. Pharm. Prod. 8(3), 113-117. DOI: 10.17795/jjnpp-9670
Falah, F., Shirani, K., Vasiee, A., Tabatabaee Yazdi, F., and Alizadeh Behbahani, B. (2021). “In vitro screening of phytochemicals, antioxidant, antimicrobial, and cytotoxic activity of Echinops setifer extract,” Biocatal. Agric. Biotechnol. 35, article no. 102102. DOI: 10.1016/j.bcab.2021.102102
Fralish, J. S., and Franklin, S. B. (2002). Taxonomy and Ecology of Woody Plants in North American Forests, John Wiley and Sons Inc., New York, NY, USA.
Gamal El-Din, H. M., Metwally, A. M., AbdAllah, A. A., and El-Bltagy, H. M. (2019). “The effect of infection by Caloglyphus berlesei on organic volatile compounds of some stored products,” Egypt. J. Nutr. Feeds 22(2), 121-132. DOI: 10.21608/ejnf.2019.103459
Ibrahim, N. A., El-Seedi, H. R., and Mohammed, M. M. D. (2007). “Phytochemical investigation and hepatoprotective activity of Cupressus sempervirens L. leaves growing in Egypt,” Nat. Prod. Res. 21(10), 857-866. DOI: 10.1080/14786410601132477
Halliwell, B. (1997). “Antioxidants and human diseases: A general introduction,” Nutr. Rev. 55, S44-S52. DOI: 10.1111/j.1753-4887.1997.tb06100.x
Hassanpouraghdam, M. B. (2011). “α-Pinene-and β-myrcene-rich volatile fruit oil of Cupressus arizonica Greene from northwest Iran,” Nat. Prod. Res. 25(6), 634-639. DOI: 10.1080/14786419.2010.531479
Hosseinihashemi, S. K., and Aghajani, H. (2017). “Chemical composition and antioxidant capacity of extracts from the wood of Berberis vulgaris stem,” Lignocellulose 6(1), 36-47.
Julian, D., and Konig, W. A. (1988). The Atlas of Spectral Data of Sesquiterpene Hydrocarbons, E. B. Verlag, Hamburg, Germany.
Kano, H., Shibutani, S., Hayashi, K., Iijima, Y., and Doi, S. (2004). “Effect of high temperature drying processes on termite resistance of sugi (Cryptomeria japonica) heartwood,” Mokuzai Gakkaishi 50(1), 91-98. (in Japanese)
Kim, J. K., Noh, J. H., Lee, S., Choi, J. S., Suh, H., Chung, H. Y., Song, Y.O., and Lee, C. (2002). “The first total synthesis of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether (TDB) and its antioxidant activity,” Bull. Korean Chem. Soc. 23(5), 661-662. DOI:10.5012/bkcs.2002.23.5.661
Kuiate, J. R., Bessière, J. M., Vilarem, G., and Amvam Zollo, P. H. (2006). “Chemical composition and antidermatophytic properties of the essential oils from leaves, flowers and fruits of Cupressus lusitanica Mill. from Cameroon,” Flavour Fragr. J. 21(4), 693-697. DOI: 10.1002/ffj.1686
Kuo, P.-C., Chen, G.-F., Yang, M.-L., Lin, Y.-H., and Peng, C.-C. (2014). “Chemical constituents from the fruits of Forsythia suspensa and their antimicrobial activity,” BioMed. Res. Int. 2014, article ID 304830. DOI: 10.1155/2014/304830
Li, W. H., Chang, S. T., Chang, S. C., and Chang, H. T. (2008). “Isolation of antibacterial diterpenoids from Cryptomeria japonica bark,” Nat. Prod. Res. 22(12), 1085-1093. DOI: 10.1080/14786410802267510
Little, E. L., Jr., and Skolmen, R. G. (1989). Common Forest Trees of Hawaii (Native and Introduced), Agriculture Handbook, USDA Service, Washington D.C., USA.
Mannai, S., Benfradj, N., Karoui, A., Salem, I. B., Fathallah, A., M’Hamdi, M., and Boughalleb-M’Hamdi, N. (2021). “Analysis of chemical composition and in vitro and in vivo antifungal activity of Raphanus raphanistrum extracts against Fusarium and Pythiaceae, affecting apple and peach seedlings,” Molecules 26(9), article no. 2479. DOI: 10.3390/molecules26092479
Matsushita, Y., Hwang, Y. H., Sugamoto, K., and Matsui, T. (2006). “Antimicrobial activity of heartwood components of sugi (Cryptomeria japonica) against several fungi and bacteria,” J. Wood Sci. 52, 552-556. DOI: 10.1007/s10086-005-0793-9
Mbaveng, A. T., Kuete, V., Mapunya, B. M., Beng, V. P., Nkengfack, A. E., Meyer, J. J. M., and Lall, N. (2011). “Evaluation of four Cameroonian medicinal plants for anticancer, antigonorrheal and antireverse transcriptase activities,” Environ. Toxicol. Pharmacol. 32(2), 162-167. DOI: 10.1016/j.etap.2011.04.006
Mikucka, W., Zielinska, M., Bulkowska, K., and Witonska, I. (2022). “Processing of distillery stillage to recover phenolic compounds with ultrasound-assisted and microwave-assisted extractions,” Int. J. Environ. Res. Public Health 19, article no. 2709. DOI: 10.3390/ijerph19052709
Mothana, A. R., Gruenert, R., Bednarski, P. J., and Lindequist, U. (2009). “Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine,” Pharmazie 64(4), 260-268. DOI: 10.1691/ph.2009.8789
Otto, A., Walther, H., and Püttmann, W. (1997). “Sesqui- and diterpenoid biomarkers preserved in Taxodium-rich Oligocene oxbow lake clays, Weisselster basin, Germany,” Org. Geochem. 26(1-2), 105-115. DOI: 10.1016/S0146-6380(96)00133-7
Pierre-Leandri, C., Fernandez, X., Lizzani-Cuvelier, L., Loiseau, A., Fellous, R., and Garnero, J. (2003). “Chemical composition of cypress essential oils: Volatile constituents of leaf oils from seven cultivated Cupressus species,” J. Essent. Oil Res. 15(4), 242-247. DOI: 10.1080/10412905.2003.9712130
Pillai, M. K., Santi, L. I., and Magama, S. (2019). “DPPH radical scavenging activity of extracts from Rhamnus prinoides,” J. Med. Plants Res. 13(15), 329-334. DOI: 10.5897/JMPR2019. 6793
Qian, J., Hou, M., Wu, X., Dai, C., Sun, J., and Dong, L. (2020). “A review on the extraction, purification, detection, and pharmacological effects of 2,3,5,4’-tetrahydroxystilbene-2-O-β-d-glucoside from Polygonum multiflorum,” Biomed. Pharmacother. 124, article no. 109923. DOI: 10.1016/j.biopha.2020.109923
Ramdani, M., Lograda, T., Chalard, P., Chalchat, J. C., and Figueredo, G. (2011). “Chemical variability of essential oils in natural populations of Cupressus dupreziana,” Nat. Prod. Commun. 6, 87-92. DOI: 10.1177%2F1934578X1100600121
Rodríguez, J. A., Theoduloz, C., Yáñez, T., Becerra, J., and Schmeda Hirschmann, G. (2006). “Gastroprotective and ulcer healing effect of ferruginol in mice and rats: assessment of its mechanism of action using in vitro models,” Life Sci. 78, 2503-2509. DOI: 10.1016/j.lfs.2005.10.018
Ruberto, G., and Baratta, M. T. (2000). “Antioxidant activity of selected essential oil components in two lipid model systems,” Food Chem. 69, 167-174. DOI: 10.1016/S0308-8146(99)00247-2
Sabeti, H. (1976). Forest, Trees and Shrubs of Iran, National Agriculture and Natural Resources Research Organization, Ministry of Agriculture and Natural Resources, Tehran, Iran.
Sabeti, H. (1966). Native and Exotic Tree and Shrubs of Iran, Tehran: Tehran Univ. Press; p. 430 (in Persian).
Sabzikar, A., Hosseinihashemi, S. K., Shirmohammadli, Y., and Jalaligoldeh, A. (2020). “Chemical composition and antimicrobial activity of extracts from thyme and rosemary against Staphylococcus aureus and Candida albicans,” BioResources 15(4), 9656-9671. DOI: 10.15376/biores.15.4.9656-9671
Saijo, H., Tsuruta, K., Kusumoto, N., Ashitani, T., and Takahashi, K. (2013). “Growth inhibition activities of Sugi bark components against Heterosigma akashiwo,” J. Wood Sci. 59, 238-242. DOI: 10.1007/s10086-013-1328-4
Smith, L. C., Ravel, J. M., Skinner, C. G., and Shive, W. (1962). “3,4-Dehydroproline, a proline antagonist,” Arch. Biochem. Biophys. 99(1), 60-64. DOI: 10.1016/0003-9861(62)90243-6
Sok, D. E., Cui, H. S., and Kim, M. R. (2009). “Isolation and boactivities of furfuran type lignan compounds from edible plants,” Recent Pat. Food, Nutr. Agric. 1(1), 87-95. DOI: 10.2174/2212798410901010087
Špaldoňová, A., Havelcová, M., Machovič, V., and Lapčák, L. (2020). “Molecular resin composition of two Taxodium taxa growing in different climate condition: Chromatographic and spectroscopic study,” Advancement in Medicinal Plant Research 8(3), 60-72. DOI: 10.30918/AMPR.83.20.029
Swearingen, J., and Bargeron, C. (2016). Invasive Plant Atlas of the United States, University of Georgia Center for Invasive Species and Ecosystem Health. http://www.invasiveplantatlas.org/
Syamsir, D. R. B. (2009). Essential Oils and Biological Activities of Three Selected Wild Alpinia Species, Master’s Thesis, Institute of Biological Sciences, Faculty of Sciences, University of Malaya, Kuala Lumpur, Malaysia.
Tepe, B., Sokmen, M., Akpulat, H. A., Daferera, D., Polissiou, M., and Sokmen, A. (2005). “Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans,” J. Food Eng. 66, 447-454. DOI: 10.1016/j.jfoodeng.2004.04.015
Trivulzio, S., Colombo, R., Rossoni, G., and Caironi, E. (1988). “5-Aminobenzimidazole inhibits gastric acid secretion in shay-rats,” Pharmaco. Res. Commun. 20(11), 975-982. DOI: 10.1016/S0031-6989(88)80125-5
Tumen, I., Senol, F. S., and Orhan, I. E. (2012). “Evaluation of possible in vitro neurobiological effects of two varieties of Cupressus sempervirens (Mediterranean cypress) through their antioxidant and enzyme inhibition actions,” Türk Biyokimya Dergisi [Turk. J. Biochem.] 37(1), 5-13. DOI:10.5505/tjb.2012.92400
USDA (2002). “Plant fact sheet,” Guide Coordination Page, http://plantmaterials.nrcs.usda.gov/intranet/pfs.html
Viuda-Martos, M., López-Marcos, M. C., Fernández-López, J., Sendra, E., López-Vargas, J. H., and Pérez-Álvarez, J. A. (2010). “Role of fiber in cardiovascular diseases: A review,” Compr. Rev. Food Sci. Food Saf. 9, 240-258. DOI: DOI:10.1111/j.1541-4337.2009.00102.x
Wang, S. Y., Wu, J. H., Shyur, L. F., Kuo, T. H., and Chang, S. T. (2002). “Antioxidant activity of abietane type diterpenes from heartwood of Taiwania cryptomerioides Hayata,” Holzforschung 56, 487-492. DOI: 10.1515/HF.2002.075
Zaidan, U. H., Zen, N. I. M., Nor, Amran, N. A., Shamsi, S., and Gani, S. S. A. (2019). “Biochemical evaluation of phenolic compounds and steviol glycoside from Stevia rebaudiana extracts associated with in vitro antidiabetic potential,” Biocatal. Agric. Biotechnol. 18, article no. 101049. DOI: 10.1016/j.bcab.2019.101049
Zare, H. (2001). “Introduction and Native Conifers in Iran,” Research Institute of Forests and Ranglands, Tehran, Iran. pp. 83-89.
Zhang, L., Zhang, Y., Li, S., and Karchesy, J. J. (2012). “Cupressus macrocarpa heartwood oil and its bioactivity against some wood decay fungi,” Adv. Mater. Res. 485, 413-416. DOI: 10.4028/www.scientific.net/AMR.485.413
Article submitted: August 25, 2022; Peer review completed: October 22, 2022; Revised version received: October 25, 2022; Accepted: October 26, 2022; Published: November 1, 2022.
DOI: 10.15376/biores.18.1.19-38
APPENDIX
Table A1. Univariate Test Results of the Effect of Fruit, Leaf, and Branchlet Hydroethanolic Extracts and Their Concentrations on the Antioxidant Activity of C. arizonica Extracts Compared with BHT and Vitamin C
