Abstract
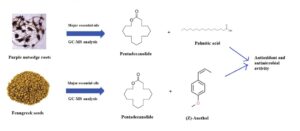
Purple nutsedge roots (Cyperus rotundus L.) and fenugreek seeds (Trigonella foenum-graecum L.) have been traditionally used as food and to treat common ailments. After extraction by solid-phase microextraction (SPME), the chemical structure of the revealed volatile fractions was researched with gas chromatography with mass spectrometry (GC-MS). The determined substances of the C. rotundus were pentadecanolide (72.0%), palmitic acid (8.2%), 16-hydroxy-6-hexadecenoic acid omega lactone (4.4%), and (Z)-anethol (3.9%). Most of the identified compounds of the T. foenum-graecum were pentadecanolide (61.3%) and (Z)-anethol (16.5%). The C. rotundus showed good antifungal activity against the yeast strands of Candida albicans and Candida krusei. Minimum inhibitory concentration (MIC) numbers were 250 and 125 µg/mL, respectively. However, the T. foenum-graecum seeds did not show any effect against the test microorganisms. The C. rotundus roots in particular exhibited good 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity with an IC50 number of 0.91 mg/mL.
Download PDF
Full Article
Chemical Composition, Antimicrobial, and Antioxidant Activities of Medicinal Plants Nutsedge and Fenugreek
Şule Ceylan,a,* Yasemin Camadan,b Özlem Saral,c and Özge Özşen Batur d
Purple nutsedge roots (Cyperus rotundus L.) and fenugreek seeds (Trigonella foenum-graecum L.) have been traditionally used as food and to treat common ailments. After extraction by solid-phase microextraction (SPME), the chemical structure of the revealed volatile fractions was researched with gas chromatography with mass spectrometry (GC-MS). The determined substances of the C. rotundus were pentadecanolide (72.0%), palmitic acid (8.2%), 16-hydroxy-6-hexadecenoic acid omega lactone (4.4%), and (Z)-anethol (3.9%). Most of the identified compounds of the T. foenum-graecum were pentadecanolide (61.3%) and (Z)-anethol (16.5%). The C. rotundus showed good antifungal activity against the yeast strands of Candida albicans and Candida krusei. Minimum inhibitory concentration (MIC) numbers were 250 and 125 µg/mL, respectively. However, the T. foenum-graecum seeds did not show any effect against the test microorganisms. The C. rotundus roots in particular exhibited good 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity with an IC50 number of 0.91 mg/mL.
DOI: 10.15376/biores.17.3.4580-4594
Keywords: Antimicrobial; Antioxidant; C. rotundus L.; Volatile fraction; T. foenum-graecum L.
Contact information: a: Department of Forest Industry Engineering, Artvin Coruh University, 08000, Artvin, Turkey; b: Department of Pharmacy Services, Artvin Coruh University, 08000, Artvin, Turkey; c: Department of Nutrition and Dietetics, Recep Tayyip Erdogan University, 53100, Rize, Turkey; d: Department of Chemistry, Eskisehir Osmangazi University, 26480, Eskisehir, Turkey;
* Corresponding author: sulecanim@hotmail.com
GRAPHICAL ABSTRACT

INTRODUCTION
Medicinal herbs play an important role in the protection of human health and the beautification of human living conditions. Such plants are used in the food, cosmetics, fragrance, paint, and pharmaceutical industries, as well as valuable food ingredients such as spices and beverages. Almost seventy-four percent of the 121 therapeutically active plant-derived phytocompounds currently used in the world were created after further studies were conducted to demonstrate their ethnomedical use (Olaoluwa et al. 2022). The use of different medicinal and herbal plants and the presence of valuable natural compounds in these plants directly affect human life. Pharmaceutical research with medicinal plants containing biologically active groups, which is a good source for new powerful drug discovery process, supports the interest in phytochemicals (Mouthe Kemayou et al. 2021; Nalawade et al. 2022). Numerous extracts from herbs have been found to have antimicrobial properties in-vitro and in-vivo, which justifies traditional medicine studies focused on the nature of the biological activities of these plants (Martínez et al. 1996). These extracts, obtained directly or indirectly from plants, are used in the structure of many pharmacologically important drugs (Alsabah et al. 2018). Despite the dependence on synthetic chemistry to produce and develop drugs today (Zainal et al. 2018), the use of herbs in disease control and treatment is still very high (Salih et al. 2017).
The number of drug-resistant bacteria across the world has increased, especially in recent years. One method to prevent antibiotic resistance in resistant bacteria is to use new substances different from existing synthetic antimicrobial compounds (Shah 2005). Systematic screening of herbs may result in the discovery of new effective compounds that will overcome the existing problem of drug resistance. It is hoped that plant components can effectively target drug-resistant bacterial pathogens (Yang et al. 2002).
Reactive oxygen species, which are present in cells and are random by-products of body metabolism, show cytotoxic properties and cause cardiovascular diseases, inflammation, and cancer, among other various diseases (Josh and Janardhanan 2000; Gomes et al. 2001). Antioxidants protect the body against damage produced by free radicals such as reactive oxygen species and act as a defense against toxicity caused by radicals (Manzi et al. 1999; Yang et al. 2002). Recently, interest in natural antioxidants obtained from various types of vegetables, fruits, plants, grains, and seeds has increased (Alara et al. 2020).
Cyperus rotundus L. (Cyperaceae family), commonly known as musta or motha, is one of the most widespread herbs in the world, and it is abundant in pan-India. C. rotundus is also known as tiger nut, purple nutsedge, red nutsedge, nutgrass, and java grass. C. rotundus grows naturally in the tropical, subtropical, and temperate zones throughout the world and is predominant in the south, center, and northeast of Tunisia (Sharma and Gupta 2007). C. rotundus is a conventional medicinal herb found within Indian, Japanese, and Chinese native medicines utilized for stomach illnesses, spasms, inflammatory bowel diseases, and menstrual irregularities (Dang et al. 2011). The extract of this plant has been used in the treatment of intestinal problems, dysentery, fever, diarrhea, and pain (Umerie and Ezeuzo 2000).
Trigonella foenum-graecum L., commonly known as methi, is a self-pollinating legume plant native to the eastern Mediterranean region and the Indian subcontinent (Petropoulos 2002). T. foenum-graecum is one of the traditional and most promising medicinal plants from the legume family.
T. foenum-graecum has been widely used for more than 2,500 years due to its medicinal and food properties (Shi et al. 2017). The seeds and leaves of this herb are broadly used for medicinal objectives, such as an anti-microbial (Subhapriya and Gomathipriya 2018), and anti-cancer (El Bairi et al. 2017), anti-inflammatory (Tavakoly et al. 2018), anti-diabetic, and antioxidant agent (Bahmani et al. 2016).
Therefore, the objective of this paper was to determine the chemical substance of the volatile fractions extracted by solid-phase microextraction (SPME) from C. rotundus roots and T. foenum-graecum seeds and determine their antimicrobial and antioxidant properties in-vitro.
EXPERIMENTAL
Materials
Chemicals
2,2-diphenyl-1-picrylhydrazil (DPPH), methanol, 2,4,6-tripyridyl-s-triazine (TPTZ), Folin-Ciocalteu’s phenol reagent, and 6-hydroxy-2,5,7, 8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Aluminum nitrate nonahydrate (Al(NO3)3.9H2O), sodium carbonate (Na2CO3), ammonium acetate (C2H2NO2), neocuproine (2,9-dimethyl-1,10-phenanthroline), and acetic acid (CH3COOH) were obtained from Merck (Kenilworth, NJ, USA).
Plant material
The dry seeds of T. foenum-graecum and the roots of C. rotundus used in the study were acquired from the local herbalist (market) of Mardin, Turkey, and identified by Dr. Hayal Akyıldırım Begen, Department of Forest Botanical Science, Artvin Coruh University, Artvin, Turkey.
These studied plants have been used as medicinal plants for a long time by the people of Mardin, and they have are edible. The seeds and roots of the studied plants were cut into small pieces with the help of a pestle and an electric grinder was used to grind them into powder. Approximately 10 g samples (roots or seeds) were used to prepare the methanolic extract. The samples were then stirred on a shaker at room temperature for 24 h. The methanolic extracts obtained were filtered using ordinary filter paper. The recovered extracts were used to reveal the antioxidant properties and the analyses for the experiment were repeated three times. Spectrophotometric measurements were used for the antioxidant activity determination, total flavonoid content, and total polyphenol content analysis. Spectrophotometric methods are widely used in this kind of natural raw material analysis. Additional volatile fractions and antibacterial effects of plant extracts were also studied.
SPME procedure
Five-gram portions of T. foenum-graecum or C. rotundus plants were cut into small pieces, placed in a 10 mL sealed bottle, and sent to the SPME device (Supelco, Bellefonte, PA, USA). A divinylbenzene/carboxy/polydimethylsiloxane coating fiber was laid to expose the volatile substances. The SPME-treated fibers were placed in a gas-chromatography (GC) injector at 250 °C for 5 min. The extraction process was carried out at 80 °C, with a waiting time of 5 min and a total extraction time of 10 min by mixing in a magnetic stirrer. Then the fiber was sent to the GC injector. Helium at a flow rate of 1 mL/min was used as the carrier gas. The injection was carried out in split mode (1:30) and at 250 °C. The results of the sample analysis were reported. The extraction, incubation, and temperature values required for the experimental conditions were made according to the study by Renda et al. (2016).
Gas chromatography-mass spectrometry (GC-MS) analysis
A Shimadzu QP2010 plus gas chromatography device (Kyoto, Japan) was used for the GC analysis. A TRB-5MS capillary column (Teknokroma Analítica, Barcelona, Spain) with dimensions of 30 m × 0.25 mm and a film thickness of 0.25 μm was also used. The GC device was connected to a mass selective detector using the split mode with a split ratio of 1:30. The furnace dimensions for analysis were made as follows. Initially, it was increased to 40 °C in 2 min and to 240 °C in 3 min, and it was kept for 4 min with the final temperature at 250 °C. A total of 55 min was spent on this analysis. The temperature values of the mass transfer section and the injector were arranged as 200 and 250 °C, respectively. Helium (99.999%) with a flow rate of 1 mL/min was used as the carrier gas. The ionization voltage value was set to 70 eV. The sensing part was applied in electronic pulse mode (EI), and the scanning mode used for mass gain was 40 to 400 m/z) (Renda et al. 2016).
Identification of constituents
The retention values of the substances were obtained by the Kováts method using the C7 to C30 alkane standards (Kováts 1958). Mass spectral values of volatile fraction components were determined using version 1.3 of the Flavors and Fragrance of Natural and Synthetic Compounds library (Shimadzu 2008).
Total Phenolic Assay
The Folin-Ciocalteu method (Slinkard and Singleton 1977) was used to find the total phenolic content of the plants. Various concentrations of gallic acid were used as a standard for this experiment at doses of 1, 0.5, 0.25, 0.125, 0.0625, and 0.03125 mg/mL. To summarize, 20 µL of methanolic extracts (1 mg/mL), 680 µL of distilled water, 20 µL of gallic acid of varying concentrations, and 400 µL of 0.5 N Folin-Ciocalteu reagents were mixed, and the resulting mixture was vortexed. After waiting for 3 min, 400 µL of Na2CO3 (10%) was added to the mixture and vortexed, then the mixture was left for 2 h to incubate at room temperature. At the end of this period, the absorbance values of the mixtures were recorded at 760 nm. The total amounts of phenolic compounds were calculated as gallic acid equivalents per gram of the samples in dry form.
Total Flavonoid Assay
The aluminum chloride assay was used to determine the total flavonoid content (Chang et al. 2002). Various concentrations of quercetin (1, 0.5, 0.25, 0.125, 0.0625, and 0.03125 mg/mL) were used as the standard. The mixture was vortexed by adding 4.3 mL of methanol, 0.5 mL of quercetin, 0.1 mL of 1 M NH4CH3COO, and 0.1 mL of 10% Al(NO3)3 into the tubes. The mixture was then allowed to stand at room temperature for 40 minutes. After this 40-minute waiting period, the absorbance of the plants at 415 nm was measured and recorded. The total flavonoid content values of the plants were calculated as mg quercetin equivalent per gram of dried samples.
Determination of Antioxidant Activity
The antioxidant contents of the plants were revealed by using the cupric reducing antioxidant capacity (CUPRAC), fluorescence recovery after photobleaching (FRAP), and DPPH methods.
In the FRAP experiment, the reduction of the yellow-colored Fe3+-TPTZ compound to the blue Fe2+-TPTZ compound was realized with the electron-donating substance in acidic conditions, and antioxidant capacity was determined with this principle (Benzie and Szeto 1999). The FRAP reagent (containing acetate buffer, TPTZ, and iron(III) chloride (FeCl3)) was prepared for the experiment. Then, 3 mL of the FRAP reagent was added to the 100 µL plant extract sample and vortexed. After 4 min of incubation, the absorbance values were measured at 593 nm. The obtained absorbances were compared with the standard curve (100 µmol/L to 1,000 µmol/L). The results were expressed as µmol FeSO4.7H2O equivalent per gram of the samples in dry form.
The CUPRAC method consists of mixing a pH=7 ammonium acetate aqueous buffer with a solution of neocuproin in alcohol with a solution of copper (II) chloride. Then, after 60 min of incubation, absorbances at 450 nm were measured and recorded (Apak et al. 2004). Considering this information, 0.2 mL sample, 1 mL of 7.5 mM neocuproine, 0.9 mL of H2O, 1 mL of 10 mM CuCl2, and 1 mL of 1M NH4Ac were placed in test tubes and the final mixtures were vortexed. The final volume was 4.1 mL. After 1 h at room temperature, absorbances were recorded at 450 nm. The results obtained were explained as Trolox equivalent antioxidant capacity (TEAC).
The scavenging effect of the DPPH radical was revealed using the Molyneux trial (Molyneux 2004). According to this method, 750 microliters were taken from the samples prepared in different concentrations, and 750 microliters of DPPH dissolved in methanol was added on it and the mixture was vortexed. Thereupon, the DPPH dissolved in methanol was added and vortexed. Afterward, the test tubes were kept at room temperature and in the dark for 50 min. At the end of this period, the spectrophotometer absorbances were determined at 517 nm. Trolox was used as the standard in this experiment and the values obtained were expressed as IC50 (mg sample per mL).
Biological Materials
A total of 13 microorganisms were utilized in this research. The microorganisms were obtained from Anadolu University Faculty of Pharmacy, commercial culture collections, the American Type Culture Collection (ATCC), United States Agricultural Research Service Culture Collection (NRRL). The bacteria samples were Gordonia rubripertincta NRRL B-3906, Pseudomonas citronellosis NRRL B-2504, Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, Streptomyces griseolus NRRL B-1062, Proteus vulgaris NRRL B-123, Bacillus velezensis NRRL B-14580, Staphylococcus epidermidis ATCC 12228, Bacillus subtilis NRRL B-4378, and Salmonella typhimurium ATCC 13311. The yeast samples were C. krusei ATCC 6258, Candida glabrata ATCC 2001, and C. Albicans ATCC 90028.
The microorganisms used for the study were stored in 15% glycerol at -85 °C. Then, malt extract agar (1.05398, Merck) and nutrient agar (1.05450, Merck) were maintained at 4 °C with gradients.
The microorganisms used in this study were selected from important human and plant pathogens that have been the subject of antimicrobial activity studies by many recent researchers.
In-vitro antimicrobial activity
To determine the antifungal and antimicrobial activities of the plant extracts in-vitro, the broth microdilution assay used by the Clinical Laboratory Standards Institute (CLSI) was applied (Amsterdam 1996). Ketoconazole and amphotericin B were used as standards for the antifungal agent, while tetracycline and chloramphenicol were used as the antimicrobial agents. The antifungal and antimicrobial agents were obtained from Merck. All the applied tests were tested in duplicate in two separate independent assays.
Broth microdilution test for bacteria
The broth microdilution experiment was performed in accordance with the CLSI M100-S16 (CLSI 2006) requirements. The minimum inhibitory concentration (MIC) value of Quercus infectoria galls was investigated by the microdilution method using 96-well microtiter plates that were obtained from Sigma-Aldrich. Overnight cultivated bacterial mixes in double strength Mueller-Hinton broth (MHB) (Merck) were rendered uniform by 108 CFU 1/mL, using the McFarland No. 0.5 standard. The test extracts were mixed in 10% dimethyl sulfoxide (DMSO) and diluted in MHB to obtain a concentration value of 15.6 to 4,000 µg/mL. The DMSO was utilized as a negative check. Then, the mixture was diluted twofold in 100 L of MHB and inoculated with the microorganism strains. It was then incubated at 37 °C for 24 h. Resazurin mixture obtained from Merck was added to confirm the MIC values. The final MIC value was applied as the lowest concentration value with a full growth value (100%). The outcomes of antibacterial assaying were compared with the results of the chloramphenicol and ampicillin standards. The final concentrations were between 0.25 and 32 µg/mL. Dimethyl sulfoxide was tested as the negative check.
Broth microdilution test for yeasts
The CLSI Broth microdilution assay also used 0.5 cells/mL to 2.5 cells/mL × 103 cells/mL (McFarland 0.5), with RPMI-1640 media (Sigma-Aldrich) and 96-well microtiter plates as mentioned in document M27-A2. The final concentration values of the Q. infectoria galls were found between 15.6 and 4000 µg/mL, and the MIC values were found at 37 °C for 24 h. The resazurin mixture was annexed to approve the MIC. The final MIC value was applied as the lowest concentration value with a full growth value (100%) (CLSI 2002).
RESULTS AND DİSCUSSİON
Chemical Composition
The outcomes of the SMPE and GC-MS analysis of the volatile fractions from the C. rotundus roots and T. foenum-graecum seeds are shown in Tables 1 and 2. In total, 32 and 30 compounds were identified and quantified for purple nutsedge roots and fenugreek seeds, respectively. The chemical ingredients were formulated based on literature data and library research (FFNSC 2008).
The test outcomes listed in Table 1 can be summarized as follows. Pentadecanolide and palmitic acid were the main substances of the C. rotundus root species. While ethyl pyruvate was eluted first from the column [Retention time (RT) of 1.331 min], 16-hydroxy-6-hexadecenoic acid omega lactone was held in the column for the longest time of all (RT of 53.940 min and Retention index (RI) of 2143). Pentadecanolide was the main substance of this volatile fraction of C. rotundus, with content of approximately 72%, followed by palmitic acid (8.2%), 16-hydroxy-6-hexadecenoic acid omega lactone (4.4%), and (Z)-anethol (3.9%). The other constituent, beta-patchoulene was detected with an average percentage of 1.3%. The rest of the ingredients generally had a content of less than 1%. The compounds were separated into 11 classes, which were ether, ketones, monoterpenes, fatty acids, monoterpenoids, alcohols, esters, sesquiterpenes, aldehydes, hydrocarbons, and others (Table 1). The research of Jirovetz et al. (2004) indicated that α-copaene (11.4%), valerenal (9.8%), caryophyllene oxide (9.7%), cyperene (8.4%), nootkatone (6.7%), and trans-pinocarveol (5.2%) are the major constituents of the volatile fraction of C. rotundus roots from south India. In another study, alpha-cyperone (11.0%), myrtenol (7.9%), caryophyllene oxide (5.4%), and β-pinene (5.3%) were identified as the main components of the C. rotundus tubers from south Africa (Dhar et al. 2017). It can therefore be concluded that the proportion of the substances or chemical combinations of the volatile fractions are different with respect to the environmental and climatic conditions. Other surveys have also demonstrated that the chemical combination of the volatile fractions of C. rotundus can be altered significantly with respect to the harvest period, the geographical zones, and the age of the herb (Kilani et al. 2008).
Table 1. Identified Substances in the Volatile Constituents of C. rotundus
aRI calculated from retention times relative to that of alkanes (C7 to C30) on the non-polar TRB-5MS column. Bold values represent the major components.
Table 2. Identified Substances in the Volatile Constituents the of T. foenum-graecum
aRI calculated from retention times relative to that of alkanes (C7 to C30) on the non-polar TRB-5MS column. Bold values represent the major components.
The assay results shown in Table 2 indicate that in the volatile fractions of the T. foenum-graecum seeds, pentadecanolide, and (Z)-anethol were the main substances of the seed species. While propylene glycol initially appeared from the column (RT of 1.115 min), civetone remained in the column the longest (RT of 53.912 min). Pentadecanolide is the main substance of this volatile fractions of T. foenum-graecum, with content of approximately (61.3%), followed by (Z)-anethol (16.5%). The other constituents of menthalactone (2.9%), palmitic acid (2.8%), lauric aldehyde (1.7%), and diethyl phthalate (1.6%) were detected as average percentages. The rest of the ingredients were generally less than 1% in content. The compounds were separated into 10 classes, which were alcohols, esters, ketones, monoterpenes, alcohols, monoterpenoids, ether, fatty acids, sesquiterpenoids, aldehydes, ketones, and others (Table 2). In previous studies, large different compounds were found in the GC-MS analyses of T. foenum-graecum seeds for the plant growing in Bangladesh and Malaysia (Moniruzzaman et al. 2015; Akbari et al. 2019). These differences may result from the kind of extraction process and the kind of solvent utilized to herb extract the oil. Furthermore, it can be concluded that the chemical combination of the herb as a whole depends on the time the plant is harvested, as well as the place of growth and harvest (de Barros Fernandes et al. 2014).
There are not many published studies that report on the definition of volatile fractions ingredients, antioxidant properties, and antibacterial properties in a single study. Therefore, it is important to explore the novel biological properties of C. rotundus roots and T. foenum-graecum seeds which have many medicinal properties (Umerie and Ezeuzo 2000; Dang et al. 2011; Bahmani et al. 2016; El Bairi et al. 2017; Shi et al. 2017; Subhapriya and Gomathipriya 2018; Tavakoly et al. 2018) to contribute to the activities noted in the literature.
Antioxidant Activity
In this paper, three primary processes, DPPH radical scavenging activity, ferric reducing power (FRAP), and CUPRAC were utilized to determine the antioxidant activity. The properties of the total flavonoids and the phenolics were also determined for the herb extracts. The total flavonoid values, total phenolic values, DPPH, CUPRAC, and FRAP results are given in Table 3. The IC50 outcomes calculated from test of the DPPH are showed in Fig. 1.
Table 3. Phenolic Contents, Flavonoid Contents, FRAP, CUPRAC, and DPPH Results for the Plant Samples
Much information can be attained by conducting a literature study concerning purple nutsedge (C. rotundus) roots or aerial parts solvent extraction or volatile fraction analysis and their biological properties. A study was carried out on the C. rotundus root in India (Kandikattu et al. 2015). The total polyphenol and total flavonoid values were calculated as 149 ± 7.5 μg GAE/mg and 390 ± 22 μg CE/mg, respectively. In addition, the DPPH activity was determined to be an IC50 value of 23.7 μg/mL. Kumar et al. (2014) reported that the total polyphenol and total flavonoid contents C. rotundus root were 192.77 ± 5.48 μg GAE/mg and 138.01 ± 5.24 μg CE/mg. The DPPH activity was determined to be an IC50 value of 86.2 μg/mL. However, this study found that the total polyphenol and total flavonoid contents were equal to 4.07 ± 0.26 mg GAE/g and 0.23 ± 0.15 mg QE/g, respectively. In addition, the DPPH activity was determined to be an IC50 value of 0.91 mg/mL. Therefore, the values found in the literature were higher than the values found in this study. The differences can be attributed to the extraction process and the contents of the herb from various zones.
Fig. 1. DPPH values for the C. rotundus and T. foenum-graecum plant extracts
According to the CUPRAC method results, the antioxidant activity was 0.18 ± 0.05 mmol TEAC/g, and according to the FRAP process outcomes, the antioxidant activity was 0.06 ± 0.00 µmol Fe/g. While the DPPH radical property determination process, which is one of the antioxidant ingredient definition processes for the C. rotundus herb, is described in the literature, there has been no reported research on the CUPRAC and FRAP processes. This study was the first known research on the antioxidant properties of C. rotundus roots analyzed by the CUPRAC and FRAP methods.
The antioxidant capacity seeds of the T. foenum-graecum were determined using a total polyphenol (38.97 ± 0.34 mg GAE/g sample), total flavonoid (14.417 ± 0.23 mg QE /g sample), and DPPH (172.6 ± 3.1 µg/mL) process, as outlined by Akbari et al. (2019). In an article by Premanath et al. (2011), the antioxidant properties of T. foenum-graecum seeds were investigated using the total polyphenol, total flavonoid, FRAP, and DPPH processes. The total polyphenol and total flavonoid contents were found to be 4.2 ± 0.15 mg GAE/g and 0.32 ± 0.02 mg QE/g, respectively. In addition, the DPPH activity and FRAP activity values were found to be 53.2 ± 0.33% and 31.0 ± 1.0 µmol Fe/g, respectively. However, this study determined total polyphenol, total flavonoid, DPPH, and FRAP values of 0.82 ± 0.52 mg GAE/g, 0.22 ± 0.01 mg QE/g, 25.67 mg/mL, and 0.01 ± 0.01 µmol Fe/g, respectively. Unlike other published studies, the CUPRAC method was used in this study, and according to this method, the antioxidant activity of the seeds of the T. foenum-graecum plant was determined as 0.02 ± 0.00 mmol TEAC/g. This study was the first to use such methods to define the antioxidant properties of T. foenum-graecum seeds by the CUPRAC method.
Antimicrobial Activity
The antimicrobial activity of the C. rotundus and T. foenum-graecum extracts that were tested against the microorganisms in this paper were quantitatively and qualitatively appraised by evaluating the existence of inhibition zones, zone diameter, and MIC ranges. Three yeast strains and 10 bacteria were used to determine the antibacterial properties of the plant extract. The microorganisms that were selected for antibacterial activity properties are among the important human pathogens and plant and biofilm-producing microorganisms. These studied microorganisms have been used in many recent studies. Table 4 demonstrates the MIC values of the C. rotundus and T. foenum-graecum by broth microdilution for isolates.
Table 4. MIC Values of Q. infectoria Against the Bacterial Strains Tested
Sa: S. aureus ATCC 6538, Pv: P. vulgaris NRRL B-123, St: S. typhimurium ATCC 13311, Se: S. epidermidis ATCC 12228, Bs: B. subtilis NRRL B-4378, Sg: S. griseolus NRRL B-1062, Pc: P. citronellosis NRRL B-2504, Bv: B. velezensis NRRL B-14580, Gr: G. rubripertincta NRRL B-3906, Ec: E. coli ATCC 8739, as yeast ; Ca: C. albicans ATCC 90028, Cg: C. glabrata ATCC 2001, Ck: C. krusei ATCC 6258, Chlor: Chloramphenicol, Tet: Tetracycline, AmfB: Amphotericin B, Ket: Ketoconazole, >: Not applicable as microorganisms are not susceptible to these samples.
At the tested concentrations, the C. rotundus exhibited good antifungal properties against the C. albicans and C. krusei with MIC values of 250 and 125 µg/mL, respectively. However, C. rotundus is not found to be very effective on gram-negative and gram-positive bacteria. Antimicrobial property research is available in the literature on the C. rotundus. Previous research (Parekh and Chanda 2006; Kabbashi et al. 2015) has found that herb extracts are more effective against gram-positive bacteria than gram-negative bacteria. There are not many studies in the literature that report on plants with antifungal effects.
The T. foenum-graecum had no effect against any of the test microorganisms at the tested concentrations in this study. However, it has been noted in the literature that the seeds of T. foenum-graecum show a great and broad effect on test microorganisms that can be used against gram-positive bacteria, gram-negative bacteria, and fungi (Premanath et al. 2011; Moniruzzaman et al. 2015).
Volatile fractions and herb extracts are potential resources of novel antimicrobial constituents, especially against bacterial agents. These natural substances may also be implemented for the treatment of diseases, which gives them effective antimicrobial properties (Torbati et al. 2013).
CONCLUSIONS
- This paper evaluated the chemical contents of the volatile fractions acquired from C. rotundus roots and T. foenum-graecum seeds and evaluated their antioxidant and antibacterial capacities. It was determined that the volatile fractions of C. rotundus and T. foenum-graecum contain numerous basic substances in great quantities. Under experimental conditions, the SPME-GCMS technique serves as a good method for the study of volatile fractions from different parts of plants and can produce significant qualitative and quantitative results.
- The outcomes of this study support the traditional usage of the studied plants. The herbal antioxidants from the studied species offer a safer alternative to synthetic antioxidants, and the volatile fractions are potential resources for novel antimicrobial constituents.
- In conclusion, significant antioxidant and antibacterial activities of the methanolic extracts of C. rotundus roots and T. foenum-graecum provide a scientific validation for the traditional use of this plant as a novel source of natural antioxidants and antimicrobial with consequent health benefits.
Conflict of Interest
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors would like to thank Hayal Akyıldırım Beğen for identifying the studied the herbs. The authors are also grateful to Artvin Çoruh University for the laboratory facilities.
REFERENCES CITED
Akbari, S., Abdurahman, N. H., Yunus, R. M., Alara, O. R., and Abayomi, O. O. (2019). “Extraction, characterization and antioxidant activity of fenugreek (Trigonella-foenum graecum) seed oil,” Materials Science for Energy Technologies 2(2), 349-355. DOI: 10.1016/j.mset.2018.12.001
Alara, O. R., Abdurahman, N. H., and Olalere, O. A. (2020). “Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design,” Journal of King Saud University-Science 32(1), 7-16. DOI: 10.1016/j.jksus.2017.08.001
Alsabah, A. S., Abd, A. H., and Al-Shammari, A. M. (2018). “Cytotoxicity of Xanthium strumarium against breast cancer cell lines,” Journal of Global Pharma Technology 10(3), 767-776.
Amsterdam, D. (1996). Susceptibility testing of antimicrobials in liquid media, in: V. Lorian (Ed.), Antibiotics in Laboratory Medicine (4th Ed.). Baltimore: Williams & Wilkins.
Apak, R., Güçlü, K., Özyürek, M., and Karademir, S. E. (2004). “Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method,” Journal of Agricultural and Food Chemistry 52(26), 7970-7981. DOI: 10.1021/jf048741x
Bahmani, M., Shirzad, H., Mirhosseini, M., Mesripour, A., and Rafieian-Kopaei, M. (2016). “A review on ethnobotanical and therapeutic uses of fenugreek (Trigonella foenum-graceum L),” Journal of Evidence-Based Complementary & Alternative Medicine 21(1), 53-62. DOI: 10.1177/2156587215583405
Benzie, I. F. F., and Szeto, Y. T. (1999). “Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay,” Journal of Agricultural and Food Chemistry 47(2), 633-636. DOI: 10.1021/jf9807768
Chang, C.-C., Yang, M.-H., Wen, H.-M., and Chern, J.-C. (2002). “Estimation of total flavonoid content in propolis by two complementary colorimetric methods,” Journal of Food and Drug Analysis 10(3), 178-182. DOI: 10.38212/2224-6614.2748
Clinical and Laboratory Standards Institute (CLSI) (2002). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Second Edition (CLSI document M27-A2), Clinical and Laboratory Standards Institute, Wayne, PA, USA.
Clinical and Laboratory Standards Institute (CLSI) (2006). Performance Standards for Antimicrobial Susceptibility Testing-Sixteenth Informational Supplement (CLSI document M100-S16), Clinical and Laboratory Standards Institute, Wayne, PA, USA
Dang, G. K., Parekar, R. R., Kamat, S. K., Scindia, A. M., and Rege, N. N. (2011). “Antiinflammatory activity of Phyllanthus emblica, Plumbago zeylanica and Cyperus rotundus in acute models of inflammation,” Phytotherapy Research 25(6), 904-908. DOI: 10.1002/ptr.3345
de Barros Fernandes, R. V., Borges, S. V., and Botrel, D. A. (2014). “Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil,” Carbohydrate Polymers 101, 524-532. DOI: 10.1016/j.carbpol.2013.09.083
Dhar, P., Dhar, D. G., Rawat, A. K. S., and Srivastava, S. (2017). “Medicinal chemistry and biological potential of Cyperus rotundus Linn.: An overview to discover elite chemotype(s) for industrial use,” Industrial Crops and Products 108(1), 232-247. DOI: 10.1016/j.indcrop.2017.05.053
El Bairi, K., Ouzir, M., Agnieszka, N., and Khalki, L. (2017). “Anticancer potential of Trigonella foenum graecum: Cellular and molecular targets,” Biomedicine & Pharmacotherapy 90, 479-491. DOI: 10.1016/j.biopha.2017.03.071
Gomes, A. J., Lunardi, C. N., Gonzalez, S., and Tedesco, A. C. (2001). “Antioxidant action of Polypodium leucotomos extract and Kojic acid: Reactions with reactive oxygen species,” Brazilian Journal of Medical and Biological Research 34(1), 1487-1494. DOI: 10.1590/s0100-879×2001001100018
Jirovetz, L., Wobus, A., Buchbauer, G., Shafi M. P., and Thampi, P. T. (2004). “Comparative analysis of the essential oil and SPME-headspace aroma compounds of Cyperus rotundus L. roots/tubers from south-India using GC, GC-MS and olfactometry,” Journal of Essential Oil Bearing Plants 7(2), 100-106. DOI: 10.1080/0972-060X.2004.10643373
Josh, N., and Janardhanan, K. K. (2000). “Antioxidant and antitumorous activity of Pleurotus florida,” Current Science 79(7), 941-943.
Kabbashi, A. S., Mohammed, S. E. A., Almagboul, A. Z., and Ahmed, I. F. (2015). “Antimicrobial activity and cytotoxicity of ethanolic extract of Cyperus rotundus L.,” American Journal of Pharmacy and Pharmaceutical Sciences 2(1), 1-13.
Kandikattu, H. K., Rachitha, P., Krupashree, K., Jayashree, G. V., Abhishek, V., and Khanum, F. (2015). “LC-ESI-MS/MS analysis of total oligomeric flavonoid fraction of Cyperus rotundus and its antioxidant, macromolecule damage protective and antihemolytic effects,” Pathophysiology 22(4), 165-173. DOI: 10.1016/j.pathophys.2015.07.001
Kilani, S., Ledauphin, J., Bouhlel, I., Sghaier, M. B., Boubaker, J., Skandrani, I., Mosrati, R., Ghedira, K., Barillier, D., and Chekir-Ghedira, L. (2008). “Comparative study of Cyperus rotundus essential oil by a modified GC/MS analysis method. Evaluation of its antioxidant, cytotoxic, and apoptotic effects,” Chemistry & Biodiversity 5(5), 729-742. DOI: 10.1002/cbdv.200890069
Kováts, E. (1958). “Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: I. Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone [Gas chromatographic characterization of organiz compounds. Part 1: Retention indices of aliphatic halides, alcohols, aldehydes and ketones],” Helvetica Chimica Acta 41(7), 1915-1932. DOI: 10.1002/hlca.19580410703
Kumar, K. H., Razack, S., Nallamuthu, I., and Khanum, F. (2014). “Phytochemical analysis and biological properties of Cyperus rotundus L.,” Industrial Crops and Products 52, 815-826. DOI: 10.1016/j.indcrop.2013.11.040
Martínez, M. J., Betancourt, J., Alonso-González, N., and Jauregui, A. (1996). “Screening of some Cuban medicinal plants for antimicrobial activity,” Journal of Ethnopharmacology 52(3), 171-174. DOI: 10.1016/0378-8741(96)01405-5
Manzi, P., Gambelli, L., Marconi, S., Vivanti, V., and Pizzoferrato, L. (1999). “Nutrients in edible mushroom: An inter-species comparative study,” Food Chemistry 65(4), 477-482. DOI: 10.1016/S0308-8146(98)00212-X
Molyneux, P. (2004). “The use of the stable free radical diphenylpicrylhyrazyl (DPPH) for estimating antioxidant activity,” Songklanakarin Journal of Science and Technology 26(2), 211-219.
Moniruzzaman, Shahinuzzaman, Haque, A., Khatun, R., and Yaakob, Z. (2015). “Gas chromatography mass spectrometry analysis and in vitro antibacterial activity of essential oil from Trigonella foenum-graecum,”Asian Pacific Journal of Tropical Biomedicine 5(12), 1033-1036. DOI: 10.1016/j.apjtb.2015.09.010
Mouthe Kemayou, G. P., Fotsing Kache, S., Dzouemo, L. C., M Happi, G., Fogue Kouam, S., and Tchouankeu, J. C. (2021). “Phytochemistry, traditional uses, and pharmacology of the genus Ekebergia (Meliaceae): A review,” Trends in Phytochemical Research 5(3), 110-125. DOI: 10.30495/tpr.2021.1928329.1204.
Nalawade, A. S., Gurav, R. V., Patil, A. R., Patwekar, M., and Patwekar, F. (2022). “A comprehensive review on morphological, genetic and phytochemical diversity, breeding and bioprospecting studies of genus Chlorophytum Ker Gawl. from India,” Trends in Phytochemical Research 6(1), 19-45. DOI:10.30495/tpr.2022.1949493.1238.
Olaoluwa, O., Taiwo, O., Nahar, L., and Sarker, S. D. (2022). “Ethnopharmacology, phytochemistry and biological activities of the African species of the genus Ficus L.,” Trends in Phytochemical Research 6(1), 46-69. DOI:10.30495/tpr.2022.1939285.1219
Parekh, J., and Chanda, S. (2006). “In-vitro antimicrobial activities of extracts of Launaea procumbens Roxb. (Labiateae), Vitis vinifera L. (Vitaceae) and Cyperus rotundus L. (Cyperaceae),” African Journal of Biomedical Research 9(2), 89-93. DOI: 10.4314/ajbr.v9i2.48780
Petropoulos, G. A. (2002). Fenugreek-The Genus Trigonella, Taylor and Francis, Abingdon, United Kingdom.
Premanath, R., Sudisha, J., Lakshmi Devi, N., and Aradhya, S. M. (2011). “Antibacterial and anti-oxidant activities of fenugreek (Trigonella foenum graecum L.) leaves,” Research Journal of Medicinal Plants 5(6), 695-705. DOI: 10.3923/rjmp.2011.695.705
Renda, G., Tosun, G., and Yayli, N. (2016). “SPME GC/MS analysis of three Ornithogalum L. species from Turkey,” Records of Natural Products 10(4), 497-502.
Salih, R. H., Odisho, S. M., Al-Shammari, A. M., and Ibrahim, O. M. S. (2017). “Antiviral effects of Olea europaea leaves extract and interferon-beta on gene expression of Newcastle disease virus,” Advances in Animal and Veterinary Sciences 5(11), 436-445. DOI: 10.17582/journal.aavs/2017/5.11.436.445
Shah, P. M. (2005). “The need for new therapeutic agents: What is in the pipeline?” Clinical Microbiology and Infection 11(3), 36-42. DOI: 10.1111/j.1469-0691.2005.01141.x
Sharma, R., and Gupta, R. (2007). “Cyperus rotundus extract inhibits acetylcholin-esterase activity from animal and plants as well as inhibits germination and seedling growth in wheat and tomato,” Life Sciences 80(24-25), 2389-2392. DOI: 10.1016/j.lfs.2007.01.060
Shi, X., Miyakawa, T., Nakamura, A., Hou, F., Hibi, M., Ogawa, J., Kwon, Y., and Tanokura, M. (2017). “Engineering a short-chain dehydrogenase/reductase for the stereoselective production of (2S,3R,4S)-4-hydroxyisoleucine with three asymmetric centers,” Scientific Reports 7(1), 13703. DOI: 10.1038/s41598-017-13978-w
Shimadzu (2008). Flavour & Fragrance Natural & Synthetic Compounds GCMS (FFNSC) library, Shimadzu, Kyoto, Japan.
Slinkard, K., and Singleton, V. L. (1977). “Total phenol analysis: Automation and comparison with manual methods,” American Journal of Enology and Viticulture 28(1), 49-55.
Subhapriya, S., and Gomathipriya, P. (2018). “Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties,” Microbial Pathogenesis 116, 215-220. DOI: 10.1016/j.micpath.2018.01.027
Tavakoly, R., Maracy, M. R., Karimifar, M., and Entezari, M. H. (2018). “Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial,” European Journal of Integrative Medicine 18, 13-17. DOI: 10.1016/j.eujim.2018.01.005
Torbati, M., Nazemiyeh, H., Lotfipour, F., Asnaashari, S., Nemati, M., and Fathiazad, F. (2013). “Composition and antibacterial activity of Heracleum transcaucasicum and Heracleum anisactis aerial parts essential oil,” Advanced Pharmaceutical Bulletin 3(2), 415-418. DOI: 10.5681/apb.2013.066
Umerie, S. C., and Ezeuzo, H. O. (2000). “Physicochemical characterization and utilization of Cyperus rotundus starch,” Bioresource Technology 72(2), 193-196. DOI: 10.1016/S0960-8524(99)00103-0
Yang, J.-H., Lin, H.-C., and Mau, J.-L. (2002). “Antioxidant properties of several commercial mushrooms,” Food Chemistry 77(2), 229-235. DOI: 10.1016/S0308-8146(01)00342-9
Zainal, I. G., Al-Shammari, A. M., and Kachi, W. (2018). “Characterization of the modified nickel-zinc ferrite nanoparticles coated with APTES by salinization reaction,” AIP Conference Proceedings 1968, 030008.
Article submitted: March 22, 2022; Peer review completed: May 21, 2022; Revised version received and accepted: June 3, 2022; Published: June 13, 2022.
DOI: 10.15376/biores.17.3.4580-4594
