Abstract
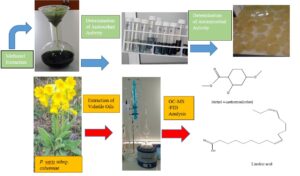
The proportions and constituents of the essential oils of flowers and leaves of Primula veris subsp. columnae were determined, and the antioxidant and the antimicrobial (antibacterial and antifungal) properties of their methanol extracts were investigated. Percentage ratios and main components of the Primula veris subsp. columnae plant, which grows naturally, were detected by extracting flower and leaf volatile oils. The components of the volatile oil were identified with Gas Chromatography Mass Detector-Flame Ionization Detector (GC-MS/FID). A total of 62 compounds were identified in flower volatile oil, and methyl 4-methoxysalicylate (37.1%) was determined as the main compound. While defining the structure of 50 compounds in leaf essential oil, linoleic acid (40.1%) was established as the main compound. As the result of the extraction of flowers and leaves with methanol, the extractive substance was obtained as 34.5% in flowers and as 28.8% in leaves. Methanol extraction and the antioxidant properties of the Primula veris subsp. columnae plant were quite high. In the antifungal and antibacterial activity test conducted on the volatile oils and methanol extracts of flowers and leaves of the Primula veris subsp. columnae species, only flower volatile oil showed weak inhibition properties.
Download PDF
Full Article
Chemical Content of Volatile Oil of Primula veris subsp. columnae, Obtaining the Methanol Extracts and their Biological Activities
Çağrı Karapınar and Mehmet Öz *
The proportions and constituents of the essential oils of flowers and leaves of Primula veris subsp. columnae were determined, and the antioxidant and the antimicrobial (antibacterial and antifungal) properties of their methanol extracts were investigated. Percentage ratios and main components of the Primula veris subsp. columnae plant, which grows naturally, were detected by extracting flower and leaf volatile oils. The components of the volatile oil were identified with Gas Chromatography Mass Detector-Flame Ionization Detector (GC-MS/FID). A total of 62 compounds were identified in flower volatile oil, and methyl 4-methoxysalicylate (37.1%) was determined as the main compound. While defining the structure of 50 compounds in leaf essential oil, linoleic acid (40.1%) was established as the main compound. As the result of the extraction of flowers and leaves with methanol, the extractive substance was obtained as 34.5% in flowers and as 28.8% in leaves. Methanol extraction and the antioxidant properties of the Primula veris subsp. columnae plant were quite high. In the antifungal and antibacterial activity test conducted on the volatile oils and methanol extracts of flowers and leaves of the Primula veris subsp. columnae species, only flower volatile oil showed weak inhibition properties.
DOI: 10.15376/biores.18.3.4475-4491
Keywords: Antimicrobial activity; Antioxidant activity; GC-MS; Primula veris subsp. columnae; Volatile oil
Contact information: Gümüşhane University, Department of Forestry and Environment Sciences, Institute of Natural Applied Science, Gümüşhane 29000 Turkey;
*Corresponding author: mehmetoz@gumushane.edu.tr
GRAPHICAL ABSTRACT

INTRODUCTION
Primula L., consisting of approximately 500 species, is the largest genus of the Primulaceae family. The species spreads commonly in the humid and colder regions of the northern hemisphere (Baasanmunkh et al. 2020). A total of 12 taxa of Primula genus belonging to 8 species have been identified in Turkey. Aside from Primula davisii W.W.Sm, most of the other taxa grow naturally in northeastern Anatolia (Terzioğlu et al. 2012). The primrose is a perennial herbaceous plant, growing 10 to 50 cm tall, with clusters of 8 to 12 golden yellow flowers and leaves gathered in a rosette shape at the base. It grows in water meadows. Volatile oil, saponin glycosides, and flavone derivatives are found in its composition. The flowers of the plant have diaphoretic, expectorant, and sedative effects, and it has been used to treat eye diseases. Its fresh leaves have been used to treat furuncles (Baytop 1999). Primula veris subsp. columnae (Ten.) Maire & Petitm. (Synonym: Primula veris subsp. suaveolens (Bertol.) Gutermann & Ehrend.) grows naturally in the provinces of Giresun, Gümüşhane, Rize, and Trabzon (Karapınar 2020).
There are several studies on the Primula genus in literature. Borisova and Popov (2012) investigated the qualitative composition and quantitative content of the flavonoids of Primula officinalis plant parts in their study. Latypova et al. (2015) examined the amount of ursolic acid (they have anti-microbial, anti-tumor, and anti-inflammatory properties) of Primula veris and Primula crocalyx plants in the research they conducted. Meos et al. (2017) investigated the vitamin C (ascorbic acid) amount of Primula veris flowers. Bączek et al. (2017) examined the phenolic components of Primula veris and Primula elatior flowers and roots, which they had collected in Poland with the HPLC-DAD method. In the study conducted on Primula veris var. columnae, Vuko et al. (2017) obtained volatile oils from the leaves, roots, and flowers of Primula veris var. columnae they collected in 2011 in Croatia and determined their components with GC-MS method. Güneş (2016) conducted the chemical composition analysis of volatile oil and chloroform extracts obtained through distillation and extraction from the flower and leaf sections of the Primula vulgaris subsp. sibthorpii plant collected in Giresun province in a post graduate thesis. Demir et al. (2019) investigated the antioxidant properties and cytotoxic effects of the extract prepared with dimethyl sulfoxide from P. vulgaris flowers.
Volatile oils, also called essential oils, are oily liquids obtained from trees and plants. Essential oils have pleasant fragrances, are water-insoluble, accumulate on the surface of the water, and can easily evaporate when left uncovered. Volatile oils are found in certain tissues or all organs of the plant, including flower, fruit, petal, leaf, bark, resin, and woody structure (Baltacı et al. 2022; Fidan et al. 2022). Monoterpene hydrocarbons, sesquiterpene hydrocarbons, and their oxygenated derivatives, aliphatic hydrocarbons, aldehydes, ketones, acids, and esters are the chemical constituents of the volatile essence of aromatic plants (Altaf et al. 2020). Gas chromatography coupled with mass spectrometry (GC-MS) leads to a high chromatographic tenacity and a peak intensity and provides compositional information for nearly all volatile and semivolatile materials, including organic acids, amino acids, etc. (Qadir et al. 2020). The preserving effects of plant spices and herbs are due to the diverse phenolics they contain, which can have antioxidative and antimicrobial properties (Abramovıč et al. 2018). Antimicrobial agents obtained from medicinal plants can be used to treat infectious diseases with fewer side effects when compared with synthetic drugs (Liu et al. 2021).
In this study, the volatile oil components of the flowers and leaves of the Primula veris subsp. columnae (P. v. subsp. columnae) species collected from Gümüşhane, Türkiye were investigated. Chemical characterization was followed by the study of the antioxidant properties of the methanol extracts and the antibacterial and antifungal properties of volatile oils and methanol extracts were determined.
EXPERIMENTAL
Plant Material
Primula veris subsp. columnae (Tutya, Primrose) flower and leaf samples were gathered in Zigana Village, Torul District, Gümüşhane Province in the altitude of 1900 m (40°38´16´´N and 39°23´30´´E). The taxonomic identification of plant materials was done by Assoc. Prof. Mutlu Gültepe, in Programme of Forestry, Dereli Vocational School, Giresun University, Giresun, Turkey. The plant was enlisted with the number KTUB Gültepe 661 with a diagnosis from the Herbarium of Karadeniz Technical University, Faculty of Science, Department of Biology. The location where flower and leaf samples of Primrose (P. v. subsp. columnae) were gathered is shown in Fig. 1.
Fig. 1. The location where the plant materials were collected
Extraction of Volatile Oils
Volatile oils were extracted with a modified Clevenger apparatus. Homogenized flower (80 g) and leaf (100 g) samples were placed into a 2 L Clevenger apparatus flask, and 1500 mL of distilled water was added. Then, 2 mL of n-hexane was added to the condenser, and the temperature of the cooler was set to 4.0 °C. Volatile oils were obtained by boiling for 4 hours on the low setting. Percentages of yield for volatile oils by weight were calculated separately for flower and leaf samples according to Eq. 1,
Y % = (Qe / Qp) x 100 (1)
where Y is the yield (%), Qe is the quantity of isolated essential oil (g), and Qp is the quantity of fresh raw plant material (g) (Öz et al. 2021).
Analysis of Volatile Oils Components with GC-MS/FID
The volatile oils obtained through water hydrodistillation in the Clevenger apparatus were filtered as dissolved in hexane and were placed in the autosampler by putting them in dark colored bottles. The evaluation of volatile oils was carried out with Gas Chromatography Mass Detector-Flame Ionization Detector (GC-MS/FID). Analyzes were conducted on the GC-FID Agilent-7890A, MS Agilent 5975C model device, and an HP-5MS model nonpolar capillary column was used for analysis (GC-MS conditions: 30. m / 0.32 mm / 0.25 µm, He, 3. K / min; Tstart: 60. °C; Tend: 240. °C). Both detectors are located in the same device. Simultaneous analysis was performed. The mass spectra analysis of the essential oils was carried out in the MS detector, and the quantitative analysis was conducted in the FID detector. The injections were applied in splitless mode at 240 °C using helium as the carrier gas with a flow rate of 1 mL/min. 1 µL volatile oil solution in hexane (GC class) was injected and stored initially at 60 ° C for 2 min, and then spectra were taken by raising the temperature to 240 °C with an increase of 3 °C/min. The identification of constituents of volatile oils was performed based on a comparison of retention indices (RI) with reference to a homologous series of n-alkanes (C6 – C32), under the same experimental status. The mass spectrum of each compound was identified through the structure clarification by comparing with the reference constituents of the NIST and Willey libraries, besides that making a comparison of their retention time either with retention times of authentic compounds or with the literature data (Öz et al. 2021).
Methanol Extraction
For analysis, 80 and 100 grams were taken from the grounded flower and leaf parts, respectively. Then, 750 mL of methanol was added to the samples and were stirred at room temperature at 200 rpm for 24 h. At the end of 24 h, they were centrifuged at 4000 rpm for 10 min by filtering through Whatman 1 filter twice, and plant extracts were acquired. The section, which stayed on the top at the end of centrifugation, was placed in a beaker and the extracts were acquired by completely evaporating methanol at 40 °C. Percentages of yield for methanol extract with respect to weight were calculated separately for flower and leaf samples by the following Eq. 2,
Y % = (Ae / Ap) x 100 (2)
where Y is the yield (%), Ae is the amount of extracted substance (g), and Ap is the amount of fresh raw plant material (g) (Karapınar 2020).
Determination of Antioxidant Activity
DPPH Free radical scavenging activity
Samples were prepared from 0.20% methanol extracts obtained from flower and leaf sections. Methanol was HPLC purity grade, and DPPH, Trolox, and L-Ascorbic acid were analytical grade. Trolox and ascorbic acid working solutions in methanol were prepared at 25, 50, 100, 200, and 400 µg/mL. The mixture was vortexed and stored in the dark for 30 min. The absorbance of the obtained solution was read in a UV-Vis spectrophotometer (Optizen MECASYS) at 517 nm. Triplicate analyses were performed for each specimen. Methanol was utilized as the blank solution. The same procedures were conducted by taking from the standards (Ascorbic acid and Trolox). Results were provided as mg AA eq./g, mg Trolox eq./g and % inhibition (Ahmed et al. 2015).
Ferric (III) ion reducing antioxidant power (FRAP)
Samples were prepared from 0.04% methanol extracts obtained from flower and leaf sections. Research standards were as follows: solutions were prepared at concentrations of 5, 10, 25, 50, 100, and 200 µL/mL. from the main stock ferric (II) sulphate heptahydrate (FeSO4. 7H2O) solution. The mixture was vortexed and kept in the dark for 30 min. The absorbance of the obtained solutions was read in a spectrophotometer at 593 nm. Triplicate analyses were performed for each specimen. Pure water was used as the blank. Total ferric reducing antioxidant capacity was determined as mg FeSO4 eq./g (Ahmed et al. 2015).
Total phenolic substance
Samples were prepared from 0.20% methanol extract obtained from the flower and leaf sections. First, 0.50 mL methanol and 200 µL Folin-Ciocalteu reagent were added to the mixture. The mixture was vortexed and incubated under room conditions for 10 min, and 600 µL 10% (m/ v) Na2CO3 solution was added. After the final mixture was vortexed again, it was incubated in the dark at room conditions for 120 min and the absorbance of the mixture at 760 nm was read at the end of the incubation period. Triplicate analyses were performed for each specimen. A mixture of 3.7 mL water, 500 µL methanol, 100 µL Folin-Ciocalteu reagent, 600 µL Na2CO3 was used as a blank. The phenolic substance amounts in the samples were declared as the total phenolic mg GA eq./g by using the correct equation of the calibration graph obtained with the solution of gallic acid (10, 20, 30, 40, 60, and 80 µg/mL) (Kasangana et al. 2015).
Total antioxidant substance
Samples were prepared from 0.20% methanol extracts obtained from flower and leaf sections. Ascorbic acid was utilized for the calibration curve. The mixture was vortexed and incubated in a 95 °C water bath with caps closed for 90 min. It was recovered from the water bath and was kept for 20 to 30 min until it reached room temperature. Distilled water was used as the blank sample. The absorbance of the obtained reaction mixtures was read as 695 nm in the spectrophotometer. Triplicate analyses were performed for each specimen. The same procedures were conducted by taking 500 µL from the standards. Total antioxidant amount from flower and leaf samples was determined as mg AA eq./g by using the correct equation of the calibration graph obtained with the solution of ascorbic acid (25, 50, 100, 150, 250, and 500 µg/mL) (Kasangana et al. 2015).
Total flavonoid substance
Samples were prepared from 0.20% methanol extracts obtained from flower and leaf sections. The mixture was vortexed and 150 µL of 0.5 M sodium nitrite solution was added and then 150 µL 0.3 M aluminum chloride was added. It was kept for 5 min. 1 mL of 1 M NaOH solution was added. The mixture was vortexed again and after storing for 10 min, and then its absorbance was read at 506 nm in the spectrophotometer. Triplicate analyses were performed for each specimen. 500 µL distilled water was used as the blank. The same procedures were carried out by taking 500 µL from the standards. The total flavonoid was determined as mg catechin eq./g by using the correct equation of the calibration graph obtained with standard solutions at 5, 10, 25, 50, and 100 mg/L (Kasangana et al. 2015).
Determination of Antimicrobial Activity
The antimicrobial (antibacterial and antifungal) activities of the methanol extracts and volatile oils were determined with two different methods in accordance with agar diffusion and disk diffusion methods (Sağdıç et al. 2006; Matuschek et al. 2014). Four different concentrations of samples (100%, 50%, 25%, and 10%) were prepared for both methods. Volatile oil samples were prepared by dissolving in hexane, methanol extracts were dissolved in methanol.
Agar diffusion method
The agar diffusion method was executed in 3 stages: preparation of microorganisms, preparation of agar medium, and incubation. The samples were prepared as 108 CFU/mL at the end of the second activation for 18 h after the first activation of the bacteria in nutrient broth (Merck) medium at 36 °C for 24 h. Yeast and molds were prepared as 108 CFU/mL at the end of the second activation for 24 h after the first activation at 25 °C for 48 h. Nutrient Agar (Merck) medium was prepared for bacteria, and Malt Extract Agar (Merck) medium was prepared for yeast-mold. Afterwards they were sterilized in the autoclave. Then, 1% of the activated microorganisms were added to the medium which was brought to casting temperature, and they were put in petri dishes to enable solidification. Wells with a diameter of 4 mm were drilled on the agar medium. A total of 20 µL from sample extracts were added to these drilled wells. Bacteria were incubated at 36 °C for 24 h, and yeast-molds were left at 25 °C for 48 h. After the incubation, the diameter of the transparent zone formed around the wells was measured (Sağdıç et al. 2006).
Disc diffusion method
Microorganisms were prepared at the density of 108 CFU/mL in disc diffusion method. Bacteria whose densities were arranged by activating on the sterile Nutrient Agar and Malt Extract Agar media were spread as 1 mL. Sterile discs were soaked with 20 µL sample extracts and placed on the media. Bacteria were incubated at 36 °C for 24 h, and yeast-molds were incubated at 25 °C for 48 h. After the incubation, the diameter of the transparent zone formed around the discs was measured (Matuschek et al. 2014).
RESULTS AND DISCUSSION
Yield of Obtained Volatile Oil
The yields of the volatile oil obtained from the flowers and leaves of P. v. subsp. columnae species were determined as 0.33% and 0.12% respectively. The volatile oil amount and ratio in flowers was detected to be higher. In a study carried out on leaves, roots, and flowers of Primula veris var. columnae in Croatia in 2011, Vuko et al. (2017) noted that the total volatile oil yield was found to be 0.03% with respect to the dry weight of the samples.
GC-MS/FID Analysis Results of Volatile Oil Components Obtained from Flower and Leaf
The GC-MS/FID analysis identified 62 compounds belonging to the flowers of the P. veris subsp. columnae, but 3 compounds could not be identified. While the structure of a total of 50 compounds belonging to the leaves of the P. veris subsp. columnae has been defined, 4 compounds could not be identified. The GC-MS/FID analyses results of the volatile oil obtained from the flowers and leaves of the P. veris subsp. columnae (Tutya, Primrose) plant are given in Table 1.
Table 1. GC-MS/FID Analysis Results of Volatile Oil Obtained from the Flowers and Leaves of Primula veris subsp. columnae
PvcF: Primula veris subsp. columnae flower; PvcL: Primula veris subsp. columnae leaf; RT: Retention time; RI: Retention index, hydrocarbons with (C6-C32) carbon numbers were taken as standard, LRI: Literature retention indices based on Adams (2007), NIST and WILLEY.
A total of 62 of the compounds with the defined structures were found to belong to P. veris subsp. columnae flowers; 50 of them belonged to P. veris subsp. columnae leaves and constitute 95.2% and 94.1% of the total sample, respectively. While the number of compounds with the unidentified structures was 3 in flower volatile oil, it was 4 in leaf volatile oil; their ratios were 3.52% and 3.66%, respectively. Results of the analyses on the volatile oil of flowers show that the number of identified compounds and the percentage of these compounds in the total sample were found to be higher than the leaf volatile oil samples. The main compounds in volatile oils, which were isolated from flowers, were determined as methyl 4-methoxysalicylate (37.1%), tricosane (23.5%), methyl 2,6-dihydroxy-4-methyl benzoate (12.2%), and docosane (3.10%). The main compounds in volatile oils, which were isolated from the leaves, were determined as linoleic acid (40.1%), tricosane (14.1%), methyl 4-methoxysalicylate (7.49%), hexadecenoic acid (9.87%), and limonene (5.76%) (Table 1).
Vuko et al. (2017) reported that the main components were methyl 4-methoxysalicylate, pentacosane, and benzoic acid, 2-hydroxy-methyl ester (methyl salicylate) in their research, which they conducted on leaves, roots, and flowers volatile oil of Primula veris var. columnae. Güneş (2016) specified that while the main components of Primula vulgaris Huds. subsp. sibthorpii flower volatile oil were tricosane and hexadecenoic acid, the main components of leaf volatile oil were tricosane and hexadecenoic acid. Yaylı et al. (2016) specified that the main components of Primula vulgaris Huds. subsp. vulgaris volatile oil are methyl-4-methoxy salicylate, (Z,Z,Z)-7,10,13-hexadecatrienal, flavone, docosane, tricosane, and tetracosane, depending on the altitude (300 to 2100 m) in their study on Primula vulgaris Huds. subsp. vulgaris (Pvv) and P. vulgaris Huds. subsp. sibthorpii (Pvs). In the same study, the main compounds of P. vulgaris Huds. subsp. sibthorpii volatile oil were stated as methyl-4-methoxy salicylate, flavone, docosane, tricosane, tetracosane, and pentacosane depending on the altitude (100-1300 m). In another study, Nan et al. (2002) stated that the main components in the Primula obconica leaves, stems and flowers volatile oils were found as methyl 2,4-dihydroxy-5-methyl benzoate, methyl 2,6-dihydroxy-4-methyl benzoate, hypnone, and methyl salicylate.
When the main components of essential oil obtained in the present study were compared with the studies in the literature, it was seen that compounds such as methyl 4-methoxysalicylate, tricosane, methyl 2,6-dihydroxy-4-methyl benzoate, docosane, methyl 4-methoxysalicylate were similar. In addition, it was understood that differences occurred in compounds such as linoleic acid, limonene, tetracosane, pentacosane, methyl salicylate, flavone, (Z,Z,Z)-7,10,13-hexadecatrienal, and hypnone. The chemical composition and the number of essential oils vary according to their type, species, growing environment, climatic characteristics, genetic differences, collection time, storage method, and analysis parameters of the plants (Öz et al. 2021; Karataş et al. 2022).
The 62 compounds with identified structures in flower samples were classified into 13 groups. The identified structures from these classes and the number of compounds they contained were determined, respectively, as 4 ketones, 7 aldehydes, 3 alcohols, 10 esters, 3 oil acids, 11 hydrocarbons, 7 monoterpenes, 6 monoterpenoids, 7 sesquiterpenes, 1 sesquiterpenoid, 1 diterpenoid, and 2 others. Fifty compounds with the identified structures in leaf samples were classified into 13 groups. The identified classes in leaf samples were, respectively, 3 ketones, 4 aldehydes, 3 alcohols, 8 esters, 1 ether, 4 oil acids, 7 hydrocarbons, 6 monoterpenes, 7 monoterpenoids, 2 sesquiterpenes, 1 sesquiterpenoid, and 4 others. In flower volatile oil samples, hydrocarbons have the highest number of compounds with 11 compounds, while in the leaf volatile oil samples, esters were the most frequent, with 8 compounds.
As the result of flower essential oil analysis, the chemical class with the highest % amount was esters with 51.6% and it was oil acids with 51.2% in leaf volatile oil analysis. While 22 compounds and 7.93% terpene class compounds were identified in flower volatile oil samples, monoterpenes (3.03%) were determined as the highest terpene class. While 16 compounds and 11.7% terpene class compounds were identified in the leaf volatile oil, the highest terpene class was found as monoterpenes (8.02%) in flowers. The chemical classification of compounds found in flowers and leaves of P. v. subsp. columnae are given in Fig. 2.
Fig. 2. Chemical classification of compounds found in flowers and leaves of Primula veris subsp. columnae
The Extractive Substance Amount Obtained as the Result of Methanol Extraction
As a result of the methanol extraction analysis, the percentage of extractive substance amount in the flower was determined as 34.5%. The extractive substance amount in the leaf was detected as 28.8%.
Antioxidant Activity Results
Antioxidant activity results of methanol extracts obtained from flowers and leaves of the P. v. subsp. columnae plant are given in Table 2.
Table 2. Antioxidant Activity of Methanol Extracts from P. veris subsp. columnae
*: Results are given as average, **±: Standard deviation, AA: Ascorbic acid, GA: Gallic acid
DPPH Free radical scavenging activity
As shown in Table 2, the radical scavenging amount (DPPH) of the samples were defined as 24.75 ± 0.10 mg AA eq./g and 25.74 ± 0.09 mg Trolox eq./g in methanol extract of flowers. The amount of DPPH was found to be 6.42 ± 0.07 mg AA eq./g and 7.28 ± 0.34 mg Trolox eq./g in leaves. DPPH % inhibition rates of the same samples were determined as between 81.97 ± 0.73 and 61.03 ± 2.64%, respectively. When the radical scavenging amount and % inhibition ratios of flower and leaf extracts were examined, the flower methanol extract demonstrated more inhibition properties when compared with the leaf. It is considered to originate from the existence of more organic compounds in the flower sections when compared with the leaf sections. In the literature, DPPH inhibition ratios were found out as 86.65% ± 1.11 and 88.46% ± 0.11 in the study on 10 and 20 Primula veris L. flower samples with 70% ethanol extract. It has been acknowledged that the inhibition ratio increased depending on the concentration (Tünde et al. 2015). In the literature, it has been stated that the main active compounds of primula flowers and roots are phenolic compounds, flavonoids (about 3% in flowers), phenolic acids, and phenolic glycosides, as well as triterpene saponins. Saponins are responsible for secretolytic and expectorant activity; phenolic compounds in Primula flowers are responsible for antioxidant, antimicrobial and cytostatic properties (Tokalov et al. 2004; Demir et al. 2014). When compared in terms of DPPH inhibition rates, it was understood that P. veris subsp. columnae species has similar values with other Primula species.
Ferric (III) ion reducing antioxidant power (FRAP)
Total ferric reduction antioxidant capacity (FRAP) amounts of the P. v. subsp. columnae samples were found out as 677.59 ± 41.30 mg FeSO4 eq./g in the flower section and 165.83 ± 2.62 mg FeSO4 eq./g in the leaf section (Table 2). In the study conducted with the fresh above ground sections of Primula veris L., the total ascorbic acid content was determined as 118 mg/100g. In the same study, the FRAP value of Primula veris L. plant was determined in terms of 757 µmol Fe II/g (Murathan 2018). When compared with Primula veris L. values in the literature, the FRAP values of P. v. subsp. columnae methanol extract were determined as significantly different, it was identified that especially flower sections have high ferric reduction properties.
Total phenolic substance
Total phenolic substance amounts prepared from 0.20% methanol extracts obtained from flower and leaf sections of P. v. subsp. columnae species have been found as 78.62 ± 2.25 mg GA eq./g in the flower section in terms of gallic acid and as 38.43 ± 0.68 mg GA eq./g in the leaf section (Table 2). In the literature, phenolic compounds, especially hyperoxide, primeverin, and primulaverin, have been specified as chemical markers for the determination of Primula species in a study on Primula veris. Bączek et al. (2017) found it as 1.73 ± 0.16 mg/g in their total phenolic substance study conducted on Primula veris L. in 2017. In a study, the total phenolic substance contents of different Primula veris L. extracts were determined as between 5.10 and 17.3 mg/g, respectively (Rudhani et al. 2017). It can be said that P. v. subsp. columnae methanol extract is significantly different in terms of phenolic substance when looking at the values of Primula veris L. plant in the literature. On this basis, it can be declared as quite rich in terms of phenolic content.
Total antioxidant substance
According to ascorbic acid standards, the total antioxidant substance was found as 127.05 ± 9.55 mg AA eq./g in the flower section and 74.63 ± 5.80 mg AA eq./g in the leaf section in samples with 0.20% methanol extracts obtained from flower and leaf sections of P. v. subsp. columnae species (Table 2). Primula veris L. is a plant species of the Primulaceae family. According to the literature data, it has been stated as a plant rich in saponins (about 60%) including primulic acid, as well as many flavonoid compounds and flavanols, which means rutin, catechin, kaempferol, and luteolin (Okrslar et al. 2007; Colombo et al. 2017). Primula veris L. is a source of phenolic and flavonoid compounds. Since they are rich in rutocide, they express effects in terms of many pharmacological hyperoxide activities, which means anti-inflammatory, antioxidant, and antimicrobial activities (Başbülbül et al. 2008).
Total flavonoid substance
The total amount of flavonoid substance was determined as 1.96 ± 0.06 mg catechin eq./g in the flower and as 1.00 ± 0.01 mg catechin eq./g in the leaf section of 0.20% methanol extract samples obtained from the flower and leaf sections of the P. veris subsp. columnae species, regarding to the catechin standards (Table 2). In a study, Rudhani et al. determined that the total flavonoid substance contents of different Primula veris L. extracts were between 12.2 and 31.4 mg/g, respectively (Rudhani et al. 2017). In another study, the total flavonoid substance contents of above ground sections of Primula veris L. were determined as 22.88±2.7 mg/g (Murathan 2018). In the literature, it has been acknowledged by the public that the leaves, roots, and flowers of Primula veris L. have various health properties lasting for a long time. Therefore, it is used as a diuretic, antimicrobial, antifungal, sedative, anti-inflammatory and expectorant remedy among the public. Since the plant is also rich in phenolic acids and flavonoids in its flowers and roots, it is used against bronchitis, cough, and flu (Başbülbül et al. 2008). When compared with the literature data, it can be said that the antioxidant properties of the P. veris subsp. columnae plant are quite high.
Antimicrobial Test Results of Volatile Oil and Methanol Extracts
In the antimicrobial activity test conducted on flower and leaf volatile oils and methanol extracts, it was determined that among them, only flower essential oil showed weak activity against Escherichia coli bacteria and it did not show any antifungal and antibacterial activity apart from that. The antimicrobial activity test conducted on flower and leaf volatile oils and methanol extracts are shown in Table 3.
Başbülbül et al. (2008) found with the well diffusion method that among the tested microorganisms: Enterococcus faecalis, Bacillus cereus, and Pseudomonas fluorescens are inhibited by all extracts in their study on water, ether, and ethanol extracts of Primula veris L. flowers. Besides, ether and water extracts had higher inhibitory activity than ethanol extract and it has been stated that none of the tested extracts showed a activity against Staphylococcus aureus, Proteus sp. and Listeria sp. Najmus-Saqib et al. (2009) stated in their study that antibacterial activity was carried out on Escherichia coli, Bacillus subtilis, Shigella flexenari, Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella typhi by using the raw extract of Primula macrophylla and agar well diffusion method, and the samples did not show any activity at the end of the study.
Yaylı et al. (2016) stated that Primula vulgaris Huds. subsp. vulgaris (Pvv) and P. vulgaris Huds. subsp. sibthorpii (Pvs) volatile oils showed weak activity against Bacillus cereus and strong activity against Mycobacterium smegmatis in their antimicrobial study with agar diffusion method. The resulting activity can be explained by the high concentration of hydrocarbons and aromatic esters found in essential oils (Yaylı et al. 2016).
In the literature, it is emphasized that carboxylic acids, aldehydes, and terpenes (from the main chemical classes of essential oils) have antimicrobial properties (Swamy et al. 2016; Baltacı et al. 2022).
Table 3. Antimicrobial Activity Test Conducted on Flower and Leaf Volatile Oils and Methanol Extracts
CONCLUSIONS
- In the conducted study, 62 volatile components were found in the flower section of the plant and 50 volatile components were found in the leaf section, which belong to acid, hydrocarbon, alcohol, aldehyde, ketone, terpenoids, and ester groups. As the result of the analyses on the volatile oil of the flowers, the number of identified compounds and the percentage of these compounds with respect to the total sample were found to be higher than the leaf volatile oil samples.
- As the result of the extraction of the flowers and leaves of the plant with methanol, it was identified that the ratio of extractive substance amount in the flower was higher than extractive substance amount in the leaf.
- When the P. veris subsp. columnae plant was examined generally, its antioxidant properties were determined as quite high. It has been revealed that the plant is rich in terms of phenolic substances in samples prepared from methanol extracts, which were obtained from the flower and leaf sections of the plant. It can be stated that its phenolic components can be investigated in future studies.
- In the antimicrobial activity test, which was carried out on flowers and leaves of the P. veris subsp. columnae species, only flower volatile oil showed weak activity against Escherichia coli bacteria. Apart from that, no activity against test microorganisms was evident in any one of the used concentrations of flower and leaf volatile oils and methanol extracts.
This study is a part of a master thesis entitled “Primula veris subsp. columnae Chemical Content of Essential Oil, Obtaining the Methanol Extract and Biological Activities” prepared by Çağrı KARAPINAR under the supervisor of Assistant Professor Dr. Mehmet ÖZ in Gümüşhane University. This research has been supported by Gümüşhane University, Scientific Research Projects Coordination Department (Project Number: 19.B0122.07.01).
REFERENCES CITED
Abramovıč, H., Abram ,V., Čuk, A., Čeh, B., Smole-Možına, S., Vıdmar, M., Pavlovıč, M., and Poklar-Ulrıh, N. (2018). “Antioxidative and antibacterial properties of organically grown thyme (Thymus sp.) and basil (Ocimum basilicum L.),” Turkish Journal of Agriculture and Forestry 42, 185-194. DOI: 10.3906/tar-1711-82
Adams, R. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, Allured Publishing Corporation (4th ed), Illinois, USA.
Ahmed, D., Khan, M. M., and Saeed, R. (2015). “Comparative analysis of phenolics, flavonoids, and antiooxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves,” Antioxidants 4, 394-409. DOI: 10.3390/antiox4020394
Altaf, A., Xinkai, Z., Min, Z., Muhammad, A., Aamir, S., Sadia, G., Quan, M., Bo, Z. X., Fujian, L., Khan, I., and Zada, A. (2020). “Study of volatiles from fresh and dried eucalyptus (Eucalyptus citriodora) and spearmint (Mentha spicata) leaves using gas chromatography (GC-FID),” Pakistan Journal of Agricultural Sciences 57(5), 1469-1479. DOI: 10.21162/PAKJAS/20.706
Baasanmunkh, S., Kovtonyuk, N. K., Oyuntsetseg, B., Tsegmed, Z., Han, İ. V., and Choi, H. J. (2020). “Diversity and distribution of the genus Primula L. (Primulaceae) in Mongolia,” Journal of Asia-Pacific Biodiversity 13, 687-700. DOI: 10.1016/j.japb.2020.09.002
Bączek, K., Przybyl, J. L., Mirgos, M., Kosakowska, O., Szymborska-Sandhu, I., and Węglarz, Z. (2017). “Phenolics in Primula veris L. and P. elatior (L.),” International Journal of Analytical Chemistry 3, 1-7. DOI: 10.1155/2017/2871579
Baltacı, C., Öz, M., Fidan, M. S., Üçüncü, O., and Karataş, Ş. M. (2022). “Chemical composition, antioxidant and antimicrobial activity of Colchicum speciosum Steven growing in Türkiye,” Pakistan Journal of Agricultural Sciences 59(5), 729-736. DOI:10.21162/PAKJAS/22.1096
Başbülbül, G., Özmen, A., Biyik, H. H., and Şen, Ö. (2008). “Antimitotic and antibacterial effects of the Primula veris L. flower extracts,” Caryologia 61(1), 88-91. DOI: 10.1080/00087114.2008.10589614
Baytop, T. (1999). Treatment with Medicinal Plants in Turkey, Nobel Tip Bookstores (2nd ed), Istanbul, Turkey.
Borisova, D. A., and Popov, D. M. (2012). “Comparative study of the qualitative composition and quantitative content of flavonoids in various organs of Primula officinalis,” Meditsinskoii Farmatsevticheskoi Khimii 8, 8-13.
Colombo, P. S., Flamini, G., Rodondi, G., Giuliani, C., Santagostini, L., and Fico, G. (2017). “Phytochemistry of european Primula species,” Phytochemistry 143, 132-144. DOI: 10.1016/j.phytochem.2017.07.005
Demir, N., Güngör, A. A., Nadaroğlu, H., and Demir, Y. (2014). “The antioxidant and radical scavenging activities of Primrose (Primula vulgaris),” European Journal of Experimental Biology 4, 395-401.
Demir, S., Turan, İ., and Aliyazıcıoğlu, Y. Y. (2019). “Antioxidant properties of Primula vulgaris flower extract and its cytotoxic effect on human cancer cell lines,” KSÜ Journal of Agriculture and Nature 22(1), 78-84, DOI: 10.18016/ ksutarimdoga.vi.460242
Fidan, M. S., Öz, M., Ücüncü, O., Baltaci, C., and Karatas, S. M. (2022). “Composition of antimicrobial and antioxidant activities and chemical components of essential oil from flowers and leaves of Pyrus elaeagnifolia Pallas in Turkey,” Fresenius Environmental Bulletin 31(4), 4106-4117.
Güneş, G. (2016). Essential Oil and Solvent Extract GC/MS Analysis of Aboveground Parts of Primula vulgaris subs. sibthorpii. Master’s Thesis, Giresun University, Giresun, Türkiye.
Karapınar, Ç. (2020). Primula veris subsp. columnae Chemical Content of Essential Oil, Obtaining the Methanol Extract and Biological Activities, Master’s Thesis, Gümüşhane University, Gümüşhane, Türkiye.
Karataş, S. M., Öz, M., Fidan, M. S., Baltaci, C., and Ücüncü, O. (2022). “Determination of chemical content and biological activities obtaining essential oil from Ribes petraeum Wulfen plant grown in Gümüşhane region,” Gümüşhane University Journal of Science and Technology 12(2), 498-511, DOI: 10.17714/gumusfenbil.997171
Kasangana, P. B., Haddad, P. S., and Stevanovic, T. (2015). “Study of polyphenol content and antioxidant capacity of Myrianthus arboreus (Cecropiaceae) root bark extracts,” Antioxidants 4(2), 410-426. DOI: 10.3390/antiox4020410
Latypova, G. M., Bubenchikova, V. N., and Kataev, V. A. (2015). “The level of ursolic acid in the plants of the genus Primula,” Farmatsiya 4, 21-24.
Liu, J., Mahmood, M. S., Abbas, R. Z., Dillawar, A., Nawaz, Z., Luqman, M., Abbas, A., Rehman, A., and Rafique, A. (2021). “Therapeutıc appraısal of ethanolıc and aqueous extracts of clove (Syzygium aromaticum) and garlıc (Allium sativum) as antımıcrobıal agent,” Pakistan Journal of Agricultural Sciences 58(1), 245-251. DOI: 10.21162/PAKJAS/21.650
Matuschek, E., Brown, D. F. J., and Kahlmeter, G. (2014). “Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories,” Clinical Microbiology and Infection 20, 255-266. DOI: 10.1111/1469-0691.12373
Meos, A., Zaharova, I., Kask, M., and Raal, A. (2017). “Content of ascorbic acid in common cowslip (Primula veris L.) compared to common food plants and orange juices,” Acta Biologica Cracoviensia Series Botanica 59(1), 113-120. DOI: 10.1515/abcsb-2016-0020
Murathan, Z. T. (2018). “Determination of the biochemical content and antioxidant properties of some medical plants grown in the Northeast Anatolia Region ecological conditions,” Journal of Balıkesir University Institute of Science and Technology 20(2), 51-60. DOI: 10.25092/baunfbed.468493
Najmus-Saqib, Q., Alam, F., and Ahmad, M. (2009). “Antimicrobial and cytotoxicity activities of the medicinal plant Primula macrophylla,” Journal of Enzyme İnhibition and Medicinal Chemistry 24(3), 697-701. DOI: 10.1080/14756360802333406
Nan, P., Peng, S., Zhang, Y., and Zhong, Y. (2002). “Composition of volatile oil of Primula obconica in central China,” Natural Product Letters 16(4), 249-253. DOI: 10.1080/10575630290020569
NIST Chemistry WebBook. (2020). “Gas Chromatography”. www. https://webbook.nist.gov/chemistry/name-ser/
Okrslar, V., Plaper, I., Kovac, M., Erjavec, A., Obermajer, T., Rebec, A., Ravnikar, M., and Zel, J. (2007). “Saponins in tissue culture of Primula veris L,” In Vitro Cellular and Developmental Biology-Plant 43, 644-651. DOI: 10.1007/s11627-007-9072-3
Öz, M., Fidan, M. S., Baltaci, C., Ücüncü, O., and Karataş, S. M. (2021). “Determination of antimicrobial and antioxidant activities and chemical components of volatile oils of Atropa belladonna L. growing in Turkey,” Journal of Essential Oil-Bearing Plants 24(5), 1072-1086. DOI: 10.1080/0972060X.2021.1987334
Qadir, A., Aqil, M., Ali, A., Ahmad, F. J., Ahmad, S., Arif, M., and Khan, N. (2020). “GC-MS analysis of the methanolic extracts of Smilax china and Salix alba and their antioxidant activity,” Turkish Journal of Chemistry 44, 352-363. DOI: 10.3906/kim-1907-5
Rudhani, I., Raci, F., Ibrahimi, H., Mehmeti, A., Kameri, A., Faiku, F., Majlinda, M., Govori, S., and Haziri, A. (2017). “Phytochemical and in vitro antioxidant studies of Primula veris L. growing wild in Kosovo,” Khimiya 26(5), 773-785.
Sağdıç, O., Aksoy, A., and Ozkan, G. (2006). “Evaluation of the antibacterial and antioxidant potentials of cranberry (Gilaburu, Viburnum opulus L.) fruit extract,” Acta Alimentaria 35(4), 487-492. DOI: 10.1556/aalim.35.2006.4.12
Swamy, M. K., Akhtar, M. S., and Sinniah, U. R. (2016). “Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review,” Evidence-Based Complementary and Alternative Medicine 1, 1-21, article ID 3012462. DOI: 10.1155/2016/3012462
Terzi̇oğlu, S., Coşkunçelebi̇, K., and Gültepe, M. (2012). “Primula × uzungolensis (Primulaceae): a new natural hybrid from NE Anatolia,” Turkish Journal of Botany 36 (1), 9-19. DOI: 10.3906/bot-1011-19
Tokalov, S. V., Kind, B., Wollenweber, E., and Gutzeit, H. O. (2004). “Biological effects of epicuticular flavonoids from Primula denticulata on human leukemia cells,” Journal of Agricultural and Food Chemistry 52(2), 239-245. DOI: 10.1021/jf0347160
Tünde, J., Eleonora, M., Laura, V., Neagu, O., and Annamária, P. (2015). “Bioactive compounds and antioxidant capacity of Primula veris L. flower extracts,” Analele Universităţii din Oradea, Fascicula: Ecotoxicologie, Zootehnie Şi Tehnologii de Industrie Alimntară 14, 235-242.
Vuko, E., Spahija, D., Bezic, N., Ruscic, M., Topic, S., and Dunkic, V. (2017). “Essential oil composition of Primula veris var. columnae,” Chemistry of Natural Compounds 53(2), 386-387. DOI: 10.1007/s10600-017-2000-9
Yaylı, N., Tosun, G., Yaylı, B., Gündoğan, Z., Coşkunçelebi, K., and Karaoğlu, Ş. A. (2016). “Altitude variation in the composition of essential oils, fatty acid methyl esters, and antimicrobial activities of two subspecies of Primula vulgaris grown in Turkey,” Natural Product Communications 11(10), 1505-1510. DOI: 10.1177/1934578X1601101020
Article submitted: February 21, 2023; Peer review completed: March 13, 2023; Revised version received: April 4, 2023; Accepted: April 21, 2023; Published: May 4, 2023.
DOI: 10.15376/biores.18.3.4475-4491
