Abstract

Activated carbon from coconut shells is a low-cost, environmentally friendly material that is available for fabricating the electrodes for electric double-layer capacitance supercapacitors. As such, activated carbon derived from coconut shells was coated with Co3O4/CeO2, and its electrical and ionic conductivity were evaluated. The ternary technique for selecting materials was systematically investigated with an economical process. The Co3O4/CeO2 coating that was formed on the activated carbon coconut shells was deemed AC-Co3O4-CeO2. The 90-05-05 composite was the best electrode for electric double-layer capacitance supercapacitors, resulting in high conductivity (0.62 x 103 S·cm2), low series resistance and internal resistance (based on the Nyquist plot), and the charge-discharge was able to reach 0.56 V for 90 seconds (1A/g). Therefore, activated carbon coconut shells coated in Co3O4/CeO2 can promote the necessary characteristics of electrodes needed for electric double-layer capacitance supercapacitors.
Download PDF
Full Article
Coating Activated Carbon from Coconut Shells with Co3O4/CeO2 for High-Performance Supercapacitor Applications: An Experimental Study
Krittiya Chopngam,a Montri Luengchavanon,b,* Matthana Khangkhamano,a Kanadit Chetpattananondh,c and Warakorn Limbut d
Activated carbon from coconut shells is a low-cost, environmentally friendly material that is available for fabricating the electrodes for electric double-layer capacitance supercapacitors. As such, activated carbon derived from coconut shells was coated with Co3O4/CeO2, and its electrical and ionic conductivity were evaluated. The ternary technique for selecting materials was systematically investigated with an economical process. The Co3O4/CeO2 coating that was formed on the activated carbon coconut shells was deemed AC-Co3O4-CeO2. The 90-05-05 composite was the best electrode for electric double-layer capacitance supercapacitors, resulting in high conductivity (0.62 x 103 S·cm2), low series resistance and internal resistance (based on the Nyquist plot), and the charge-discharge was able to reach 0.56 V for 90 seconds (1A/g). Therefore, activated carbon coconut shells coated in Co3O4/CeO2 can promote the necessary characteristics of electrodes needed for electric double-layer capacitance supercapacitors.
Keywords: Coconut shell; Activated carbon; Specific surface area; Supercapacitor; Electrodes
Contact information: a: Department of Mining and Materials Engineering, Faculty of Engineer, Prince of Songkla University, Hatyai, Songkhla 90110 Thailand; b: Sustainable Energy Management Program, Wind Energy and Energy Storage Systems Centre (WEESYC), Faculty of Environmental Management, Prince of Songkla University, Hatyai, Songkhla 90110 Thailand; c: Department of Electrical Engineering, Faculty of Engineer, Prince of Songkla University, Hatyai, Songkhla 90110 Thailand; d: Division of Health and Applied Sciences, Thailand Center of Excellence for Innovation in Chemistry, Center of Excellence for Trace Analysis and Biosensor, Faculty of Science, Prince of Songkla University, Hatyai, Songkhla 90110 Thailand; *Corresponding Author: montri.su@psu.ac.th
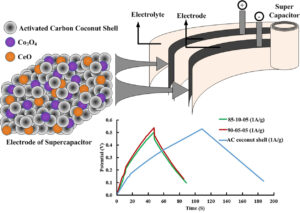
GRAPHICAL ABSTRACT
INTRODUCTION
Supercapacitors are highly efficient novel green energy storage devices. According to the electrochemical energy storage mechanism, a supercapacitor is, in the terms of electric double-layer capacitors (EDLCs), the best for electrical storage. The EDLCs primarily store energy by the adsorption of electrostatic charges on the porosity of the electrode surface. Due to the fact that EDLCs can be operated under a charge-discharge process that does not activate the chemical charges of substances, these processes enable long service life. While electrode materials are important in determining high-efficiency supercapacitors, the high performance of EDLCs should involve the use of good electrode materials that have a high specific surface area, high porosity structure, and high electrical conductivity (Zhai et al. 2021). The electrons, i.e., energy, are stored in a carbon-based electrode by the collection of opposite charges on either side of the electrode/electrolyte that is separated by an electric double layer structure (Sesuk et al. 2019).
Biomass-waste materials applied as an electrode for supercapacitors, based on activated carbon (AC) from coconut shells, have been reported to result in higher quality electrodes compared to other sources (Sesuk et al. 2019). Southern Thailand has an agriculture-based economy that is abundant in coconuts. Coconut waste could be an available raw material, with a focus on coconut shells. Biomass transforming technologies includes the conversion into activated carbons, which can be utilized for multiple applications (Nguyen et al. 2021; Yağmur and Kaya 2021; Yusuf et al. 2021). In this investigation, activated carbon from coconut shells was formed into the electrode for a supercapacitor. Activated carbon from coconut shells can generate a high surface area (Dandekar et al. 2005), a highly porous composite, and high KOH electrolyte activation. This combination can show a high rate of performance in terms of symmetric supercapacitors (Yin et al. 2016).
Many researchers have used Co3O4 and CeO2 composites for fabricating electrodes in supercapacitors that could enable high performance electrochemistry. Additionally, the Co3O4 and CeO2 composites have been increasingly prepared with nanoparticles or fibre and the pure chemistry for highly-efficient supercapacitors (Aravinda et al. 2013; Kim et al. 2016; Wei et al. 2017; Li et al. 2018). The nanoparticle composites represent high cost production, and the reliance on pure chemistries results in low environment-friendliness.
However, activated carbon from coconut shells has a low electrical conductivity and low ionic transfer, which are necessary properties of a supercapacitor. Cobalt oxide (Co3O4) has been reported to have high electrochemical activity, good electrocatalytic properties (Nguyen et al. 2021), and increases the electrical conductivity of active materials (Pandolfo et al. 2010). In addition, cerium oxide (CeO2) has shown the ability to easily generate oxygen vacancies to further improve ionic conductivity; CeO2 can reverse the oxidation state of Ce4+ and Ce3+, which can transform these two stable ions and therefore promote rapid electron migration and ion diffusion (Pant et al. 2019).
This study investigated a novel and low-cost electrode composite for a symmetrical supercapacitor. The novel electrode composites used activated carbon from coconut shells and improved the electrical and ionic conductivity by coating it with Co3O4 and CeO2. The ternary technique was used to determine the optimal ratios among AC, Co3O4, and CeO2 for high efficiency, environment-friendliness, and low cost, based on EDLC supercapacitor applications.
EXPERIMENTAL
Materials
In this investigation, the coconut shells used as raw materials to produce the activated carbon were obtained from Green Carbon Hatyai Co., Ltd, (Thailand), which was followed by physical activation. The coconut shells were activation-heated at a temperature of 1000 °C, for 40 min under an O2 gas mix super-heated steam flow. After that, the samples were cooled to room temperature. The activated carbon was washed with deionized water until the pH value was 7, followed by heat drying at a temperature of 90 °C for 12 h. The activated carbon was pulverized in a ball mill, and then sieved to obtain particles with sizes between 10 μm to 15 μm. The proximate analysis revealed 87.61 wt% fixed carbon that fabricated high carbon in the AC-coconut shell (Abdeljaoued et al. 2018), as shown in Table 1.
Table 1. Proximate Analysis Results of the Coconut Shells
Fig. 1. Schematic for preparation of (AC-Co3O4-CeO2) electrode
Preparation of Supercapacitors
Figure 1 shows a schematic of the electrode solution, which was prepared by mixing wt% of activated carbon (AC) from a coconut shell mixture, (Co3O4) cobalt (II, II) oxide, and (CeO2) cerium (IV) oxide (AC-Co3O4 -CeO2) in a 0.85 to 0.05 to 0.10 ratio (85-10-05) and a 0.90 to 0.05 to 0.05 ratio (90-05-05) as a conductive agent and 5 wt% of polytetrafluoroethylene (PTFE) as a binder (Alfa Aesar, Haverhill, MA) in 10 mL of ethanol. This solution was then mixed via ultrasonic treatment for 30 min and ground in a ball mill until a homogeneous solution was formed for 24 h. The slurry was used evenly with a doctor blade for painting on a 1 cm x 1 cm silver sheet with a thickness of approximately 200 μm. Carbon electrodes which contained activated materials were available. Thereafter, the electrodes were dried at a temperature of 90 °C for 6 h under vacuum to remove the solvent and eliminate any superfluous anhydrous ethanol. A 0.1 Mol KOH aqueous electrolyte solution was adopted as the electrolyte for the supercapacitors.
Experimental Techniques
The activated carbon samples were characterized via scanning electron microscope SEM. The analysis was conducted with a Quanta 400 (SEM-Quanta) used for observing the surface morphology of the activated carbons. To determine the surface area and pores of the activated carbons, nitrogen adsorption-desorption isotherms were measured and carried out at a temperature of 77 K by employing an ASAP 2460, ASAP 2060, (Micromeritics, Norcross, GA) device; the specific surface areas were calculated using the Brumauer–Emmett–Teller (BET) method.
The X-ray diffraction (XRD) patterns were generated with a Empyrean, PAN analytical diffractometer (WI-RES-XRD EMPYREAN-001, Malvern Panalytical, Malvern, United Kingdom) using Cu-Kα radiation within a 2θ range of 10° to 80° )λ = 0.154 nm(.
The electrochemical measurements, e.g., cyclic voltammetry (CV) and charge-discharge characteristics, were performed using a workstation (Metrohm Autolab B.V., Utrecht, Netherlands) with a typical three-electrode cell set-up connected to a lab system (potentiostat/galvanostat with NOVA 1.11 software). An Ag/AgCl electrode served as a reference electrode, and a platinum wire served as a counter electrode. The four-point probes measured the electrical DC conductivity using a Mitsubishi Chemical Analytech, Loresta-GX (MCP-T700, Tokyo, Japan). The electrolyte was a 0.1 Mol KOH aqueous solution. To investigate the electrochemical performance of the activated carbons, the voltage window and scan rate were set to be -0.2 to 0.6 V and 10, 20, 50, and 100 mV/s, respectively.
Electrochemical impedance spectroscopy (EIS) was performed using a sine wave of 10 mV over a frequency range of 0.1 Hz to 100 kHz controlled by a Metrohm Autolab B.V. (Utrecht, Netherlands). The proximate analysis used a LECO Macro TGA (TGA 701, St. Joseph, MI). Fourier Transform Infrared Spectrometer (FTIR) was used to analyse composites (Vertex 70, Bruker, Germany).
Ternary Techniques
Figure 2 shows the ternary technique used to determine the ratios of AC, Co3O4, and CeO2, respectively. The AC materials must be primarily used in the composite, while the Co3O4 and CeO2 were a minor portion of the composite based on the wt% for the electrode in the supercapacitor, which selected all composites on the top of the ternary analysis. The ternary was determined in the first phase using an orange colour circle and the second phase using a purple colour circle to measure the electrical conductivity via DC four-point probes for comparison.
Fig. 2. The ternary technique used to determine the ratio of AC, Co3O4, and CeO2 in the composite
RESULTS AND DISCUSSION
Figure 3 shows the electrical DC conductivity of composites of various AC-Co3O4-CeO2 ratios (wt%) that were measured and compared to pure AC materials and the supercapacitor electrode standard (Green Cap) produced from nano-graphite. Pure AC is the activated carbon from coconut shells, which had low conductivity when compared to standard electrode supercapacitors. The properties of the electrodes belonging to a supercapacitor should be highly conductive in order to reduce loss in the power source (Zhai et al. 2021). The inclusion of Co3O4 and CeO2 can increase the activated electric and ionic conductivities of a material (Kim et al. 2015; Xing et al. 2020). Therefore, Co3O4 and CeO2 were selected to coat pure AC to provide high conductivity; the optimal systematic ratio was determined via the ternary technique, as shown in Fig. 2.
During the first phase, the electrical DC conductivity of composites with a 90 to 05 to 05, 80 to 10 to 10, 70 to 20 to 10, and 70 to 10 to 20 ratio (AC to Co3O4 to CeO2) was measured. The composites with a 90 to 05 to 05 and 80 to 10 to 10 ratio showed the highest conductivity values (0.62 x 103 S·cm2 and 0.52 x 103 S·cm2, respectively). During the second phase, the electrical DC conductivity of the composites with a 85 to 10 to 05, and 85-05-10 ratio was measured, which resulted in conductivities of 0.46 x 103 S·cm2 and 0.44 x 103 S·cm2, respectively. The composite with a 90 to 05 to 05 ratio resulted in a conductivity of 0.62 x 103 S·cm2, which was close to the electrode standard, with a conductivity of 0.70 x 103 S·cm2. The composites with 85 to 10 to 05 and 85 to 05 to 10 ratios did not result in a greater conductivity value than the 90 to 05 to 05 composite. Generally, the electrical conductivity depended on the amount of Co3O4 in the composite, but small amounts of Co3O4 and CeO2 can induce catalytic activity and promote electron transfer (Khan et al. 2016; Yu et al. 2018). The carbon coated with Co3O4 and CeO2 can provide high electrical conductivity and greatly promote charge transfer (Li et al. 2019b). However, this investigation was focused on using the maximum amount of AC from coconut shells and minimal additional chemicals, since this study is based on being environmentally friendly and low cost. Therefore, the composites with a 90 to 05 to 05 and 85 to 10 to 05 ratios were selected for further study in terms of suitability as an electrode for EDLC supercapacitors.
Fig. 3. The composites of various AC-Co3O4-CeO2 ratios (wt%) were measured for electrical DC conductivity compared to pure AC materials and the supercapacitor electrode standard (Green Cap)
Fig. 4. XRD patterns of the AC-coconut shell composites at ratios of 90 to 05 to 05 and 85 to 10 to 05
Figure 4 compares the XRD patterns for pure AC-coconut shell and composites with a 90 to 05 to 05 and 85 to 10 to 05 ratio. Fig 4a and 4b shows the diffraction peaks of composites with a 85 to 10 to 05 and 90 to 05 to 05 ratio at 2θ values of 31.266°, 36.841°, 59.348°, 68.618°, 78.393°, and 90.955° related to the (220), (311), (511), (531), (622), and (642) planes of Co3O4 (ICSD:01-078-1969), respectively. These composites revealed diffraction peaks at 2θ values of 28.548°, 33.082°, 47.485°, and 76.704°, corresponding to the (111), (200), (220), and (331) planes of CeO2 (ICSD:01-075-9470), respectively. In addition, these composites exhibited diffraction peaks at 2θ values of 17.990°, 29.391°, and 33.215° corresponding to the (101), (200), and (112) planes of KCo (HCOO)3 (ICSD:01-080-4584), respectively. Furthermore, these composites also showed diffraction peaks at 2θ values of 35.468°, 53.478°, and 57.008° corresponding to the (311), (422), and (511) planes of Fe3O4 (ICSD:01-080-4584), respectively. The XRD of the AC-coconut shell (as shown in Fig. 4c) reveals that the diffraction peaks of carbon at 2θ values of 26.586° and 44.664° correspond to the (002) and (101) planes of carbon (ICSD:01-083-6084), respectively. These carbon peaks also appeared in the XRD patterns of the composites with 85 to 10 to 05 and 90 to 05 to 05 ratios.
The graphene oxide (C) and Fe3O4 can be fabricated for nano-composites from coconut shells (Rukman et al. 2019). Coconut shell contains soil elements, e.g., oxygen (O), carbon (C), hydrogen (H), calcium (Ca), and potassium (K) (Sujiono et al. 2020). Therefore, the XRD patterns of the composites with 85 to 10 to 05 and 90 to 05 to 05 ratios generated peaks for KCo (HCOO)3, which activated the Co3O4, H, and K for a chemical reaction in the electrode composites.
Fig. 5. FTIR patterns of the AC-coconut shell composites at ratios of 90 to 05 to 05 and 80 to 10 to 05
Figure 5 shows the FTIR spectra of original AC-coconut shell modified by Co3O4 and CeO2 in terms of ratios of 90 to 05 to 05, and 80 to 10 to 10. The aromatic structure of AC-coconut shell was shown to be around 1577 cm−1 (C=C vibration) and 1184 cm−1 (C-C stretching). The C-O stretching was found at around 1215 cm−1. Furthermore, the AC-coconut shell showed the broad peak at around 3435 cm−1 and peak at around 1633 cm−1, corresponding to the stretching vibration and the bending mode of hydroxy groups, respectively. These two peaks dropped after being doped with Co3O4 and CeO2, specifically at ratios of 90 to 05 to 05. It appears that the Co3O4 and CeO2 bonded with the structure of AC-coconut shell (Jayakumar et al. 2019). The bridging vibration of O-Co-O bond was indicated clearly at around 662 cm−1 in both cases of adding Co3O4. The CeO2 stretching vibration in the structure and Ce-O bending vibration were represented in the same wavelength range of around 570 cm−1 (Prabaharan et al. 2017: Jayakumar et al. 2019).
The FTIR spectra can indicate the modification of AC-coconut shell, which shows similar adsorption peaks, revealing original and modified activated carbon by coated Co3O4 and CeO2. However, the composites are modified by Co3O4 and CeO2, which destroys the organic structure. Further, the acidic group was enlarged during the chemical modification process.
Table 2 shows the surface area parameters of the AC coconut shell compared to the electrode material standard and the 90-05-05 and 85-10-05 composites. The surface area and pore volume properties were ranked as follows: the electrode standard was greater than the AC coconut shell, which was greater than the 90-05-05 composite, which was greater than the 85-10-05 composite. The average pore width properties were ranked as follows: 90-05-05 composite, was greater than the electrode standard, which was greater than the 90-05-05 composite, which was greater than the AC coconut shell, respectively. The electrode standard had a higher surface area and pore volume since it was produced from nano-graphite materials.
Table 2. Surface Area Parameters of the Activated Carbon (AC) Coconut Shell Compared to the Electrode Material Standard (Green Cap) and the 90 to 05 to 50 and 85 to 10 to 05 Composites
The AC coconut shell had a surface area of 2100 m2/g, a pore volume of 0.153 cm3/g, and an average pore width of 22.34 Å, which was dependent on the procedure used to prepare the materials (Suleman et al. 2015). The AC coconut shell composite coated in Co3O4 and CeO2 had lower a surface area and pore volume, but a higher average pore width. These properties may change with different coated particle sizes. The breadth of the particle size distribution can also be affected by the total space, with research demonstrating an increase in the particle size distribution breadth reduces the total space while producing broader void size distributions. Since the particle size can affect the total void volume and void sizes, the particle size distribution can be fabricated to influence the resistance and efficiency of devices using composite electrodes, as shown in Fig. 6 (Rennie et al. 2016).
Fig. 6. Particle size effect on the surface area parameters: (a) mono-size particle; and (b) a broad particle distribution (Rennie et al. 2016)
Figure 7 shows the SEM images of the electrode composites, revealing the porosity and homogenous nature of the composites. Figures 7a to 7c show the SEM images of the AC coconut shell, which had a small and homogenous particle size and a square shape, with a particle size less than 10 μm. Figures 7d to 7f show the SEM images of the 85-10-05 composite, which exhibited a homogenous particle size, a big square shape, and a small spherical shape, with a particle size is 10 μm. Figures 7g to 7i show the SEM images of the 90-05-05 composite which exhibited a homogenous particle size, a big square shape, and a small spherical shape, with a particle size ranging from 10 to 20 μm. The SEM images of the AC coconut shell, 85-10-05 composite, and 90-05-05 composite were related to surface area parameters (Table 2) and the Co3O4/CeO2 coated composites were affected by different particle sizes (Fig. 7).
As the average particle size (3.5 μm to 18.1 μm) in the EDLCs electrodes increased, the internal resistance of the electrode increased, and the rate capacitance decreased. This behavior was generated from the dense packing of the AC found in composites with smaller particle sizes, as the volume of the space substantially increased as the particle size decreased (Yoshida et al. 1996). The trends of resistance and specific capacitance were affected by a wider range of average particle sizes (0.02 to 20 μm). It was found that the electrode of the EDLCs with larger particle sizes showed poorer capacitance retention with an increased discharge rate (Portet et al. 2008). The reducing particle size can easily conduct ionic transfer by reducing the diffusion length of the ions (Tanaka et al. 2012). The 85-10-05 and 90-05-05 composites also produced particle sizes in a range of 10 to 20 μm.
Fig. 7. SEM images of the electrode composites: (a, b, and c) AC coconut shell at x350, x1500, and x 3000 magnification, respectively; (d, e, and f) 85-10-05 composite at x350, x1500, and x3000 magnification, respectively; and (g, h, and i) 90-05-05 composite at x350, x1500, and x3000 magnification, respectively
Figure 8 shows the Nyquist plots comparing the AC coconut shell, 85-10-05 composite, and 90-05-05 composite in terms of performance as the electrode in the EDLC cell. The 85-10-05 composite, 90-05-05 composite, and AC coconut shell had similar interval resistance trends; however, the 90-05-05 composite was found to have lower resistance. The semicircle is displayed in the high-frequency region, i.e., the magnified region near the origin in the inset of Fig. 8, and a linear section represented the low-frequency range, which was found to be linear for the 85-10-05 and 90-05-05 composites. The linear section at low frequencies of Nyquist plot reflects capacitance properties (Fig. 8). This result indicated a degree of inhomogeneity in the double layer region that is frequently seen when using porous electrodes (Song et al. 1999).
Fig. 8. Nyquist plots representing the data from the EIS experiments; magnified high-frequency region (inset)
The inset of Fig. 8 shows the values of the series resistance (Rs) that were related to the point where the spectra crosses the real axis. These resistances are generated due to electrolyte conductivity that remained the same in each cell. The Rs values of the 90-05-05 composite, 85-10-05 composite, and AC coconut shell were determined to be 11 Ω, 16 Ω, and 18 Ω, respectively. The semicircle indicated at higher frequencies, internal resistance (Ri) is produced via the reactions between the pores of the electrode with the ions conducted in the electrolyte, which can be referred to as the collective distributed resistance (Pandolfo et al. 2010). The AC coconut shell and 85-10-05 composite show semicircles larger than the 90-05-05 composite. Table 2 shows that the pore volume of AC coconut shell was greater than that of 90-05-05 composite, which was greater than that of the 85-10-05 composite, the latter about two-fold. This may be caused by a minor increase in the pore volume that substantially influenced the Ri values.
Figure 9 shows the electrochemical performance of the AC coconut shell, 85-10-05 composite, and 90-05-05 composite evaluated via cyclic voltammetry. Figure 9a shows the cyclic voltammogram (CV) curves, which revealed a large area, indicating better capacitive behaviors. Figure 9b shows the reduction peaks at 0.48 V, 0.50 V, 0.50 V, and 0.52 V, respectively. Figure 9c shows the reduction peaks at 0.48 V, 0.50 V, 0.52 V, and 0.54 V, respectively. Notably, the 90-05-05 composite indicated better reduction peak potentials than the 90-05-05 composite, which was primarily due to the synergies between the active chemicals in the Co3O4 and CeO2 after coating. Moreover, the close contact between the Co3O4/CeO2 and the AC coconut shell layer can act to smooth the charge transfer and enhance the oxygen reduction reaction (ORR) activity. This activity on Co3O4/CeO2 could improve electrical conductivity and high oxygen-storage capacity (Li et al. 2019a). The CV of the 85-10-05 composite and 90-05-05 composite revealed a narrow area and fabricated higher peaks in comparison to the CV of the AC coconut shell, in which the Co3O4/CeO2 reacted clearly with the reduction peaks in the AC composite.
Fig. 9. Cyclic voltammogram for (a) the AC coconut shell; (b) the 85-10-05 composite; and (c) the 90-05-05 composite in a KOH solution at various scan rates
Figure 10 shows the charge-discharge characteristics for different composites using a current density of 1 A/g. The voltage potentials corresponded to the CV between -0.2 and 0.6 V, which demonstrated that the charge time drastically affected the various materials. The peak of the 85-10-05 composite, AC coconut shell, and 90-05-05 composite was reached at 5.20 V, 5.50 V, and 5.68 V, respectively. The 90-05-05 composite was found to have the charging potential sufficient to reach a higher voltage. The AC coconut shell composite, when coated with Co3O4/CeO2, can decrease the charge-discharge time due to the sluggish kinetics of the reduction reaction to fast potential change (Li et al. 2019a; Pant et al. 2019). Many factors, e.g., conductivity, specific surface area, and pore size, can affect the electrochemical capacitance of an electrode material (Gao et al. 2012). Co3O4 is a spinel oxide whose cation production can be fabricated to produce Co+2(Co+3)2(O-2)4. The Co+2 ions are colonized in the tetrahedral site, and the Co+3 ions are colonized in the octahedral site. These two ion charge states can exchange in a small amount among the two sites, which describe the electric properties of stoichiometric Co3O4. In addition, Co3O4 is an intrinsic p-type semiconductor with electric conductivity, which is mostly due to its being a hole-free carrier (Makhlouf et al. 2013). The increasing amount of Co3O4 in the AC composite can be evaluated for conductivity but may not depend on the quantity. While CeO2 is stable, it can react to reversible oxidation states of Ce4+ and Ce3+ to transform into each other easily and rapidly, generating rapid electron migration and ion diffusion (Xing et al. 2020). The increasing CeO2 can connect the higher ionic transfer between the electrode and electrolytes (KOH) of the EDLCs. Therefore, a Co3O4/CeO2 coating on AC coconut shells that can promote a faster charge potential and the voltage capacity needed to be a high-performance EDLC.
Fig. 10. Charge-discharge characteristics for various composites using a current density of 1 A/g
CONCLUSIONS
- The development of electrical double layer capacitance (EDLC) supercapacitors using activated carbon (AC) coconut shells has been investigated by many researchers. While nano-AC composites can be utilized for high performance EDLCs, such composites rely on high-technology machinery, a multi-step procedure, and have a considerable cost. Based on the low cost and environmentally friendly nature of this study, the composite must primarily use AC coconut shells.
- Since the best choice for developing an AC electrode EDLCs supercapacitor is using an applied coating, the ternary technique was used for the systematic selection of the lowest amount of oxide element. Co3O4 and CeO2 were selected to cause high ionic and electrical conductivity. The ratio of the Co3O4/CeO2 coating to AC coconut shells are loaded in wt%, as determined by the ternary analysis in terms of AC to Co3O4 to CeO2.
- The 85-10-05 composite and 90-05-05 composite were selected since they used small amounts of Co3O4 and CeO2, which led to high electrical direct current (DC) conductivity. The 90-05-05 composite had higher performance, with a high electrical DC conductivity of 0.62×103 Scm2 and a surface area of 245 m2/g.
- The 90-05-05 composite also exhibited a low resistance from the Nyquist plot based on a series resistance (Rs) value of 11 Ω, and the internal resistance (Ri) generated a smaller semicircle. The CV of the 90-05-05 composite showed high peaks of electrochemical reduction in all rate scans. In addition, the charge-discharge characteristics of the 90-05-05 composite was able to reduce the charge-discharge time to 90 s with a higher potential of 5.60V (at 1 A/g).
- The AC coconut shell was coated with Co3O4/CeO2, which resulted in electrical and ionic conductivity. As such, a small amount of Co3O4/CeO2 coating on AC coconut shells is low in cost and can easily be processed for producing electrodes for EDLC supercapacitors.
ACKNOWLEDGMENTS
This research was financially supported by the Higher Education, Center of Excellence in Materials Engineering (CEME), the Energy Technology Research Center (ETRC), the Graduate Engineering Scholarship and the Graduate school scholarship from the Prince of Songkla University. The authors would also like to thank the Department of Mining and Materials Engineering, Faculty of Engineering, Prince of Songkla University for facility support.
REFERENCES CITED
Abdeljaoued, A., Querejeta, N., Durán, I., Álvarez-Gutiérrez, N., Pevida, C., and Hachemi Chahbani, M. (2018). “Preparation and evaluation of a coconut shell-based activated carbon for CO2/CH4 separation,” Energies 11, 1748. DOI: 10.3390/en11071748
Aravinda, L. S., Udaya Bhat, K., and Bhat, B. R. (2013). “NanoCeO2/activated carbon based composite electrodes for high performance supercapacitor,” MaterialsLetters 112, 158-161. DOI: 10.1016/j.matlet.2013.09.009
Dandekar, M. S., Arabale, G., and Vijayamohanan, K. (2005). “Preparation and characterization of composite electrodes of coconut-shell-based activated carbon and hydrous ruthenium oxide for supercapacitors,” Journal of Power Sources 141(1), 198-203. DOI: 10.1016/j.jpowsour.2004.09.008
Gao, Y., Pandey, G. P., Turner, J., Westgate, C. R., and Sammakia, B. (2012). “Chemical vapor-deposited carbon nanofibers on carbon fabric for supercapacitor electrode applications,” Nanoscale Research Letters 7(1), 1-8. DOI: 10.1186/1556-276X-7-651
Jayakumar, G., Irudayaraj, A. A., and Raj, A. D. (2019). “Investigation on the synthesis and photocatalytic activity of activated carbon–cerium oxide (AC–CeO2) nanocomposite” Appl. Phys A 125, 742. DOI: 10.1007/s00339-019-3044-4
Khan, S. A., Khan, S. B., and Asiri, A. M. (2016). “Electro-catalyst based on cerium doped cobalt oxide for oxygen evolution reaction in electrochemical water splitting,” Journal of Materials Science: Materials in Electronics 27(5), 5294-5302. DOI: 10.1007/s10854-016-4427-3
Kim, J., Ghouri, Z. K., Khan, R. Z., An, T., Park, M., and Kim, H.-Y. (2015). “Experimental study on synthesis of Co/CeO 2-doped carbon nanofibers and its performance in supercapacitors,” Carbon Letters 16(4), 270-274. DOI: 10.5714/CL.2015.16.4.270
Kim, M., Choi, J., Oh, I., and Kim, J. (2016). “Design and synthesis of ternary Co3O4/carbon coated TiO2 hybrid nanocomposites for asymmetric supercapacitors,” Physical Chemistry Chemical Physics 29, 1-9. DOI: 10.1039/C6CP03064E
Li, X., You, S., Du, J., Dai, Y., Chen, H., Cai, Z., Ren, N., and Zou, J. (2019a). “ZIF-67-derived Co3O4@carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions,” Journal of Materials Chemistry A 7(45), 25853-25864. DOI: 10.1039/C9TA08926H
Li, X., Qi, M., Sun, X., Weir, M. D., Tay, F. R., Oates, T. W., Dong, B., Zhou, Y., Wang, L., and Xu, H. H. K. (2019b). “Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities” Acta Biomaterialia 94, 627-643. DOI: 10.1016/j.actbio.2019.06.023
Li, Y. M., Yi, T. F., and Luo, S. (2018). “Facile synthesis of tremelliform Co3O4@CeO2 hybrid electrodes grown on Ni foam as high-performance electrodes for supercapacitors,” Materials Letters 233, 220-223. DOI: 10.1016/j.matlet.2018.09.020
Makhlouf, S. A., Bakr, Z. H., Aly, K. I., and Moustafa, M. S., (2013). “Structural, electrical and optical properties of Co3O4 nanoparticles,” Superlattices and Microstructures 64, 107-117. DOI: 10.1016/j.spmi.2013.09.023
Nguyen, T. T., Huynh, L. T. N., Pham, T. N., Tran, T. N., Ho, T. T. N., Nguyen, T. D., Nguyen, T. T. T., Vo, T. K. A., Pham, G. V., Le, V. H., et al. (2021). “Enhanced capacitive deionization performance of activated carbon derived from coconut shell electrodes with low content carbon nanotubes–graphene synergistic hybrid additive,” Materials Letters 292, 1-5. DOI: 10.1016/j.matlet.2021.129652
Pandolfo, A. G., Wilson, G. J., Huynh, T. D., and Hollenkamp, A. F. (2010). “The influence of conductive additives and inter‐particle voids in carbon EDLC electrodes,” Fuel Cells 10(5), 856-864. DOI: 10.1002/fuce.201000027
Pant, B., Park, M., and Park, S.-J. (2019). “TiO2 NPs assembled into a carbon nanofiber composite electrode by a one-step electrospinning process for supercapacitor applications,” Polymers 11(5), 1-13. DOI: 10.3390/polym11050899
Portet, C., Yushin, G., and Gogotsi, Y. (2008). “Effect of carbon particle size on electrochemical performance of EDLC,” Journal of The Electrochemical Society 155(7), 1-7. DOI: 10.1149/1.2918304
Rennie, A. J. R., Martins, V. L., Smith, R. M., and Hall, P. J. (2016). “Influence of particle size distribution on the performance of ionic liquid-based electrochemical double layer capacitors,” Scientific Reports 6(1), 1-10. DOI: 10.1038/srep22062
Prabaharan, D. D. M., Sadaiyandi, K., Mahendran, M., and Sagadevan, S. (2017). “Precipitation method and characterization of cobalt oxide nanoparticles,” Appl. Phys. A 123, 264. DOI: 10.1007/s00339-017-0786-8
Rukman, N. K., Jannatin, M., Supriyanto, G., Fahmi, M. Z., and Ibrahim, W. A. W. (2018). “GO-Fe3O4 nanocomposite from coconut shell: Synthesis and characterization,” in: Proceedings of the 12th Congress of Indonesian Soc. for Biochemistry and Molecular Biology in Conjunction with the 2nd International Conference “Collaboration Seminar of Chemistry and Industry (CoSCI)” and AnMicro Workshop, Jawa Timur, Indonesia, pp. 1-8.
Sesuk, T., Tammawat, P., Jivaganont, P., Somton, K., Limthongkul, P., and Kobsiriphat, W. (2019). “Activated carbon derived from coconut coir pith as high performance supercapacitor electrode material,” Journal of Energy Storage 25, 1-9. DOI: 10.1016/j.est.2019.100910
Song, H.-K., Jung, Y.-H., Lee, K.-H., and Dao, L. H. (1999). “Electrochemical impedance spectroscopy of porous electrodes: The effect of pore size distribution,” Electrochimica Acta 44(20), 3513-3519. DOI: 10.1016/S0013-4686(99)00121-8
Sujiono, E. H., Zurnansyah, Zabrian, D., Dahlan, M. Y., Amin, B. D., Samnur, and Agus, J. (2020). “Graphene oxide based coconut shell waste: synthesis by modified Hummers method and characterization,” Heliyon. 6(8), 1-8. DOI: 10.1016/j.heliyon.2020.e04568
Suleman, M., Kumar, Y., and Hashmi, S.A. (2015). “Flexible electric double-layer capacitors fabricated with micro-/mesoporous carbon electrodes and plastic crystal incorporated gel polymer electrolytes containing room temperature ionic liquids,” Journal of Solid State Electrochemistry 19(5), 1347-1357. DOI: 10.1007/s10008-014-2731-5
Tanaka, S., Nakao, H., Mukai, T., Katayama, Y., and Miyake, Y. (2012). “An experimental investigation of the ion storage/transfer behavior in an electrical double-layer capacitor by using monodisperse carbon spheres with microporous structure,” The Journal of Physical Chemistry C 116(51), 26791-26799. DOI: 10.1021/jp308415s
Wei, C. Z., Liu, K. F., Tao, J., Kang, X. T., Hou, H. Y., Cheng, C., and Zhang, D. J. (2017). “Self-template synthesis of hybrid porous Co3O4-CeO2 hollow polyhedrons for high performance supercapacitors,” Chem. Asian J 13(1), 111-117. DOI: 10.1002/asia.201701582
Xing, H., Long, G., Zheng, J., Zhao, H., Zong, Y., Li, X., Wang, Y., Zhu, X., Zhang, M., and Zheng, X. (2020). “Interface engineering boosts electrochemical performance by fabricating CeO2@CoP Schottky conjunction for hybrid supercapacitors,” Electrochimica Acta 337, 1-10. DOI: 10.1016/j.electacta.2020.135817
Yağmur, H. K., and Kaya, İ. (2021). “Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue,” Journal of Molecular Structure 1232, 1-12. DOI: 10.1016/j.molstruc.2021.130071
Yin, L., Chen, Y., Li, D., Zhao, X., Hou, B., and Cao, B. (2016). “3-dimensional hierarchical porous activated carbon derived from coconut fibers with high-rate performance for symmetric supercapacitors,” Materials & Design 111, 44-50. DOI: 10.1016/j.matdes.2016.08.070
Yoshida, A., Nonaka, S., Aoki, I., and Nishino, A. (1996). “Electric double-layer capacitors with sheet-type polarizable electrodes and application of the capacitors,” Journal of Power Sources 60(2), 213-218. DOI: 10.1016/S0378-7753(96)80013-9
Yu, H., Xu, J., Liu, Z., Li, Y., and Jin, Z. (2018). “Functionalization of sheet structure MoS2 with CeO2–Co3O4 for efficient photocatalytic hydrogen evolution,” Journal of Materials Science 53(21), 15271-15284. DOI: 10.1007/s10853-018-2687-4
Yusuf, J. Y., Soleimani, H., Yahya, N., Sanusi, Y. K., Kozlowski, G., Öchsner, A., Adebayo, L. L., Wahaab, F. A., Sikiru, S., and Balogun, B. B. (2021). “Electromagnetic wave absorption of coconut fiber-derived porous activated carbon,” Boletín de la Sociedad Española de Cerámica y Vidrio In Press, 1-11. DOI: 10.1016/j.bsecv.2021.02.003
Zhai, Z., Ren, B., Xu, Y., Wang, S., Zhang, L., and Liu, Z. (2021). “Nitrogen self-doped carbon aerogels from chitin for supercapacitors,” Journal of Power Sources 481, 1-8. DOI: 10.1016/j.jpowsour.2020.228976
Article submitted: June 11, 2021; Peer review completed: September 18, 2021; Revised version received: October 11, 2021; Accepted: October 12, 2021; Published: October 14, 2021.
DOI: 10.15376/biores.16.4.8022-8037
