Abstract
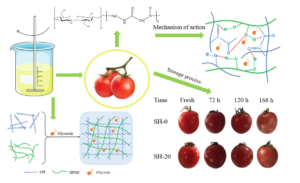
Coating a film on the surface of fruits to prolong the shelf life is an often-used method. However, wax coating is not sustainable and environmentally compatible. In this study, soybean protein isolate (SPI) and hydroxypropyl methyl cellulose (HPMC) were dissolved to form a coating solution with glycerin added as a plasticizer. The results showed that the tensile strength (TS) of the films increased from 6.52 to 20.76 MPa and the elongation at break (EAB) decreased from 68.07% to 12.67% when HPMC content increased from 0% to 20%, respectively. The intermolecular forces between the SPI and HPMC molecules made the polymers film-forming and the obtained film more continuous and stronger. The obtained film was tested on grapes and cherry tomatoes, which tightly coated with the film without any cracks. This greatly delayed their deterioration. By comparing the mass loss, total soluble solids, hardness, titratable acids, and pH values, the results showed that the coated grapes and cherry tomatoes exhibited higher freshness than the bare ones. This study fabricated an environmentally friendly coating that could prolong the shelf life of fruits, which will potentially promote the healthy development of the fruit industry.
Download PDF
Full Article
Coating of Fruit with an Edible Soybean Protein Isolate Film Doped with Hydroxypropyl Methyl Cellulose for Improved Preservation
Tinghao Zhang, Yalu Yun, Menghui Chu, Xiaowen Bai, Jiawu Sun, Yumeng Zhang, and Lijuan Wang *
Coating a film on the surface of fruits to prolong the shelf life is an often-used method. However, wax coating is not sustainable and environmentally compatible. In this study, soybean protein isolate (SPI) and hydroxypropyl methyl cellulose (HPMC) were dissolved to form a coating solution with glycerin added as a plasticizer. The results showed that the tensile strength (TS) of the films increased from 6.52 to 20.76 MPa and the elongation at break (EAB) decreased from 68.07% to 12.67% when HPMC content increased from 0% to 20%, respectively. The intermolecular forces between the SPI and HPMC molecules made the polymers film-forming and the obtained film more continuous and stronger. The obtained film was tested on grapes and cherry tomatoes, which tightly coated with the film without any cracks. This greatly delayed their deterioration. By comparing the mass loss, total soluble solids, hardness, titratable acids, and pH values, the results showed that the coated grapes and cherry tomatoes exhibited higher freshness than the bare ones. This study fabricated an environmentally friendly coating that could prolong the shelf life of fruits, which will potentially promote the healthy development of the fruit industry.
DOI: 10.15376/biores.17.2.2563-2575
Keywords: SPI/HPMC coating; Coating fruits; Preservation
Contact information: Key Laboratory of Bio-based Materials Science and Technology of Ministry of Education, Northeast Forestry University, 26th Hexing Road, Xiangfang District, Harbin 150040, P. R. China; Corresponding author: nefuwlj@nefu.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
Fresh fruit is an important source of nutrients such as minerals, vitamins, and dietary fiber. However, fruits are rich in nutrients and high in water content, which are easily affected by physiological metabolic activities and microbial infection after harvest, resulting in water loss, shrinkage, softening, and mildew. In 2000, China produced approximately 28 percent of the world’s 470 million tons of fruit. Nowadays, China produces 270 million tons of fruit each year, which is nearly half of the world’s total fruit production. While China leads the world in fruit and vegetable yields, preservation and processing methods are often neglected. Compared with developed countries, the preservation of fruits and vegetables in China faces many problems, and the annual loss rate is as high as 25% to 30%, which is undoubtedly a huge economic loss (Thakur et al. 2018). At present, waxing is an internationally permitted fresh-keeping method with mature technology and regulations (Li et al. 2018). The wax forms a protective film on the surface of the fruit, which protects the skin, improves its gloss, and prevents moisture from evaporating and retains the fruit’s freshness. In addition, waxing forms a barrier against bacteria, such that it can prevent degradation and attack by insects. However, wax is often difficult to clean on the peel and it may have to be eaten after being peeled, which wastes epidermal nutrients. Wax is also a type of petroleum processing product, which is non-renewable and causes carbon emissions. Therefore, the development of environmentally friendly, edible, anticorrosive coatings have broad application prospects (Khan et al. 2021; Pirozzi et al. 2021).
A fresh-keeping coating can improve the quality of fruits as an ideal method. Currently, biodegradable films are mainly composed of polysaccharides, proteins, and lipids. These compounds are evenly coated on the surface of the fruit to form a polymer film with a network structure after drying. The formed film can maintain fruit freshness by inhibiting water evaporation and respiration, reducing the consumption of nutrients, and preventing bacterial invasion (Ediyilyam et al. 2021). Therefore, coatings are a kind of food packaging material with great potential for development because of their strong preservation abilities, edibility, degradability, and environmentally-friendly nature (Pan et al. 2020). Hydroxypropyl methyl cellulose (HPMC) and food preservatives were developed as edible coatings for antifungal ingredients. They were tested as coatings on clementine oranges, and all the coatings effectively reduced the mass loss without adversely affecting the sensory flavor and appearance of the fruit (Valencia-Chamorro et al. 2011). Saki et al. (2019) coated fresh figs with chitosan and thymol and found that the coating was very effective for fruit preservation. Although the above methods can prolong the shelf life of fruits, the brittleness of the film restricts its development and application in fruit preservation. Biodegradable food packaging films were prepared by adding ethanol red grape seed extract and volatile oil of purple spikelet to chitosan and gelatin films (Shahbazi 2017). Meindrawan et al. (2018) developed a coating of carrageenan combined with nano zinc oxide, which resulted in an obvious increase in tensile strength and the water barrier, but little change in the elongation of the film. Meindrawan et al. (2020) explored the possibility of improving the quality and shelf life of snake fruit with gluconan-beeswax-chitosan edible coating. This treatment successfully reduced the growth of Staphylococcus aureus and Escherichia coli, but the effect of preservation needed to be strengthened. Chitosan, as used in the cited studies, is a natural and renewable material that is widely used in fruit coatings. Its price is around 200 RMB per kilogram. Moreover, it is soluble in acid solution but not water.
Therefore, there is an urgent need to develop a low-cost edible coating in the field of fruit preservation. Through numerous studies, soybean protein isolate (SPI) has been found to be inexpensive, edible, biodegradable, film forming, and an antioxidant. The price is around 20 RMB per kilogram, which is much lower than that of chitosan. Furthermore, it is easily soluble in water. Therefore, SPI can be used as a substrate material for fruit coating, but its mechanical strength and moisture resistance are poor. Shon and Choi (2011) found an SPI coating to have antioxidant and moisture resistance properties on fruit. However, the coating is prone to cracking due to the poor mechanical strength of SPI (Nishinari et al. 2014; Salgado et al. 2020), which affects the fruit preservation. This is attributed to the combination of intermolecular disulfide and hydrogen bonds, hydrophobic interactions, and electrostatic forces during film formation (Sothornvit and Krochta 2001). A large number of studies have shown that the SPI has better emulsification when mixed with other substances, giving rise to better emulsification, thermal stability, and oxidation resistance (Koshy et al. 2015). Martelli-Tosi et al. (2017) prepared a composite film with improved vapor barrier properties with chitosan and SPI, but the oxidation resistance still needed improvement. Composite films were prepared by soybean straw and SPI/straw for fruit preservation. The prepared film had higher mechanical resistance, lower elongation at break (EAB) values, and lower solubility in water. The addition of 1% fiber had no obvious effect on the permeability of the film.
To solve the shortcomings of SPI, HPMC was used in this work as a film-forming material. As a cellulose ether, HPMC has good water solubility, dispersity, thickening, water retention, and film formation. In the production of protein film (Ding et al. 2015; Zhang et al. 2019a), HPMC is often used to improve the tensile strength (TS) and EAB values. In this study, SPI and HPMC were blended to prepare the film because of the good film formation of HPMC, which improve the original single anti-corrosion effect and mechanical strength. The performance and structure of the SPI/HPMC coating were studied, and the preservation effect of cherry tomatoes and grapes was evaluated.
Materials
The HPMC (AR) was supplied by Shandong Head Co., Ltd. (Shandong, China). The food grade SPI was obtained from Hebei Baiyou Biotechnology Co. (Hebei, China). The water content of SPI and HPMC is 0.29% and 0.13%, respectively. The glycerin (AR) was acquired from Shanghai Beite Chemical Co. (Shanghai, China). The fresh grapes and cherry tomatoes were bought from a local supermarket (Harbin, China).
Preparation of the Films
In this study, the SPI/HPMC composite films were prepared under different conditions because the coating is not easy to characterize. Six grams of SPI powder were gradually poured into 100 mL of distilled water and stirred at 55 °C for 20 min. Then 0%, 5%, 10%, 15%, 20%, and 25% HPMC (w/w, SPI base) and 1.8 g of glycerol were added and stirred for 20 min. The pH was adjusted to 9, the temperature was set to 85 °C, and the solution was further stirred for 20 min. Finally, the solution was poured into a plastainer by the tape casting method and dried at 50 °C for 24 h. The obtained films were labeled as SH-0, SH-5, SH-10, SH-15, SH-20, and SH-25, respectively.
The films were anchored to the sample platform of the electron microscope. After spraying the films with gold, the morphology of the surface and the cross section of the films was photographed under 500 × magnification at a 5000 V acceleration voltage using a Quanta 200 scanning electron microscope (SEM) (FEI, Hillsboro, OR, USA).
The Fourier-transform infrared spectroscopy (FTIR) spectra were recorded on an infrared spectrometer (Nicolette 6700; Thermo Fisher Scientific, Waltham, MA, USA) over the range from 4000 to 600 cm-1 to determine the functional groups of the film samples.
The film (15.00 mm × 15.00 mm) was scanned by an X’Pert3 Powder X-ray diffractometer (Malvern Panalytical, Malvern, UK) at a scanning rate of 5 °C min-1 within the scope of 2θ = 5° to 50° at a voltage of 40 kV and a current of 30 mA.
Performance Tests
The film was cut to 20 mm × 40 mm and examined with a UV-Vis spectrophotometer (UV-2600; Shimadzu, Kyoto, Japan), and the transmittance was analyzed in the wavelength range of 200 to 800 nm. The TS and EAB values of the sample films were examined with an XLW-PC auto tensile tester (Labthink, Jinan, China) with a 50 mm fixture spacing and a 300 mm min-1 strain rate. The sample films (15 mm × 80 mm) were conditioned at 75% relative humidity (RH) using saturated lithium chloride (LiCl) solution for 24 h at 25 °C, and the average thickness of the film was obtained with a DC11ZXBS film thickness tester (Mitutoyo, Kanagawa, Japan). The parallel experiments were performed five times.
The water vapor permeability (WVP) was determined by the gravimetric method at 75% RH (25 °C). A 5 cm diameter sample was cut and sealed at the top of a weighing flask that contained 23 g anhydrous calcium chloride (0% RH). The weighing bottle was placed in a 75% RH (25 °C) dryer (saturated sodium chloride solution) and weighed periodically. Each film was determined three times in parallel. The WVP of the sample was calculated according to Eq. 1,
(1)
where W is the increased weight of weighing bottle (g), x is the average film thickness (m), t is the duration (s) of weight gain of the weighing bottle, A is the permeation area of the film (m2), and ΔP is the difference in the WVP between the two sides of the film (1753.5 Pa, 20 °C).
The oxygen permeability (OP) was determined according to the GB/T 19789 standard (2005). The films (8 cm × 9 cm) were anchored to the Perme OX2/230 oxygen transmittance tester (Labthink, Jinan, China) by the test principle of the isobaric method. The OP coefficient was measured and calculated to evaluate the OP of the films. Each film was proceeded in triplicate.
Tests of Cherry Tomatoes and Grapes Preservation
Cherry tomatoes and grapes with similar maturity in the same batch were rinsed with water, then soaked in sodium hypochlorite (NaClO) solution with an effective chlorine content of approximately 5,040 mg L-1 for 10 min, and finally rinsed with water. The treated fruits were immersed in a film-forming solution for 1 min, suspended in air at 25 °C by ropes, weighed, and photographed periodically. The pH values of the grape and cherry tomatoes were determined by a pH meter (Zhejiang Lichen Instrument Technology Co., Shaoxing, China). The hardness was determined with a GY-4 fruit hardness Tester (YUCHENGTECH, Guangzhou, China) according to the NY/T 2009-2011 standard (2011). The soluble solids in the cherry tomatoes and grapes were measured by a refractometer (WAY-2WAJ; NANBEI, Shanghai, China). The titratable acid (TA) content in fruit can be calculated by measuring the amount of sodium hydroxide (NaOH) according to the principle of acid-base titration with the known concentration of NaOH solution (0.1 mol L-1).
Statistical Analysis
The reported result of each experiment was the average of three parallel data. The Duncan multi-range test was performed on discrepancies among the mean values of the functional figure of the films by SPSS 17.0 software (IBM, Armonk, NY, USA) to determine the significance of each average attribute value (p<0.05).
RESULTS AND DISCUSSION
Morphological Analysis
As shown in Fig. 1, the film without the HPMC exhibited a uniform state, with particles obviously distributed in the films with the higher HPMC content. When the HPMC content was increased from 5% to 15%, the composite film surface was homogeneous, which indicated that the SPI and HPMC were strongly crosslinked. As shown in Fig. 2, hydrogen bonds formed between the hydroxyl groups on the structure of HPMC and the active groups of the protein. Therefore, appropriate amounts of HPMC could enhance the intermolecular force to make the compatibility better. When the HPMC content reached 25%, the surface and cross section became rough, which was due to excessive HPMC hindered the interaction between the proteins, and the surface and cross section of the film appeared as a multiphase structure. Furthermore, a large amount of HPMC was embedded in the film, which made the film rigid and prevented molecular arrangement. This generated pores in the film cross section during quenching.
Fig. 1. SEM images of the surface and cross section of SH-0, SH-5, SH-15 and SH-25
Fig. 2. The schematic diagram of the molecular cross-linking mechanism in the SHx films
FTIR Analysis
The infrared spectra of the SH-0, SH-20, SPI, and HPMC films are shown in Fig. 3. The spectrum of the SH-20 film shows a band at 3273 cm-1, which was caused by O-H tensile vibration. The band value at 2932 cm-1 was obtained from the absorption peak of C-H, which all come from primary structure of SPI. The band at 1626 cm-1 was the stretching vibration of the C=O bond. The band at 1536 cm-1 came from the bending vibration of N-H. The bands of 1399 and 1043 cm-1 corresponded to the C-C bending vibration and the C-O-C tensile vibration of the HPMC molecules, respectively. Since HPMC contains a large amount of hydroxypropyl groups in the system after film formation, there are many hydrogen bond interactions between the molecules, so the O-H and N-H stretching and bending vibration characteristic bands tend to be obvious. Moreover, the characteristic absorption peak of HPMC disappeared or weakened after the film formation, and the characteristic peak with the maximum strength at 1371 cm-1 also disappeared, indicating that there is chemical cross-linking and strong fusion between the HPMC and SPI.
Fig. 3. The FTIR spectra of the HPMC, SPI, SH-0, and SH-20 films
X-ray Diffraction (XRD) Analysis
The XRD patterns of the pure SPI and SPI/HPMC films are shown in Fig. 4.
Fig. 4. The XRD patterns of the SHx films
The peak shape of the pure SPI was similar to that of the SPI/HPMC films, which indicated that the addition of HPMC did not change the crystal structure of the SPI film. In addition, the peak of 8.4° could be seen from the SH-5 film, which indicated that the crystallization region of HPMC still was present. When the addition amount of HPMC was 15%, the peak of 8.4° weakened or even disappeared because there was strong fusion between the SPI and HPMC. Under this condition, the SPI and HPMC had excellent compatibility. At the same time, the pure SPI film and the SPI/HPMC film both had wide characteristic diffraction peaks at 20.0°, which proved that the SPI and HPMC films had amorphous properties.
Light Barrier Properties of the Films
Prolonged exposure to ultraviolet (UV) rays and sunlight can cause fruit to oxidize and even spoil, so shading is crucial to keep fruit fresh. The UV-Vis spectra are shown in Fig. 5. The pure SPI film had the highest transmittance. When the HPMC content increased from 0% to 10%, the transmittance at 600 nm decreased from 83% to 42%. This was attributed to the strong interaction between the SPI and HPMC, which made the internal structure of the film tidier and more compact, resulting in poor transmittance. When the HPMC content was 15%, the optical transmittance of the film increased, and then decreased with increasing HPMC content. It was worth noting that the transmittance of the film was almost 0 up to 300 nm (Fig. 5), which had a good ability to block UV rays. In general, the films displayed favorable shading properties.
Fig. 5. The UV-visible spectra of the SHx films
Mechanical and Barrier Properties
As shown in Table 1, when the HPMC content increased from 0% to 20%, the TS values of the SPI/HPMC films increased slightly, while the EAB values gradually decreased. Since the molecular chain was completely extended in the HPMC solution, intermolecular hydrogen bonds interacted with the amino group on the SPI molecule, which improved the internal network structure of the film. With the enhancement of various molecular interactions between the HPMC and SPI, the internal network structure of the film became regular, the intermolecular fluidity became poor, the ductility decreased, and the EAB decreased. Although the HPMC reduced the EAB values of the films, it had a good elongation in the experimental range. In addition, the TS of the SH-15 film was similar to that of the SH-25 film, which reduced the production cost of the film to a certain extent. The results showed that SH-20 exhibited better performances, therefore, was used for the coating.
Table 1. Mechanical and Barrier Properties of the SHx films
The respiration of the fruit continually depletes stored organic matter and speeds up the life processes of the fruit. If the respiration is too strong, then the stored organic matter will be consumed too much. This will cause the fruit quality to decline, which is adverse to fruit storage. Table 1 showed that with the increased HPMC content, the WVP value and the OP value of the film decreased significantly. This is due to the hydrophobic methoxyl group on the residue of D-glucopyranoside, the structural unit of HPMC. Hydroxyl groups on hydroxypropyl group could form hydrogen bonds with the protein molecular -NH2+ and -COO- groups to form a relatively stable network structure. This would expose the hydrophobic groups in the film, thus improving the hydrophobicity of the structure and reducing the WVP value of the film. Moreover, the OP value of the SPI/HPMC film was lower than that of the pure SPI film, which is due to the dense grid structure of protein film. The structure of the film led to the obstruction of oxygen through the film, which enhanced the oxygen barrier ability of the film. The results showed that the SPI/HPMC film had strong oxygen and moisture resistance, which could also block external bacteria and play a role in fruit preservation.
Application in Cherry Tomatoes and Grapes Preservation
As shown in Fig. 6, in the same batch of cherry tomatoes and grape ripening conditions, the coated specimens were better preserved than the uncoated specimens. With the prolongation of storage time, the mass loss rate increased for both the coated and uncoated fruits. The mass loss rate of the blank control increased the fastest, reaching the highest after 7 d of storage, which was 8.05% for the cherry tomatoes and 13.57% for the grapes. The weight loss rates of the coated cherry tomatoes and grapes were 6.3% and 11.73%, respectively. Compared with the blank control, the weight loss rate of the two fruits treated with the SH-20 coating was significantly reduced.
As shown in Table 2, after storage at 25.0 °C for 7 d, the pH value of the cherry tomatoes increased to 4.62, the hardness decreased to 1.70 kgf cm-2, the soluble solids content decreased to 2.50%, and the TA content decreased to 0.17%. In the coated group, pH value was 4.73, the hardness was 2.30 kgf cm-2, the soluble solids content was 4.90%, and the TA content decreased to 0.29%. The pH value of the grapes decreased to 3.67, the hardness decreased to 1.86 kgf cm-2, the soluble solids content decreased to 4.80%, and the TA content decreased to 0.32%. In the coated group, pH was 3.78, the hardness was 3.21 kgf cm-2, the soluble solids content was 8.80%, and the TA content decreased to 0.44%. In contrast, the hardness and soluble solids content decreased significantly after the SH-20 coating of both fruits, and the TA content of the coated group did not change within the range. The results showed that the composite coating could effectively inhibit the fruit hardness decline, and the effect of preservation was remarkable. Table 3 provides the comparison of hardness and weight loss of different coatings. The data showed that the hardness of the fruits coated with the SPI/HPMC film was improved by 74.99%. The weight loss decreased by 1.75% compared with the uncoated group, which was superior to other coating materials. Therefore, the SPI/HPMC composite film showed its development potential in the field of fruit preservation coating. Photos of the coated and uncoated cherry tomatoes and grapes over the 7 d can be seen in Fig. 7.
Fig. 6. The relationship of mass loss rate to time of the coated and uncoated fruits
Table 2. Total Soluble Solids Content, Hardness Values, TA content, and pH Values of the Fresh and 7 d Uncoated and Coated Cherry Tomatoes and Grapes
Table 3. Hardness and Weight Loss of the Uncoated and Coated Fruits with Different Film-Forming Materials
Fig. 7. The relationship of the coated and uncoated cherry tomatoes (a) and grapes (b) appearance changes to time.
CONCLUSIONS
- In this study, hydroxypropylmethyl cellulose (HPMC) was added to soy protein isolate (SPI) to prepare a barrier coating for fruit. The obtained film coating had excellent mechanical properties, great film-forming stability, and achieved good results in the preservation of the fruit film.
- Through a series of characterization methods, it was shown that there was good compatibility between SPI and HPMC, and the architecture was stable and continuous. The oxygen permeability (OP) and water vapor permeability (WVP) values of the film did not change much, but they were much smaller than those of a single system. The films had a good blocking effect on ultraviolet (UV) light to reduce the fruit deterioration.
- The results showed that the introduction of HPMC improved the intermolecular force, made the spatial arrangement and mechanical properties of the system more stable, and improved the tensile strength (TS) and elongation at break (EAB) values of the film. The HPMC was found to have excellent film-forming stability when the film-forming material was applied to the coating. Compared to the film-forming without adding the HPMC, the film-forming material was tightly wrapped on the coating without cracks and it had a longer service life. The coating developed in this study has repeatability and can be used with coated fruit to provide a green and new coating technology for the field of antidegradation.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the National Innovation Experiment for university students at Northeast Forestry University (NEFU) (Grant No. 202010225132).
ABBREVIATIONS
soybean protein isolate (SPI); hydroxypropyl methyl cellulose (HPMC); tensile strength (TS) ; elongation at break (EAB); scanning electron microscope (SEM); Fourier-transform infrared spectroscopy (FTIR); relative humidity (RH); lithium chloride (LiCl); water vapor permeability (WVP); oxygen permeability (OP); sodium hypochlorite (NaClO); sodium hydroxide (NaOH)
REFERENCES CITED
Álvarez, A., Manjarres, J. J., Ramírez, C., and Bolívar, G. (2021). “Use of an exopolysaccharide-based edible coating and lactic acid bacteria with antifungal activity to preserve the postharvest quality of cherry tomato,” LWT 151, article no. 112225. DOI: 10.1016/j.lwt.2021.112225
Ding, C., Zhang, M., and Li, G. (2015). “Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film,” Carbohydrate Polymers 119, 194-201. DOI: 10.1016/j.carbpol.2014.11.057
Ediyilyam, S., George, B., Shankar, S. S., Dennis, T. T., Wacławek, S., Černik, M., and Padil, V. V. T. (2021). “Chitosan/gelatin/silver nanoparticles composites films for biodegradable food packaging applications,” Polymers 13(11), 1680-1697. DOI: 10.3390/polym13111680
GB/T 19789 (2005). “Packaging materials- Test method for oxygen gas permeability characteristics of plastic film and sheting- Coulometric sensor,” Standardization Administration of China, Beijing, China.
Khan, M. R., Di Giuseppe, F. A., Torrieri, E., and Sadiq, M. B. (2021). “Recent advances in biopolymeric antioxidant films and coatings for preservation of nutritional quality of minimally processed fruits and vegetables,” Food Packaging and Shelf Life 30, 100752. DOI: 10.1016/j.fpsl.2021.100752
Koshy, R. R., Mary, S. K., Thomas, S., and Pothan, L. A. (2015). “Environment friendly green composites based on soy protein isolate – A review,” Food Hydrocolloids 50, 174-192. DOI: 10.1016/j.foodhyd.2015.04.023
Li, X., Zhu, X., Wang, H., Lin, X., Lin, H., and Chen, W. (2018). “Postharvest application of wax controls pineapple fruit ripening and improves fruit quality,” Postharvest Biology and Technology 136, 99-110. DOI: 10.1016/j.postharvbio.2017.10.012
Ma, J., Zhou, Z., Li, K., Li, K., and Zhang, H. (2021). “Novel edible coating based on shellac and tannic acid for prolonging postharvest shelf life and improving overall quality of mango,” Food Chemistry 354, article no. 129510.
Martelli-Tosi, M., Assis, O. B. G., Silva, N. C., Esposto, B. S., Martins, M. A., and Tapia-Blacido, D. R. (2017). “Chemical treatment and characterization of soybean straw and soybean protein isolate/straw composite films,” Carbohydrate Polymers 157, 512-520. DOI: 10.1016/j.carbpol.2016.10.013
Meindrawan, B., Suyatma, N. E., Wardana, A. A., and Pamela, V. Y. (2018). “Nanocomposite coating based on carrageenan and zno nanoparticles to maintain the storage quality of mango,” Food Packaging and Shelf Life 18, 140-146. DOI: 10.1016/j.fpsl.2018.10.006
Meindrawan, B., Ofe, O., Susanto, C. S., Ayman, A., Mangindaan, D., and Kasih, T. P. (2020). “Glucomannan–beeswax–chitosan antimicrobial edible coating to maintain the storage quality of salak fruit (Salacca zalacca),” Macromolecular Symposia 391. DOI: 10.1002/masy.201900164
Nishinari, K., Fang, Y., Guo, S., and Phillips, G. O. (2014). “Soy proteins: A review on composition, aggregation and emulsification,” Food Hydrocolloids 39, 301-318. DOI: 10.1016/j.foodhyd.2014.01.013
NY/T 2009-2011 (2011). “Determination of fruit hardness,” China Agricultural Press, Beijing, China.
Pan, Q., Zhou, C., Yang, Z., He, Z., Wang, C., Gu, H., Qu, Y., and Li, P. (2020). “Research progress of preservative coating of natural polymer materials,” E3S Web of Conferences 213, 02041. DOI: 10.1051/e3sconf/202021302041
Pirozzi, A., Ferrari, G., and Donsì, F. (2021). “The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables,” Coatings 11(8), 990-1017. DOI: 10.3390/coatings11080990
Saki, M., ValizadehKaji, B., Abbasifar, A., and Shahrjerdi, I. (2019). “Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage,” Journal of Food Measurement and Characterization 13(2), 1147-1158.
Salgado, P. R., D’Amico, D. A., Seoane, I. T., Montes, I. M., Mauri, A. N., and Cyras, V. P. (2020). “Improvement of water barrier properties of soybean protein isolate films by poly(3‐hydroxybutyrate) thin coating,” Journal of Applied Polymer Science 138(5), 49758. DOI: 10.1002/app.49758
Shahbazi, Y. (2017). “The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging,” International Journal of Biological Macromolecules 99, 746-753. DOI: 10.1016/j.ijbiomac.2017.03.065
Shon, J.-H., and Choi, Y.-H. (2011). “Effect of edible coatings containing soy protein isolate (SPI) on the browning and moisture content of cut fruit and vegetables,” Journal of Applied Biological Chemistry 54(3), 190-196. DOI: 10.3839/jabc.2011.032
Sothornvit, R., and Krochta, J. M. (2001). “Plasticizer effect on mechanical properties of β-lactoglobulin films,” Journal of Food Engineering 50(3), 149-155. DOI: 10.1016/S0260-8774(00)00237-5
Thakur, R., Pristijono, P., Bowyer, M., Singh, S. P., Scarlett, C. J., Stathopoulos, C. E., and Vuong, Q. V. (2019). “A starch edible surface coating delays banana fruit ripening,” LWT 100, 341-347. DOI: 10.1016/j.lwt.2018.10.055
Thakur, R., Pristijono, P., Golding, J. B., Stathopoulos, C. E., Scarlett, C. J., Bowyer, M., Singh, S. P., and Vuong, Q. V. (2018). “Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina),” Scientia Horticulturae 237, 59-66. DOI: 10.1016/j.scienta.2018.04.005
Valencia-Chamorro, S. A., Palou, L., del Río, M. Á., and Pérez-Gago, M. B. (2011). “Performance of hydroxypropyl methylcellulose (HPMC)-lipid edible coatings with antifungal food additives during cold storage of ‘Clemenules’ mandarins,” LWT – Food Science and Technology 44(10), 2342-2348. DOI: 10.1016/j.lwt.2011.02.014
Zhang, L., Kou, X., Huang, X., Li, G., and Ye, J. (2021). “Peach-gum: A promising alternative for retarding the ripening and senescence in postharvest peach fruit,” Postharvest Biology and Technology 161, article no. 111088.
Zhang, L., Lu, Y.-Q., Yu, Y.-X., Li, Q., Qian, J.-Y., and He, X.-L. (2019a). “Effect of hydroxypropyl methylcellulose molecular weight on supramolecular structures and properties of HPMC/sodium citrate photophobic films,” International Journal of Biological Macromolecules 137, 1013-1019. DOI: 10.1016/j.ijbiomac.2019.07.064
Zhang, W., Zhao, H., Zhang, J., Sheng, Z., Cao, J., and Jiang, W. (2019b). “Different molecular weights chitosan coatings delay the senescence of postharvest nectarine fruit in relation to changes of redox state and respiratory pathway metabolism,” Food Chemistry 289, 160-168. DOI: 10.1016/j.foodchem.2019.03.047
Article submitted: January 28, 2022; Peer review completed: March 12, 2022; Revised version received and accepted: March 16, 2022; Published: March 17, 2022.
DOI: 10.15376/biores.17.2.2563-2575
