Abstract
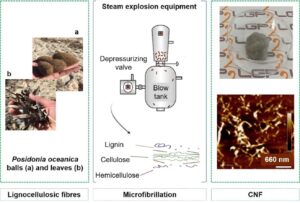
Posidonia oceanica is the dominant sea grass in the Mediterranean Sea. This biomass has great potential for use as a novel lignocellulosic material on an industrial scale. In this work, an innovative approach was applied to produce cellulose nanofibril (CNF) from Posidonia. First, fibres were isolated by a delignification-bleaching process, followed by refining and TEMPO-mediated oxidation to facilitate their further microfibrillation. Cellulose nanofibril suspensions were then produced by steam explosion or grinding (Masuko supermasscolloider). Next, CNF gel-like suspensions were characterized by several techniques such as morphological analysis (Morfi, optical microscopy, atomic force microscopy, transmission electron microscopy) and turbidity measurements. Nanopapers, prepared by filtration, were used to perform tensile tests. Finally, the efficiency of the combination of TEMPO-mediated oxidation and steam explosion was discussed. Obtained results show that the steam explosion process allows the production of CNF with a width between 4 and 10 nm and properties close to those obtained by the grinding process.
Download PDF
Full Article
Combination of Steam Explosion and TEMPO-mediated Oxidation as Pretreatments to Produce Nanofibril of Cellulose from Posidonia oceanica Bleached Fibres
Malek Khadraoui,a,b Ramzi Khiari,a,c,d,* Nicolas Brosse,e Latifa Bergaoui,b and Evelyne Mauret a,*
Posidonia oceanica is the dominant sea grass in the Mediterranean Sea. This biomass has great potential for use as a novel lignocellulosic material on an industrial scale. In this work, an innovative approach was applied to produce cellulose nanofibril (CNF) from Posidonia. First, fibres were isolated by a delignification-bleaching process, followed by refining and TEMPO-mediated oxidation to facilitate their further microfibrillation. Cellulose nanofibril suspensions were then produced by steam explosion or grinding (Masuko supermasscolloider). Next, CNF gel-like suspensions were characterized by several techniques such as morphological analysis (Morfi, optical microscopy, atomic force microscopy, transmission electron microscopy) and turbidity measurements. Nanopapers, prepared by filtration, were used to perform tensile tests. Finally, the efficiency of the combination of TEMPO-mediated oxidation and steam explosion was discussed. Obtained results show that the steam explosion process allows the production of CNF with a width between 4 and 10 nm and properties close to those obtained by the grinding process.
DOI: 10.15376/biores.17.2.2933-2958
Keywords: Posidonia oceanica; Cellulose nanofibril; Steam explosion; Grinding; TEMPO-mediated oxidation; Quality index
Contact information: a: Univ. Grenoble Alpes, CNRS, Grenoble INP (Institute of Engineering Univ. Grenoble Alpes), LGP2, F-38000 Grenoble, France; b: Carthage University, National Institute of Applied Sciences and Technology, EcoChimie Laboratory, Tunis, Tunisia; c: University of Monastir, Faculty of Sciences of Monastir, Laboratory of Environmental Chemistry and Clean Process (LCE2P- LR21ES04), 5019 Monastir, Tunisia; d: Higher Institute of Technological Studies of Ksar Hellal, Department of Textile, Ksar Hellal, Tunisia; e: Université de Lorraine – Faculté des Sciences et Technologies, Laboratoire D’Etudes et de Recherche sur le Matériau Bois, 54506, Vandœuvre-Lès-Nancy Cedex, France;
*Corresponding authors: khiari_ramzi2000@yahoo.fr; Evelyne.mauret@grenoble-inp.fr; Univ. Grenoble Alpes, CNRS, Grenoble INP (Institute of Engineering Univ. Grenoble Alpes), LGP2, F-38000 Grenoble, France
GRAPHICAL ABSTRACT

INTRODUCTION
Cellulosic fibres are used in various fields, such as pulp and paper industries, and for several applications in packaging, automotive industry, building construction, etc. (Belgacem and Gandini 2011; Khiari and Belgacem 2017).
Of all the available wide variety of lignocellulosic sources, wood is the most commonly used in the world (Klemm et al. 2005). However, in forest-deficient areas, the utilization of other types of fibres has been developed. Currently, great attention is focused on the exploitation of residues and agricultural crops as alternative sources of cellulosic fibres: kenaf (Villar et al. 2009; Valdebenito et al. 2017), hemp bast (Puangsin et al. 2012), flax (Cao et al. 2007; Alila et al. 2013), wheat straw (Josset et al. 2014), sisal (Siqueira et al. 2010), rice straw (Jiang and Hsieh 2013), bamboo residue (Liu et al. 2010), bagasse (Bhattacharya et al. 2008; Afra et al. 2013; Grande et al. 2018), date palm tree (Benhamou et al. 2014), alfa (Marrakchi et al. 2011), corn cobs and husk (Shogren et al. 2011), sugar beet pulp (Habibi and Vignon 2008; Li et al. 2014), banana rachis and culinary banana peel (Zuluaga et al. 2009; Khawas and Deka 2016), and Posidonia oceanica (Bettaieb et al. 2015).
This study deals with the investigation of fragments of Posidonia (Posidonia oceanica), an abundant marine plant in the Mediterranean Sea. The debris from this marine biomass are released by the sea in the form of balls and/or leaves and are accumulated in large quantities on the coasts during each summer season, leading to regular beach cleanings. Therefore, the valorisation of this waste as a source of fibres can be of interest.
Some studies have already been conducted on the delignification of Posidonia to produce cellulose, cellulose derivatives (Aguir and M’Henni 2006; Khiari et al. 2010), or composites (Rammou et al. 2021). Other researchers suggest the exploitation of Posidonia waste as an adsorbent material for dyes (Ncibi et al. 2006, 2007; Guezguez et al. 2009; Dural et al. 2011; Douissa et al. 2013). These materials have also been studied to produce a renewable and biodegradable nanomaterial called nanocellulose (Bettaieb et al. 2015). Since the 1980s, cellulose nanofibril (CNF, also called nanofibrillated cellulose) has been produced, traditionally by the application of specific mechanical treatments, such as microfluidization (Zimmermann et al. 2004), homogenization (Dinand et al. 1999; Dufresne et al. 2000), grinding (Abe et al. 2007; Afra et al. 2013; Nair et al. 2014), or cryo-cruching (Dufresne et al. 1997; Chakraborty et al. 2005; Alemdar and Sain 2008). Although these processes allow for the preparation of CNF exhibiting good properties, a major problem in all cases, regardless of the processes applied, is the high energy requirement (Spence et al. 2011). This is why great attention has been paid to the development of pretreatments leading to a reduction of the energy cost by facilitating the mechanical microfibrillation.
The most common pretreatments are biological and chemical: enzyme-assisted hydrolysis (Henriksson et al. 2007; Pääkkö et al. 2007), TEMPO-mediated oxidation (Saito et al. 2006), carboxymethylation (Heinze and Koschella 2005), and/or solvent-assisted pretreatments (Sirviö et al. 2015). In addition, other studies have been oriented towards the use of extrusion with the objective of increasing the concentration of the CNF gel-like suspensions (Ho et al. 2015; Rol et al. 2017).
Steam explosion (SE), which is considered to be inexpensive and clean, has also attracted several researchers for the production of CNF (Cherian et al. 2010; Deepa et al. 2011; Diop et al. 2015; Bansal et al. 2016; Phinichka and Kaenthong 2018). This is a thermomechanical process that allows the fragmentation and/or break-up of the lignocellulosic material by the combination of several factors, namely temperature in the reactor and high shear forces resulting from the sudden pressure drop when the treated material is blown into the final tank. The steam explosion process was firstly introduced for wood disintegration in the manufacture of panels (Mason 1926). Moreover, to produce fermentable sugars and alcohol from wood, Babcock and Kenvii (1932) used this process as a pretreatment of the raw material. In severe conditions, such as long residence time in the reactor or with the use of chemicals, this process leads to the depolymerisation of some lignin and the hydrolysis of hemicelluloses to elementary sugars and water-soluble oligomers (Fernández-Bolaños et al. 2001; Sun et al. 2005). Recently, this thermo-mechanical process has gained attention again for the separation of cellulose fibres from different lignocellulosic sources (Kaushik and Singh 2011; Bansal et al. 2016; Saelee et al. 2016; Luo et al. 2018). At the same time, this treatment was proposed for producing CNF from different lignocellulosic materials (Table 1).
Table 1. Various Processing Conditions for Fibres and CNF Production Using Steam Explosion Apparatus or Modified Autoclaves System
Various processing conditions of steam explosion were used to separate and/or defibrillate the fibres. However, several authors have worked with modified autoclave systems that do not allow applying high steam pressure, constituting a real limitation. Subsequently, the depressurization stage is not well controlled and its intensity not sufficient. In the literature, it was referred that steam explosion is composed by two steps: steam cracking and explosive decompression. Steam cracking consists in the penetration of water into the material in the form of steam under high pressure and at a high temperature. During the second stage called “explosive decompression”, the wet saturated lignocellulosic material “bursts” as a result of the sudden release of pressure in the reactor. It was shown that the pressure difference between the inside of the reactor and atmospheric pressure is proportional to the intensity of the shear forces applied to the biomass. So, taking advantage of this effect, the authors decided to use steam explosion for microfibrillation.
This paper proposes to study the steam explosion process SE to produce CNF from Posidonia oceanica (balls and leaves). A steam explosion pilot with a well-controlled decompressive stage was used. It was coupled to a conventional chemical treatment, i.e., TEMPO-mediated oxidation. Then, the obtained CNF was characterized and compared to TEMPO-oxidized CNF produced by conventional grinding. The objective is to understand the impact of the steam explosion on CNF morphologies (optical microscopy, atomic force microscopy, transmission electron microscopy, turbidity, and nanosized fraction) and mechanical properties (Young’s modulus). Finally, the results were compared with available data from the literature dealing with wood, non-wood, and agricultural crop-based sources. The novelty of this study was to use steam explosion for the first time as the main process for microfibrillation of delignified and bleached Posidonia fibres (combined or not to a chemical pretreatment). This is could be considered as a short-time process with less reduced environmental impact. The difference with the previous studies is that the explosion conditions were different mostly realized in autoclaves, not in steam explosion pilots with a well-controlled decompressive stage.
EXPERIMENTAL
Materials and Chemical Products
Posidonia oceanica balls (POB) and leaves (POL) were used as a starting raw material to produce CNF fibres. These wastes were collected in Monastir, Tunisia in December 2020. After a washing step, to eliminate sand and contaminants, the material was dried under ambient conditions for one month (average relative humidity: 71%; average temperature: 26 °C). All materials and chemicals were used as received from the suppliers without further purification.
Delignification and Bleaching of Posidonia oceanica Fibres
Bleached fibres from Posidonia balls (F-POB) and leaves (F-POL) were obtained after two operations, namely, delignification of the raw material and bleaching of the fibrous suspension. Delignification was carried out using soda in the presence of a small quantity of anthraquinone. This method has been commonly used for pulping non-wood plants (Antunes et al. 2000). More precisely, the delignification of the Posidonia was performed according to the procedure described by Khiari et al. (2010). For this purpose, 60 g (dry material) of raw material was treated at 160 °C with an alkali charge (sodium hydroxide) of 20% (w/w, based on dry material) and a liquor to solid ratio fixed at 10. Additionally, 0.1% (w/w, dry material) of anthraquinone was added as a catalyst. Pulping was carried out in electrically-heated rotating autoclaves (one-litre volume) for 120 min. Afterwards, the pulp was washed and filtered using a nylon mesh (100 μm) and finally was stored in a freezer.
After delignification, the bleaching step was completed with sodium chlorite (NaClO2) in a buffer medium. This treatment mainly consisted of two phases: a sodium chlorite bleaching phase followed by an alkaline extraction. For the bleaching phase, 50 g of the delignified pulp were treated in a heat-sealable bag with buffer solution (pH <4.7) prepared from sodium acetate trihydrate and acetic acid. Then 3.95 g of NaClO2 dissolved in 100 mL of deionized water were added and the bag was filled with water to 1 kg. Then, it was kept in a water bath for 90 min at 80 ° C and shaken manually every 5 min. At the end, sodium sulfite was added to neutralize the chlorine dioxide, and then the pulp was filtered with a nylon sieve of 40 μm mesh size and washed with water.
An alkaline extraction is then performed to facilitate lignin extraction. In a heat-sealable bag, the treated pulp is introduced into 400 mL of a sodium hydroxide solution (3% w/w). The bag was filled up to 1 kg with deionized water, then closed, kept in a water bath at 70 °C for 60 min and stirred every 5 min. Afterwards, the pulp was filtered with a nylon sieve of 40 μm mesh size and washed with water. Those two steps were repeated 5 times.
CNF Production
Fibres refining
Using the PFI mill, as per ISO 5264-2 (2011) standard, 300 mL of an aqueous suspension containing 30 g of dry bleached pulp (10% w/w) were refined until a drainage index of 82 °SR (Schopper Riegler degree), which corresponds to 6000 and 4000 revolutions of the mill for F-POB and F-POL, respectively.
TEMPO-mediated oxidation of fibres
Chemical modification of the fibres by TEMPO (2,2,6,6-tetramethylpiperidine-1-oxide radical)-oxidation was performed to selectively convert the primary hydroxyl of cellulose to carboxylate functions (Saito et al. 2006; Isogai et al. 2011). The bleached and refined pulp at a concentration of 1% (w/w) was treated according to the protocol proposed by Saito et al. (2006). The TEMPO/NaBr/NaClO system was used at 25 °C with 0.1/1/6.22 mmol of the reactants per gram of cellulose (mmol/g), respectively. The ensuing TEMPO-oxidized cellulose fibres were filtered and washed using deionized water until the filtrate conductivity reached a value below 5 μS cm-1.
Microfibrillation by steam explosion or grinding
Steam explosion experiments were conducted at LERMAB, Nancy, France. These experiments involved three main steps. First, 100 g of dry fibres were placed in a 4.8-L reactor that once closed, was saturated with steam until the target couple of temperature/pressure (170 °C/8 bar) was reached. These conditions were kept for 30 s. The release regulator was then opened, and the material was subjected to a sudden pressure drop (depressurization) during its rapid ejection from the reactor to be finally recovered in the blow tank. These conditions were selected after a preliminary study. In particular, the low residence time was chosen to limit the degradation of cellulose and hemicelluloses.
Fig. 1. Schematic diagram of the proposed pathways to produce CNF from POB and POL
In this study, the main objective of the steam explosion was to take advantage of the sudden drop of pressure and to assess if a possible effect on the fibres (expected breakage of the cell wall) may be sufficient to produce CNF from TEMPO-oxidized fibres.
Grinding was also done using the ultrafine friction grinder Supermasscolloider (model MKZA6-2, disk model MKG-C 80, Masuko Sangyo Co., Ltd., Saitama, Japan), available at LGP2, Grenoble, France. The ultrafine grinder was used at 1500 rpm to treat the fibre suspension. The nominal gaps between the rotating and the static stones were -5 for 10 passes and -10 for 15 passes. Figure 1 presents the proposed routes leading to the preparation of CNF using the different selected processes (cooking, bleaching, TEMPO oxidation, steam explosion, or grinding). Table 2 summarizes all sample labels.
Table 2. Abbreviations of the Different Samples
Characterisation
Several methods and techniques were used to characterize the obtained fibres as well as the produced CNF.
Carboxylic acid content
Conductometric titrations were used to determine the carboxylate content of the TEMPO-oxidized fibres (Besbes et al. 2011). To this purpose, TEMPO-oxidized fibres (0.5 g dry matter) were suspended in 10 mL of HCl (0.1 mol L-1) in such a way that the pH reached 3. Afterwards, the suspension was titrated with NaOH (0.01 mol L-1). Titration curves were used to calculate the carboxyl content according to the following Eq. 1,
(1)
where CNaOH is the concentration of NaOH (mmol L-1), V1 is the equivalent volume of NaOH solution added to titrate the strong acid corresponding to the excess of HCl (L), V2 is the equivalent volume of NaOH solution added to titrate the weak acid corresponding to the carboxyl content (L), and w is the dry weight of sample (g).
Morphological properties
The morphological properties of the prepared samples were investigated. To determine the size distribution and fines content of the suspensions, a MorFi LB-01 fibre analyser (Techpap, Gières, France) was used. Suspensions were diluted to 0.3 g L-1. The fibres/fines limit were set at 200 μm in length, and the analysis time was set at 5 min. The measurements were triplicated.
Optical microscopy was performed in transmission mode, using an Axio Imager M1 optical microscope (Carl Zeiss, Munich, Germany). For each prepared CNF gel-like suspension (at a concentration of 0.5 wt%) at least 10 images were taken.
Atomic force microscopy is a method for visualizing the topography of the sample surface or for characterizing the physical properties of materials with a resolution between 0.1 and 10 nm (under the best conditions for microscopic observation). Atomic force microscopic (DI, Veeco, Instrumentation Group, Plainview, NY, USA) observations were thus performed by using CNF gel-like suspensions diluted at 10-4% (w/w) with deionized water. A droplet of the prepared sample was deposited on a mica disc and then allowed to dry under ambient conditions for 24 h.
Transmission electron microscopic analysis was performed using a JEOL 200CX transmission electron microscope at 80 kV. The images were obtained after depositing approximately 0.5 μL of the diluted suspension onto a carbon-coated 300-mesh copper grid.
Fourier transform infrared spectroscopy (FTIR) and crystallinity index (CI)
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) was used to characterize the prepared papers from refined pulp and TEMPO-oxidized pulp (prepared by filtration under vacuum: see material and method, section Quality index).
The spectra were obtained using a spectrophotometer (Spectrum 65 FT-IR, Perkin-Elmer, Waltham, MA, USA). It covered the spectral range from 600 to 4000 cm-1. The acquisition conditions of this analysis were 16 scans and 2 cm-1 resolution.
The crystalline structure of the prepared samples was studied using an X-ray diffractometer (XRD) (D8-Advance, Bruker, Billerica, MA, USA) at room temperature with a monochromatic CuKα radiation source (λ = 0.154 nm). Experiments were performed in step-scan mode with a 2θ angle ranging from 5° to 60° with a step of 0.04 and a scanning time of 5.0 min. The CI was calculated using deconvolution method (amorphous subtraction method).
Quality index
A quality index (Desmaisons et al. 2017) was used to facilitate the monitoring of nanocellulose production at laboratory and industrial scales. The simplified quality index (QI*) was based on four parameters, namely nanosized fraction, turbidity, average microparticle size (µm2) of CNF gel-like suspensions, and Young’s modulus of nanopapers. The QI* is expressed according to Eq. 2,
QI *= 0.3 × x1 – 0.03 × x2 – 0.072 × x32 + 2.54 × x3 – 5.34 Ln(x4) + 58.62 (2)
where x1 represents the nanosized fraction (%), x2 is the turbidity (NTU), x3 is the Young’s modulus (GPa), and x4 is the average microparticle size (µm2).
The nanosized fraction in CNF gel-like suspensions was measured according to Naderi et al. (2015). This method allows determining the quantity of particles at the nanometric scale in the CNF gel-like suspensions by a gravimetric method. At first, the CNF gel-like suspensions were diluted to 0.02% (w/w) and centrifuged at 1000 g for 15 min using a centrifuge (Sigma 3-18 KS, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany). The concentrations in the supernatant before (Cbc) and after centrifugation (Cac) were used to calculate the nanosized fraction according to Eq. 3,
(3)
where the concentration is expressed in percentage (w/w).
A turbidimeter (AL250T-IR, Aqualytic, Dortmund, Germany) was used to measure the turbidity of the diluted CNF suspensions (0.1% w/w). Turbidity is related to the size, number, and refractive index of the visible suspended particles. The presence of nanoparticles that are not detectable makes the value of the turbidity close to zero. The average of the turbidity values was obtained from the measurement of five different samples.
The CNF gel-like suspensions were diluted to 0.5 wt%, and the resulting samples were observed with an optical microscope (Axio Imager M1, Carl Zeiss, Munich, Germany). Images were taken at 20x magnification and analysed with Fiji software (ImageJ, Java 1.8.0_172, Madison, WI, USA). They were converted to 8-byte type and thresholded before assessing the average length of the visible particles remaining in the suspension after steam explosion or grinding. At least five pictures were taken, and the most representative ones were used.
Nanopapers with basis weight of approximately 60 g m-2 were finally produced using a standard sheet former (Rapid-Köthen, Noviprofibre, Eybens, France) as per ISO 5269-2 (2004) standard and equipped with a nylon sieve of 1 μm mesh size (Buisine, Clermont de l’Oise, France). The suspensions were filtered under vacuum, and the obtained wet mats were dried for 12 min at 90 °C. For each CNF suspension, five nanopapers were produced and stored at 23 °C and 50% relative humidity (RH) for at least 48 h before further characterisation. The thickness of the nanopapers was measured using a micrometer (Adamel Lhomargy, Sms-Labo, Saint-Baldoph, France) at different positions on the nanopaper, and an average value was used for further calculation (m).
Finally, the Young’s modulus of the nanopapers were measured. The samples (width of 15 mm and length between jaws of 100 mm) were tested using a vertical extensometer an (Instron 5965, Instron, Norwood, MA, USA) with a capacity of 5 kN and a tensile velocity of 10 mm/min.
RESULTS AND DISCUSSION
Fibre Properties
Carboxylic acid content
As previously explained, bleached and refined fibres, R-FOB and R-FOL, were submitted to a TEMPO-mediated oxidation (TEMPO/NaBr/NaClO).
Table 3. Carboxylic Content of Various Biomasses Treated under Different Conditions by TEMPO-mediated Oxidation
This reaction was intended to modify the cellulose and to convert the primary alcohols of carbons from glucose rings to carboxylic acid cycles. Under the tested conditions, the treated samples were produced with important carboxylic contents, which are 0.97 mmol g-1 and 1.07 mmol g-1 for T-RFOB and T-RFOL, respectively.
The present results agree with previous studies (Bettaieb et al. 2015). Table 3 summarizes the carboxylic acid content of various biomasses treated under different conditions by TEMPO-mediated oxidation, showing that the amounts of acidic groups ranged from 0.3 to 1.75 mmol g-1. This range depended on many factors, especially the chemical composition of the starting materials, the different treatments undergone by the fibres during the bleaching step, and finally the quantities of TEMPO/NaBr/NaClO reagents. However, it is worth noting that 0.3 mmol g-1 of ionic groups seemed to be a minimal level to perceive an effect on the microfibrillation behavior (Besbes et al. 2011).
Morphological properties
Fibre morphology was assessed for the unrefined and refined pulps as well as for TEMPO-oxidized fibres by using SEM analysis and MorFi Lab. From SEM observations, it appears that the length of Posidonia oceanica fibres was remarkably lower than that observed for other common plants. MorFi analysis confirmed this observation: the average length and width as well as the percentage in fine elements are summarized in Table 4.
Table 4. Morphological Properties and Crystallinity of Bleached F-POB and F-POL, Refined R-FOB and R-FOL, and TEMPO-oxidized Fibres T-RFOB and T-RFOL
It is worth noting that the unrefined fibre length, whatever the starting raw material, was relatively low compared to other vegetal sources: Amaranthus caudatus L. (Fiserova et al. 2006), orache (Fiserova et al. 2006), Jerusalem artichoke (Fiserova et al. 2006), Cannabis sativa (Dutt et al. 2008), or Cynara cardunculus L. (Antunes et al. 2000; Abrantes et al. 2007). In contrast, the width seemed to be comparable with most wood, non-wood, as well as non-perennial fibres. It is also important to mention that the unrefined POL was characterized by a high content of fine elements (60% in length). Refining treatment induced variations of the fibre morphology, which were more noticeable for POL. Furthermore, the decrease of the fibre length was negligible for POB despite the large increase in fine element content. Contrastingly, for POL, the fibre length decreased from 484 to 381 µm. This behavior could be explained by the difference between the intrinsic strength of the fibres in the starting materials (Khiari et al. 2010; Bettaieb et al. 2015). TEMPO-oxidation importantly affected fibre morphology by weakening/fragilizing the cell wall structure. It was found in previous studies that TEMPO-mediated oxidation initiated microfibrillation of the fibres by the incorporation of carboxylic groups and fragilization of fibres by breaking of cellulose chains (Tarrés et al. 2017). These phenomena could explain the decrease of width and length of fibres after TEMPO-oxidation.
XRD and FTIR analysis
X-ray diffraction analysis was used to investigate the effect of the applied treatment on fibre crystallinity, namely the different polymorphic forms, the ratio of crystalline to amorphous regions, and the degree of crystallinity. The XRD patterns of all the tested samples are shown in Figs. 2a and 2b. It can be deduced from this figure that all the samples showed three main peaks at 2θ = 16.5°, 22.6°, and 34.5° corresponding respectively to the (110), (200), and (004) crystalline diffraction planes of cellulose type I (Khiari et al. 2010; Khiari and Belgacem 2020). The appearance of the peaks at 2θ =16.5° and 2θ = 22.6° in all XRD patterns confirmed that cellulose was of type I.
Fig. 2. (a) X-ray diffractogram of Posidonia balls; (b) X-ray diffractogram of Posidonia leaves; (c) ATR spectra of Posidonia balls; (d) ATR spectra of Posidonia leaves
Moreover, peaks around 25° reflect the presence of mineral compounds, namely quartz (SiO2) and Ca(1-x) Mg(x) CO3. The presence of these mineral salts is typical in sea biomass and related to the high ash content (Bettaieb et al. 2015). The results, reported in Table 4, show that the crystallinity index depended on the treatment conditions: CrI increased with refining and TEMPO-oxidation treatment. This increase reached 30% for the refining treatment. During this operation, which was performed in an intense way, the final Schopper Riegler degree reached approximately 80, a considerable fraction of hemicelluloses would thus have been released from the fibres. This would explain the increase in CrI.
The ATR analysis results of R-FOB, T-RFOB, R-FOL, and T-RFOL are shown in Figs. 2c and 2d. The spectra display characteristic cellulose absorption bands, such as the −CH stretching bands at 2900 cm-1 and the −CH2 band at 2950 cm-1, as well as the broad peak between 3000 and 3650 cm-1 due to –OH stretching. The band around 1645 cm-1 corresponds to the −OH bending mode of adsorbed water. The presence of different peaks at 1602 and 1409 cm-1 can also be noticed in the figure, which correspond to the vibrations of carboxylate groups (–COO–) (Saini et al. 2016; Michel et al. 2020), thus clearly confirming the oxidized state of the bleached fibres from Posidonia after TEMPO oxidation.
Characterisation of CNF
Non-oxidized fibres produced by steam explosion
Cellulose fibres produced by SE using non-oxidized Posidonia fibres are shown in Fig. 3. An important number of coarse elements was observed by optical microscopy (Fig. 3.1). Likewise, even if the transmission electron microscope (TEM) images (Fig. 3.2) showed some individualised nanofibrils of cellulose with a width between 2 and 8 nm for Posidonia balls (3 and 13 nm for Posidonia leaves), elements with a diameter of more than 100 nm were also present.
Fig. 3. Morphological aspect of non-oxidized nanofibres of cellulose produced using a steam explosion: 1) optical observations, 2) TEM observations
From this morphological analysis, it can then be concluded that coupling the two processes of refining and SE does not constitute an efficient way to produce CNF with a narrow width distribution.
Turbidity of the gel-like suspensions was equal to 402 and 388 NTU for Posidonia balls and leaves, respectively. These relatively high values confirmed the presence of large particles in the non-oxidized suspensions, as already discussed. Nevertheless, the suspensions presented a nanosized fraction of approximately 54%, which still reflected the presence of nanofibril of cellulose. Nanopapers exhibited Young’s modulus of 3.0 and 5.8 GPa for Posidonia balls and leaves, respectively. Such a result was lower than the CNF obtained from the same raw material by grinding (Bettaieb et al. 2015). Consequently, it can be concluded that steam explosion without any chemical pretreatment did not allow an effective individualisation of the cellulose nanofibres.
Oxidized-CNF produced by grinding or steam explosion
The CNF gel-like suspensions obtained from oxidized pulps, ground or steam exploded, were fully characterized. Figure 4 shows the different CNF gel-like suspensions images obtained from optical microscopy, atomic force microscopy (AFM), and transmission electron microscopy (TEM). For CNF gel-like suspensions obtained by grinding, optical microscopy (Fig. 4a.1) revealed the presence of a reduced number of coarse particles compared to Fig. 3.1. This confirmed the effectiveness of microfibrillation for TEMPO-oxidized Posidonia fibres by grinding. The AFM and TEM images (Fig. 4a.2 and Fig. 4a.3) also show the production of cellulose nanofibrils. The mean width of the prepared nanofibrils, between 2 and 7 nm from balls (3 and 8 nm from leaves), was low and in agreement with the literature (Table 5).
Table 5. Width Values of CNF Produced via Masuko and TEMPO-mediated Oxidation and Steam Explosion Process
Fig. 4. 1) optical, 2) AFM, and 3) TEM observations of the oxidized CNF; a) for ground CNF and b) for steam exploded CNF
Regarding the now oxidized-CNF obtained by steam explosion, optical microscopy (Fig. 4b.1) showed a higher number of coarse particles compared to the ground gel-like suspension. The AFM images (Fig. 4b.2) highlight the presence of nanometric cellulose fibril, which is also revealed by TEM images (Fig. 4b.3) showing individualised nanofibrils together with some aggregates. The mean width of the observed nanofibrils of cellulose from Posidonia balls was between 3 and 11 nm (4 and 13 nm from Posidonia leaves). These results are comparable with previous studies results (see Table 5).
Table 6. Quality Indexes for the Different CNF Obtained in this Work Compared to Data from the Literature
Quality index
To compare the produced CNF in a more thorough way, the quality indexes were assessed. The obtained data are summarized in Table 6, which also includes data from previously published works, for comparison purposes.
Table 6 shows that the quality indexes (QI*) of the CNF produced in this work were in line with data from the literature, independently of the process and the applied pretreatment, except for non-oxidized steam exploded fibres, which presented, as expected, lower values of QI*. For the latter, the quality indexes were comprised between 52 and 57 for Posidonia balls and leaves, respectively. These values were lower than that encountered for gel-like suspensions obtained by grinding of enzymatically treated fibres, generally higher than 65 (Desmaisons et al. 2017).
Compared to previous studies, the four measured parameters were close, apart from turbidity that seemed to be higher than that reported for TEMPO-oxidized wood, i.e., 50 NTU. This can be explained by the heterogeneity of suspensions, due to the presence of residual fibres in a non-negligible quantity in the prepared samples, which may negatively impact the mechanical properties of nanopapers. Indeed, Young’s moduli do not exceed 12 GPa, and it is important to mention that the values were relatively low for SE-TRFOB and SE-TRFOL (where steam explosion was used as the main microfibrillation process). Probably for exploded samples, some failure zones were created during nanopaper preparation, due to the presence of more residual fibres. Therefore, the existence of these coarse elements limits the mechanical properties, but it is still important.
In contrast, the microparticle sizes and nanosized fractions evolved in a classical way depending on the treatment intensity.
CONCLUSIONS
- During this work, cellulose nanofibril (CNF) suspensions were successfully produced from a marine biomass waste, namely Posidonia balls and leaves, after delignification and bleaching, by combining TEMPO-oxidation as a chemical pretreatment with the ultra-fine grinding or steam explosion.
- The obtained CNF was extensively characterized using complementary methods. Steam explosion made it possible to produce CNF with morphological and mechanical properties comparable to those conventionally produced by grinding.
- Promising results were obtained when steam explosion was applied to non-oxidized fibres. Indeed, considering the mild conditions used in this work (170 °C, 30 s), an improvement in the quality of the gel-like suspensions may be expected by applying a more severe steam explosion.
- Finally, this work confirms that Posidonia oceanica could be promising alternative biomass for manufacturing CNF products in the future.
ACKNOWLEDGMENTS
This work was financially supported by the “PHC Utique” program of the French Ministry of Foreign Affairs and Ministry of Higher Education, Research and Innovation and the Tunisian Ministry of Higher Education and Scientific Research in the CMCU (project number TN 18G1132// FR 39316VF). The LGP2 is part of the LabEx Tec 21 (Investissements d’Avenir – grant agreement n°ANR-11-LABX-0030) and of PolyNat Carnot Institute (Investissements d’Avenir – grant agreement n° ANR-16-CARN-0025-01). This research was made possible thanks to the facilities of the TekLiCell platform funded by the Région Rhône-Alpes (ERDF: European Regional Development Fund).
In addition, the authors would like to thank Thierry Encinas from CMTC – Grenoble, France for the XRD analysis and Christine Lancelon-Pin from CERMAV Grenoble, France for the TEM observations.
REFERENCES CITED
Abe, K., Iwamoto, S., and Yano, H. (2007). “Obtaining cellulose nanofibers with a uniform width of 15 nm from wood,” Biomacromolecules 8(10), 3276-3278. DOI: 10.1021/bm700624p.
Abou-Zeid, R. E., Kamal, K. H., Abd El-Aziz, M. E., Morsi, S. M., and Kamel, S. (2020). “Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions,” International Journal of Biological Macromolecules. DOI: 10.1016/j.ijbiomac.2020.11.063.
Abouzeid, R. E., Khiari, R., Beneventi, D., and Dufresne, A. (2018). “Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering,” Biomacromolecules 19(11), 4442-4452. DOI: 10.1021/acs.biomac.8b01325.
Abraham, E., Deepa, B., Pothan, L. A., Jacob, M., Thomas, S., Cvelbar, U., and Anandjiwala, R. (2011). “Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach,” Carbohydrate Polymers 86(4), 1468-1475. DOI: 10.1016/j.carbpol.2011.06.034
Abrantes, S., Amaral, M. E., Costa, A, P., and Duarte, A. P. (2007). “Cynara cardunculus L. alkaline pulps: Alternatives fibres for paper and paperboard production,” Bioresource Technology 98(15), 2873-2878. DOI: 10.1016/j.biortech.2006.09.052.
Afra, E., Yousefi, H., Hadilam, M. M., and Nishino, T. (2013). “Comparative effect of mechanical beating and nanofibrillation of cellulose on paper properties made from bagasse and softwood pulps,” Carbohydrate Polymers 97(2), 725-730. DOI: 10.1016/j.carbpol.2013.05.032.
Agblevor, F. A., Ibrahim, M. M., and El-Zawawy, W. K. (2007). “Coupled acid and enzyme mediated production of microcrystalline cellulose from corn cob and cotton gin waste,” Cellulose 14(3), 247-256. DOI: 10.1007/s10570-006-9103-y.
Aguir, C., and M’Henni, M. F. (2006). “Experimental study on carboxymethylation of cellulose extracted from Posidonia oceanica,” Journal of Applied Polymer Science 99(4), 1808-1816. DOI: 10.1002/app.22713.
Al-Ahmed, Z. A., Hassan, A. A., El-Khouly, S. M., and El-Shafey, S, E. (2020). “TEMPO-oxidized cellulose nanofibers/TiO2 nanocomposite as new adsorbent for Brilliant Blue dye removal,” Polym. Bull. 77(12), 6213-6226. DOI: 10.1007/s00289-019-03068-4.
Alemdar, A., and Sain, M. (2008). “Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls,” Bioresource Technology 99(6), 1664-1671. DOI: 10.1016/j.biortech.2007.04.029.
Alila, S., Besbes, I., Vilar, M. R., Mutjé, P., and Boufi, S. (2013). “Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study,” Industrial Crops and Products 41, 250-259. DOI: 10.1016/j.indcrop.2012.04.028.
Alves, L., Ferraz, E., Lourenço, A. F., Ferreira, P. J., Rasteiro, M. G., and Gamelas, J. A. F. (2020). “Tuning rheology and aggregation behaviour of TEMPO-oxidised cellulose nanofibrils aqueous suspensions by addition of different acids,” Carbohydrate Polymers 237, article no. 116109. DOI: 10.1016/j.carbpol.2020.116109.
Antunes, A., Amaral, E., and Belgacem, M. N. (2000). “Cynara cardunculus L.: Chemical composition and soda-anthraquinone cooking,” Industrial Crops and Products 12(2), 85-91. DOI: 10.1016/S0926-6690(00)00040-6.
Babcock, L. W. (1932). “Method of producing fermentable sugars and alcohol from wood,” US Patent 1,855,464A.
Bakkari, M. E., Bindiganavile, V., Goncalves, J., and Boluk, Y. (2019). “Preparation of cellulose nanofibers by TEMPO-oxidation of bleached chemi-thermomechanical pulp for cement applications,” Carbohydrate Polymers 203, 238-245. DOI: 10.1016/j.carbpol.2018.09.036
Bansal, M., Chauhan, G. S., Kaushik, A., and Sharma, A. (2016). “Extraction and functionalization of bagasse cellulose nanofibres to Schiff-base based antimicrobial membranes,” International Journal of Biological Macromolecules 91, 887-894. DOI: 10.1016/j.ijbiomac.2016.06.045.
Belgacem, M. N., and Gandini, A. (2011). Monomers, Polymers and Composites from Renewable Resources, Elsevier.
Benhamou, K., A. Dufresne, A., Magnin, A., Mortha, G., and Kaddami, H. (2014). “Control of size and viscoelastic properties of nanofibrillated cellulose from palm tree by varying the TEMPO-mediated oxidation time,” Carbohydrate Polymers 99, 74-83. DOI: 10.1016/j.carbpol.2013.08.032.
Besbes, I., Vilar, M. R., and Boufi, S. (2011). “Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: Preparation, characteristics and reinforcing potential,” Carbohydrate Polymers 86(3), 1198-1206. DOI: 10.1016/j.carbpol.2011.06.015.
Bettaieb, F., Khiari, R., Dufresne, A., Mhenni, M. F., Putaux, J. L., and Boufi, S. (2015). “Nanofibrillar cellulose from Posidonia oceanica: Properties and morphological features,” Industrial Crops and Products 72, 97-106. DOI: 10.1016/j.indcrop.2014.12.060.
Bhattacharya, D., Germinario, L. T., and Winter, W. T. (2008). “Isolation, preparation and characterization of cellulose microfibers obtained from bagasse,” Carbohydrate Polymers 73(3), 371-377. DOI: 10.1016/j.carbpol.2007.12.005.
Cao, X., Dong, H., and Li, C. M. (2007). “New nanocomposite materials reinforced with flax cellulose nanocrystals in waterborne polyurethane,” Biomacromolecules 8(3), 899-904. DOI: 10.1021/bm0610368.
Chakraborty, A., Sain, M., and Kortschot, M. (2005). “Cellulose microfibrils: A novel method of preparation using high shear refining and cryocrushing,” Holzforschung 59(1), 102-107. DOI: 10.1515/HF.2005.016.
Chen, Y., Geng, B., Ru, J., Tong, C., Liu, H., and Chen, J. (2017). “Comparative characteristics of TEMPO-oxidized cellulose nanofibers and resulting nanopapers from bamboo, softwood, and hardwood pulps,” Cellulose 24(11), 4831-4844. DOI: 10.1007/s10570-017-1478-4.
Cherian, B. M., Leão, A. L., de Souza, S. F., Thomas, S., Pothan, L. A., and Kottaisamy, M. (2010). “Isolation of nanocellulose from pineapple leaf fibres by steam explosion,” Carbohydrate Polymers 81(3), 720-725. DOI: 10.1016/j.carbpol.2010.03.046.
Cherian, B. M., Pothan, L. A., Nguyen-Chung, T., Mennig, G., Kottaisamy, M., and Thomas, S. (2008). “A novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization,” J. Agric. Food Chem. 56(14), 5617-5627. DOI: 10.1021/jf8003674.
Chirayil, C. J., Mathew, L., and Thomas, S. (2014). “Review of recent research in nano cellulose preparation from different lignocellulosic fibers,” Rev. Adv. Mater. Sci. 37, 20-28.
Deepa, B., Abraham, E., Cherian, B. M., Bismarck, A., Blaker, J. J., Pothan, L. A., Leao, A. L., de Souza, S. F., and Kottaisamy, M. (2011). “Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion,” Bioresource Technology 102(2), 1988-1997. DOI: 10.1016/j.biortech.2010.09.030.
Desmaisons, J., Boutonnet, E., Rueff, M., Dufresne, A., and Bras, J. (2017). “A new quality index for benchmarking of different cellulose nanofibrils,” Carbohydr. Polym. 174, 318-329.
Dinand, E., Chanzy, H., and Vignon, R. M. (1999). “Suspensions of cellulose microfibrils from sugar beet pulp,” Food Hydrocolloids 13(3), 275-283. DOI: 10.1016/S0268-005X(98)00084-8.
Diop, C. I. K., Lavoie, J.-M., and Huneault, M. A. (2015). “Structural changes of Salix miyabeana cellulose fibres during dilute-acid steam explosion: Impact of reaction temperature and retention time,” Carbohydrate Polymers 119, 8-17. DOI: 10.1016/j.carbpol.2014.11.031.
Douissa, N. B., Bergaoui, L., Mansouri, S., Khiari, R., and Mhenni, M. F. (2013). “Macroscopic and microscopic studies of methylene blue sorption onto extracted celluloses from Posidonia oceanica,” Industrial Crops and Products 45, 106-113. DOI: 10.1016/j.indcrop.2012.12.007.
Dufresne, A., Cavaillé, J.-Y., and Vignon, M. R. (1997). “Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils,” Journal of Applied Polymer Science 64(6), 1185-1194. DOI: 10.1002/(SICI)1097-4628(19970509)64:6<1185::AID-APP19>3.0.CO;2-V.
Dufresne, A., Dupeyre, D., and Vignon, M. R. (2000). “Cellulose microfibrils from potato tuber cells: Processing and characterization of starch–cellulose microfibril composites,” Journal of Applied Polymer Science 76(14), 2080-2092. DOI: 10.1002/(SICI)1097-4628(20000628)76:14<2080::AID-APP12>3.0.CO;2-U.
Dural, M. U., Cavas, L., Papageorgiou, S. K., and Katsaros, F. K. (2011). “Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: Kinetics and equilibrium studies,” Chemical Engineering Journal 168(1), 77-85. DOI: 10.1016/j.cej.2010.12.038.
Dutt, D., Upadhyaya, J. S., Tyagi, C. H., Kumar, A., and Lal, M. (2008). “Studies on Ipomea carnea and Cannabis sativa as an alternative pulp blend for softwood: An optimization of kraft delignification process,” Industrial Crops and Products 28(2), 128-136. DOI: 10.1016/j.indcrop.2008.02.001.
Fernández-Bolaños, J., Felizón, B., Heredia, A., Rodrı́guez, R., Guillén, R., and Jiménez, A. (2001). “Steam-explosion of olive stones: Hemicellulose solubilization and enhancement of enzymatic hydrolysis of cellulose,” Bioresource Technology 79(1), 53-61. DOI: 10.1016/S0960-8524(01)00015-3.
Fukuzumi, H., Saito, T., Iwata, T., Kumamoto, Y., and Isogai, A. (2009). “Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation,” Biomacromolecules 10(1), 162-165. DOI: 10.1021/bm801065u.
Grande, R., Trovatti, E., Pimenta, M. T. B., and Carvalho, A. J. F. (2018). “Microfibrillated cellulose from sugarcane bagasse as a biorefinery product for ethanol production,” Text. Tech Science Press. Accessed March 31, 2020. https://www-ingentaconnect-com.gaelnomade-2.grenet.fr/content/tsp/jrm/2018/00000006/00000002/art00008.
Guezguez, I., Dridi-Dhaouadi, S., and Mhenni, F. (2009). “Sorption of Yellow 59 on Posidonia oceanica, a non-conventional biosorbent: Comparison with activated carbons,” Industrial Crops and Products 29(1), 197-204. DOI: 10.1016/j.indcrop.2008.05.002.
Heinze, T., and Koschella, A. (2005). “Carboxymethyl ethers of cellulose and starch – A review,” Macromolecular Symposia 223(1), 13-40. DOI: 10.1002/masy.200550502.
Henriksson, M., Henriksson, G., Berglund, L. A., and Lindström, T. (2007). “An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers,” European Polymer Journal 43(8), 3434-3441. DOI: 10.1016/j.eurpolymj.2007.05.038.
Ho, T. T. T., Abe, K., Zimmermann, T., and Yano, H. (2015). “Nanofibrillation of pulp fibers by twin-screw extrusion,” Cellulose 22(1), 421-433. DOI: 10.1007/s10570-014-0518-6.
Hongrattanavichit, I., and Aht-Ong, D. (2020). “Nanofibrillation and characterization of sugarcane bagasse agro-waste using water-based steam explosion and high-pressure homogenization,” Journal of Cleaner Production 277, article no. 123471.
Isogai, A., Saito, T., and Fukuzumi, H. (2011). “TEMPO-oxidized cellulose nanofibers,” Nanoscale 3(1), 71-85. DOI: 10.1039/C0NR00583E.
Jiang, F., and Hsieh, Y.-L. (2013). “Chemically and mechanically isolated nanocellulose and their self-assembled structures,” Carbohydrate Polymers 95(1), 32-40. DOI: 10.1016/j.carbpol.2013.02.022.
Josset, S., Orsolini, P., Siqueira, G., Tejado, A., Tingaut, P., and Zimmermann, T. (2014). “Energy consumption of the nanofibrillation of bleached pulp, wheat straw and recycled newspaper through a grinding process,” Nordic Pulp & Paper Research Journal 29(1), 167-175. DOI: 10.3183/npprj-2014-29-01-p167-175.
Kaushik, A., and Singh, M. (2011). “Isolation and characterization of cellulose nanofibrils from wheat straw using steam explosion coupled with high shear homogenization,” Carbohydrate Research 346(1), 76-85. DOI: 10.1016/j.carres.2010.10.020.
Khawas, P., and Deka, S. C. (2016). “Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment,” Carbohydrate Polymers 137, 608-616. DOI: 10.1016/j.carbpol.2015.11.020.
Khiari, R., and Belgacem, M. N. (2017). “21 – Potential for using multiscale Posidonia oceanica waste: Current status and prospects in material science,” Lignocellulosic Fibre and Biomass-Based Composite Materials, Woodhead Publishing Series in Composites Science and Engineering, Woodhead Publishing, M. Jawaid, P. Md Tahir, and N. Saba (eds.), 447-471.
Khiari, R., and Belgacem, M. N. (2020). “Date palm nanofibres and composites,” Date Palm Fiber Composites: Processing, Properties and Applications, Composites Science and Technology, M. Midani, N. Saba, and O. Y. Alothman (eds.), Springer, Singapore, pp. 185-206.
Khiari, R., Marrakchi, Z., Belgacem, M. N., Mauret, E., and Mhenni, F. (2011). “New lignocellulosic fibres-reinforced composite materials: A stepforward in the valorisation of the Posidonia oceanica balls,” Composites Science and Technology 71(16), 1867-1872. DOI: 10.1016/j.compscitech.2011.08.022.
Khiari, R., Mhenni, M. F., Belgacem, M. N., and Mauret, E. (2010). “Chemical composition and pulping of date palm rachis and Posidonia oceanica – A comparison with other wood and non-wood fibre sources,” Bioresource Technology 101(2), 775-780. DOI: 10.1016/j.biortech.2009.08.079.
Klemm, D., Heublein, B., Fink, H.-P., and Bohn, A. (2005). “Cellulose: Fascinating biopolymer and sustainable raw material,” Angewandte Chemie International Edition 44(22), 3358-3393. DOI: 10.1002/anie.200460587.
Labidi, K., Cao, Z., Zrida, M., Murphy, A., Hamzaoui, A. H., and Devine, D. M. (2019). “Alfa fiber/polypropylene composites: Influence of fiber extraction method and chemical treatments,” Journal of Applied Polymer Science 136(18), article no. 47392. DOI: 10.1002/app.47392.
Levanič, J., Šenk, V. P., Nadrah, P., Poljanšek, I., Oven, P., and Haapala, A. (2020). “Analyzing TEMPO-oxidized cellulose fiber morphology: New insights into optimization of the oxidation process and nanocellulose dispersion quality,” ACS Sustainable Chem. Eng. 8(48), 17752-17762. DOI: 10.1021/acssuschemeng.0c05989.
Liu, D., Zhong, T., Chang, P. R., Li, K., and Wu, Q. (2010). “Starch composites reinforced by bamboo cellulosic crystals,” Bioresource Technology 101(7), 2529-2536. DOI: 10.1016/j.biortech.2009.11.058.
Liu, X., Sun, X.-J., Zhang, L., Qing, Y., Yan, N., Chen, J., and Wu, Y. (2019). “Obtaining nanofibers from lignocellulosic residues after bioethanol production,” Cellulose 26(6), 3725-3734. DOI: 10.1007/s10570-019-02333-z.
Lu, P., Liu, R., Liu, X., and Wu, M. (2018). “Preparation of self-supporting bagasse cellulose nanofibrils hydrogels induced by zinc ions,” Nanomaterials 8(10), 800. DOI: 10.3390/nano8100800.
Luo, H., Zhang, H., Yue, L., Pizzi, A., and Lu, X. (2018). “Effects of steam explosion on the characteristics of windmill palm fiber and its application to fiberboard,” Eur. J. Wood Prod. 76(2), 601-609. DOI: 10.1007/s00107-017-1259-7.
Manhas, N., Balasubramanian, K., Prajith, P., Rule, P., and Nimje, S. (2015). “PCL/PVA nanoencapsulated reinforcing fillers of steam exploded/autoclaved cellulose nanofibrils for tissue engineering applications,” RSC Advances 5(31), 23999-24008. DOI: 10.1039/C4RA17191H.
Marrakchi, Z., Khiari, R., Oueslati, H., Mauret, E., and Mhenni, F. (2011). “Pulping and papermaking properties of Tunisian alfa stems (Stipa tenacissima)—Effects of refining process,” Industrial Crops and Products 34(3), 1572-1582. DOI: 10.1016/j.indcrop.2011.05.022
Mason, W. H. (1926). “Process and apparatus for disintegration of wood and the like,” US Patent 1,578,609.
Michel, B., Bras, J., Dufresne, A., Heggset, E. B., and Syverud, K. (2020). “Production and mechanical characterisation of TEMPO-oxidised cellulose nanofibrils/β-cyclodextrin films and cryogels,” Molecules 25(10), article no. 2381. DOI: 10.3390/molecules25102381
Nair, S. S., Zhu, J. Y., Deng, Y., and Ragauskas, A. J. (2014). “Characterization of cellulose nanofibrillation by micro grinding,” J. Nanopart. Res. 16(4), article no. 2349. DOI: 10.1007/s11051-014-2349-7
Ncibi, M. C., Mahjoub, B., and Seffen, M. (2006). “Studies on the biosorption of textile dyes from aqueous solutions using Posidonia oceanica (L.) leaf sheath fibres,” Adsorption Science & Technology 24(6), 461-474. DOI: 10.1260/026361706780154383
Ncibi, M. C., Mahjoub, B., and Seffen, M. (2007). “Kinetic and equilibrium studies of methylene blue biosorption by Posidonia oceanica (L.) fibres,” Journal of Hazardous Materials 139(2), 280-285. DOI: 10.1016/j.jhazmat.2006.06.029
Nechyporchuk, O., Pignon, F., and Belgacem, M. N. (2015). “Morphological properties of nanofibrillated cellulose produced using wet grinding as an ultimate fibrillation process,” J. Mater. Sci. 50(2), 531-541. DOI: 10.1007/s10853-014-8609-1
Okita, Y., Saito, T., and Isogai, A. (2010). “Entire surface oxidation of various cellulose microfibrils by TEMPO-mediated oxidation,” Biomacromolecules 11(6), 1696-1700. American Chemical Society. https://doi.org/10.1021/bm100214b.
Pääkkö, M., Ankerfors, M., Kosonen, H., Nykänen, A., Ahola, S., Österberg, M., Ruokolainen, J., Laine, J., Larsson, P. T., Ikkala, O., and Lindström, T. (2007). “Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels,” Biomacromolecules 8(6), 1934-1941. DOI: 10.1021/bm061215p.
Phinichka, N., and Kaenthong, S. (2018). “Regenerated cellulose from high alpha cellulose pulp of steam-exploded sugarcane bagasse,” Journal of Materials Research and Technology 7(1), 55-65. DOI: 10.1016/j.jmrt.2017.04.003.
Puangsin, B., Fujisawa, S., Kuramae, R., Saito, T., and Isogai, A. (2012). “TEMPO-mediated oxidation of hemp bast holocellulose to prepare cellulose nanofibrils dispersed in water,” J. Polym. Environ. 21(2), 555-563.
Qin, S., Wang, Y., Xing, Y., Zhao, P., Bu, L., Sun, D., and Jiang, J. (2015). “Comparison of enzymatic hydrolysis of bamboo using steam explosion and acid sulfite, alkali, and alkaline sulfite pretreatments,” BioResources 10(4), 7580-7590.
Rammou, E., Mitani, A., Ntalos, G., Koutsianitis, D., Taghiyari, H. R., and Papadopoulos, A. N. (2021). “The potential use of seaweed (Posidonia oceanica) as an alternative lignocellulosic raw material for wood composites manufacture,” Coatings 11(1), 69. DOI: 10.3390/coatings11010069.
Rol, F., Karakashov, B., Nechyporchuk, O., Terrien, M., Meyer, V., Dufresne, A., Belgacem, M. N., and Bras, J. (2017). “Pilot-scale twin screw extrusion and chemical pretreatment as an energy-efficient method for the production of nanofibrillated cellulose at high solid content,” ACS Sustainable Chem. Eng. 5(8), 6524-6531. DOI: 10.1021/acssuschemeng.7b00630.
Rol, F., Banvillet, G., Meyer, V., Petit-Conil, M., and Bras, J. (2018). “Combination of twin-screw extruder and homogenizer to produce high-quality nanofibrillated cellulose with low energy consumption,” J. Mater. Sci. 53(17), 12604-12615. DOI: 10.1007/s10853-018-2414-1.
Rol, F., Saini, S., Meyer, V., Petit-Conil, M., and Bras, J. (2019). “Production of cationic nanofibrils of cellulose by twin-screw extrusion,” Industrial Crops and Products 137, 81-88. DOI: 10.1016/j.indcrop.2019.04.031.
Rol, F., Rouilly, A., and Bras, J. (2020). “Thermo-compression of cellulose nanofibrils,” Cellulose 27(1), 25-40. DOI: 10.1007/s10570-019-02720-6.
Saelee, K., Yingkamhaeng, N., Nimchua, T., and Sukyai, P. (2016). “An environmentally friendly xylanase-assisted pretreatment for cellulose nanofibrils isolation from sugarcane bagasse by high-pressure homogenization,” Industrial Crops and Products 82, 149-160. DOI: 10.1016/j.indcrop.2015.11.064.
Saini, S., Quinot, D., Lavoine, N., Belgacem, M. N., and Bras, J. (2016). “β-Cyclo-dextrin-grafted TEMPO-oxidized cellulose nanofibers for sustained release of essential oil,” Journal of Materials Science 7(52), 3849-3861. DOI: 10.1007/s10853-016-0644-7.
Saito, T., Nishiyama, Y., Putaux, J.-L., Vignon, M., and Isogai, A. (2006). “Homo-geneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose,” Biomacromolecules 7(6), 1687-1691. DOI: 10.1021/bm060154s.
Salvadó, J., Velásquez, J. A., and Ferrando, F. (2003). “Binderless fiberboard from steam exploded Miscanthus sinensis: Optimization of pressing and pretreatment conditions,” Wood Sci. Technol. 37(3), 279-286. DOI: 10.1007/s00226-003-0186-4.
Shogren, R. L., Peterson, S. C., Evans, K. O., and Kenar, J. A. (2011). “Preparation and characterization of cellulose gels from corn cobs,” Carbohydrate Polymers 86(3), 1351-1357. DOI: 10.1016/j.carbpol.2011.06.035.
Siqueira, G., Tapin-Lingua, S., Bras, J., da Silva Perez, D., and Dufresne, A. (2010). “Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers,” Cellulose 17(6), 1147-1158. DOI: 10.1007/s10570-010-9449-z.
Siqueira, P., Siqueira, É., De Lima, A. E., Siqueira, G., Pinzón-Garcia, A. D., Lopes, A. P., Segura, M. E. C., Isaac, A., Pereira, F. V., and Botaro, V. R. (2019). “Three-dimensional stable alginate-nanocellulose gels for biomedical applications: towards tunable mechanical properties and cell growing,” Nanomaterials 9(1), 78. DOI: 10.3390/nano9010078.
Sirviö, J. A., Visanko, M., and Liimatainen, H. (2015). “Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose,” Green Chem. 17(6), 3401-3406. DOI: 10.1039/C5GC00398A.
Solikhin, A., Hadi, Y. S., Massijaya, M. Y., and Nikmatin, S. (2019). “Production of microfibrillated cellulose by novel continuous steam explosion assisted chemo-mechanical methods and its characterizations,” Waste Biomass Valor. 10(2), 275-286. DOI: 10.1007/s12649-017-0066-z.
Spence, K. L., Venditti, R. A., Rojas, O. J., Habibi, Y., and Pawlak, J. J. (2011). “A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods,” Cellulose 18(4), 1097-1111. DOI: 10.1007/s10570-011-9533-z.
Sun, X. F., Xu, F., Sun, R. C., Geng, Z. C., Fowler, P., and Baird, M. S. (2005). “Characteristics of degraded hemicellulosic polymers obtained from steam exploded wheat straw,” Carbohydrate Polymers 60(1), 15-26. DOI: 10.1016/j.carbpol.2004.11.012.
Tanpichai, S., Witayakran, S., and Boonmahitthisud, A. (2019). “Study on structural and thermal properties of cellulose microfibers isolated from pineapple leaves using steam explosion,” Journal of Environmental Chemical Engineering 7(1), article no. 102836. DOI: 10.1016/j.jece.2018.102836.
Tarrés, Q., Boufi, S., Mutjé, P., and Delgado-Aguilar, M. (2017). “Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibers for the production of nanopapers: Morphological, optical, thermal and mechanical properties,” Cellulose 24(9), 3943-3954. DOI: 10.1007/s10570-017-1394-7.
Valdebenito, F., Pereira, M., Ciudad, G., Azocar, L., Briones, R., and Chinga-Carrasco, G. (2017). “On the nanofibrillation of corn husks and oat hulls fibres,” Industrial Crops and Products 95, 528-534. DOI: 10.1016/j.indcrop.2016.11.006.
Villar, J. C., Revilla, E., Gómez, N., Carbajo, J. M., and Simón, J. L. (2009). “Improving the use of kenaf for kraft pulping by using mixtures of bast and core fibers,” Industrial Crops and Products 29(2), 301-307. DOI: 10.1016/j.indcrop.2008.06.002.
Yamashiki, T., Matsui, T., Saitoh, M., Okajima, K., Kamide, K., and Sawada, T. (1990). “Characterisation of cellulose treated by the steam explosion method. Part 1: Influence of cellulose resources on changes in morphology, degree of polymerisation, solubility and solid structure,” British Polymer Journal 22(1), 73-83. DOI: 10.1002/pi.4980220111.
Yuan, B. Z., Han, G. T., Pan, Y., and Zhang, Y. M. (2013). “The effect of steam explosion treatment on the separation of lotus fiber,” Advanced Materials Research 750-752, 2307-2312. DOI: 10.4028/www.scientific.net/AMR.750-752.2307.
Zimmermann, T., Pöhler, E., and Geiger, T. (2004). “Cellulose fibrils for polymer reinforcement,” Advanced Engineering Materials 6(9), 754-761. DOI: 10.1002/adem.200400097.
Zuluaga, R., Putaux, J. L., Cruz, J., Vélez, J., Mondragon, I., and Gañán, P. (2009). “Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features,” Carbohydrate Polymers 76(1), 51-59. DOI: 10.1016/j.carbpol.2008.09.024.
Article submitted: December 22, 2021; Peer review completed: March 20, 2022; Revised version received and accepted: March 29, 2022; Published: April 6, 2022.
DOI: 10.15376/biores.17.2.2933-2958
