Abstract
The presence of lignin sheaths and the crystalline structure of cellulose are two key barriers in the development of corn stover (CS). To remove these two barriers and improve the digestibility of carbohydrates in CS, CS (pretreated by H2O2, H2SO4, NaOH, an enzyme, steam explosion [SE], and SE combined with NaOH [SE-NaOH]) was hydrolyzed. The total reducing sugar yield (Ytrs), accessibility of the enzyme to the substrate (Kobs,0), and gradual loss of the enzyme activity (Ki) were compared by regression analysis of the kinetic model, scanning electron microscopy, Fourier transform infrared spectroscopy, and X-ray diffraction analysis. The pretreatment dramatically increased the Ytrs and Kobs,0, and remarkably decreased the Ki. The maximum increase in the Ytrs (106.57%) was obtained after the saccharification reaction of the CS pretreated by SE-NaOH. Physicochemical characterizations of the CS pretreated by SE-NaOH showed that the SE-NaOH pretreatment effectively reduced the lignin sheath, decreased cellulose crystallization, and created favorable conditions for enzymatic diffusion and penetration.
Download PDF
Full Article
Combination Pretreatment of Steam Explosion and NaOH Enhances Enzymatic Saccharification of Corn Stover
Lu Liu,a Zhicai Zhang,b,c,* Jia Wang,a Quanshan Sun,a Wenjing Shi,a and Xiaocui Liu a
The presence of lignin sheaths and the crystalline structure of cellulose are two key barriers in the development of corn stover (CS). To remove these two barriers and improve the digestibility of carbohydrates in CS, CS (pretreated by H2O2, H2SO4, NaOH, an enzyme, steam explosion [SE], and SE combined with NaOH [SE-NaOH]) was hydrolyzed. The total reducing sugar yield (Ytrs), accessibility of the enzyme to the substrate (Kobs,0), and gradual loss of the enzyme activity (Ki) were compared by regression analysis of the kinetic model, scanning electron microscopy, Fourier transform infrared spectroscopy, and X-ray diffraction analysis. The pretreatment dramatically increased the Ytrs and Kobs,0, and remarkably decreased the Ki. The maximum increase in the Ytrs (106.57%) was obtained after the saccharification reaction of the CS pretreated by SE-NaOH. Physicochemical characterizations of the CS pretreated by SE-NaOH showed that the SE-NaOH pretreatment effectively reduced the lignin sheath, decreased cellulose crystallization, and created favorable conditions for enzymatic diffusion and penetration.
Keywords: Corn stover; Pretreatment; Hydrolysis; Kinetic
Contact information: a: School of Food Science and Biotechnology, Jiangsu University, Zhenjiang 212013, P. R. China; b: Institute of Agro-production Processing Engineering, Jiangsu University, Zhenjiang 212013, P. R. China; c: Zhenjiang Yemaikang Food Bio-biotechnology Co., Ltd., Zhenjiang 212013, P. R. China; *Corresponding author: zhangzhicai@ujs.edu.cn
INTRODUCTION
Corn stover (CS) is one of the most abundant agricultural residues in China. Bioconversion of CS to biofuel can alleviate the energy crisis caused by the depletion of fossil energy and reduce environmental pollution caused by the combustion of fossil energy (Yang et al. 2015; Chang et al. 2016). The bioconversion process of lignocellulose includes four steps: pretreatment of the CS, saccharification of the cellulose, fermentation of the saccharification products, and biorefining of the biofuel. As a critical processing step for bioethanol and bio-based material production, pretreatment results in a high sugar yield via the bioconversion route (Zhao et al. 2016). The primary objective of pretreatment is to reduce the impeding effects of the lignin and hemicellulose, destroy the cellulose crystallinity, decrease the degree of polymerization (DP) of the cellulose, and increase the accessible surface area (Sun et al. 2015).
Numerous approaches, including physical, chemical, physicochemical, and biological pretreatments, have been applied to pretreat lignocellulose (Alvira et al. 2010). Long-term research has been conducted on the enzymatic pretreatment strategy of CS and a complete microbial pretreatment system has been developed (Fan et al. 2019). The saccharification reaction of cellulose in pretreated CS is a dynamic reaction process. To explore its behavioral mechanism (dynamic model mechanism), it is necessary to investigate the factors and laws affecting the efficiency of enzymatic hydrolysis.
It has been reported that the total reducing sugar yield (Ytrs) is closely related to the changes in the compositions and morphologies of the lignocellulose after pretreatment, which was determined through scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy (Li et al. 2016). The pretreatment of lignocellulose can increase the Ytrs by depolymerizing the lignin and hemicellulose fractions and disrupting the crystalline structure of the cellulose (Behera et al. 2014).
Li et al. (2016) has reported that sulfuric acid pretreatment can result in achieving a maximum glucose yield (94.2%), and a distinct change in the composition and crystal texture of the solid residuals was found by XRD and SEM analyses. Some studies have shown with SEM micrographs and XRD crystallinity measurements that the increase in the accessible surface area of cellulose is more important than the removal of lignin when the goal is to achieve high sugar yields (Rollin et al. 2011). Fourier transform infrared spectroscopy has been used to characteristically assess the structural changes in cellulose and lignin before and after pretreatment (Ma et al. 2015). Physical treatment is more effective than typical chemical methods at decomposing the crystal structure of CS (Chang et al. 2012). Chemical treatment can effectively degrade lignin. However, microbial fermentation and enzymatic hydrolysis are also more efficient at converting CS into reducing sugars and low-molecular weight carbohydrates compared with physicochemical pretreatment (Huang et al. 2017).
To further enhance the pretreatment efficiency, it is necessary to compare the mechanical differences of these methods using a kinetic model. This is because the hydrolysis of cellulose is done in a heterogeneous reaction system and the reaction rate constant is affected by many factors, including diffusion, mass transfer, adsorption, transfer, desorption, and enzyme denaturation of the reactant molecules, product molecules, and catalyst surface (Bansal et al. 2009). Therefore, the conventional Michaelis-Menten equation cannot be used to describe the hydrolysis reaction of cellulose and the model needs to be modified to describe the enzymatic reaction in heterogeneous systems (Yang and Fang 2015).
Kopelman (1988) stated that the kinetics of heterogeneous reactions do not follow the classic kinetic model. A heterogeneous reaction system includes the following characteristics: 1) the rate constant changes with time; 2) the rate constant is related to the reaction order and spectrum dimension; and 3) the kinetics of the enzymatic reaction belong to the fractal dynamic model. Väljamäe et al. (2003) used fractal-like kinetics to study the hydrolysis process of Trichoderma reesei and suggested that fractal-like kinetics are suitable for the enzymatic hydrolysis of cellulose. Huang (1975) studied the hydrolysis of substrates at various concentrations with different concentrations of insoluble enzymes, proposed a kinetic model for rapid adsorption, and discussed methods for determining the kinetic parameters. Zhang et al. (2017) applied the impeded Michaelis model (IMM) to fit the enzymatic hydrolysis of cellulose.
In the present study, the IMM was further used to fit the kinetic process of cellulose hydrolysis from CS pretreated by physicochemical and biological methods and to determine the mechanical differences between these methods.
EXPERIMENTAL
Materials
Corn stover was purchased from a local farm (Henan, China), dried at 105 °C to a constant weight, ground into a fine powder, and sieved through a 0.25-mm sieve. The ground CS powder consisted of 26.2% hemicellulose, 32.1% cellulose, and 15.4% lignin. A commercial T. reesei cellulase was supplied by Ningxia NCM Biotechnology Ltd. (Ningxia, China). The activities of the carboxymethyl-cellulase, filter paper enzyme, and β-glucosidase were 6.11 × 104 U/mL, 844 FPU/mL, and 10.9 U/mL, respectively. All of the chemicals were of standard analytical grade and used as received without further purification.
Pretreatment
Acid, H2O2, alkaline, and enzyme pretreatments
The CS was pretreated with sulfuric acid, H2O2, NaOH, and lignin peroxidase according to previously described methods (Chen et al. 2009; Tai and Keshwani 2014; Ramadoss and Muthukumar 2015; Zhang et al. 2015). After the CS was washed, the pretreated CS was dried at 80 °C to a constant weight and preserved at room temperature.
Steam explosion pretreatment
The dried CS was rapidly heated by high-pressure steam in the absence of any chemicals. The pressure inside of the digester was 1.5 MPa and the steam mixture was held for 400 s. The effects of the operating parameters during steam explosion (SE), such as the pressure (0.6 MPa, 0.8 MPa, 1.2 MPa, 1.5 MPa, and 2 MPa) and reaction time (200 s, 400 s, and 600 s), were investigated.
SE-NaOH pretreatment
The dried CS pretreated by SE under the optimum conditions was washed, dried at 80 °C, and then treated with NaOH (SE-NaOH). The pretreated biomass was washed with distilled water until a neutral pH was achieved. Subsequently, the CS was dried at 80 °C to a constant weight and preserved at room temperature.
Enzymatic Saccharification
All of the saccharification tests of the pretreated CS were conducted in 100-mL Erlenmeyer flasks. Briefly, 1 g of pretreated CS was added to 20 mL of 0.1 M acetate buffer containing 3% cellulase (pH = 4.4). After the mixture was fully blended, the initial concentration of the reducing sugar was denoted as C0. The saccharification reaction was conducted at 47 °C in a water shaking bath (160 rpm) for 32 h. Subsequently, samples were taken at 1 h, 2 h, 4 h, 8 h, 16 h, and 32 h. Each sample was immediately cooled in an ice bath to room temperature to terminate the reaction. The Ytrs in each flask was determined according to the 3,5-dinitrosalicylic acid method (Miller 1959) and denoted as C1. The Ytrs was calculated according to Eq. 1,
Ytrs (%) = (C1 – C0) × V/G × 100% (1)
where Ytrs is the total reducing sugar yield, V is the volume of the reaction solution (mL), G is the weight of the total dry substrate (g), and C0 and C1 are the reducing sugar concentrations (g/mL) at 0 h and t h of reaction, respectively.
IMM
According to the IMM (Yang and Fang 2015; Zhang et al. 2017), the pretreatment efficiency depends upon the Kobs,0 (h-1) and Ki (h-1). The variable Kobs,0 expresses the comprehensive action of the initial accessibility of cellulase to the substrate and the activity of the cellulase. The variable Kobs,0 was calculated as follows,
Kobs,0 = k2(E0) / Km (2)
where E0 is the initial enzyme concentration (mol/L), k2 is the rate constant from the enzyme-substrate complex to the product, and Km (mol/L) is the Michaelis constant.
The correlation between the Ytrs, reaction time (t, h), and Kobs,0 was as follows,
![]() (3)
(3)
where α is a constant, and Ki is the coefficient of the time-dependent inactive enzyme.
The coefficient Ki can be calculated according to the constant α and the following equation:
![]() (4)
(4)
Equation 3 can be solved to give:
![]() (5)
(5)
Equation 5 then can be rearranged as:
![]() (6)
(6)
Equation 6 was applied to fit the experimental results by plotting −t/n(1 – Ytrs) versus t. The coefficient of the t and constant term are α/Kobs,0 and 1/Kobs,0, respectively. The value for Ki was calculated with Eq. 4. All of the determinations were repeated at least three times, and the results were presented as the means with the standard errors. The regressions and analysis of variance (ANOVA) were performed using SPSS 17.0 software (SPSS Company, Chicago, USA).
Physical and Chemical Characterizations of the CS
SEM analysis
Photographs of the samples with different pretreatments were acquired by SEM analysis. Before observation, the samples were passed through an 80-mesh screen sieve and then pasted on the side of the glass using a double-sided adhesive. The tissues were randomly selected for image testing at different magnifications, such as 500 and 2000 times.
FTIR analysis
To analyze the changes in the chemical components of the differently pretreated CS, the FTIR spectra of the CS were obtained by a Nicolet iS50 spectrometer (Massachusetts, USA). The samples were passed through a 100-mesh screen sieve. The FTIR spectra were generated on the FTIR spectrometer using KBr translucent disks containing 1% sample in the absorbance mode. The data was recorded within the region of 4000 cm-1 to 400 cm-1 at a resolution of 4 cm-1 with 128 scans (Jiang et al. 2015).
XRD analysis
The crystallinity index (CrI) has been used to describe the relative amount of crystalline material in cellulose. CrI was analyzed by XRD analysis (D8 ADVANCE, Karlsruhe, Germany) with Cu Kα radiation (λ = 1.54 nm) and operated at 30 kV. The samples were passed through a 100-mesh screen sieve, and the scattering angle ranged from 10° to 40° with a scan rate of 2°/min and step size of 0.02°.
The CrI was calculated according to the method developed by Segal et al. (1959),
CrI (%) = (I002 – Iam) / I002 × 100% (7)
where I002 is the intensity of the crystalline portion in the biomass (cellulose), and Iam is the intensity of the amorphous portion (such as the cellulose, hemicellulose, and lignin).
RESULTS AND DISCUSSION
The IMM contains two parameters, Kobs,0 and Ki, which are calculated with the α. The variable Kobs,0 is considered to be the initial activity of the cellulase and accessibility of cellulase to the substrate, and Ki shows the loss and inactivation of the enzymatic activity in a heterogeneous system caused by the inhibitory reaction of inert and non-reactive substances. The IMM can clearly reflect the effects of the structural characteristics and residual lignin on saccharification of cellulase in pretreated CS. In the present study, the relationship between the Ytrs and structural characteristics of the pretreated CS with various methods was determined by comparing the accessibility of cellulase to the substrate and enzymatic loss in the process of cellulose hydrolysis. Therefore, IMM analysis was introduced in the present study.
Effect of the SE Pressure and Maintenance Time on the Ytrs
To determine the effectiveness of the SE pretreatment, the saccharification results of the CS pretreated with SE under different conditions are shown in Table 1. The Ytrs at the different time points showed a positive correlation with the pressure and maintenance time in the SE process. The Kobs,0, Ki, and ANOVA results were fitted by Eq. 6 (Table 1).
Table 1. Ytrs of the CS Pretreated with SE under Various Conditions after Saccharification
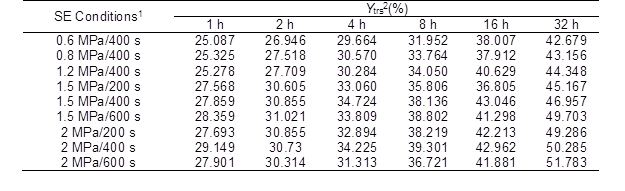
1: Pressure/maintenance time of SE; and 2: Ytrs is the total reducing sugar yield at 32 h
Table 2 shows that the R2 values of the data for all of the conditions were higher than 0.968 and were close to the adjusted R2 values (p < 0.001, and F-value > 100). These results indicated that the IMM analysis could fit the experimental results well and it possessed a high interpretation rate and reliability.
The Ytrs at 32 h and Kobs,0 in Table 1 increased from 426.8 mg/g to the maximum value of 453.5 mg/g and from 0.242 h-1 to the maximum value of 0.330 h-1, respectively, and Ki was not significantly changed when the pressure increased from 0.6 MPa to 1.5 MPa. The significant change in the Kobs,0 and insignificant change in the Ki indicated that more of the internal cellulose was exposed on the surface because of the SE process, and destruction of the crystalline structure of the cellulose decreased the DP of the cellulose and increased the accessible surface area, which led to an increased enzyme accessibility. Li and Chen (2014), Zhao and Chen (2013), and Kojiro et al. (2010) have shown that in the SE process, the saturated steam can penetrate the pores of CS and tear all of the pores in a rapid decompression process, and the exposure of cellulose breaks the straw organization, which results in a huge hole and the exposure of most fiber bundle sheaths. These conclusions supported the findings of the current study. At the same pressure, an increase in the SE maintenance time had a minor effect on the Ki; thus, the SE pretreatment was conducted under the conditions of 1.5 MPa and 400 s.
Table 2. Regressive Analysis of the Ytrs Obtained from the CS Pretreated by SE under Various Conditions versus Time after Saccharification
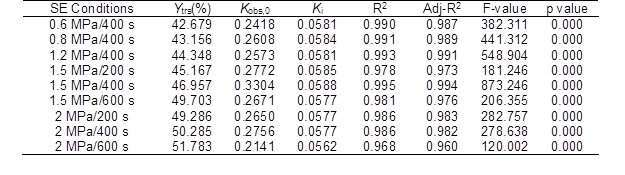
Adj-R2 – adjusted R2
To analyze the effects of the pressure and time on the SE process, the Ytrs at 32 h was used as the dependent variable, the pressure (P) and time (t) were used as the independent variables, and Eq. 8 was developed,
![]()
where α and β are the coefficients of the eP and t, respectively, and γ is a constant.
The model fitting F-value (80.078) and p value (0.000) in Table 3 showed that there was a correlation between the Ytrs and P, that t was extremely significant, and that the reliability of the analysis was extremely high. The R2 value was 0.964, which was higher than the R2 value of 0.80 (Biswas et al. 2015). The adjusted R2 value (0.952) was close to the R2 value. The above-mentioned results suggested that the predictability and adaptability of this model was relatively reliable. The coefficients of the eP and t were significant (p < 0.01) (Table 3). Among them, the coefficients of the eP and t were greater than 0, which revealed that the Ytrs had a significant positive correlation with the P and t. Therefore, the small coefficient of t showed that the effect of the SE maintenance time on the Ytrs was small in the experimental scope. The eP value (13.976) showed that the Ytrs and SE pressure exponentially correlated, and that the effect of the pressure on the Ytrs was extensive. A higher pressure resulted in a higher Ytrs. Table 3. Regression Analysis of Each Item between the Ytrs, P, and T

F-value = 80.078, significance: p = 0.000, R2 = 0.964, and adj-R2 = 0.952
Comparison of the Various Pretreatment Methods
Table 4 shows the Ytrs of the CS pretreated with physiochemical or biological methods after saccharification. Compared with the untreated CS, the Ytrs of the pretreated CS after saccharification significantly increased. The maximum Ytrs obtained from the CS pretreated with SE-NaOH increased by 106.57%.
Table 4. Ytrs of the CS Pretreated with Various Methods after Saccharification
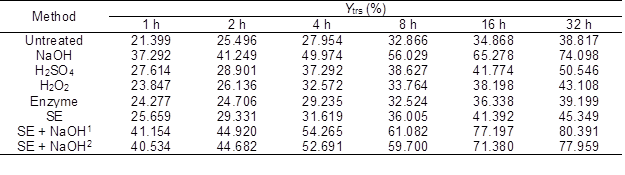
Enzyme: lignin peroxidase; SE + NaOH1: the pressure of SE was 1.5 MPa; and SE + NaOH2: the pressure of SE was 2.0 MPa
Table 5 indicates that the Kobs,0, Ki, and ANOVA results in Table 4 were fitted by Eq. 6. The R2 values for all of the data were higher than 0.936 and were close to the adjusted R2 values (p < 0.01, F-value > 100). These results showed that the IMM analysis could fit the experimental results of the various pretreatment methods, which suggested a high interpretation rate and reliability. Moreover, there was a smaller difference in the Kobs,0 between the CS pretreated by H2SO4 and H2O2 and the untreated CS compared with that between the NaOH and SE-pretreated CS and the untreated CS. However, the difference in the Ki between the CS pretreated by H2SO4, H2O2, and NaOH and the untreated CS was larger than that between the CS pretreated with SE and enzyme and the untreated CS. These results were supported by previous studies (Xiao et al. 2014; Singh et al. 2015). The results further proved that SE could destroy the crystalline structure, decrease the DP of the cellulose, and increase the accessibility of cellulase to cellulose in the substrate. However, chemical pretreatment could decrease the loss and inactivation of the cellulase. The maximum Kobs,0 and minimum Ki were obtained from the saccharification reaction of the NaOH-pretreated CS. To further increase the Ytrs, the combination of the SE and NaOH pretreatments was used to pretreat the CS. The maximum Ytrs (803.91 mg/g) and Kobs,0 (0.3953 h-1) and the minimum Ki (0.0545 h-1) of the saccharification reaction were obtained from the CS pretreated under the conditions of 1.5 MPa and 400 s.
Table 5. Regressive Analysis of the Ytrs Obtained from the CS Pretreated with Various Methods versus Time after Saccharification
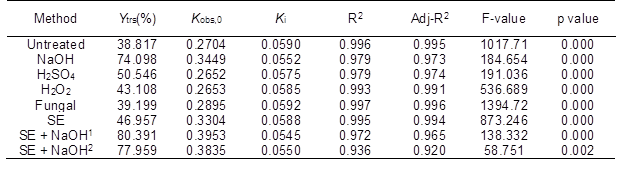
To further confirm their relationship, a linear regression analysis was conducted using Ytrs as the dependent variable, and Kobs,0 and Ki as the independent variables. The following equation was developed,
Ytrs = αKobs,0 + βKi + γ (9)
where α and β are the coefficients of the Kobs,0 and Ki, respectively, and γ is a constant.
Table 6. Regression Analysis between the Ytrs, Kobs,0, and Ki
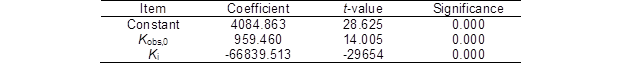
F-value = 1242.858, p = 0.000, R2 = 0.994, and adj-R2 = 0.993
Table 6 shows that the model fitting F-value and p value were 1242.858 and 0.000, respectively, which indicated that the correlation of the Ytrs with the Kobs,0 and Ki was significant, and the reliability of the analysis was high. The R2 value was 0.994, which was higher than the R2 value of 0.80 (Biswas et al. 2015). The adjusted R2 value (0.993) was close to the R2 value. These results suggested that the predictability and adaptability of this model were relatively more reliable. The coefficients for all of the parameters in Table 6 were significant (p = 0.000). Among them, the coefficient of the Kobs,0 was positive, and the coefficient of the Ki was negative; this indicated that the Ytrs significantly and positively correlated with the accessibility of the enzyme to the substrate (Kobs,0), while it significantly and negatively correlated with the gradual loss of the enzyme activity (Ki). Therefore, it was necessary to increase the accessibility for cellulase to the substrate and decrease the loss and inactivation of the enzyme to obtain the maximum saccharification efficiency. The same results obtained using a fractal model fitting empirical data of enzymatic saccharification of acid pretreated corn stover Wojtusik et al. (2016) and steam-exploded sugarcane bagasse (Aguiar et al. 2013).
SEM Analysis
Figure 1a shows that the untreated CS had smooth and well-ordered fibers, and the pores were irregularly distributed on the surface. These pores were believed to be the binding sites of cellulase. Lignin wraps cellulose and acts as a physical barrier, which hinders the accessibility of cellulase to cellulose.
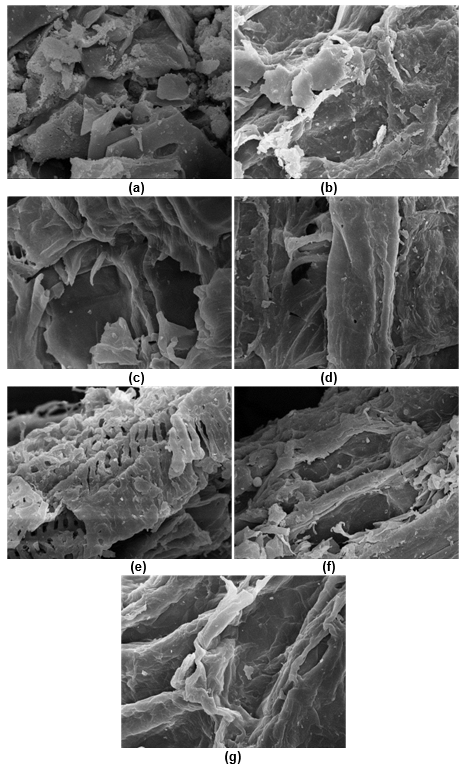
Fig. 1. Microscopic pictures of the various CS pretreatments: (a) untreated(5000×); (b) enzyme(2000×); (c) H2O2(2000×); (d) H2SO4(2000×); (e) NaOH(2000×); (f) SE(2000×); and (g) SE-NaOH(2000×)
Most studies have found that lignin negatively impacts biofuel production in the saccharification process (Zeng et al. 2014). Lignin causes irreversible adsorption on cellulase, which greatly reduces its effectiveness (Fang et al. 2015). Figure 1 reveals that fractures only occurred on the surface of the untreated CS, that it was not disrupted on a deep level, and that the cellulose-hemicellulose-lignin structure remained stubborn.
Figure 1b shows that the substrate of the lignin that had been exposed to peroxidase pretreatment had undulating rough fibers and more lignin remained on the surface. However, the chemical pretreatment had a greatly destructive effect on the CS structure (Figs. 1c to 1e). In the H2O2-pretreated CS, the vascular bundles of the CS swelled at high temperatures and were partially degraded (Fig. 1c). The lignin residue was almost invisible on the surface of the CS pretreated with H2SO4 (Fig. 1d) and NaOH (Fig. 1e). The vascular bundle was exposed, especially during the NaOH pretreatment (Fig. 1e). After treatment, many pores and hollow network structures were formed. Consequently, the modified biomass structure resulted in favorable conditions for increased accessibility of cellulase to cellulose.
Figure 1f shows that residual lignin and many destroyed vascular bundles were on the surface of the SE-pretreated CS. This could have been the reason why the saturated steam penetrated the CS pores, tore the vascular bundle during rapid decompression, caused huge voids, and exposed the cellulose cavity. The SE-NaOH-pretreated CS showed the best effect. The lignin was almost completely removed from the CS surface and vascular bundles could be observed in a strip-like arrangement. The above-mentioned structural characteristics were explained by the fact that the SE-NaOH-pretreated CS had a high accessibility and low enzyme adsorption rate after enzymatic hydrolysis. This could have been because of the role that chemical high-temperature cooking plays in the degradation of hemicellulose and lignin, as well as the softening and exposing of cellulose (Yang et al. 2017).
XRD Analysis
Figure 2 shows the XRD images of the raw materials and pretreated samples. The peak intensities at 2θ values of 16° (002) and 22.8° (101) in all of the pretreated CS samples increased compared with that of the raw material, and the CrI of the pretreated samples also increased compared with that of the raw material.
The increased peak intensity at a 2θ value of 22.8° indicated that the chemical pretreatment changed the crystalline structure of the crystallization zone into irregular and non-crystalline structures (Figs. 2d, 2e, and 2f). Moreover, only the chemically pretreated CS showed a diffraction peak of the (040) crystal surface at a 2θ value of 35°, which suggested that the chemical pretreatment greatly affected the crystalline structure of the cellulose.
The cellulose in lignocellulosic biomass can be divided into crystalline structures and amorphous structures (Perez-Pimienta et al. 2015). The content of amorphous cellulose is positively related with the CrI value. Table 7 shows that the CrI value of the cellulose in the untreated CS (18.1%) was lower than that in the other pretreated CS. The increase in the CrI implied that pretreatment led to the conversion from crystalline cellulose to amorphous cellulose. The crystalline structure of cellulose and the lignin content have been reported to be two key factors that impact the Ytrs of pretreated lignocellulosic biomass after saccharification.
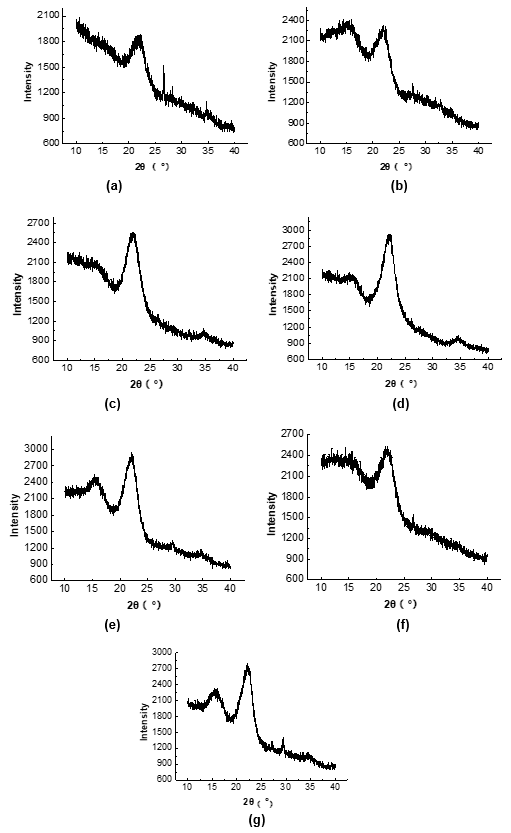
Fig. 2. XRD images of the (a) untreated; (b) enzyme; (c) H2O2; (d) H2SO4; (e) NaOH; (f) SE; and (g) SE-NaOH
To analyze the effects of the two factors on the Ytrs, non-linear regression analysis was performed using the Ytrs at 32 h in Table 4 as the dependent variable and the CrI and Clignin in Table 7 as the independent variables with Eq. 10,
![]()
where α and β are the coefficients of the e27×CrI and Clignin, respectively, Clignin is the content of the substrate (%), and γ is a constant.
Table 7. CrI and Lignin Content of the CS with Pretreated Various Methods
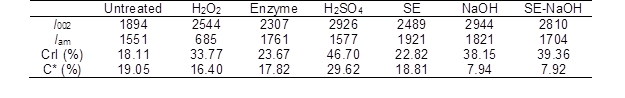
*: C is the lignin content of the pretreated CS
The F-value and p value were 42.709 and 0.002, respectively, which indicated that the correlation of the Ytrs with the CrI and Clignin was significant, and the reliability of the analysis was high (Table 8). The adjusted R2 value (0.913) was close to the R2 value (0.942), which showed that the model had a good adaptability.
The coefficients for all of the parameters in Table 8 were significant (p < 0.01). The coefficient of e27×CrI was positive, which showed that the Ytrs had a positive correlation with the CrI. The Ytrs exponentially increased with an increase in the CrI. The phenomenon may be related to recrystallization when cellulose dried into water and crystallized cellulose is left after amorphous cellulose dissolved into water in the pretreatment process. The coefficient of the Clignin was negative, which suggested that the Ytrs had a negative correlation with the Clignin. Therefore, less residual lignin resulted in a better enzymatic hydrolysis effect and a higher Ytrs. Consequently, it was speculated that the effect of the CrI on cellulose saccharification was greater than that of the residual lignin. Table 8. Regression Analysis of the Correlation between the Ytrs, CrI, and Clignin

F-value = 32.636, p = 0.003, R2 = 0.942, and adj-R2 = 0.913
FTIR Analysis
The FTIR analysis was performed to evaluate the changes in the functional groups of the cellulose before and after the different pretreatments. The peak at 3404 cm-1 refers to the stretching vibration of the -OH group. Figure 3 shows that after the pretreatment, the intensity of this peak decreased, which indicated that pretreatment could destroy some hydrogen bonds in the cellulose. This was consistent with the increased Ytrs after pretreatment. The peaks at 1165 cm-1 and 1059 cm-1 were assigned to the β-1,4-glycosidic bonds present in the cellulose, which is a strong signal hinting at a high cellulose composition (Muñoz et al. 2018). The intensities of the two peaks at 1165 cm-1 and 1059 cm-1 after the various pretreatments did not change much (Fig. 3), which indicated that the pretreatments did not destroy the primary structure of the cellulose.
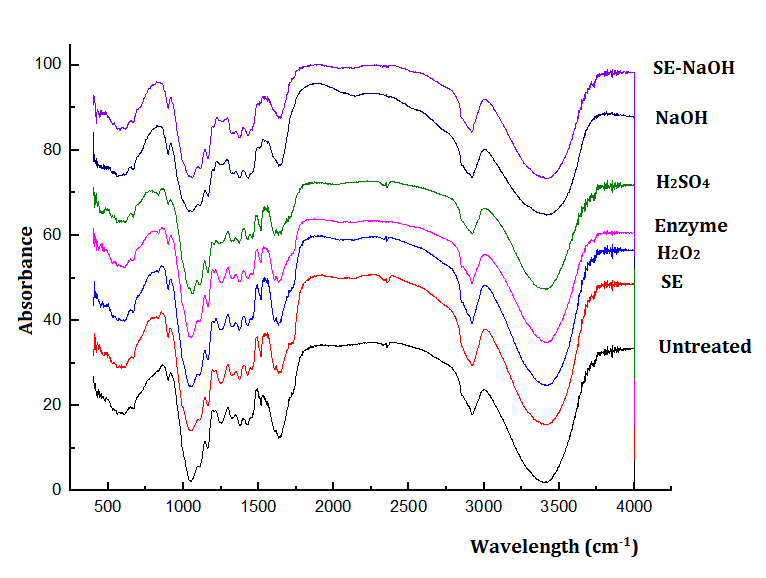
Fig. 3. FTIR spectra of the various pretreatments
The peak at 1731 cm-1 referred to the stretching vibration of the C=O group in the hemicellulose. After the NaOH and SE-NaOH pretreatments, the peak at 1731 cm-1 disappeared. The absence of a band at 1731 cm-1 could have been because of the removal of the C=O group (Kapoor et al. 2015), which indicated the removal of the acetyl and uronic ester groups from the hemicellulose. The peak intensities were changed after the other pretreatments. The peaks at 1515 cm-1 and 1650 cm-1 to 1515 cm-1 referred to the peaks of lignin aromatic ring vibration and aromatic ring C=C stretching in lignin. The disappearance of the peaks at 1515 cm-1 or 1650 cm-1 to 1515 cm-1 (Fig. 3) revealed that lignin was removed after chemical pretreatment, especially for the SE-NaOH pretreatment. According to the literature, phenolic lignin has more essential inhibitory effects compared with non-specific adsorption on cellulase (Xu et al. 2015). This was consistent with the increased availability of enzymes versus substrates, and the increased cellulase saccharification resulted in an increase in the Kobs,0 and Ytrs.
CONCLUSIONS
Corn stover was subjected to sequential steam explosion (SE) and NaOH pretreatment. The SE-NaOH pretreatment was found to be an effective method to improve the yield of total reducing sugars (Ytrs) by 106.6% after saccharification of the CS. Under the experimental conditions, the Ytrs and SE pressure had an exponential correlation and the pressure had a strong effect on the Ytrs. A higher pressure resulted in a higher Ytrs.
The chemical and SE pretreatments mainly increased the Ytrs by removing lignin and destroying the crystalline structure of the cellulose, respectively, according to the impeded Michaelis model (IMM), scanning electron microscopy (SEM), and X-ray diffraction (XRD) analyses. The Ytrs exponentially increased with an increase in the crystallinity index (CrI) and was negatively correlated with the Clignin.
The SE-NaOH pretreatment removed most of the lignin in the samples and destroyed the crystalline structure of the cellulose.
ACKNOWLEDGEMENTS
This work was financially supported by the China Postdoctoral Science Foundation (No. 2015M571691) and the National Natural Science Foundation of China (No. 31101269).
REFERENCES CITED
Aguiar, R. S., Silveira, M. H. L., Pitarelo, A. P., Corazza, M. L., and Ramos, L. P. (2013). “Kinetics of enzyme-catalyzed hydrolysis of steam-exploded sugarcane bagasse,” Bioresour. Technol. 147, 416-423. Doi:10.1016/j.biortech.2013.08.067
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresour. Technol. 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093
Bansal, P., Hall, M., Realff, M. J., Lee, J. H., and Bommarius, A. S. (2009). “Modeling cellulase kinetics on lignocellulosic substrates,” Biotechnol. Adv. 27(6), 833-848. DOI: 10.1016/j.biotechadv.2009.06.005
Behera, S., Arora, R., Nandhagopal, N., and Kumar, S. (2014). “Importance of chemical pretreatment for bioconversion of lignocellulosic biomass,” Renew. Sust. Energ. Rev. 36, 91-106. DOI: 10.1016/j.rser.2014.04.047
Biswas, R., Teller, P. J., and Ahring, B. K. (2015). “Pretreatment of forest residues of Douglas fir by wet explosion for enhanced enzymatic saccharification,” Bioresour. Technol. 192, 46-53. DOI: 10.1016/j.biortech.2015.05.043
Chang, J., Cheng, W., Yin, Q., Zuo, R., Song, A., Zheng, Q., Wang, P., Wang, X., and Liu, J. (2012). “Effect of steam explosion and microbial fermentation on cellulose and lignin degradation of corn stover,” Bioresour. Technol. 104, 587-592. DOI: 10.1016/j.biortech.2011.10.070
Chang, Z., Cai, D., Wang, Y., Chen, C., Fu, C., Wang, G., Qin, P., Wang, Z., and Tan, T. (2016). “Effective multiple stages continuous acetone-butanol-ethanol fermentation by immobilized bioreactors: Making full use of fresh corn stalk,” Bioresour. Technol. 205, 82-89. DOI: 10.1016/j.biortech.2016.01.034
Chen, M., Zhao, J., and Xia, L. (2009). “Comparison of four different chemical pretreatments of corn stover for enhancing enzymatic digestibility,” Biomass Bioenerg. 33(10), 1381-1385. DOI: 10.1016/j.biombioe.2009.05.025
Fan, Y., Zhang, Z., Wang, F., Li, J., Hu, K., and Du, Z. (2019). “Lignin degradation in corn stover catalyzed by lignin peroxidase from Aspergillus oryzae broth: Effects of conditions on the kinetics,” Renew. Energ. 130, 32-40. DOI: 10.1016/j.renene.2018.06.037
Fang, C., Schmidt, J. E., Cybulska, I., Brudecki, G. P., Frankær, C. G., and Thomsen, M. H. (2015). “Hydrothermal pretreatment of date palm (Phoenix dactylifera L.) leaflets and rachis to enhance enzymatic digestibility and bioethanol potential,” Biomed Res. Int. 2015. DOI: 10.1155/2015/216454
Huang, A. A. (1975). “Kinetic studies on insoluble cellulose-cellulase system,” Biotechnol. Bioeng. 17(10), 1421-1433. DOI: 10.1002/bit.260171003
Huang, W., Wang, E., Chang, J., Wang, P., Yin, Q., Liu, C., Zhu, Q., and Lu, F. (2017). “Effect of physicochemical pretreatments and enzymatic hydrolysis on corn straw degradation and reducing sugar yield,” BioResources 12(4), 7002-7015. DOI: 10.15376/biores.12.4.7002-7015
Jiang, H., Han, B., and Ge, J. (2015). “Enhancement in the enzymatic digestibility of hybrid poplar with poor residual hemicelluloses after Na2SO3 pretreatment,” Bioresour. Technol. 180, 338-344. DOI: 10.1016/j.biortech.2014.12.103
Kapoor, M., Raj, T., Vijayaraj, M., Chopra, A., Gupta, R. P., Tuli, D. K., and Kumar, R. (2015). “Structural features of dilute acid, steam exploded, and alkali pretreated mustard stalk and their impact on enzymatic hydrolysis,” Carbohydr. Polym. 124, 265-273. DOI: 10.1016/j.carbpol.2015.02.044
Kojiro, K., Miki, T., Sugimoto, H., Nakajima, M., and Kanayama, K. (2010). “Micropores and mesopores in the cell wall of dry wood,” J. Wood Sci. 56(2), 107-111. DOI: 10.1007/s10086-009-1063-z
Kopelman, R. (1988). “Fractal reaction kinetics,” Science 241(4873), 1620-1626. DOI: 10.1126/science.241.4873.1620
Li, G., and Chen, H. (2014). “Synergistic mechanism of steam explosion combined with fungal treatment by Phellinus baumii for the pretreatment of corn stalk,” Biomass Bioenerg. 67, 1-7. DOI: 10.1016/j.biombioe.2014.04.011
Li, P., Cai, D., Luo, Z., Qin, P., Chen, C., Wang, Y., Zhang, C., Wang, Z., and Tan, T. (2016). “Effect of acid pretreatment on different parts of corn stalk for second generation ethanol production,” Bioresour. Technol. 206, 86-92. DOI: 10.1016/j.biortech.2016.01.077
Ma, L., Cui, Y., Cai, R., Liu, X., Zhang, C., and Xiao, D. (2015). “Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob,” Bioresour. Technol. 180, 1-6. DOI: 10.1016/j.biortech.2014.12.078
Miller, G. L. (1959). “Use of dinitrosalicylic acid reagent for determination of reducing sugar,” Anal. Biochem. 31(3), 426-428. DOI: 10.1021/ac60147a030
Muñoz, A. H. S., Guerrero, C. E. M., Ortega, N. L. G., Vaca, J. C. L., Vargas, A. A., and Canchola, C. C. (2018). “Characterization and integrated process of pretreatment and enzymatic hydrolysis of corn straw,” Waste Biomass Valori. (70), 1-15. DOI: 10.1007/s12649-018-0218-9
Perez-Pimienta, J. A., Lopez-Ortega, M. G., Chavez-Carvayar, J. A., Varanasi, P., Stavila, V., Cheng, G., Singh, S., and Simmons, B. A. (2015). “Characterization of agave bagasse as a function of ionic liquid pretreatment,” Biomass Bioenerg. 75, 180-188. DOI: 10.1016/j.biombioe.2015.02.026
Ramadoss, G., and Muthukumar, K. (2015). “Influence of dual salt on the pretreatment of sugarcane bagasse with hydrogen peroxide for bioethanol production,” Chem. Eng. J. 260, 178-187. DOI: 10.1016/j.cej.2014.08.006
Rollin, J. A., Zhu, Z., Sathitsuksanoh, N., and Zhang, Y.-H. P. (2011). “Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia,” Biotechnol. Bioeng. 108(1), 22-30. DOI: 10.1002/bit.22919
Segal, L., Creely, J. J., Martin Jr., A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer,” Text. Res. J. 29(10), 786-794. DOI: 10.1177/004051755902901003
Singh, J., Suhag, M., and Dhaka, A. (2015). “Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review,” Carbohydr. Polym. 117, 624-631. DOI: 10.1016/j.carbpol.2014.10.012
Sun, S.-L., Sun, S.-N., Wen, J.-L., Zhang, X.-M., Feng, P., and Sun, R.-C. (2015). “Assessment of integrated process based on hydrothermal and alkaline treatments for enzymatic saccharification of sweet sorghum stems,” Bioresour. Technol. 175, 473-479. DOI: 10.1016/j.biortech.2014.10.111
Tai, C., and Keshwani, D. (2014). “Impact of pretreatment with dilute sulfuric acid under moderate temperature on hydrolysis of corn stover with two enzyme systems,” Appl. Biochem. Biotech. 172(5), 2628-2639. DOI: 10.1007/s12010-013-0721-1
Väljamäe, P., Kipper, K., Pettersson, G., and Johansson, G. (2003). “Synergistic cellulose hydrolysis can be described in terms of fractal-like kinetics,” Biotechnol. Bioeng. 84(2), 254-257. DOI: 10.1002/bit.10775
Wojtusik, M., Zurita, M., Villar, J.-C., Ladero, M., and Garcia-Ochoa, F. (2016). “Enzymatic saccharification of acid pretreated corn stover: Empirical and fractal kinetic modelling,” Bioresour. Technol. 220, 110-116. Doi:10.1016/j.biortech.2016.08.069
Xiao, X., Bian, J., Li, M.-F., Xu, H., Xiao, B., and Sun, R.-C. (2014). “Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment,” Bioresour. Technol. 159, 41-47. DOI: 10.1016/j.biortech.2014.02.096
Xu, H., Yu, G., Mu, X., Zhang, C., DeRoussel, P., Liu, C., Li, B., and Wang, H. (2015). “Effect and characterization of sodium lignosulfonate on alkali pretreatment for enhancing enzymatic saccharification of corn stover,” Ind. Crop. Prod. 76, 638-646. DOI: 10.1016/j.indcrop.2015.07.057
Yang, C.-Y., and Fang, T. J. (2015). “Kinetics for enzymatic hydrolysis of rice hulls by the ultrasonic pretreatment with a bio-based basic ionic liquid,” Biochem. Eng. J. 100, 23-29. DOI: 10.1016/j.bej.2015.04.012
Yang, H., Li, J., Xu, J., and Mo, L. (2017). “The critical analysis of catalytic steam explosion pretreatment of corn stalk, lignin degradation, recovery, and characteristic variations,” BioResources 12(1), 344-361. DOI: 10.15376/biores.12.1.344-361
Yang, M., Zhang, J., Kuittinen, S., Vepsäläinen, J., Soininen, P., Keinänen, M., and Pappinen, A. (2015). “Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone-butanol-ethanol fermentation,” Bioresour. Technol. 189, 131-137. DOI: 10.1016/j.biortech.2015.04.008
Zeng, Y., Zhao, S., Yang, S., and Ding, S.-Y. (2014). “Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels,” Curr. Opin. Biotech. 27, 38-45. DOI: 10.1016/j.copbio.2013.09.008
Zhao, C., Shao, Q., Ma, Z., Li, B., and Zhao, X. (2016). “Physical and chemical characterizations of corn stalk resulting from hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment,” Ind. Crop. Prod. 83, 86-93. DOI: 10.1016/j.indcrop.2015.12.018
Zhao, J., and Chen, H. (2013). “Correlation of porous structure, mass transfer and enzymatic hydrolysis of steam exploded corn stover,” Chem. Eng. Sci. 104, 1036-1044. DOI: 10.1016/j.ces.2013.10.022
Zhang, Z., Xia, L., Wang, F., Lv, P., Zhu, M., Li, J., and Chen, K. (2015). “Lignin degradation in corn stalk by combined method of H2O2 hydrolysis and Aspergillus oryzae CGMCC5992 liquid-state fermentation,” Biotechnol. Biofuels 8, 183. DOI: 10.1186/s13068-015-0362-4
Zhang, Z. C., Li, J. H., and Wang, F. (2017). “Kinetics of cellulase saccharification of corn stover after pretreatment by lignin peroxidase and H2O2,” BioResources 12(3), 5462-5486. DOI: 10.15376/biores.12.3.5462-5486
Article submitted: August 30, 2018; Peer review completed: November 18, 2018; Revised version received and accepted: December 15, 2018; Published: December 19, 2018.
DOI: 10.15376/biores.14.1.1157-1173
