Abstract
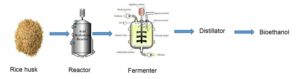
The effects of acid and alkali hydrolysis, as well as rice husk varieties (pure and mixed), on bioethanol production using saccharification and fermentation, were investigated in this study. Microbes such as Saccharomyces cerevisiae are currently used for fermenting agricultural wastes to bioethanol, an environmentally friendly alternative to petroleum energy sources. Rice husks were ground to a fine powder, then hydrolyzed with acid and alkali, and incubated for five days. A refractometer was used to perform a sugar reduction test, which determined the presence of fermentable sugar in the media. The parameters revealed that the variety of rice husk used did not affect the ethanol percent yield, which was 14.8 ± 0.5% and 15.0 ± 0.5% for the pure and mixed varieties, respectively; however, there was a substantial difference in the percentage yield in the method of pre-treatment. The percentage yield of ethanol in the acid pre-treated sample was higher (14.8 ± 0.5% and 15.0 ± 0.5%) than that of the alkali (6.1 ± 0.5% and 4.8 ± 0.5%). The presence of alcohol in the sample was confirmed by FTIR analysis, while GC-MS identified the specific compounds and their percentage composition – ethanol (9.67%). This suggests that using H2SO4 in the hydrolysis of rice husk is a promising and effective method for producing bioethanol.
Download PDF
Full Article
Conversion of Waste Products Derived from Rice Processing Industry Into Bioethanol
Rose E. Kukwa,a,c,* Donald T. Kukwa,a,b Winifred U. Nande,c and Michael T. Tse a
The effects of acid and alkali hydrolysis, as well as rice husk varieties (pure and mixed), on bioethanol production using saccharification and fermentation, were investigated in this study. Microbes such as Saccharomyces cerevisiae are currently used for fermenting agricultural wastes to bioethanol, an environmentally friendly alternative to petroleum energy sources. Rice husks were ground to a fine powder, then hydrolyzed with acid and alkali, and incubated for five days. A refractometer was used to perform a sugar reduction test, which determined the presence of fermentable sugar in the media. The parameters revealed that the variety of rice husk used did not affect the ethanol percent yield, which was 14.8 ± 0.5% and 15.0 ± 0.5% for the pure and mixed varieties, respectively; however, there was a substantial difference in the percentage yield in the method of pre-treatment. The percentage yield of ethanol in the acid pre-treated sample was higher (14.8 ± 0.5% and 15.0 ± 0.5%) than that of the alkali (6.1 ± 0.5% and 4.8 ± 0.5%). The presence of alcohol in the sample was confirmed by FTIR analysis, while GC-MS identified the specific compounds and their percentage composition – ethanol (9.67%). This suggests that using H2SO4 in the hydrolysis of rice husk is a promising and effective method for producing bioethanol.
DOI: 10.15376/biores.18.1.1836-1847
Keywords: Rice husk; Hydrolysis; Fermentation; Bioethanol
Contact information: a: Department of Chemistry, Benue State University, Makurdi, Nigeria; b: Department of Chemical Engineering Durban University of Technology, Durban, South Africa; c: Centre for Food Technology and Research, Benue State University, Makurdi, Nigeria;
* Corresponding author: erdoo.rose@gmail.com
GRAPHIC ABSTRACT

The management of solid waste from the agriculture and food processing industries, which causes environmental contamination, is one of the most serious concerns at hand today. The need for an alternative energy source that is sustainable, renewable, economically viable, environmentally friendly, and does not compete with human food sources has also been sparked by the high cost of producing fossil fuels, their inability to be replenished, and the rising demand for food and energy due to the growing human population. As a result, the use of rice husk for future supplies of ethanol is a means to lessen reliance on petroleum-based fuels, mitigate the effects of global warming, and efficiently manage agricultural waste.
Ethanol is a clear, colourless, volatile, inflammable liquid produced either by synthesis or fermentation. Synthetic ethanol is produced by the hydration of ethylene, which is a petrochemical process with a high negative impact on the carbon economy. Fermentative ethanol, on the other hand, is produced from biomass, which can either be starchy or lignocellulosic material. Biomass-based ethanol, commonly referred to as bioethanol, is a renewable fuel, which is produced by the microbial degradation of starch and structural polymers such as cellulose, hemicellulose, and lignin (Awoyale and Lokhat 2019). Ethanol from the petrochemical industry or bioethanol from biomass fermentation both bear the same chemical formula, C2H5OH, and are distilled at the same constant boiling temperature of 78.5 °C. Ethanol, and hence bioethanol, has an octane rating of 99.5 while the premium motor spirit (PMS) has an octane rating of 91 to 95. A blend of bioethanol and PMS achieves oxygenated fuel compositions to lower automotive emissions, and it boosts the octane ratings, allowing for higher engine compression ratios, which improves engine economy and performance (Ibeto et al. 2011). This has elicited the use of renewable feedstock such as corn cobs, cassava peels, yam peels, mango peels, sorghum straw, pineapple peels, rice husks, and more recently, microalgae biomass to make bioethanol (Aruwajoye et al. 2020a,b; Somda et al. 2011). Although bioethanol is one of the most promising fossil fuel alternatives, its viability as a bulk commodity is heavily dependent on the pre-treatment stage and process technology.
Bioethanol has been categorized as having at least four generations. The first generation, from edible biomass, poses a great danger to food availability. The second generation, which is made from agricultural wastes such as rice husks, removes the danger of food insecurity (Naik et al. 2010; Ben-Iwo et al. 2016). The use of microalgae and genetically modified plants for the manufacture of bioethanol (the third and fourth generations, respectively) is currently being researched (Kukwa and Chetty 2020; Kukwa and Chetty 2022). Rice is the most essential grain in terms of nutritional and caloric content, as well as the world’s most widely consumed staple food, particularly in Asia and Africa. Oryza sativa (Asian rice) and Oryza glaberrima (African rice) are the two known species of cereal rice crop (Wambugu et al. 2021). It is the world’s third most produced commodity, after sugarcane and maize, and Nigeria is eighth in producing over 1.19 million tonnes of husk, which is primarily burned, releasing CO2, a global problem (Ben-Iwo et al. 2016). Benue State, Nigeria, produces millions of tons of husk garbage that pollutes the air and water due to poor disposal methods such as burning. The need for an alternative energy source that is sustainable, renewable, economically competitive, ecologically benign, and does not compete with human food supplies has been sparked by the rising demand for energy as a result of the growing human population. Because of its high availability, cheap cost, and lack of rivalry with human food sources, using rice husk for future ethanol supply is a strategy to minimize reliance on petroleum-based fuels, mitigate the greenhouse effect, and effectively manage agricultural waste.
Some studies have been published on the subject of bioethanol production from biomass, including rice husks. Saha et al. (2005) used 15% w/v H2SO4, equivalent to 1.0% v/v H2SO4 aqueous solution to pretreat the milled rice hull. Karimi et al. (2006) employed 0.5 % H2SO4 to convert rice straws to soluble sugars via hydrolysis. At 15 bar pressure and the hydrolysis retention time (HRT) of 10 minutes, the milled rice straw gave a glucose yield of 28.9 g/kg. Dagnino et al. (2013) optimized acid-pretreated milled rice hulls for bioethanol production using 0.3% w/v H2SO4 and produced 0.11 g ethanol/g of rice hulls, accounting for 84 % conversion efficiency. Cacua et al. (2018) pretreated rice husk with a 2.0% w/v NaOH solution and subsequently hydrolyzed it with acid cellulase (CFB3S). The alcoholic fermentation of total reducing sugars (TRS) obtained in the enzymatic hydrolysis was carried out with Saccharomyces cerevisiae, giving 4.70 g bioethanol/100 g of rice husk and a yield of 15.67 %. The goal of this study was to produce bioethanol from rice husk via acidic and alkaline (5M H2SO4 and 5M NaOH) hydrolysis using Saccharomyces cerevisiae. Unlike many related studies, this study evaluated the minor chemical components of the resulting hydrolysate and many solution properties.
Materials and Methods
Sample and reagents procurement
MIKAP Nigeria Limited (MIVA Rice, Makurdi, Nigeria) provided the pure variety rice husk, while the Wurukum Rice mill (Makurdi, Nigeria) provided the mixed variety rice husk. Both samples were kept in clean polythene bags. Reagents such as Benedict’s solution, sodium hydroxide (NaOH), sulphuric acid (H2SO4), and Saccharomyces cerevisiae (Baker’s yeast) were purchased from Emole Nigeria Limited (Makurdi, Nigeria). The work was carried out in the Chemistry Department at Benue State University, Makurdi.
Pre-treatment and Hydrolysis of rice husk substrate
The pure and mixed variety rice husk substrates were sun-dried for three days to reduce moisture content and make them more millable. The samples were then oven dried for 15 min at 75 °C before being pulverized in a hammer mill. Subsequently, the milled rice husk was sieved with a 0.5 mm sieve. A 30 g portion of the crushed substrate was mixed in 180 mL of 5 M NaOH and 5M H2SO4 was used to form a slurry. The reactions were carried out in duplicate in an autoclave at 121 °C and a pressure of 15.0 bar in a borosilicate glass vessel resistant to pressure and temperature. Both pure rice husks and mixed rice husks were subjected to this technique. The conditions were chosen based on prior research (Sarkar et al. 2012). The hydrolysate was allowed to cool to 30 °C before adjusting the pH to 5 with a controlled volume of 5 M H2SO4; and 5M NaOH, using a pH meter, and was filtered through a sintered glass filter (Kroumov et al. 2006). The filtrate was tested for the presence of reducing sugars using Benedict’s method (Onwuakor et al. 2017).
Preparation of fermentation medium
Twenty grams portion of Saccharomyces cerevisiae was added to the mix. Saccharomyces cerevisiae was mixed with 50 mL of distilled water at room temperature. The slurry was stirred for 5 min and left to stand for 2 h before it was added to the hydrolysate, which was already mixed with the solution (Ezejofor et al. 2018).
Fermentation of the hydrolysate
This procedure was carried out as described by Gupta and Verma (2015). Conical flasks were labelled with the hydrolysis technique and sample; they were then plugged with cotton wool and covered in aluminium foil and sterilized for 30 min at 121 °C and 15 bar pressure. Approximately, 1.5 mL of Saccharomyces cerevisiae was used to inoculate the flasks. The flasks were corked with sterilized cotton wool, mixed well, and incubated for five days at room temperature (28 °C). Shaking the flasks periodically ensured a homogeneous solution and uniform distribution of the organisms in the substrate mixture (Oyeleke and Jibrin 2009).
Distillation of fermented hydrolysate
This was accomplished by distilling the mixture and separating the resulting alcohol. The fermented liquid was moved to a round bottom flask and positioned on a heating mantle attached to a distillation column filled with running tap water. At intervals, the resulting ethanol was collected in a collecting flask linked to the other end of the distillation column. The boiling temperature, flash point, viscosity, and specific gravity of the distilled alcohol were all determined (Ezejofor et al. 2018).
Physicochemical Analysis of Ethanol in the Distillate
Distillation temperature and pH, reducing sugar content, percentage yield of ethanol produced, specific gravity, the density of the ethanol, viscosity, flash point, and refractive index were some of the physicochemical parameters of the bioethanol that were measured in this study.
Determination of temperature and pH
A thermometer was used to determine the sample’s temperature, and the pH was allowed to equilibrate for 5 min. It was calibrated with a pH 5.0 buffered solution. The electrode of the pH meter was placed into the ethanol in a beaker to obtain a reading, and the pH value was read on the meter’s screen. When the figures were stable, a reading was taken.
Determination of reducing sugar concentration and refractive index
Following hydrolysis, the concentration of total reducing sugars /refractive index was determined using abbe 60/DR refractometer, Bellingham + Stanley Ltd. UK, which reads sample values directly from the scale graticule in either refractive index or % sucrose (obrix). The presence of the reducing sugars was first determined using a benedict solution, brick red precipitate indicated a positive result.
The total reducing sugar concentration of the hydrolysate as well as the refractive index of the produced ethanol was determined using the refractometer as described by James (1995). A few drops of the sample were transferred into the glass slide of the instrument and the temperature was maintained at 40 °C to keep the temperature uniform. The eyepiece of the refractometer was used to view the dark portion and was adjusted to be in line with the intersection of the cross. The pointer on the scale pointed to the refractive index at no parallax error; the process was repeated and the mean value was recorded as the refractive index. The readings for the reducing sugar concentration were expressed in percent brix.
Determination of percentage yield
The Pearson (1976) method was used to determine how much alcohol had been produced. The ratio of the volume of ethanol made to the quantity of the fermented substrate distilled was used. To make ethanol, 100 mL of the distilled fermented rice husk hydrolysate was poured into two large bottom distillation flasks. Each flask was filled with 50 mL of distilled water and filled to the top. It took 5 mL of NaOH and 1.0 mL of H2SO4 to adjust the pH of the sample. In the next step, the flask was connected to the condenser, and the thermometer was inserted in place while the flask was on the heating mantle. A thermometer was used to monitor the temperature as the mixture was boiled. During this time, the recovery outlet tube (through which the alcohol flows) was put into a receiver flask. The tube passed through a stopper with a big barrier. The receiver flask was capped so that the amount of alcohol that comes out will be as low as possible. It stopped when the temperature started to rise, and the base of the condenser started to get a little wet. The amount of alcohol was then measured. Equation 1 was used to figure out how much alcohol was produced.
(1)
Determination of the specific gravity of alcohol produced
The distillate’s specific gravity was determined using a specific gravity bottle (density bottle). The bottle was dried in the oven, cooled, and weighed (W1). It was weighed after being filled with 10 mL of water (W2). The ethanol sample was then added, weighed, and recorded (W3). The specific gravity of ethanol was estimated using the same formula as James and presented in Eq. 2 (Pearson 1976).
(2)
where W1 is the weight of the empty specific gravity bottle (g), W2 is the weight of the specific gravity bottle + water (g), and W3 is the weight of the specific gravity bottle + produced ethanol (g).
Determination of the viscosity of ethanol produced
Exactly 50 mL of ethanol was added into A-arm of U-tube capillary viscometer through the orifices to the marked point. A sucker was used to lift the sample to the B-arm of the capillary to the marked point. A stopwatch was used to regulate the time it took for the ethanol to return (flow) to the mark under the B-arm, and the time was noted. The viscosity calibration curve was then used to convert viscosity in seconds to centistokes (Fan 2001).
Determination of the flash point of ethanol produced
This test was carried out using a flash point apparatus. The cup in the apparatus was dried. About 50 mL portion of the sample was transferred into the flash point cup. The cup was fixed into position in the apparatus assembled with a thermometer, and the apparatus was switched on; the heat was controlled by a steady stirrer to maintain a uniform temperature while passing a small flame across the material every five seconds. The temperature at which the vapour first flashes with a blue flame was recorded as the flash point of the ethanol produced.
Fourier-transform infrared spectroscopy (FTIR) analysis
FTIR analysis was carried out for the detection of functional groups present in the distillate. The FTIR spectra were obtained using Agilent Technology Cary 630 FTIR of the National Research Institute for Chemical Technology, Zaria. The spectra identified were numbered, the wave numbers saved, and printed.
Gas chromatography-mass spectrometry (GC-MS) analysis
A gas chromatograph from Agilent USA connected to a mass spectrophotometer (5975C) with a triple-axis detector equipped with an auto-injector (10 µL syringe) was used, and helium gas was used as a carrier gas.
All chromatographic separation was performed on the capillary column having the specification: length, 30 m; internal diameter, 0.2 µm; thickness, 250 µm; treated with phenyl methyl siloxane. Other GC-MS conditions are ion source temperature (EI) at 2500 °C, interface temperature at 3000 °C, pressure at 16.2 psi, out time at 1.8 mm, 1.0 µL injector in split mode with split ratio 1:50 with injection temperature of 3000 °C; the column temperature started at 350 °C for 5 min and changed to 1500 °C at the rate of 40 °C/min. The temperature was again raised to 2500 °C at the rate of 200 °C/min and held for 5 min. The total elution time was 47.5 min. MS Solution software was used to control the system and to acquire the data. Identification of the compounds was carried out by comparing the mass spectra obtained with those of the standard mass spectra from NIST library (NISTII).
Table 1 summarizes the results of the physicochemical measurements, including temperature and pH, reducing sugar content, percentage yield, specific gravity, viscosity, flash point, and refractive index of the ethanol produced.
Temperature and pH
The distillation temperature of the generated alcohol was found to be constant at 78.5 °C for the acid hydrolyzed samples and only showed a small fluctuation during the second distillation. The distillation temperature of the alkali hydrolyzed samples followed a similar trend, with a small increase from 79.9 °C to 80.9 °C. The small increase or fluctuation could be the result of some impurities due to higher boiling alcohols as well as the distillation method used.
Table 1. Physicochemical Analysis of the Produced Bioethanol
Reducing Sugar Concentration and Refractive Index
The concentration of reducing sugar in the hydrolysates was determined to be 7.12% brix and 7.34% brix for the pure and mixed variety rice husks, respectively, of the acid-hydrolyzed samples. Similar values of 8.01% brix and 7.50% brix were observed for the alkali hydrolyzed samples of the pure and mixed husks, respectively.
The refractive index of the produced alcohol from the acid hydrolysate was determined to be 1.36 ± 0.10 and 2.10 ± 0.10 for the pure and mixed rice husks varieties, respectively, and 1.39 ± 0.10 and 2.18 ± 0.10 for the alkali hydrolyzed samples of the pure and mixed husks, respectively. There was a noticeable difference in the acid and alkali hydrolyzed samples. This trend was also observed in the varieties of rice husks.
There was a linear relationship between the total reducing sugar concentration of a solution and the refractive index of that solution (Meyrowtiz 1955). The “abbe 60/DR refractometer” used in this study gave values for the total reducing sugar concentration directly from the scale graticule in obrix. Benedict’s test was carried out to detect the presence of reducing sugar (and was merely a qualitative analysis). Depending on the colour exhibited on the addition of the solution, one can estimate the concentration of the reducing sugar in the solution; green/yellow shows that the amount of the reducing sugar is low and red if it is high. The refractometer, however, gives the exact reducing sugar concentration of the solution.
The acid pretreatment of the rice husks de-lignifies the lignin to release cellulose and also could lead to the formation of byproducts. The use of concentrated acid promotes the formation of inhibitor compounds that affect microbial activity. The residual acid in acid hydrolysis is not usually recycled; it is only neutralized, precipitating high amounts of salt, which can also have an inhibitory effect (Dussán et al. 2014). Depending on the hydrolysis retention time (HRT), the hydrolysate may contain some black residues of carbon implying the degradation of sugar, thereby reducing the total reducing sugars of the solution (Sandesh et al., 2017). Similarly, the solubilized lignin in alkali-pretreated rice husks presents very high inhibitory potentials to enzyme activity; this can explain the low alcohol yield for the alkali-pretreated sample (Nwankwo et al. 2021).
Percentage Yield
Pure and mixed rice husk samples that were pre-treated with acid yielded 14.8% and 15.0%, respectively. This result was in good agreement with earlier studies by Cacua et al. (2018), which yielded 15.7% alcohol. However, pre-treatment with alkali, the pure and mixed rice husk samples yielded 6.10% and 4.80% respectively. This shows that the variety of rice had little or no effect on the yield of ethanol; rather, the method of pre-treatment had a great influence on the yield of bioethanol. The acid pre-treatment was found to be more favourable in terms of the percentage yield of ethanol produced. Though, the refractive index for the alkali hydrolyzed samples were higher than that for the acid hydrolyzed samples, the percent alcohol for the acid-treated samples was higher, indicating that not much of the sugars in the alkali hydrolyzed samples were converted to alcohol. This may be due to NaOH solubilising lignin, which may have acted as an enzyme inhibitor during the enzyme fermentation of the alkali hydrolysate (Nwankwo et al. 2021).
Specific Gravity and Viscosity of Ethanol Produced
Ethanol made with acid or alkali hydrolysis did not have a big difference in its specific gravity. The specific gravities of the pure rice husks and the mixed variety did not show much difference. There were also no substantial differences in the viscosity of bioethanol as both the acid and alkali pre-treated samples of the pure and mixed rice husk had corresponding viscosity of 1.15± 0.80 -1.90 ± 0.80 (mPa.S), as presented in Table 1.
Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
FTIR analysis identified the presence of inter-molecular bonded alcohol. The FTIR result revealed two more functional groups at different frequency ranges — alkyne and alkene with absorbance at 2117 cm-1 and 1636 cm-1, respectively. Alkynes are transformed into alcohols via transfer hydrogenation, whereas alkenes are converted via dehydration (Geethu et al. 2004). The functional groups are identified in Table 2.
Table 2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Produced Alcohol
Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
GC-MS analysis further identified the specific compounds and their percentage compositions. Other products at various peaks which could serve as precursors for the production of ethanol as well as by-products obtained from the production of ethanol were also identified. The identified components, retention time, percentage area, molecular weight, formula, and applications are presented in Table 3.
Table 3. Chemical Composition and Characteristics of Produced Bioethanol
The GC-MS analysis identified 10 chemical components. The identified components, their retention time, molecular formula, molecular weight, and application are given in Table 3. Some identified compounds were diacetato [1,2-bis (dicyclohexylphosphino)-ethane], 1,3,5,7-tetramethyl-silane, formic acid hydrazide, 2-chloro-2-nitro-propane, 2-furanmethanol, cis-vaccenic acid, fumaric acid, cyclohexyl-methyl hexadecyl ester, and 2-octyl-ethanol. Diacetato[1,2-bis(dicyclohexyl-phosphino)ethane] was found to be 1.74% with a well-known function of coupling azoles with aromatic esters, as suggested by Matsushita et al. (2018), which reacts with water to produce alcohols. Formic acid hydrazide (33.1%) serves as a precursor in the preparation of 1,2,4-triazole derivatives (products formed from the formation of bioethanol). 2-Chloro-2-nitro-propane is used as a fuel; 2-furan methanol is a secondary alcohol; and cis-vaccenic acid is a product formed in the acetate fermentation process using rice as a feedstock, as noted by Fujimori et al. (2009). The distillate also contained fumaric acid, used as food acidulates in the manufacture of polyhydric alcohols. Cyclohexyl-methyl hexadecyl ester is a primary alcohol used as fuel and solvent, and 2-octyl-ethanol is known for its use as a solvent and as a substitute for fossil fuels. The analysis showed that the percentage of ethanol in the distillate was 9.62%. This is within the range as most yeast can tolerate alcohol concentrations between 10% to 15% as reported by Alba-Lois and Segal-Kischinevzky (2010).
CONCLUSIONS
- This study determined that the acid hydrolysis method was superior to alkali hydrolysis in the production of bioethanol from rice husk. The acid hydrolysis was found to be more favourable in terms of the percentage yield of ethanol produced. Parameters such as the boiling point, refractive index, viscosity, and specific gravity of the bioethanol were more in favour of the acid-hydrolyzed sample. It is important to explain here that only 5M acidic and 5M alkaline conditions were used for the pre-treatment (other concentrations were not considered).
- The total reducing sugar present in the hydrolysate was determined to be 7.12% brix and 7.34% brix for the acid-hydrolyzed samples of the pure and mixed variety rice husks respectively and 8.01% brix and 7.50% brix for the alkali pre-treated samples of the pure and mixed husks respectively
- The variety of rice husk used was shown to have little or no effect on the ethanol percent yield, which was 14.80 ± 0.50% and 15.00 ± 0.50% for the acid-pretreated sample of the pure and mixed varieties, respectively.
- Fourier transform infrared (FTIR) analysis confirmed the presence of alcohol while gas chromatography – mass spectrometry (GC-MS) identified the specific compounds and their percentage composition, including ethanol (9.67%).
- These results suggest that using H2SO4 in the hydrolysis of rice husk is a promising and effective method for producing bioethanol. As a result, rice husk confirms its viability in the manufacture of bioethanol by acid hydrolysis, which not only reduces greenhouse gas emissions but also acts as a viable alternative to fossil fuels and effectively manages agricultural waste through recycling.
ACKNOWLEDGEMENTS
The authors acknowledge the Centre for Food Technology and Research and Chemistry Department at Benue State University for their support and for providing an enabling environment for this research work.
REFERENCES CITED
Alba-Lois, L., and Segal-Kischinevzky, C. (2010). “Beer and wine makers,” Nature Education 3(9), 17:1-6.
Aruwajoye, G. S., Kassim, A., Saha, A. K., and Kana, E. B. G. (2020a). “Prospects for the improvement of bioethanol and biohydrogen production from mixed starch-based agricultural wastes,” Energies 13, 6609, 1-22. DOI: 10.3390/en13246609
Aruwajoye, G. S., Sewsynker-Sukai, Y., and Kana, E. B. G. (2020b). “Valorisation of cassava peels through simultaneous saccharification and ethanol production: Effect of prehydrolysis time, kinetic assessment and preliminary scale up,” Fuel 278, article 118351. DOI: 10.1016/j.fuel.2020.118351
Awoyale, A. A., and Lokhat, D. (2019). “Harnessing the potential of bio-ethanol production from lignocellulosic biomass in Nigeria—A review,” Biofuels, Bioproducts and Biorefining 13, 192-207. DOI: 10.1002/bbb.1943
Ben-Iwo, J., Manovic, V., and Longhurst, P. (2016). “Biomass resources and biofuels potential for the production of transportation fuels in Nigeria,” Renewable and Sustainable Energy Reviews 63, 172-192. DOI: 10.1016/j.rser.2016.05.050
Cacua, A., Gelvez, J. J., Rodríguez, D. C., and Parra, J. W. (2018). “Production of bioethanol from rice husk pretreated with alkalis and hydrolyzed with acid cellulase at pilot scale,” Journal of Physics: Conf. Series 1126 (012034). DOI: 10.1088/1742-6596/1126/1/012034
Dagnino, E. P., Chamorro, E. R., Romano, S. D., Felissia, F. E., and Area, M. C. (2013). “Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production,” Industrial Crops and Products 42, 363-368. DOI: 10.1016/j.indcrop.2012.06.019
Dussán, K. J., Silva, D. D.V., Moraes, E. J. C., Arruda, P.V., and Felipe, M. G. A. (2014).
“Dilute-acid hydrolysis of cellulose to glucose from sugarcane bagasse,” Chemical Engineering Transactions 38, 433-438.
Ezejofor, T. N., Enenebeaku, U. E., Enenebeaku, C. K., Nwankwo, M. U., and Ogbonnaya, C. I. A. (2018). “Comparative study of bio-ethanol yield from yam, potato, watermelon and pineapple peels using different concentration of hydrochloric acid,” World News of National Sciences16, 18-32.
Fan, T. (2001). “Viscosity measurement using Cannon-Fenske viscometers,” http://www.prrc.nmt.edu/groups/petrophysics/media/pdf/viscometer.pdf
accessed June 5, 2022.
Fujimori, M., Keita, Y., Hidetsugu, G., and Yoshinori, T. (2009). “Existence of cis–vaccenic acid in rice vinegar lipids and its origin,” Journal of the Brewing Society of Japan 104(2), 123-130. DOI: 10.6013/jbrewsocjapan.104.123
Geethu, M. G., Sucthra, P. S., Kavitha, C. H., Aswathy, J. M., Dinesh, B., and Murugan, K. (2004). “Fourier transform infrared spectroscope analysis of different solvent extracts of water hyacinth (Leichormia crassipes) on allopathic approach,” World Journal Pharmacy and Pharmaceutical Sciences 3(6), 1256-1266.
Gupta, A., and Verma, J. P. (2015). “Sustainable bio-ethanol production from agro-residues: A review,” Renewable and Sustainable Energy Reviews 41, 550-567. DOI: 10.1016/j.rser.2014.08.032
Ibeto, C. N., Ofoefule, A. U., and Agbo, K. E. (2011). “A global overview of biomass potentials for bioethanol production: A renewable alternative fuel,” Trends in Applied Sciences Research 6, 410-425. DOI:10.17311/tasr.2011.410.425
James, C. S. (1995). Experimental Methods on Analytical Chemistry of Foods, New York: Chapman and Hall.
Karimi, K., Kheradmandinia, S., and Taherzadeh, M. J. (2006). “Conversion of rice straw to sugars by dilute-acid hydrolysis,” Biomass and Bioenergy 30, 247-253. DOI: 10.1016/j.biombioe.2005.11.015
Kroumov, A. D., Modenes, A. N., and Tait, D. M. C. (2006). “Development of new unstructured model for simultaneous saccharification and fermentation of starch to ethanol by recombinant strain,” Journal of Biochemical Engineering 28, 243-255. DOI: 10.1016/j.bej.2005.11.008
Kukwa, D. T., and Chetty, M. (2020). “Microalgae: The Multifaceted Biomass of the 21st Century. In Biotechnological Applications of Biomass (pp. 29),” Intechopen.
DOI: 10.5772/intechopen.94090
Kukwa, D. T., and Chetty, M. (2022). “Biomass Production and Simultaneous Minerals Sequestration from Brewery Wastewater with concomitant Lipid Accumulation using algae,” Chemical Engineering Transactions, 96, 475-480.
DOI: 10.3303/CET2296080
Lu, X., Zhang, Y., and Angelidaki, I. (2009). “Optimization of H2SO4-catalyzed hydrothermal pretreatment of rapeseed straw for bioconversion to ethanol: Focusing on pretreatment at high solids content,” Bioresource Technology 100(12), 3048-3053. DOI: 10.1016/j.biortech.2009.01.008
Matsushita, K., Takise, R., Hisada, T., Suzuki, S., Isshiki, R., Itami, K., Muto, K., and Yamaguchi, J. (2018). “Pd‐catalyzed decarbonylative C‐H coupling of azoles and aromatic esters,” Chemistry – An Asian Journal 13(17) DOI: 10. 1002/asia.201800478
Meyrowtiz, R. (1955). “A compilation and classification of immersion media of high index of refraction,” American Mineralogist 40, 398.
Naik, S. N., Goud, V. V., Rout, P. K., and Dalai, A. K. (2010). “Production of first- and second-generation biofuels: A comprehensive review,” Renewable Sustainable Energy Reviews 14(2), 578-597. DOI: 10.1016/J.RSER.2009.10.003
Nwankwo, M. O., Shirsha, J. J., Onah, D. C., and Lakabra, D. Y. (2021). “Alkaline
pre-treatment and enzymatic saccharification of rice husks for bioethanol production,” Journal of Research in Agriculture and Animal Science 8(9), 14-18.
Onwuakor, C. E., Hans-Anukam, U., and Uzokwe, M. J. (2017). “Production of ethanol and biomass from rice husk using cultures of Aspergillus flavus, Aspergillus eamarii and Saccharomyces cerevisiae,” American Journal of Microbiological Research 5(4), 86-90. DOI: 10.12691/ajmr-5-4-3
Oyeleke, S. B., and Jibrin, N. M. (2009). “Production of bioethanol from guinea corn husk and millet husk,” African Journal of Microbiology Research 3(4), 147-152.
Pearson, D. C. (1976). Determination of Alcohol Content. The Chemical Analysis of Foods, Churchill Livingstone Edinburgh, London and New York, pp. 329-330.
Saha, B. C., Iten, L. B., Cotta, M. A., and Wu, Y. V. (2005). “Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol,” Biotechnol. Prog. 21(3), 816-822. DOI: 10.1021/bp049564n
Sandesh, K., Sana, A., Harshakiran S., Anju P., and Vaman Rao, C. (2017). “Optimization of pretreatment of Saccharum spontaneum (Kans grass) biomass for production of alcoholic biofuels,” Research Journal of Pharmaceutical, Biological and Chemical Sciences 8, 117-126.
Sarkar, N., Ghosh, S. K., Bannerjee, S., and Aikat, K. (2012). “Bioethanol production from agri-cultural wastes: An overview,” Renewable Energy 37(1), 19-27.
Somda, M. K., Savadogo, A., Ouattara, C. A. T., Ouattara, A. S., and Traore, A. S. (2011). “Improvement of bioethanol production using amylasic properties from Bacillus licheniformis and yeasts strains fermentation for biomass valorization,” Asian Journal of Biotechnology, 3254-261. DOI: 10.3923/ajbkr.2011.254.261
Wambugu, P. W., Ndjiondjop, M. N., and Henry, R. (2021). “Genetics and genomics of African rice (Oryza glaberrima Steud) domestication,” Rice 14(6), 1-14. DOI: 10.1186/s12284-020-00449-6.
Article submitted: July 11, 2022; Peer review completed: Aug. 28, 2022; Revised version received and accepted: January 9, 2023; Published: January 20, 2023
DOI: 10.15376/biores.18.1.1836-1847
