Abstract
The chemical modification of wood can be directed to improve various properties, e.g., the dimensional stability, hardness properties, and/or durability properties, against weathering. In this study, a Romanian softwood species Abies alba L. was treated and chemically modified using two cyclic acid anhydrides, i.e., maleic and succinic, to improve its interfacial properties relative to unmodified wood. Structural changes, with focus on the evolution of crystalline part in wood after chemical modification, the water absorption, and the water repellent efficiency, were determined. Maleic anhydride exhibited a lower reactivity towards wood substrate than succinic anhydride, presumably because of their different chemical structure (maleic anhydride is very sensitive to the presence of water). It was found that the percentage of water absorption was diminished, primarily after the succinic anhydride treatment. The chemically modified wood was characterized via Fourier transform-infrared spectroscopy and wide-angle X-ray diffraction methods. The crystalline part from wood structure was evidenced in relation to the employed anhydride in chemical modification approach.
Download PDF
Full Article
Crystalline Structure of Cellulose in Wood after Chemical Modification Using Cyclic Acid Anhydrides (Maleic and Succinic)
Carmen-Alice Teacă *
The chemical modification of wood can be directed to improve various properties, e.g., the dimensional stability, hardness properties, and/or durability properties, against weathering. In this study, a Romanian softwood species Abies alba Mill. was treated and chemically modified using two cyclic acid anhydrides, i.e., maleic and succinic, to improve its interfacial properties relative to unmodified wood. Structural changes, with focus on the evolution of crystalline part in wood after chemical modification, the water absorption, and the water repellent efficiency, were determined. Maleic anhydride exhibited a lower reactivity towards wood substrate than succinic anhydride, presumably because of their different chemical structure (maleic anhydride is very sensitive to the presence of water). It was found that the percentage of water absorption was diminished, primarily after the succinic anhydride treatment. The chemically modified wood was characterized via Fourier transform-infrared spectroscopy and wide-angle X-ray diffraction methods. The crystalline part from wood structure was evidenced in relation to the employed anhydride in chemical modification approach.
DOI: 10.15376/biores.18.2.2535-2550
Keywords: Wood; Acid anhydride; Chemical modification; Crystalline part; FT-IR spectroscopy; X-ray diffraction
Contact information: Center of Advanced Research in Bionanoconjugates and Biopolymers, “Petru Poni” Institute of Macromolecular Chemistry, 41A Grigore-Ghica Vodă Alley, 700487 Iaşi, Romania; Corresponding author: cateaca@icmpp.ro; cateaca14@yahoo.com
GRAPHIC ABSTRACT

INTRODUCTION
Wood represents a complex and versatile natural material that can be modified using many efficient strategies to change its properties via improvement of its strength and resistance properties for both indoor and outdoor applications (Teacă and Tanasă 2020). Thus, high-value wood products, i.e., modified wood products and wood-based polymer composites, are largely employed in different applications including housing, building and construction, and transport.
There are many approaches related to wood modification. Some of them were introduced in the last decades and are still applied, while other modification strategies were investigated and successfully implemented in recent years (Yamaguchi et al. 1994; Norimoto 2001; Hill 2006; Rowell 2006; Suurnakki et al. 2006; Kudanga et al. 2010; Rowell 2012; Gerardin 2016). The last-mentioned methods refer to physical and mechanical approaches, e.g., plasma and densification procedures, chemical, and biological (enzyme-assisted or enzymatic grafting of different functional molecules to the wood surface to confer hydrophobic properties), application of thin films as coatings, and deposition of nanoparticles by sol-gel techniques. All of these methodologies aim to modify the wood structure in a way that allows a sustainable service life of the resulted modified wood without any environmental damaging impact in the form of toxicity in relation to all issues dealing with use, recycling, or disposal of such products.
Improvement in various wood properties, e.g., dimensional stability and durability, under different exposure conditions, is acquired through applying suitable and efficient modification procedures. Amongst them, chemical modification, impregnation, and coating treatment approaches are often employed (Lu et al. 2000; Papadopoulos and Hill 2002; Papadopoulos 2006; Bodîrlău et al. 2008; Bodîrlău and Teacă 2009; Jebrane et al. 2009; Roşu et al. 2010; Xiao et al. 2012; Baur and Easteal 2013; Thybring 2013; Ringman et al. 2014; Nagarajappa and Pandey 2016; Zelinka et al. 2016). These aim to confer wood resistance against the following: microorganisms’ action, which causes biological degradation; solar radiation, primarily UV light; humid environments; damaging chemical reagents; extreme varying temperature values (heating, cooling); damaging mechanical stresses; and harmful fire conditions.
Usually, there are treatments that do not change the molecular structure of the wood constituent polymers, e.g., impregnation procedures, also named passive modification approaches for wood treatment. These employ different chemicals, e.g., monomers, polymers, resins, and waxes, which diffuse into the wood cell walls or cell lumen with an implicitly positive effect on improvement wood resistance against water by increasing wood density.
An application of thin films as coatings relies on the formation of physical barrier layers (through the addition of appropriate agents that efficiently protect wood surfaces against water, biological organisms, fire, or UV radiation exposure) as a final stage of wood-processing technology, which makes wood durable for outdoor applications when exposure to environmental conditions is an inherent process. In this context, employment of new protective alternatives derived from natural resources, e.g., resins, waxes, and biopolymers, for sustainable wood treatment approaches is of real importance considering economic and environmental issues (costs, risks on recycling, and/or disposal measures) (Teacă et al. 2019).
Wood modification through applying chemical treatment employs different reagents. These approaches include silane treatment (Ichazo et al. 2001), acrylation (Li and Matuana 2003), benzoylation (Xie et al. 2009; Farsi 2010), acid anhydride treatment – maleic anhydride (Essoua et al. 2015), acetylation (Hill 2006; Rowell 2012), and succinylation (Doczekalska et al. 2007; Bodîrlău and Teacă 2009). In addition, the treatment can use a mixture of citric acid and glycerol (Essoua et al. 2016). The last mentioned treatment was applied and successfully introduced in order to improve the durability of softwood siding products used in outdoor applications.
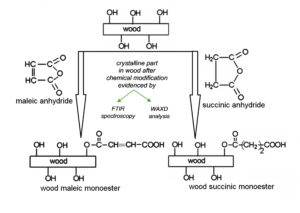
The acid anhydride treatment of wood can be schematically represented as shown in Fig. 1. The chemical structure of wood polymer components is changed, which further influences various properties, e.g., the hydrophilic behavior. Wood acetylation occurs through the replacement of hydrophilic hydroxyl groups with hydrophobic acetyl groups and the release of acetic acid (Rowell 2012). Modified wood structures with a single ester functional group and a free carboxyl group are the results after a partial reaction of wood with cyclic anhydrides. Carboxylic acid groups are formed through the esterification reaction attaching to the wood. After treatment using acid anhydrides, wood also suffers a dimensional change due to the swelling of the cell walls.
Fig. 1. A schematic representation of the reactions between wood and acid anhydrides: acetic (a); maleic (b); and succinic (c) – with the formation of the corresponding wood monoester [re-drawn from Teacă et al. (2018) and Teacă and Tanasă (2020)]
Each of reactions shown in Fig. 1 predicts the formation of a mole equivalent of carboxylic acid in addition to the ester formation. Carboxylic acid formation also could occur by hydrolysis of the anhydride if water is present. In this case, maleic anhydride could be easily hydrolyzed to maleic acid, which is prone to the crystallization process. There are cases when the freed-up carboxylic acid group (for example, when using polycarboxylic acid such as citric acid for wood esterification-based bulk impregnation) can be easily dehydrated when it is alongside to another such group (Essoua et al. 2016; Berube et al. 2017), leading to the formation of another anhydride group under sufficient heating and time conditions. Therefore, an extensive crosslinking can occur. This can positively affect wood sensitivity toward the presence of water, which will be expressed by decreasing hydrophilic character.
In most cases, chemically modified wood has a lower capacity for water absorption, with reduced equilibrium moisture content at a specified atmospheric relative humidity, in comparison with non-modified wood (Adebawo et al. 2016). Replacement of some hydroxyl groups from the wood constituent polymers via reaction with acid anhydrides determines changes in the hygroscopic properties with positive effects on the surface energy and polarity of the modified wood substrates. In composite formulations, when wood is combined with different non-polar polymer matrices, the interfacial properties (wetting, adhesion, surface tension, and porosity) are of real importance in relation with the water repellence and other interactions at the interface.
The chemical composition of different lignocellulosic sources and the crystallinity of the corresponding cellulose represent important features when different properties, e.g., physical, chemical, and mechanical, of cellulose-based materials, are envisaged for specific applications. Therefore, they need to be evaluated in terms of the processing of their biomass-derived sources, e.g., forestry-wood, agricultural feedstocks, agro-industrial residues, when intended for various applications, primarily production of biofuels (Ragauskas et al. 2006), chemicals, and materials (Klemm et al. 2005; Ragauskas et al. 2006; Zhou et al. 2011; Laurichesse and Avérous 2014; Isikgor and Becer 2015). In this context, it is well known that lignocellulosic fibers with a high crystallinity have a good dimensional stability, high density, and tensile strength, but a reduced swelling ability and low chemical reactivity. This is the primary issue that must be addressed for improving their processing ease.
Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction as solid-state characterization methods represent useful tools for evidencing changes in the crystalline structure for different types of cellulose substrates, including wood and wood-based materials. In the present study, a Romanian softwood species Abies alba Mill. (European silver fir) was treated and chemically modified using two cyclic acid anhydrides, i.e., maleic (coded as MA) and succinic (coded as SA), to improve its interfacial properties relative to unmodified wood. Structural changes that occurred in the wood after chemical modifications were investigated using FT-IR spectroscopy and wide-angle X-ray diffraction (WAXD) methods. The water absorption and water repellent efficiency (WRE) were also evaluated. The structure of the crystalline part from the wood was evidenced in relation to the employed anhydride in chemical modification approaches.
EXPERIMENTAL
Materials
Softwood (Abies alba Mill.) samples with dimensions of 80 mm x 10 mm x 2 mm (length x width x thickness) were obtained from a Romanian local source of softwood logs. Initial somewhat fresh 10-year-old logs (Christmas fir tree) had a moisture content around 80%. Wood samples employed in the study were prepared after the logs’ drying process through their being kept at ambient temperature in the laboratory for one year. When wood samples were subjected to esterification reaction, their moisture content was 5 to 6 %. The resulting wood samples were washed with distilled water for 1 h under constant stirring at a temperature of 80 °C, and oven-dried at 100 °C until a constant weight was achieved. Xylene, as an analytical grade reagent, was supplied by Chemical Company, Iasi, Romania and used as received. Maleic anhydride (MA) and succinic anhydride (SA) with a purity greater than 98% were supplied by Fluka, Neu-Ulm, Germany.
Chemical Modification of Wood Using Cyclic Acid Anhydrides
Prior to the chemical modification, the softwood sawdust samples were extracted with xylene for 8 h. The extracted wood samples were further oven-dried for 24 h under vacuum, at a temperature of 70 °C, to obtain a constant weight. For chemical treatment, the dried softwood samples were soaked in acid anhydrides (MA and SA), which had been dissolved previously in xylene. These were heated for 1 h at 90 °C, under continuous stirring. The concentration level of acid anhydride in the xylene solution was designed to be 60%, 80%, and 120% (w/w). After chemical treatment, the resulting wood samples (coded as: W-MA60, W-MA80, and W-MA120 and W-SA60, W-SA80, and W-SA120, respectively) were removed from the xylene solutions and cooled to room temperature. A new extraction with xylene for 8 h was performed to remove the non-reacted acid anhydride and lastly the wood samples were vacuum oven-dried for 24 h at 70 °C to reach a constant weight. A total of 20 samples for each anhydride treatment were designated in this study (10 samples for each concentration value and 10 non-treated samples – W).
FTIR Spectroscopy Investigation
The FTIR-attenuated total reflectance (ATR) spectra were recorded using a spectrophotometer Vertex 70 model (Bruker, Ettlingen, Germany) in the range of 4000 to 400 cm-1 with a 4 cm-1 resolution and scan rate of 32. The spectrophotometer was equipped with the MIRacleTM ATR accessory designed for single or multi-reflection ATR. The ATR crystal plate is made from diamond (1.8 -mm diameter), and solid materials can be put into intimate physical contact with the sampling area through high-pressure clamping, yielding high-quality, reproducible spectra.
WAXD Analysis
The X-ray diffraction patterns were recorded in reflection mode in the angular range 3° to 30° (2θ) at a speed of 2 °min-1, and at ambient temperature, using a Bruker AD8 Advance X-ray diffractometer (Ettlingen, Germany) with Cu Kα radiation operating at 40 kV and 35 mA.
Water Absorption Determination
Five wood samples for each treatment were first placed in an oven set at a temperature of 60 °C, under reduced pressure, for 8 h. The oven-dried weight (WD – expressed in g) was determined and used to calculate the water absorption values as shown in Eq. 1,
WA (%) = [(W – WD) / WD] x 100 (1)
where W is the weight (g) of the sample after soaking in deionized water at a temperature of 30 °C (time ranged from 5 h up to 188 h).
The water repellent efficiency (WRE) was determined using Eq. 2,
WRE (%) = [(WAU – WAM) / WAU] x 100 (2)
where WAU is the water absorption of the unmodified wood sample and WAM is the water absorption of the modified wood sample (Adebawo et al. 2016).
RESULTS AND DISCUSSION
FTIR Spectroscopy Investigation
The crystallinity index of cellulose is a parameter commonly used to quantify the amount of crystalline cellulose present in wood and cellulosic materials. It has been widely used to evidence its structural changes after different treatments, e.g., physical, chemical, and biological. As such, FTIR spectroscopy is the simplest method for crystallinity index determination with relative values, because the resulting spectra always contain contributions from both the crystalline and amorphous regions present in wood substrates. In this study, FTIR spectroscopy was used to determine changes in the crystalline part in the softwood samples after chemical treatment with acid anhydrides in comparison with those non-treated samples (O’Connor et al. 1958; Nelson and O’Connor 1964; Hatakeyama et al. 1976). Spectra recorded for investigated wood samples are shown in Fig. 2.
Fig. 2. FTIR spectra recorded for wood samples: without treatment (W), and after chemical treatment [W-SA (60 to 120), and W-MA (60 to 120)]
Fig. 3. FTIR spectra recorded for wood samples: without treatment (W), and after chemical treatment [W-SA (60 to 120)] in the 800 to 1800 cm-1 spectral region
The increase of the number of maleic/succinyl groups can be easily observed in the FTIR spectra. Three major strong peak regions can be observed in the modified wood samples (as shown in Figs. 3 and 4). These features can be compared to their controls as follows: carbonyl (C=O) stretching region (observed at and near to 1730 cm-1), carbon–hydrogen (C–H) bonds at approximately 1375 cm-1 to 1370 cm-1, and carbon–oxygen (C–O) stretching region at 1245 to 1000 cm-1 (Hon 1996; Matsuda 1996; Rowell 2012). The last-mentioned spectral range was due to carbonyl deformation in the ester bonds in lignin and xylan (from the hemicelluloses component) with some differences between the wood samples when different cyclic acid anhydrides, SA or MA, were used for treatment. The spectral regions observed at 1720 cm-1 and lower than this may be attributed to the carboxyl groups, which are also expected to be present for wood samples under study.
Fig. 4. The FTIR spectra recorded for wood samples: without treatment (W), and after chemical treatment [W-MA (60 to 120)] in the 800 to 1800 cm-1 spectral region
The main information about the esterification was in the spectral range 2000 to 1500 cm−1. From spectral data, there can be mentioned the following absorbance bands:
The peak at 1730 cm−1 is specific to the holocellulose component in the wood structure (cellulose and hemicelluloses). As evidenced by the spectral bands shown above, after chemical modification, the carboxylic acid groups were more prominent in the cases considered. These absorbance values are characteristic for -C=O from resulted carboxylic acid groups. A higher intensity for these bands was evidenced when SA was employed for wood esterification in comparison with MA.
When MA was employed, a new peak appeared at 1162 cm−1 (W-MA60 and W-MA80), and at 1159 cm−1 (W-MA120). These are characteristic to C-O-C bridges resulting from the esterification of the -OH groups in wood polysaccharides through bonding with MA. When MA hydrolysis may occur due to the inherent moisture present in the reaction system, the resulted corresponding organic acid, maleic acid, not only can crystallize, but it can also disrupt the cross-linked structure of lignin that is removed as depolymerized and esterified lignin. Moisture can also depolymerize hemicelluloses from wood. All these hydrolyzed components are removed from wood samples by extraction with solvent.
It was evidenced that wood esterification using MA (system without solvent) can facilitate the fibrillation when moisture is present not only through the introduction of carboxyl groups, but also by removal of part of the lignin (Iwamoto and Endo 2015). Wood esterification using MA without solvent can assist its mechanical fibrillation to realize ultrathin lignocellulose nanofibers (LCNFs), but moisture is required not only for a high degree of esterification, but also for the removal of lignin. As the moisture content of wood is higher, the resulting esterified wood evidences a decrease in the amount of maleic acid ester, an increase in the amount of glucose (that means an increase in cellulose content), and a decrease in its lignin and hemicellulose contents (Iwamoto et al. 2019).
Some indexes for evidence of structural changes that occurred in wood samples after chemical treatment can be calculated from the FTIR spectral absorbance values and are presented in Table 1.
Table 1. Structural Indexes Calculated from FTIR Spectra for Studied Wood Samples
The total crystallinity index (TCI) is defined as the A1370/A2900 absorbance ratio, where the band at 1370 cm-1 is attributed to C-H deformation (bending) and the band at 2900 cm-1 is attributed to C-H and CH2 stretching. The lateral order index (LOI), defined as A1420/A900, is evaluated using the band at 1420 cm-1 (attributed to the CH2 scissoring or symmetric bend in cellulose) and the band at 900 cm-1 (attributed to the C1 group in cellulose) (Hurtubise and Krassig 1960; Nelson and O’Connor 1964). Additionally, it has been shown that the LOI is a better indicator of changes in crystallinity than the TCI for lignocellulose samples (Nelson and O’Connor 1964).
The absorbance values at 1420 and 900 cm-1 are sensitive to the amount of crystalline part versus amorphous part in the wood material, i.e., the broadening of these bands reflects a more disordered structure (Nelson and O’Connor 1964). The crystallinity index slightly increased through chemical modification of wood, which indicated a relatively ordered structure for modified wood samples comparative with the non-modified ones. The results also evidence that the carbonyl index (CI) values considerably increased after chemical treatment, primarily following modification using succinic anhydride, in comparison to maleic anhydride. The hydroxyl index (HI – attributed to the OH groups stretching) presents a different evolution in relation with acid anhydride employed for wood modification as follows: when using succinic anhydride, the HI decreases with increasing concentration and comparatively with non-modified wood samples; and when using maleic anhydride, the HI increases with increasing concentration and comparatively with non-modified wood samples. The moisture index increased with increasing concentration for both anhydrides, with a slight difference as a function of the anhydride type being more evident when succinic anhydride was used.
WAXD Analysis
TheWAXD method is most frequently used in cellulose science to identify the cellulose allomorphs, as well as to measure the crystallinity of the cellulose component in cellulosic materials. Due to its simplicity, the Segal method – peak height method -has been widely used to measure the relative crystalline (or amorphous) fraction in cellulosic materials (Segal et al. 1959).
Fig. 5. WAXD curves recorded for the wood samples: without treatment (W), and after chemical treatment [W-SA (60 to 120), and W-MA (60 to 120)]
As shown in Fig. 5, the diffractograms from the WAXD data recorded for the wood samples, non-modified, and modified samples evidence that the esterification did not change the cellulose I crystal structure. The diffraction peaks at 2θ angles of 15.7°, approximately 22°, and 35° originate from (1-10) and (110), (200), and (004) planes of crystalline cellulose phase Iβ. A broad contribution underlying the crystalline peaks originates from amorphous scattering (Park et al. 2010).
The crystallinity index (CrI) was calculated from the WAXD data for wood samples under study (as presented in Table 2), using the method based on the ratio of the height of the (200) peak (I200), representing both the crystalline and amorphous parts, and the height of the minimum (IAM) between the (200) and (110) peaks, which is for the amorphous part only; this is the simplest and most frequently used technique (Segal et al. 1959).
Table 2 shows some inherent differences between the wood samples under study. Nevertheless, these differences were also related to the type of acid anhydride used for modification and its concentration level. The CrI increased in the wood samples after their chemical treatment using maleic anhydride compared with the non-modified samples. This evolution was possibly caused by the hydrolysis of maleic anhydride into maleic acid by the moisture inherently present in the reaction system. Maleic acid is prone to a crystallization process, but it also can further hydrolyze part of the lignin and hemicellulose from wood. When succinic anhydride was used, the CrI slightly decreased with an increasing concentration level.
Table 2. Crystallinity Index Calculated from the WAXD Data Recorded for Wood Samples (Segal et al. 1959)
Water Absorption Investigation
The results related to the water absorption experiments are presented in Fig. 6. As shown, the water absorption decreased as the concentration increased for the succinic anhydride treatment. A particular behavior was noticed for the wood samples treated with SA (120%). Treatment using anhydrides is based on bulking the wood cell walls to maintain wood in the swollen state as long as the chemical is retained. During such esterification treatment, the wood nearly swelled to its initial volume due to replacement of the –OH groups with corresponding ester groups from the anhydride used for modification. Esterified wood will absorb water with difficulty compared to the initial wood without treatment. Maleic anhydride exhibited lower reactivity towards the wood substrate compared to succinic anhydride, presumably due to their different chemical structure in close relation with their different-followed mechanism for esterification. The reaction between the cyclic acid anhydrides and the wood structure, catalyzed by temperature, can form two different types of ester bonds: mono-ester (as presented in Fig. 1) and di-ester bonds (not exemplified here).
Fig. 6. Water absorption evolution for the wood samples, both non-modified and modified, for a soaking time range of 5 h up to 188 h
The significant lower reactivity of the evolving carboxylic group after ring-opening, in comparison to the anhydride group is the reason why monoester formation occurs in a much higher degree than diester formation, which results in cross-linking (Matsuda 1987). Maleic anhydride hydrolysis due to its inherent sensitivity towards presence of moisture in the reaction system reduces the amount available for wood esterification. Thus, it can be expected that there will be consequent formation of maleic acid, which crystallizes and can further hydrolyze part of the lignin (namely, depolymerized and esterified lignin) and hemicellulose from wood. In the present study, both cyclic anhydrides added to the wood underwent a reaction below 100 °C, and the ester content agrees well with the monoester form (Matsuda 1987). Moreover, in the presence of a relative high level for environmental humidity, the resulting esterified wood samples can become susceptible to a partial de-esterification reaction due to the cleavage of ester linkage attached as monoester to the wood matrix. Such a decrease in the ester content can increase the hygroscopicity of wood samples. The hydrophobicity was more evident when SA was employed for wood esterification, in comparison with MA. In the case of SA, it was also possible to achieve the lengthening of the chain consisting, initially, in the attachment of monoester molecules to wood active hydroxyl groups and, later, successive monoesters to the carboxyl groups can be formed as a result of these reactions (Doczekalska et al. 2007). Possible occurrence of all these above-mentioned can result in a more hydrophilic structure after wood esterification with MA.
The water repellent efficiency (WRE) calculated values are presented in Table 3 (data given only for the wood samples modified using succinic anhydride). For the maleic anhydride treatment, the calculated WRE had negative values due to the presence of MA, which does not react with the wood structure and interacts with the liquid water with the formation of maleic acid. This determines a further breakdown of wood polymer components, starting from the weakest to strongest, i.e., primarily hemicelluloses (because of the presence of acetyl groups in their chemical structure), followed by the amorphous parts of the cellulose and lignin polymers (Bodîrlău et al. 2008).
Table 6. WRE Values for Wood Samples Modified Using SA
CONCLUSIONS
- The chemical treatments using acid anhydrides induced structural changes in the wood samples. The spectral changes that were evidenced through Fourier transform infrared (FTIR) spectroscopy and wide-angle X-ray diffraction (WAXD) methods confirmed the changes in the wood chemical structure via their reaction with cyclic acid anhydrides.
- The stronger carbonyl band at 1720 cm-1 and lower than this value was observed for chemically modified wood as the acid anhydride concentration increased, for both employed treatments. This enhanced carbonyl absorption peak can be ordinarily attributed to the pendant carboxylic acid groups that resulted from ring-opening of the anhydride during its interaction with wood polysaccharides components. The C–H absorbance band at 1370 cm-1 (–/C–/CH3), and –C–/O–/ stretching band at approximately 1210 cm-1 confirmed the esterification of the -OH groups in lignin.
- Changes in the crystalline part in the softwood samples after chemical treatment with cyclic acid anhydrides in comparison with the non-modified samples were evaluated by calculating various indexes, including the crystallinity index. The effect of succinic anhydride on the wood structural changes was more prominent compared with the maleic anhydride treatment.
- The water absorption experiments evidenced a different behavior of the wood samples depending on the anhydride used for treatment and its concentration level, as well as on the soaking time. The water absorption decreased as the concentration increased for the SA treatment. The water absorption behavior for the modified wood samples using MA was different because of the greater affinity of MA for water molecules. The WRE values were dependent on the soaking time for the modified wood samples and the anhydride concentration level.
- Maleic anhydride exhibited lower reactivity towards the wood substrate compared to succinic anhydride, presumably due to their different chemical structure and different mechanism during wood modification.
ACKNOWLEDGMENTS
The author dedicates this work to the memory of her colleague and friend, Dr. Ruxanda Bodîrlău (April 6, 1953 – June 26, 2016), and thanks her for her discretion, dedication, and enthusiasm for as long as they have been working together.
REFERENCES CITED
Adebawo, F. G., Naithani, V., Sadeghifar, H., Tilotta, D., Lucia, L. A., Jameel, H., and Ogunsanwo, O. Y. (2016). “Morphological and interfacial properties of chemically-modified tropical hardwood,” RSC Adv. 6(8), 6571-6576. DOI: 10.1039/C5RA19409A
Baur, S. I., and Easteal, A. J. (2013). “Improved photoprotection of wood by chemical modification with silanes: NMR and ESR studies,” Polym. Adv. Technol. 24, 97-103. DOI: 10.1002/pat. 3056
Berube, M.-A., Schorr, D., Ball, R. J., Landry, V., and Blanchet, P. (2017). “Determination of in situ esterification parameters of citric acid-glycerol based polymers for wood impregnation,” J. Polym. Environ. 26(3), 970-979. doi:10.1007/s10924-017-1011-8
Bodîrlău, R., Teacă, C.-A., and Spiridon, I. (2008). “Chemical modification of beech wood: Effect on thermal stability,” BioResources 3(3), 789-800. DOI: 10.15376/biores.3.3.789-800
Bodîrlău, R., and Teacă, C.-A. (2009). “Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides,” Rom. J. Phys. 54(1-2), 93-104.
Doczekalska, B., Bartkowiak, M., and Zakrzewski, R. (2007). “Modification of sawdust from pine and beech wood with the succinic anhydride,” Holz. Roh Werkst. 65(3), 187-191. DOI: 10.1007/s00107-006-0152-6
Essoua, E. G. G., Blanchet, P., Landry, V., and Beauregard, R. (2015). “Maleic anhydride treated wood: Effects of drying time and esterification temperature on properties,” BioResources 10(4), 6830-6860. DOI: 10.15376/biores.10.4.6830-6860
Essoua, E. G. G., Blanchet, P., Landry, V., and Beauregard, R. (2016). “Pine wood treated with a citric acid and glycerol mixture: Biomaterial performance improved by a bio-byproduct,” BioResources 11(2), 3049-3072. DOI: 10.15376/biores.11.2.3049-3072
Farsi, M. (2010). “Wood–plastic composites: Influence of wood flour chemical modification on the mechanical performance,” J. Reinf. Plast. 29(24), 3587-3592. DOI: 10.1177/07316844103787 79
Gérardin, P. (2016). “New alternatives for wood preservation based on thermal and chemical modification of wood – A review,” Ann. For. Sci. 73(3), 559-570. DOI: 10.1007/s13595-015-0531-4
Hatakeyama, H., Nagasaki, C., and Yurugi, T. (1976). “Relation of certain infrared bands to conformational changes of cellulose and cellulose oligosaccharides,” Carbohydr. Res. 48(2), 149-158. DOI: 10.1016/S0008-6215(00)83211-5
Hill, C. A. S. (2006). Wood Modification: Chemical, Thermal and Other Processes, John Wiley & Sons Ltd., Chichester, West Sussex, England.
Hurtubise, F. G., and Krassig, H. (1960). “Classification of fine structural characteristics in cellulose by infared spectroscopy. Use of potassium bromide pellet technique,” Anal. Chem. 32(2), 177-181. DOI: 10.1021/ac60158a010
Ichazo, M. N., Albano, C., Gonzalez, J., Perera, R., and Candal, M. V. (2001). “Polypropylene/wood flour composites: Treatments and properties,” Compos. Struct. 54(2–3), 207-214. DOI: 10.1016/S0263-8223(01)00089-7
Isikgor, F. H., and Becer, C. R. (2015). “Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers,” Polym. Chem. 6(25), 4497-4559. DOI: 10.1039/c5py00263j
Iwamoto, S., Saito, Y., Yagishita, T., Kumagai, A., and Endo, T. (2019). “Role of moisture in esterification of wood and stability study of ultrathin lignocellulose nanofibers,” Cellulose 26, 4721-4729. DOI: 10.1007/s10570-019-02408-x
Iwamoto, S., and Endo, T. (2015). “3 nm thick lignocellulose nanofibers obtained from esterified wood with maleic anhydride,” ACS Macro Lett. 4, 80-83. DOI: 10.1021/mz500787p
Jebrane, M., Sèbe, G., Cullis, I., and Evans, P. D. (2009). “Photostabilization of wood using aromatic vinyl esters,” Polym. Degrad. Stab. 94, 151-157. DOI: 10.1016/j.polymdegradstab. 2008.11.013
Klemm, D., Heublein, B., Fink, H. P., and Bohn, A. (2005). “Cellulose: Fascinating biopolymer and sustainable raw material,” Angew. Chem. Int. Ed. 44(22), 3358-3393. DOI: 10.1002/anie.200460587
Kudanga, T., Prasetyo, E. N., Sipila, J., Guebitz, G. M., and Nyanhongo, G. S. (2010). “Reactivity of long chain alkylamines to lignin moieties: Implications onhydrophobicity of lignocellulose materials,” J. Biotechnol. 149, 81-87. DOI: 10.1016/j.jbiotec.2010.06.020
Laurichesse, S., and Avérous, L. (2014). “Chemical modification of lignins: Towards biobased polymers,” Prog. Polym. Sci. 39(7), 1266-1290. DOI: 10.1016/j.progpolymsci.2013.11.004
Li, Q., and Matuana, L. M. (2003). “Surface of cellulosic materials modified with functionalized polyethylene coupling agents,” J. Appl. Polym. Sci. 88, 278-286. DOI: 10.1002/app.11681
Lu, J. Z., Wu, Q., and McNabb, Jr., H. S. (2000). “Chemical coupling in wood fiber and polymer composites: A review of coupling agents and treatments,” Wood Fiber Sci. 32(1), 88-104.
Matsuda, H. (1987). “Preparation and utilization of esterified woods bearing carboxyl groups,” Wood Sci. Technol. 21(1), 75-88. DOI: 10.1007/bf0034971
Matsuda, H. (1996). “Chemical modification of solid wood,” in: Chemical Modification of Lignocellulosic Materials, D.N.S. Hon, (ed.) Marcel Dekker, NY, USA, pp. 159-183.
Nagarajappa, G. B., and Pandey, K. K. (2016). “UV resistance and dimensional stability of wood modified with isopropenyl acetate,” J. Photochem. Photobiol. B: Biol. 155, 20-27. DOI: 10.1016/j.jphotobiol.2015.12.012
Nelson, M. L., and O’Connor, R. T. (1964). “Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II,” J. Appl. Polym. Sci. 8(3), 1325-1341. DOI: 10.1002/app.1964.070080 323
Norimoto, M. (2001). “Chemical modification of wood,” in: Wood and Cellulose Chemistry, 2nd Edition, D. N. S. Hon, and N. Shiraishi, (eds.), Marcel Dekker, NY, USA, pp. 573-598.
O’Connor, R. T., DuPré, E. F., and Mitcham, D. (1958). “Applications of infrared absorption spectroscopy to investigations of cotton and modified cottons. Part I: Physical and crystalline modifications and oxidation,” Text. Res. J. 28(5), 382-392. DOI: 10.1177/004051755802800503
Papadopoulos, A. N. (2006). “Chemical modification of pine wood with propionic anhydride: Effect on decay resistance and sorption of water vapour,” BioResources 1, 67-74. DOI: 10.15376/biores.1.1.67-74
Papadopoulos, A. N. and Hill, C. A. S. (2002). “The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana,” Holz. Roh. Werkst. 60, 329-332. DOI: 10.1007/s00107-002-0327-8
Park, S., Baker, J. O., Himmel, M. E., Parilla, P. A., and Johnson, D. K. (2010). “Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance,” Biotechnology Biofuels 3(1), 1-10. DOI: 10.1186/1754-6834-3-10
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., Frederick, Jr., W. J., Hallett, J. P., Leak, D. J., Liotta, C. L., et al. (2006). “The path forward for biofuels and biomaterials,” Science 311(5760), 484-489. DOI: 10.1126/science.1114736
Ringman, R., Pilgard, A., Brischke, C., and Richter, K. (2014). “Mode of action of brown rot decay resistance in modified wood: A review,” Holzforschung 68(2), 239-246. DOI: 10.1515/hf-2013-0057
Roşu, D., Teacă, C.-A., Bodîrlău, R., and Roşu, L. (2010). “FTIR and color change of the modified wood as a result of artificial light irradiation,” J. Photochem. Photobiol. B: Biol. 99(3), 144-149. DOI: 10.1016/j.jphotobiol.2010.03.010
Rowell, R. M. (2012). “Chemical modification of wood,” in: Handbook of Wood Chemistry and Wood Composites, 2nd Ed., R. M. Rowell (ed.), CRC Press, Boca Raton, FL, USA, pp. 537-598. DOI: 10.1201/b12487
Rowell, R. M. (2006). “Chemical modification of wood: A short review,” Wood Mat. Sci. Eng. 1(1), 29-33. DOI: 10.1080/17480270600670923
Segal, L., Creely, J. J., Martin, Jr., A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,” Text. Res. J. 29(10), 786-794. DOI: 10.1177/004051755902901003
Suurnakki, A., Buchert, J., Gronqvist, S., Mikkonen, H., Peltonen, S., and Viikari, L. (2006). “Bringing new properties to lignin rich fiber materials,” VTT Symposium 244, 61-70.
Teacă, C.-A., Tanasă, F., and Zănoagă, M. (2018). “Multi-component polymer systems comprising wood as bio-based component and thermoplastic polymer matrices – An overview,” BioResources 13(2), 4728-4769. DOI: 10.15376/biores.13.2.Teaca
Teacă, C.-A., Roşu, D., Mustată, F., Rusu, T., Roşu, L., Roşca, I., and Varganici, C.-D. (2019). “Natural bio-based products for wood coating and protection against degradation: A review,” BioResources 4(2), 4873-4901. DOI: 10.15376/biores.14.2.Teaca
Teacă, C.-A., and Tanasă, F. (2020). “Wood surface modification—Classic and modern approaches in wood chemical treatment by esterification reactions,” Coatings 10(7), article 6293. DOI: 10.3390/coatings10070629
Thybring, E. E. (2013). “The decay resistance of modified wood influenced by moisture exclusion and swelling reduction,” Int. Biodeter. Biodegrad. 82, 87-95. DOI: 10.1016/j.ibiod.2013.02.004
Xiao, Z., Xie, Y., Adamopoulos, S., and Mai, C. (2012). “Effects of chemical modification with glutaraldehyde on the weathering performance of Scots pine sapwood,” Wood Sci. Technol. 46, 749-767. DOI: 10.1007/s00226-011-0441-z
Xiao, Z. F., Xie, Y. J., and Mai, C. (2012). “The fungal resistance of wood modified with glutaraldehyde,” Holzforschung 66, 237-243. DOI: 10.1515/HF.2011.138
Xie, H., Jarvi, P., Karesoja, M., King, A., Kilpelainen, I., and Argyropoulos, D. S. (2009). “Highly compatible wood thermoplastic composites from lignocellulosic material modified in ionic liquids: Preparation and thermal properties,” J. Appl. Polym. Sci. 111, 2468-2476. DOI: 10.1002/app.29251
Yamaguchi, H., Maeda, Y., and Sakata, I. (1994). “Bonding among woody fibers by use of enzymatic phenol dehydrogenative polymerization,” Mokuzai Gakkaishi 40, 185-190.
Zelinka, S. L., Ringman, R., Pilgård, A., Thybring, E. E., Jakes, J. E., and Richter, K. (2016). ”The role of chemical transport in the brown-rot decay resistance of modified wood,” Int. Wood Prod. J. 7(2), 66-70. DOI: 10.1080/20426445.2016.1161867
Zhou, C.-H., Xia, X., Lin, C.-X., Tong, D.-S., and Beltramini, J. (2011). “Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels,” Chem. Soc. Rev. 40(11), 5588-5611. DOI: 10.1039/c1cs15124j
Article submitted: June 29, 2022; Peer review completed: October 2, 2022; Revised version received and accepted: January 30, 2023; Published: February 3, 2023.
DOI: 10.15376/biores.18.2.2535-2550
