Abstract
Deacidification refers to chemical treatments meant to slow down the acid hydrolysis and embrittlement of books and paper documents that had been printed on acidic paper. From the early 1800s up to about 1990, papermakers used aluminum sulfate, an acidic compound, in most printing papers. Certain deacidification methods use non-aqueous media to distribute alkaline mineral particles such as MgO within the pages of the treated books. Evidence is considered here as to whether or not the proximity of alkaline particles within such documents is sufficient to neutralize the acidic species present. Because much evidence suggests incomplete neutralization, a second focus concerns what to do next in cases where books already have been treated with a non-aqueous dispersion system. Based on the literature, the neutralization of acidic species within such paper can be completed by partial moistening, by high humidity and pressure, by water condensation, as well as by optional treatments to enhance paper strength and a final drying step.
Download PDF
Full Article
Deacidification of Acidic Books and Paper by Means of Non-aqueous Dispersions of Alkaline Particles:A Review Focusing on Completeness of the Reaction
Martin A. Hubbe,a,* Richard D. Smith,b Xuejun Zou,c Svetozar Katuscak,d Antje Potthast,e and Kyujin Ahn f
Deacidification refers to chemical treatments meant to slow down the acid hydrolysis and embrittlement of books and paper documents that had been printed on acidic paper. From the early 1800s up to about 1990, papermakers used aluminum sulfate, an acidic compound, in most printing papers. Certain deacidification methods use non-aqueous media to distribute alkaline mineral particles such as MgO within the pages of the treated books. Evidence is considered here as to whether or not the proximity of alkaline particles within such documents is sufficient to neutralize the acidic species present. Because much evidence suggests incomplete neutralization, a second focus concerns what to do next in cases where books already have been treated with a non-aqueous dispersion system. Based on the literature, the neutralization of acidic species within such paper can be completed by partial moistening, by high humidity and pressure, by water condensation, as well as by optional treatments to enhance paper strength and a final drying step.
DOI: 10.15376/biores.12.2.Hubbe
Keywords: Acids; Embrittlement; Deacidification of paper and books; Accelerated aging protocols; Capillary condensation; Diffusion; pH of paper; Paper degradation
Contact information: a: Department of Forest Biomaterials, North Carolina State University, Campus Box 8005, Raleigh, NC 27695-8005 USA; b: Wei T’o Associates, Inc., 21750 Main Street, Unit 27, Matteson, IL 60443 USA; c: FPInnovations, 570 Saint-Jean Blvd., Pointe-Claire, QC, Canada H9R 3J9; d: Department of Wood, Cellulose and Paper, Institute of Natural and Synthetic Polymers, STU, Bratislava, SK 81237, EU; e: Univ. Nat. Resources & Life Sci., Dept. Chem., Division of Chem of Renewable Resources, Konrad Lorenz Str 24, A-3430 Tulln, Austria; f: Archival Preservation and Restoration Center, National Archives of Korea, 30 Dawangpangyo-ro 851beon-gil, Sujeong-gu, Seongnam-si, Gyeonggi-do, Korea (13449); *Corresponding author: hubbe@ncsu.edu
Contents

INTRODUCTION
A large proportion of books and other paper documents stored in libraries, museums, and private collections have become so brittle over the course of time that they no longer can be checked out to readers (Smith 1969, 1987; Cunha 1987, 1989). Though there are a number of contributing factors, it is well known that one of the dominant mechanisms tending to shorten the useful life of paper documents is acid-catalyzed hydrolysis, a process that attacks the cellulose chains in the fibers that make up paper (Williams 1971; Wilson and Parks 1979; Arney and Chapdelaine 1981; Baty et al. 2010). Millions of tons of acidic paper had been produced worldwide for printing applications during the previous two centuries, and it is still being produced in Asia and other continents (Drewes and France 2012). Starting in about 1980, the paper industry went through a major shift in its manufacturing practices, such that most books nowadays are printed on paper that was made under near-neutral to weakly alkaline conditions (Hubbe 2005). As a consequence, the books are much less likely to suffer from embrittlement during ordinary storage. Meanwhile, it has been estimated that 20 to 40% of Indian publications and 2 to 3% of papers from the United States require deacidification, since some publishers source papers from outside the United States (Drewes and France 2012).
The focus of this review article is on the completeness of certain technologies intended to overcome problems arising from the acidic nature of paper within many existing books. The term “non-aqueous dispersions” will be used in this article to denote such treatments, which typically employ a low-energy liquid (often fluorinated or silicone-based compounds) to distribute MgO or other alkaline particles within the pages of the treated books. The carrier liquid is subsequently drained and evaporated, leaving behind the particles distributed among the fibers of the paper. This review will consider evidence from the literature regarding whether or not such treatment actually neutralizes the acidic species present on and within the fibers of a paper-based document. In the context of this article, the words “completeness” or “completion” will refer to the degree to which acidic groups present within a treated book or other paper article have undergone a reaction such as RCOOH ® RCOO– ½Ca2+, where R may refer to H, CH3, or other structures that make up other organic acids often present in paper materials. Alternatively, the neutralization may involve inorganic species such as sulfuric acid, for which the reaction could be written as H2SO4 ® CaSO4, or related reactions.
This article has a primary focus on MgO as a deacidification agent. The reason for this focus is that processes based on MgO particles (BookkeeperÒ and ZfB:2) are presently the most widely employed in the world for deacidification of books. Since its adoption in 1994 by the US Library of Congress (Buchanan et al. 1994), the use of MgO particles suspended in non-aqueous fluid has spread worldwide in the form of compressor devices, thousands of hand sprayers, and many chamber deacidification machines. Since then, non-aqueous dispersion type deacidification facilities have been deployed in many nations, especially for the BookkeeperÒ process (Preservation Technologies 2017). More recent implementation of such deacidification technology, in the form of the ZfB:2 process, is based in Germany (Zentrum für Bucherhaltung 2017). Thus it is important to address concerns about the treatment’s efficacy, possible incompleteness, and whether there is likely to be an acid paper core or acid parts in the book microstructure.
Before one can have reactions between alkaline particles and acidic moieties within paper, the particles first need to be well distributed in the paper. Questions concerning the nonuniform distribution of alkaline MgO particles in deacidified books already were being asked as early as 1993 when carrying out tests to meet the Library of Congress (LoC) requirements (Buchanan et al. 1994). It was suggested that non-aqueous dispersion deacidification treatment can yield nonuniform results. The LoC Requirement of 1994 (updated 2004) concerning the uniformity is as follows: “The Alkaline Reserve Uniformity for a given paper type shall vary from specified optimal concentrations by no more than 20% between books and by no more than 20% within individual pages” (Buchanan et al. 1994). The process is specified to result in an alkaline reserve of not less than 1.5% with stable and uniform distribution. Uniformity of distribution of alkaline particles in paper also has been considered by Whitmore (1994); Wagner et al. (2008), and Ahn et al. (2012b). Nonuniformity of distribution of alkaline particles on the paper surface and in the cellulose micro- and nanostructure of books, as well as any incompleteness in the neutralization of acidic materials in the paper, may affect many issues of concern to conservators. The uniformity of deacidification treatment can be expected to affect a book’s lifetime, since failure would be expected to occur at the weakest part of a paper structure. Other issues include the appearance of the paper surface or tactile properties of the surface such as a gritty texture. Nonuniformity of treatment implies uncertainty about concentrations of acids within paper, as well as the possibility of continuing acid hydrolysis in the acidic parts of the incompletely deacidified paper and books. Concerns about the efficacy and longevity of paper and books that contain acidic parts or microstructures have been widely considered (Bansa 1990; Buchanan et al. 1994; Pauk 1996; Banik 2005; Bielikova 2005; Blüher et al. 2006; Hanus et al. 2006, 2008; Bielikova and Katuscak 2009; Katuscak et al. 2009b; Baty et al. 2010; Burd 2012; Jablonsky et al. 2012b,c, 2013; Johnson et al. 2012; Hubbe 2015; Ahn et al. 2013; Vizarova et al. 2016a,b).
Much less attention has been paid in the literature to the question of whether and to what extent the insertion of alkaline particles into an acidic paper document, by means of non-aqueous dispersion treatment, achieves neutralization of acidic species present in the paper. The first to call attention to this issue appears to have been Whitmore (1994), as part of a report prepared for the US Library of Congress. It has been widely assumed that, over long periods of storage on library shelves, some kind of molecular migration and neutralization reactions between acidic and alkaline entities in the material would take place (Baty et al. 2010; Burd 2012). However, as will be discussed in the present review article, there has been a lack of research findings that strongly support such an assumption.
Various researchers have attempted to estimate how much money is represented by the ongoing process of acid hydrolysis within tens of millions of bound and unbound paper documents in various collections throughout the world. For instance, Cunha (1987) estimated that 4.7% of the value of books in a typical library is lost each year due to aging and embrittlement. Smith (1987) estimated that the Library of Congress was losing $179,000 each day due to acid hydrolysis of books during storage. In cases where only the information is regarded important, rather than the book itself, options can include digital scanning (McCrady 1990), or even the reprinting of a book onto archival-quality alkaline paper. Though details of cost are generally beyond the scope of this article, it will be assumed that the unit cost of deacidification is likely to be lower than most other available options (Smith 1987).
Another way to prolong the life of books, which does not involve deacidification, involves storing them under conditions of dry refrigeration. Such conditions have been predicted to greatly slow down the degradation process of the acidic paper (Zou et al. 1996a; Smith 2004; Balažic et al. 2007). But even when such practices are implemented, the books are not protected when being checked out and used by a library client.
Based on the reviewed publications there is reason to expect that deacidification reactions can be brought to completion by a suitable post-treatment. Such a process might involve homogenization of alkaline reserve to all parts of books endangered by acid hydrolysis, including neutralization of acidic species present in the paper’s microstructure. Such treatments, depending how they are done, may provide an opportunity to implement other preservation functions required by conservators, such as strengthening and multi-thread protection of paper, inks, and book bindings against oxidation, photo-oxidation, biodegradation, and other optional treatments. If performed in a well-designed conservation process, this could also minimize wasteful repeated manipulation of future monofunctional operations, reduce costs in libraries, and increase the longevity and usability of books (Blüher et al. 2006; Katuscak et al. 2009a,b; Katuscak 2011; Ahn et al. 2012a; Drewes and France 2012; Katuscak and Konvit 2013; Vizarova et al. 2016b).
Deacidification Practices
As a means to address the problems just described, deacidification practices already have been implemented for many years to overcome or at least slow down the effects of acid hydrolysis during storage of books and other printed matter. Such technology has been explained and compared in earlier review articles (Porck 1996; Kaminska and Burgess 1994; Liénardy 1994; Kaminska 1995; Blüher and Vogelsanger 2001; Cedzova et al. 2006; Ramin et al. 2009; Baty et al. 2010). Starting in the 1930s, the storage-stability of paper has been quantified by means of accelerated aging tests (Rasch 1931; Rasch and Scribner 1933; Porck 2000; Zervos and Moropoulou 2006; Zervos 2010). Barrow (1953, 1965) was among the first to quantify the effects of paper’s acidity on its rate of strength loss. He also developed procedures to treat paper with aqueous solutions of alkaline reserve agents, such as soluble carbonates and bicarbonates (Barrow 1965; Moll 1965). Aqueous deacidification systems continue to be highly regarded for the treatment of acidic paper-based documents, especially for the treatment of unbound printed or written documents (Wächter et al. 1998; Sundholm and Tahvanainen 2003a,b; Baty et al. 2010).
Available methods and technology for individual sheets of paper (archive documents) using water-based processes (Blüher et al. 2006; Smith 2011; Katuscak et al. 2012) can assure high efficacy, longevity, and usability through both completeness of neutralization and multifunctional preservation completeness. The aqueous processes can assure (1) homogeneous distribution of alkali in the cellulose micro- and nano-structure, as well as (2) completeness in terms of the preservation functions that are necessary for a particular type of acid documents, such as information, color, ink or print letters, characters or images fixation, strengthening brittle paper, biocidic protection, etc. The preservation (“deacidification”) water bath also can contain fixation, strengthening, and other conservation compounds. Interactions between water and paper are considered in depth in the book by Banik and Brückle 2011).
The deacidification of bound books presents additional challenges. The soaking of a book in an aqueous solution swells the paper sheets. This can cause some bindings to break (Smith 1992; Kellerman 1999) or the pages to become wavy (Brückle and Banik 2011b). In addition, the drying of a bound volume, while preserving its flatness, is more difficult and time-consuming (Kelly and Fowler 1978; Liénardy 1994; Jablonsky et al. 2009a,b; Hubacek et al. 2011). Accordingly, some libraries and deacidification services have instituted rebinding of books as a routine practice associated with deacidification (Kellerman 1999). Such practices increase the complexity and cost of deacidification of books. Thus, the most widely used deacidification methods that have been applied to whole books have been based on non-aqueous treatments, of which there are several competing systems (Liénardy 1994; Blüher and Vogelsanger 2001; Cedzova et al. 2006; Baty et al. 2010). These have become known as mass-deacidification programs. The two general classes of such treatments are summarized in Table 1.
Table 1. Main Classes of Non-aqueous Mass Deacidification Systems that have been Used for Bound Books
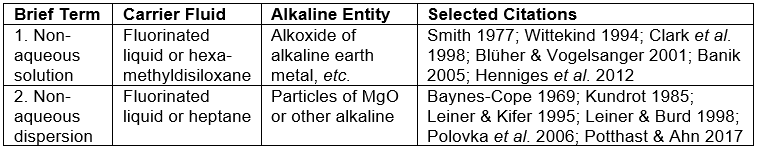
Notes: An example of a highly fluorinated liquid is 3M Performance Fluid PF-5060; the product literature says it has zero adverse effect on the ozone layer; hexa-methyldisiloxane (HMDS) is used in organic synthesis, analytical chemistry, and in paper deacidification. For non-aqueous dispersion systems, a highly fluorinated liquid is used in the Bookkeeper® process, and heptane is used in the ZfB:2 process.
Of the principal classes of the deacidification system listed in Table 1, the first of them has a long track record of beneficial usage (Smith 1977; Scott 1987; Morrow 1988; Wittekind 1994; Baty et al. 2010), though there also have been issues related to the uniformity and completeness of treatment in some cases (Kruth 1988). The second option listed in Table 1, which will be the main focus of the present article, is unique in that it does not involve either solubilization of the treatment agent or its direct reaction with acidic entities in the paper during the treatment. Rather, by means of non-interactive solvent, the dry particles are distributed throughout the book (Kundrot 1985; Leiner and Kifer 1995; Leiner and Burd 1998). The concept is that, over time, the presence of the alkaline particles will bring about neutralization of any acidity within the paper. Commercially available non-aqueous dispersion programs include BookkeeperÒ (Preservation Technologies), ZfB:2 (Zentrum für Bucherhaltung), the previously available LibertecÒ, as well as products that have been sold for small-scale treatments under the trade name KrylonÒ.
When evaluating stored paper-based documents, the most important attribute from the perspective of archival institutions is longevity (Drewes and France 2012). Longevity-related data are typically determined by accelerated aging tests, followed by evaluation of selected properties reflecting paper stability, such as folding endurance or molar mass of cellulose. The values after accelerated aging are compared between treated and untreated species, and the relative increase in performance can be given as an efficacy factor, indicating how much longer the paper will last compared to the untreated reference. The advantage of the efficacy factor is that it is largely independent of the details of the analytical method used to assess paper stability.
Efficacy factors have been used to compare deacidified paper specimens with untreated specimens exposed to the same conditions of accelerated aging (Buchanan et al. 1994; Liénardy 1994; Whitmore 1994; Banik 2004, 2005; Banik et al. 2006; Hanus et al. 2008; Ramin et al. 2009; Katuscak 2006, 2009a, 2012; Ahn et al. 2013; Potthast and Ahn 2017). As will be detailed later in this article, relative to the other tested methods of whole-book deacidification, the non-aqueous dispersion treatment often have been found to be among the least effective. Earlier evaluations, also based on accelerated aging tests, have yielded a range of results for non-aqueous dispersion treatment (Pauk 1996; Vrska et al. 2004; Balažic et al. 2007).
During the period 2010 to 2012, a large evaluation of all important deacidification processes was carried out in the US and in Europe, including synthesis of all available objective data and meta-analysis (Ahn et al. 2012a). The results included a ranking of the six best mass deacidification processes (Katuscak et al. 2010, 2012). The sequence of the processes was judged to range from the best available technology (BAT) for deacidification of books – PapersaveÒ (non-aqueous solution), CSC BooksaverÒ (non-aqueous solution), and SoBu, down to the least effective BookkeeperÒ (non-aqueous dispersion) process. Paradoxically, though the non-aqueous dispersion method with MgO particles has become the most widely used method in the world, except for Europe, the study results showed that it can, at least sometimes, provide incomplete deacidification. It achieved low long-term efficacy of the mechanical permanence factors, as measured by the testing laboratories, implying that the treatment was less effective or even ineffective.
Doubts about Criteria Used to Predict Permanence
Concerns about composition-based criteria
Some investigators have raised questions about the criteria often used in predicting the storage-stability of paper (ISO 1994; Shahani 1995; Havermans 2002; Library of Congress 2004; Area and Cheradame 2011). One such criterion involves the pH of the paper (ANSI 1984; Daniels 1996; Ramin et al. 2009), a quantity that can be determined either by placing a drop of water on the paper surface (TAPPI Method T529), or by extracting the paper with aqueous solution (TAPPI Methods T435, T509; ISO 6588 2012), or possibly by redispersing some of the paper in water and then measuring the pH. Since all such measures involve liquid water, ordinary pH tests cannot answer the question as to whether a given deacidification treatment had already brought about neutralization of acidic sites within the paper prior to the test itself or whether such neutralization became completed only at the time of pH testing.
Another criterion often used is that archival-quality paper should contain a well-distributed alkaline reserve of a suitable compound, such as CaCO3 or MgCO3 throughout the material (Bukovský 2005; Ramin et al. 2009; Smith 2011; Ahn et al. 2012b, 2013; ISO 10716 1994b). An alkaline reserve level of 2% is specified in the ISO and ANSI standards for permanence of paper (ANSI 1984; ISO 1994a; Shahani 1995). The level of alkaline reserve can be determined by treating the paper sample with a standard amount of hydrochloric acid, then back-titrating with base (Liers 1999). It has been pointed out that, in order for the alkaline reserve species to be useful, they must somehow interact with acidic species that either were in the paper to begin with or were subsequently brought there by either hydrolysis reactions within the paper or by air pollution (Smith 1987; ASTM 2002b). Notably, deacidification involving non-aqueous dispersion treatment is totally reliant upon such dry-state interactions within the paper as a primary mechanism of deacidification (Whitmore 1994). Other widely used deacidification technologies, both non-aqueous and aqueous, employ soluble alkaline species that can be expected to migrate freely into small pores within the material, offering a chance to react directly with exposed acidic compounds present in the paper sheet.
Doubts also have been raised about the conditions used for accelerated aging tests. The conditions of accelerated aging, usually involving temperatures of 80 °C or higher, are quite likely to result in different chemical reactions and different degradation products in comparison to natural aging (Luner 1969; Kruth 1988). Some of the most widely used accelerated aging tests involve the presence of water vapor (e.g. TAPPI Method T544; ISO 5630-3). At a specified relative humidity value, the proportion of water molecules increases with the temperature of the air. On the one hand, the presence of water may be required in order to best simulate the process of normal aging, while speeding it up for the sake of making an early prediction (Zou et al. 1996a,b). A complication arises, however, if the paper to be evaluated contains both acidic and alkaline species within its microstructure. In such cases, there is reason to wonder whether or not accelerated aging with high temperature and with relative humidity value in the range 25 to 65%, which represents the range that has been used for accelerated aging tests, might inadvertently bring about completion of reaction between adjacent acidic and alkaline entities within the paper microstructure (Hubbe 2015). This topic will be considered in more detail later in this article.
Thermodynamics does not determine the rate of decomposition
Though books are thermodynamically unstable, many of them have the potential to last for at least a thousand years (Balažic et al. 2007; Ramin et al. 2009; Sundholm and Tahvanainen 2004; Vrska et al. 2004; Jeong et al. 2014). Light a match, and the same book may be consumed in minutes. Analogously, the “slow fires” of acid hydrolysis and oxidation can gradually break down the chemical structure of books, especially if acidity is present (Aspler 1989). Baking powder offers a common example of a product that comprises a meta-stable mixture of acidic and alkaline compounds (Ciullo 1994). Add water, and reaction takes place immediately (Derr et al. 2000). By contrast, when it is put on a shelf, the reaction may be so slow that it can be ignored in a busy kitchen (Ishii 1950; Wolke 2002). Such phenomena will be considered in more detail later in the article. How slowly do non-aqueous dispersion deacidification reactions take place, and what to do about it, are questions that will be addressed in this article. These questions are pertinent with respect to books that have been deacidified by non-aqueous dispersion treatments that distribute alkaline particles within the microstructure of the material.
BACKGROUND
To provide context for addressing questions about completion of deacidification, this section will summarize some key points about paper’s acidic nature, as well as the most important methods that have been used to deal with the consequences of such acidity.
Acidic Paper and the More Rapid Degradation of Books
Observations of paper aging and acidity
People have been concerned about the premature degradation of stored books and the poor quality of the paper for a long time (Murray 1824; Johnson 1891; MacAlister 1898). Gradually it became known that acidity is a key factor in shortening the useful life of books (Kohler and Hall 1925; Barrow 1965, 1974; Moll 1965; Koura and Krause 1978; Sclawy and Williams 1981). Barrow compared books manufactured during the years between 1500 and 1950 and observed a disturbing decline in folding endurance, especially during the period of manufacture between about 1670 and 1900. During the same period, the typical pH values of manufactured books became lower than 6 and continued to decrease to a range between 4.5 and about 5. Wilson and Parks (1980) reported related findings. A correlation between pH and the rate of strength loss was found for both accelerated and natural aging tests of the same books. Katuscak et al. (2010, 2012), who studied more recent specimens, observed a coincidence of low pH and low folding endurance of printing papers produced during the decades between 1900 and 1990. By contrast, some books from previous centuries have been found to remain in relatively good condition, and such findings were generally correlated to higher pH values (Stephens et al. 2008a). Especially low values of pH happened to be measured for samples manufactured in the 1960s and 1970s (Katuscak et al. 2012). But the situation was very different for the samples that had been manufactured in the 1980s and 1990s, for which the average pH of the paper was near neutral and the number of double-folds before breakage was dramatically higher. Not only were those relatively recent samples newer, but they also were not degrading as fast. What happened, in order to bring about that change, was almost certainly the emergence of alkaline papermaking practices, which in just a few years has come to be dominant for the production of printing papers (Hubbe 2005).
In addition to acidity present in paper at the time of its manufacture, acids are formed continually both in acidic and alkaline cellulose material due to hydrolysis or oxidation reactions, which will be described later. Also, some acidity accumulates through absorption of pollutants from the air, particularly in urban areas (Smith 1987). However, the detailed effects are different, depending on whether the paper’s microstructure is acidic or alkaline: The acids arising in the alkaline part of the microstructure, which contains alkaline reserve, presumably can be continually neutralized. The degradation in the alkaline paper continues very slowly, and most probably the effects of the likely predominant peeling reaction can be neglected (Green et al. 1977; Ahn et al. 2012c, 2013; Testova et al. 2014). Peeling reactions cause only splitting off of a single monomer unit per reaction, thus decreasing the degree of polymerization (DP) by 1, for example from DP = 1000 to DP = 999 per 1 split, or to the DP = 998 per 2 splits, etc. Such a process would result in a negligible difference of the DP, longevity, brittleness, folding endurance, usability, and other mechanical and chemical quantities of paper or books. Another alkaline degradation mechanism, the beta-elimination reaction, could be detected in the deacidified book papers (Ahn et al. 2012c, 2013). However, it had little influence on the molar mass of cellulose in the paper compared to the beneficial effects obtained by deacidification treatment.
In any acidic paper, even if it contains alkaline particles on its surface or within some of the larger pore spaces, there may be an interior acidic region (i.e. an acid core) or acid remaining in microstructures of the paper after nonuniform or incomplete deacidification (Buchanan et al. 1994; Katuscak et al. 2012; Hubbe 2015). Within such zones the pH would tend to be further decreased by continually arising acids. Acid hydrolysis can cause rapid random cellulose degradation, in which a handful of breaks in a chain can reduce the cellulose DP in paper from an initial high value to the range 300 to 700, at which point the paper is subject to crumbling (Zou et al. 1996a; Smith 2004). Zou et al. (1996a) observed a dramatic drop in tensile strength and folding endurance after the DP of cellulose chains within paper fell below about 700. Likewise, Smith (2004) suggested that the range of 400 to 500 is a critical range of DP, beyond which paper falls apart when folded. According to Jeong et al. (2014) the critical value for DP was found to be about 1000 in the case of Hanji papers. Thus, the level of DP needed to maintain the strength of paper may depend on the type of paper. Zervos and Moropoulou (2005) found a high correlation between the DP of cotton cellulose and strength properties of paper made from the fibers. When considering such data, however, it should be kept in mind that the correlation between cellulose DP and paper strength can be different for different test procedures. Such results must be considered cautiously, since paper’s strength also can be highly affected by localized damage that within the fibers during pulping operations (Gurnagul et al. 1992).
Moisture content
The mechanism of acid hydrolysis, leading to the breakdown of cellulose, requires the presence of water molecules (Williams 1971; Baty et al. 2010). The fact that acid hydrolysis can take place at typical humidities and temperatures found in libraries provides evidence that bulk liquid water is not required. Rather, the amount of adsorbed or hydrated water associated with the cellulosic material (Joubert et al. 1959) appears to be sufficient. On the other hand, many studies have reported increasing rates of hydrolytic decomposition with increasing relative humidity (Zou et al. 1996a; Baty et al. 2010; Zervos 2010). Also, the activation energy for the hydrolysis reaction appears to become lower when the humidity is higher (Gray 1969, 1977). Some investigators have maintained that water molecules need to be present during accelerated aging in order to achieve a degradation process having a predictive value for natural aging (Graminski et al. 1979; Wilson and Parks 1983; Shahani et al. 2001). Luner (1969) urged caution in interpreting results from accelerated aging tests in general based on a concern that the mode of failure is likely to be different at elevated temperatures.
Catastrophic failure and auto-catalysis
A frightening aspect of the aging of books is a tendency for catastrophic failure to occur after the aging process has reached a certain stage of decomposition. Rather than taking place at a steady rate, with easily predicted results, many researchers have found evidence that acid-catalyzed breakdown of books often takes place with increasing speed (Calvini et al. 2007, 2008; Zervos 2007). This situation has been attributed to the production of acetic and formic acids during acid hydrolysis of some of the hemicellulose macromolecules or other components of the paper (Polovka et al. 2006; Zervos 2007; Jablonsky et al. 2012a). The term “autocatalysis” can be applied to this situation, since the acetic and formic acids produced by the paper’s decomposition further raise the level of acidity. The fact that books often degrade faster than separate sheets of similar paper (Shahani et al. 1989; Shahani 1995) has been attributed to entrapment of acidic decomposition products within stored books.
It is also important to keep in mind that acids are formed continually in paper by oxidation with oxygen, ozone, nitrogen, sulfur oxides, and other oxidants, in both acidic and alkaline cellulosic material, including paper and books during the whole paper lifetime (Katuscak et al. 2006, 2009b, 2016; Vizarova et al. 2016a,b). Acids arising from oxidation presumably can be continually neutralized by alkaline reserve, assuming that contact is achieved locally between the alkaline and acidic substances. However, the neutralization of acids does not stop their formation. Continuing generation of acidic species in paper might help to explain observations of a gradual drift to lower surface pH values over time (Ahn et al. 2011).
Key Factors Affecting Books and Papers
Alum and wood-derived acidity
Aluminum sulfate (or “papermaker’s alum) is often the major source of acidity in typical paper products (Kohler and Hall 1925; Arnson and Stratton 1983; Wittekind 1994). Alum was used in manufacturing most printing paper from the early 1800s up to about 1990 (Hubbe 2005; Cedzova et al. 2006). Its purpose included the “setting” of rosin products in the paper, rendering the paper suitably resistant to the penetration of aqueous fluids. In addition, in the case of paper containing groundwood fibers or other mechanical pulp fibers, acidity was contributed by fatty acids and resin acids from the wood (Back and Allen 2000). The latter effect may explain why some researchers have been especially concerned about the acid-catalyzed hydrolytic degradation during storage of books containing mechanically pulped fibers (Barrow 1953; Bukovský 1999; Kacik et al. 2009). Though alum and natural acidic compounds present in wood appear to play a major role in reaching an initial damaging level of breakdown of cellulose, it appears that much of their effect is indirect. As already noted, acid-catalyzed hydrolysis of the ester groups from the hemicellulose components of the fibers in paper gives rise to acetic acid, which can further lower the pH and accelerate the decomposition process.
Acidic pollutants in the air
Though acid rain has become a well-known phenomenon in modern times, air pollution of various types has been known for a long time to hurt the storage stability of books ( Smith 1987; Dupont et al. 2002). A test method has been established to evaluate such effects (ASTM D 6833-02, 2002b). Acid gases such as SO2 and NO2 (Smith 1969; Dupont et al. 2002) have been implicated in the damage to books, especially in certain urban libraries.
An especially damaging form of air pollution involves oxidation of the paper (Arney et al. 1979; Selli et al. 1998). Ozone coming from electrical equipment is a likely source of such oxidation in libraries (Andretta et al. 2016). Daniels (1996) showed that such effects can be accelerated by the presence of iron and copper in paper. Whitmore and Bogaard (1995) and Stephens et al. (2009) showed that oxidation tends to make the paper more susceptible to mechanical breakdown during subsequent acid hydrolysis.
Deacidification Treatments
To provide context for later discussion concerning non-aqueous dispersion-type deacidification, some points about deacidification processes in general will be summarized here. Such processes will be roughly divided as aqueous systems, non-aqueous systems involving dissolved alkali species, and non-aqueous systems involving suspensions of particles, i.e. non-aqueous dispersion systems.
Many of the acids likely to be present in paper, which may be inorganic acids such as sulfuric acid, or organic acids such as fatty acids, acetic acid, of formic acid, all can be classified as Brønsted acids (Pearson 1969; Hrivnak 2007; Cox 2013). Such acids can interact with water to form the H3O+ hydrated ion, which becomes the active species for many subsequent reactions. In addition, various aluminum compounds, which are often classified as Lewis acids, can contribute to acidity in the paper by consuming OH– ions. It follows logically that aqueous solutions can be used to neutralize acidic species present in paper (Barrow 1965; Kolar and Novak 1996; Wächter et al. 1998; Sundholm and Tahvanainen 2003a,b; Smith 2011). Bredereck et al. (1990) compared a number of different alkaline compounds to use for aqueous deacidification; it was concluded that solutions of either Mg(HCO3)2 or a mixture of Mg and Ca bicarbonates is the best choice for treatment of acidic paper. According to Middleton et al. (1996), for deacidification to be complete, the protonated forms of carboxylic acids all need to be converted to their respective conjugate bases, e.g. the Ca or Na salts of carboxylic acids. A fuller discussion of these issues is provided by Smith (2011).
In addition to direct neutralization of acidic species present in the paper, aqueous treatments (as in the case of non-aqueous treatments) can be used to leave behind an alkaline reserve. In other words, the paper is provided with a capacity to consume newly-formed acidity or acidic compounds that may reach the paper through the air or due to reactions within the material. As an example, a compound such as sodium bicarbonate may precipitate out of solution and remain in the paper when it is dried after the treatment. Another potential advantage of aqueous treatments is that they can be used to wash the paper free of dirt, as well to remove products of hydrolytic decomposition, which may include acidic compounds (Tang 1981; Bogaard and Whitmore 2001; Zervos 2007; Zervos and Alexopoulou 2015).
Certain potentially unfavorable aspects of aqueous-based deacidification appear to have provided the main motivation for widespread adoption of non-aqueous deacidification systems. For instance, if a book is dropped into a sink of water, the paper can become wavy (Brückle and Banik 2011b) and a lengthy drying period may be needed (Smith 1992). Each individual page tends to expand when it is wetted by water, increasing its thickness (Scallan and Tigerström 1992; Enomae and Lepoutre 1998). Such swelling may exceed the binder’s capacity, causing the book to break (Kellerman 1999). When deacidifying individual sheets of paper documents, such development of waviness can be avoided by drying the sheets individually against a smooth surface (Hubbe and Bowden 2009) or by other means of restraint during drying (Brückle and Banik 2011b). In the case of books, freeze-drying has been used successfully to avoid deformation of pages (Liénardy 1994; Jablonsky et al. 2009a; Hubacek et al. 2011). To overcome these issues concerning the aqueous treatment of bound books, it is common practice to remove and replace the original binder for the book, allowing the pages to be treated and dried individually. Advantages of this approach include optional washing of the pages, the ability to directly neutralize acidic groups present in the material, the ability to apply pH-buffering agents and/or alkaline particles, the ability to treat the paper with reducing agents such as borohydride, and the opportunity to apply dissolved starch or other hydrophilic polymers able to increase the strength of the paper. The down-side of such an approach is that it requires a considerable expense and time to disassemble and reassemble each treated book.
Other concerns about aqueous treatment include possible bleeding or dissolution of water-susceptible print colorants (Banik and Brückle 2011), a tendency of paper to become more bulky (thicker pages) after a cycle of wetting and drying (Bristow 1971; Banik and Brückle 2011), and the sticking together of pages after redrying of bound volumes, especially if the paper had been coated during its manufacture (Piltonen et al. 2013). For all of these reasons, when bound volumes need to be acidified, non-aqueous deacidification programs or treatments involving freeze drying processes generally have been selected.
Non-aqueous Media for Deacidification Systems
To avoid the issues described above (such as possible bleeding of colorants, waviness of paper, or sticking together of pages), researchers have been motivated to consider non-aqueous fluids as a possible means of transporting alkaline agents into paper documents. One of the key attributes of such a medium is that it ought not to solubilize common types of ink (Wittekind et al. 1994). Some of the earliest non-aqueous treatments were done with methanol, which did not completely avoid ink solubilization and also is quite flammable (Baynes-Cope 1969). Sequeira et al. (2006) describe more recent work employing isopropanol. Another desirable attribute of a liquid medium for deacidification is that the fluid ought to completely wet the paper with a near-zero contact angle (Hubbe et al. 2015) such that it can readily penetrate small pores in the material. Both objectives – noninteraction with inks and excellent wetting – have been demonstrated in systems employing low-surface tension perfluorinated liquids (Smith 1977; Leiner and Kifer 1995; Dupont et al. 2002; Polovka 2006). Clark et al. (1998) studied the use of hexamethyldisiloxane (HMDS) as a low-energy suspending medium with proven utility for deacidification. European mass deacidification processes are mainly being carried out with hexamethyldisiloxane (for non-aqueous solution treatments) or heptane (for non-aqueous dispersions of MgO) (Zentrum für Bucherhaltung 2017). A perfluorinated liquid is employed as the carrier in the case of the widely-used BookkeeperÒ process (Preservation Technologies 2017).
Even after committing oneself to carrying out non-aqueous deacidification, there are several general approaches that can be used. For instance, certain alkaline agents may be soluble within the selected non-aqueous fluid; in principle such soluble agents could be used to directly neutralize acidic species within the paper document. Alternatively, the medium might consist of a gas or plasma. And finally, the alkaline agent could be dispersed as small particles in the medium.
Non-aqueous Treatments with Soluble Alkaline Agents
Certain organometallic compounds can be dissolved in low-energy liquids, allowing them to be distributed in monomeric form into paper documents. Such compounds also typically have low volatility. Methylmagnesium carbonate is an example of such a compound that can be deposited within the paper upon evaporation of the carrier liquid, bringing about deacidification of the paper (Kelly et al. 1977; Green and Leese 1991; Bukovský 1999; Andres et al. 2008). Because the alkaline agent is dissolved, and because the low-energy liquid can be expected to completely wet the document, it is reasonable to hypothesize that acid groups located throughout the structure will be neutralized. At least there is potential for the alkaline agent to diffuse efficiently to all parts of the paper and fiber that can be wetted by the liquid. A likely exception is that acidic groups within the cell walls of the cellulosic fibers may be inaccessible due to pore closure during drying (Scallan 1990). In addition, Kelley and Fowler (1978) noted a likely problem in such systems when the alkaline compound is not sufficiently soluble in the carrier fluid; in such cases the alkaline agent may lag behind the liquid front as the paper is being permeated, thus possibly failing to deacidify the most interior parts of the documents.
Certain metal alkoxides meet the criteria of being soluble in low-energy liquids, and they also have the potential advantage of having a relatively high reactivity with water. The agent can react with residual water in the paper, thereby neutralizing acid groups and becoming firmly insolubilized (Smith 1970). Baynes-Cope (1969) reported early research carried out in 1961 related to such a system. That work revealed that magnesium methoxide can be highly effective as a deacidification agent. However, the material was judged to be not stable enough for practical usage.
During the period between 1992 and 1995 (see weito.com) the National Library of Canada employed a system based on a mixture of magnesium methylcarbonate and magnesium methoxide in a fluorocarbon solvent (Smith 1970, 1977, 1988; Scott 1987; Morrow 1988). A related system, using alkoxides in a hexamethyldisiloxane carrier liquid, was developed by Battelle Laboratories (Wittekind 1994); this system has been mainly implemented in Germany and Switzerland (PapersaveÒ and Papersave SwissÒ). Reaction of the alkoxide (magnesium and titanium ethoxide, METE) with residual water in the paper (maybe 0.5% after a pre-drying step) converts the organomagnesium and titanium to, e.g., Mg(OH)2; the Mg(OH)2 formed initially or during a subsequent conditioning period is able to react with CO2 from the air, forming MgCO3 (Polovka et al. 2006). For the Booksaver process, for which no drying step is usually needed, the active species is n-propoxycarbonate (Henniges et al. 2004, 2012).
Figure 1 indicates the main steps in the type of treatments just described for PapersaveÒ. It should be noted that the purpose of the initial drying step is not to remove every molecule of water. Rather, the goal is to have an optimized moisture content so that the reactive compound or compounds are able to diffuse essentially all the way to the cellulose fiber surfaces and then be converted to the hydroxide form when they encounter water molecules associated with those surfaces. Such a sequence can be expected to favor efficient uptake of the oxide and its byproducts both at the fiber surfaces and within pores.
In principle, the systems just described share a number of desirable attributes: Because they rely on non-aqueous carriers (e.g. fluorocarbons or hexamethyldisilazane) having no hydrogen bonding ability, no distortion of the paper is expected. Because the compound is converted into Mg(OH)2 or MgCO3, an alkaline reserve material becomes deposited within the paper. The first versions of these systems sometimes exhibited visible powder deposits or sometimes not enough alkaline reserve; however, continuing developments have made the systems suitable for most kinds of books, and they have become the most widely used systems in Germany and Switzerland (Booksaver®, Papersave®, Papersave Swiss®). Coated papers can represent a particular challenge due to the very fine porosity of the surface layers, which can slow down the permeation of liquids (MacInnes and Barron 1992).
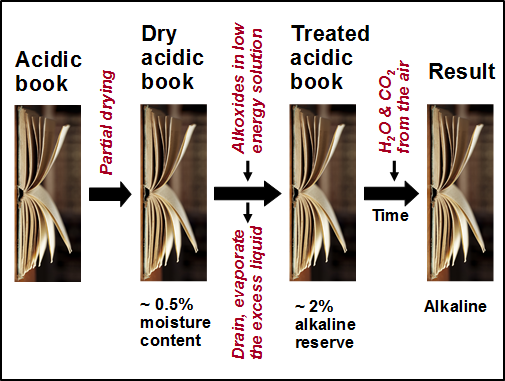
Fig. 1. Schematic procedure for deacidification of bound volumes by treatment with an alkoxide-type reagent dissolved in a low-energy liquid
Gas-phase treatments
Certain organic amine compounds have potential to be applied to paper in gaseous form, such that they have potential to react directly with acidic species present in the paper. For instance, Walker (1977) found that paper may be effectively deacidified by morpholine treatment. Koura and Krause (1980), described a similar approach using ammonia gas. Both agents have a relative strong surface tension, which raises concerns about possible solubilization of inks. An inherent drawback of any treatment that relies completely upon treatment with a volatile organic alkali such as ammonia or morpholine is that essentially no alkaline reserve is left behind in the paper (Cheradame et al. 2003).
A gaseous di-ethyl zinc deacidification system was implemented during the period from 1982 to 1993 for deacidification of books and papers held by the Library of Congress (Sparks 1987; Dufour and Havermans 2001; Cheradame et al. 2003). As in the metal alkoxide systems just described, the DEZ system entails interaction of the reagent with moisture in the paper to become transformed to Zn(OH)2 or the corresponding oxide form (MacInnes and Barron 1992). According to the cited work, distribution of ZnO particles within the documents was quite uniform. Due to operational difficulties, possibly related to the high reactivity of DEZ, the system is no longer being used.
Recently Li et al. (2014) described a newly developed system in which a plasma was formed from a mixture of argon gas and saturated Ca(OH)2 solution. This is an example of a cold-plasma system, meaning that a minor fraction of free-radical ionized species is present in a mixture together with ordinary gas molecules. The plasma was directed toward sheets of acidic paper. The authors reported an increase in the pH of the paper, whereas other properties of the paper were unaffected. A concern about plasma-based systems is that they have been found to be one-sided, mainly affecting the side of an object to which the beam is directed (Mukhopadhyay and Fangueiro 2009). In any case, more research is needed to explore the mechanisms and potential utility of plasma treatments to bring about deacidification.
Non-reactive, Non-aqueous Dispersion Treatments
Since about 1994 the US Library of Congress has been relying mainly on a system in which suspensions of MgO particles in a non-aqueous fluid are used to meet their requirements for deacidification (Leiner and Kifer 1995; Leiner and Burd 1998). The books are treated under ambient temperature and pressure while being held so that their pages are fanned open. The suspension is sprayed inward in such a way as to favor adequate treatment of the spine area (Leiner and Burd 1998), which otherwise might be the least accessible part of a bound book. Polovka et al. (2006) demonstrated that the deposited MgO particles were able to be converted to Mg(OH)2 when present in the paper, and that the hydroxide was further able to interact with CO2 from the air, yielding MgCO3. The earliest related system may have been that described by Baynes-Cope (1969), who employed a mixture of barium hydroxide and methanol to treat acidic books. The cited work describes the employed mixture as a solution, though subsequent work has shown only weak solubility of barium hydroxide in methanol (Gryglewicz 1999).
Particle distribution
Because the particles employed in the non-aqueous dispersion system are typically about a micrometer in size, one can expect that the particles will be effectively excluded from the interior of fiber cell walls. In fact, studies have shown that the particles mainly become situated on the external surfaces of sheets of paper, rather than in the interior of the sheets (Ramin et al. 2009). When non-aqueous dispersion treatment was applied to one side of paper sheets by spray application, none of the applied magnesium could be detected on the opposite sides of those specimens (Stauderman et al. 1996). The last-cited authors also found that the distribution of particles on the treated surface could be nonuniform, though their findings might have been associated with the hand-spray procedures that they employed in that work. For mechanized, mass-deacidification treatment, Wagner et al. (2008) observed a suitably uniform distribution of particles, from the perspective of the surface of the paper, based on imaging with laser ablation inductivity coupled plasma mass spectroscopy. Wojciak (2015) found that a more uniform distribution can be achieved by using nano-sized particles.
Zumbühl and Wuelfert (2001) evaluated the role of perfluorinated dispersants employed in a major non-aqueous dispersion deacidification program. The surfactant was reported to play an important role in uniform distribution of the particles.
Assumed chemical reactions in non-aqueous dispersion treatment
Once the MgO particles have been distributed onto or into the pages of a book, a series of reactions has been assumed to occur over an unspecified time period. These reactions are represented here by Eqs. 1 through 3:
MgO + H2O ® Mg(OH)2 (1)
Mg(OH)2 + CO2 (from the air) ® MgCO3 + H2O (2)
MgCO3 + (Acidic species in the paper) ® Neutralization (3)
Presumably the reaction of MgO with H2O could already take place during the deacidification treatment, since there is no oven-drying step to remove equilibrated water before non-aqueous deacidification according to descriptions of the procedures used (Buchanan et al. 1994; Burd 2012). In addition, once the treated books are returned to usual conditions of storage, where humidities of at least 50% are common, the conditions for completion of the reaction in Eq. 1 would be improved due to higher water content (typically within the range 5 to 10% in a book under typical conditions). A study by Newberg et al. (2011) using X-ray photoelectron spectroscopy (XPS) showed that such a reaction was favored by increasing humidity, and that Mg(OH)2 tended to form initially at the surface of MgO. Notably, the formation of hydroxide already was important at a relative humidity of 10%, which is well below the range experienced during typical storage of books. Work by Sutcu et al. (2009) showed that the (1 1 1) crystal face of MgO particles was most susceptible to hydration by exposure to water vapor and a relative humidity of 88% in the cited work. Research by Baumann et al. (2015) has shown that the rate of dissolution of MgO particle increases with their decreasing size, and that dissolution takes place more slowly if a stable layer of Mg(OH)2 has formed on the particle surfaces.
The further reaction of the material to form MgCO3 (Eq. 2) also is supported by the literature. Vandeperre and Al-Tabbaa (2007) found that MgO could be converted to MgCO3 at either 65% or 98% relative humidity in the presence of CO2 in the air. Both reactions, including the hydration and then then the carbonation, were supported by infrared absorption analysis by Polovka et al. (2006). Those researchers utilized 81% and 98% relative humidity conditions and ambient levels of CO2 at a temperature of 80 °C and 5 days of exposure. Only minor changes were noted at lower temperatures, including 35 °C, so it is unclear whether or not the reaction with CO2 (Eq. 2) would be brought to completion under ordinary conditions of deacidification, conditioning, and storage.
Equation 3, as shown above, is in fact the central question of the present discussion. Presumably, if MgCO3 particles present in dry paper were effective in neutralizing acidic species also in the sheet, then the same could be assumed for any remaining MgO or Mg(OH)2 species. In an effort to be more specific, suppose that acetic acid is the acidic species in the sheet that interacts with MgCO3 particles. A balanced equation then can be written in the form,
MgCO3 + 2HOAc ® Mg(OAc)2 + H2O + CO2 ↑ (4)
where the upward arrow suggests that the CO2 may be released to the atmosphere. Similarly, balanced equations can be written that result in hypothetical bicarbonate species:
MgCO3 + HOAc ® MgHCO3(OAc) (5)
or 2MgCO3 + 2HOAc ® Mg(HCO3)2 + Mg(OAc)2 (6)
Though Eqs. 4 through 6 appear to be plausible, there is as yet no firm evidence whether or not such reactions occur after the non-aqueous dispersion deacidification treatment of paper. At 80 °C Polovska et al. (2006) found evidence of conversion of carboxylic acid groups to the carboxylate form, which is consistent with the completion of a deacidification reaction under the high relative humidity conditions employed. However, to achieve clear effects corresponding to completing of the neutralization reaction, the cited authors had to employ not only high temperature but also high humidity (81% or more) during a 5-day time of exposure. Since those conditions are quite unlike what has been used for deacidification, they do not settle the question of whether Eqs. 3, 4, 5, of 6 take place during non-aqueous dispersion treatment, subsequent conditioning, or subsequent storage of books.
Based on the mechanisms just described, Fig. 2 presents the main steps involved in a non-aqueous dispersion deacidification treatment. As depicted, the book to be treated is assumed to contain about 4 to 10% moisture as a consequence of equilibration with ambient air in typical ranges of relative humidity. The deacidification treatment involves MgO particles dispersed in a low-energy liquid, which is then drained from the paper material, followed by evaporation. As depicted, the alkaline particles left behind in the paper after such treatment can be roughly 2%, depending on the targeted level. After reaction of the MgO with water (perhaps adsorbed water already present in the paper), and with CO2 from the air, it is expected that much or all of the MgO will have become converted to Mg(OH)2 or MgCO3 by the time the post-conditioning period is done and the books are ready to be returned to library stacks.
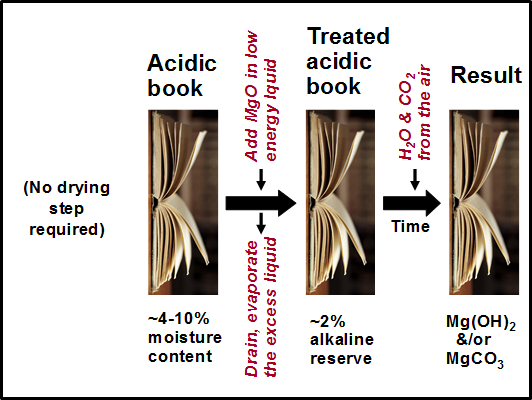
Fig. 2. Main steps in the non-aqueous dispersion deacidification of bound volumes
Proximity suggesting eventual interaction
Various evidence in the published literature supports an assumption that acidic and alkaline substances within dry material such as paper will be able to react with each other, over time, to bring about neutralization. In particular, certain acidic substances appear to be capable of migrating from one point to another. Barrow (1953) reported that organic acid materials were able to migrate from one sheet to another in bound documents having different composition in their covers vs. their interiors. For example, the cover of an 1857 booklet, which contained mechanical pulp fibers, was found to have influenced the properties of adjacent sheets of inherently higher-quality paper. The first three sheets below the cover had folding endurance values of 16, 116, and 328, sequentially. In the case of a 1760 record book having a groundwood cover the first three pages in the interior had folding endurances of zero, 9, and 10. Likewise, it has also been shown that fatty acids are able to migrate from the interior to the outsides of paper sheets (Swanson and Cordingly 1959).
Based on the migration mechanism just suggested, Fig. 3 presents a hypothesis regarding the function of alkaline particles in a bound volume that has been subjected to non-aqueous dispersion deacidification treatment. The left frame of the figure represents the auto-catalytic role of acidic species such as acetic acid (without assuming that acetic acid is the major species in a given case) when they are held within a bound volume during storage. The expanding arrow represents the migration of the acidic compound, resulting in hydrolysis of polysaccharides (cellulose and hemicellulose) or hydrolytic deacetylation of hemicellulose and the release of increasing amounts of acetic acid. The right-hand frame depicts the idea of one such acidic molecule colliding with an active site on an alkaline particle, thus potentially breaking the catalytic cycle. The process depicted in Fig. 3, if valid, suggests that smaller or better distributed alkaline particles (with a fixed total mass) might be more effective at neutralizing mobile acidic species that migrate from other sites within the paper material.
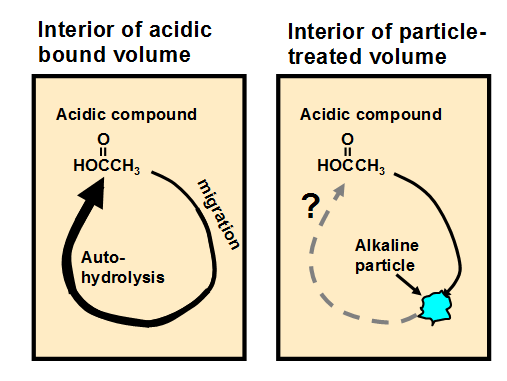
Fig. 3. Potential role of alkaline particles in breaking the cycle of auto-catalytic hydrolysis when acidic compounds such as acetic acid are able to hydrolyze hemicellulose in the fibers, thus releasing more acetic acid
The hypothetical mechanism just described depends on an assumption that acidic species such as the protonated forms of acetic acid and formic acid are able to move from one point to another at a sufficient rate to make a difference. Such migration and effectiveness is supported by various publications (Barrow 1953; Nerin et al. 1998; Wenzl and Lankmayr 2000; Banik 2005; Area and Cheradame 2011). But the present review of the literature did not reveal evidence of corresponding migration of alkaline species. The vapor pressure of inorganic compounds such as MgO and CaCO3 can be expected to be extremely low at typical conditions of storage of books (Lide and Kehiaian 1994). In addition, conjugate bases that might be present in paper (e.g. sodium acetate or calcium acetate), due to their polar nature, can be expected to have much lower vapor pressures than their protonated counterparts (e.g. acetic acid) (Barsanti et al. 2009). Another example of the very low vapor pressures inherent in ionic compounds is provided by ionic liquids (Earle and Seddon 2000). Based on these considerations, there is only limited evidence that the presence of alkaline particles within paper would result in sufficient migration of alkaline species toward acidic sites to bring about neutralization (see later discussion concerning litmus paper tests).
Evidence of efficacy based on paper strength
As already noted, the value of a stored book depends on its usability over an extended time (Drewes and France 2012; Johnson et al. 2012). While estimating the absolute longevity in years is of questionable reliability (Shahani 1995), a meaningful evaluation can be made by testing the relative increase of stability of the paper in comparison with acidic non-deacidified paper from the same source. This has been called efficacy and used for the quantitative evaluation of deacidification process (Buchanan et al. 1994; Katuscak et al. 2010, 2012; Drewes and France 2012).
Table 2. Results of Accelerated Aging and Strength or DP Evaluation Testing of Paper Deacidified by the Non-aqueous Dispersion Treatment System *
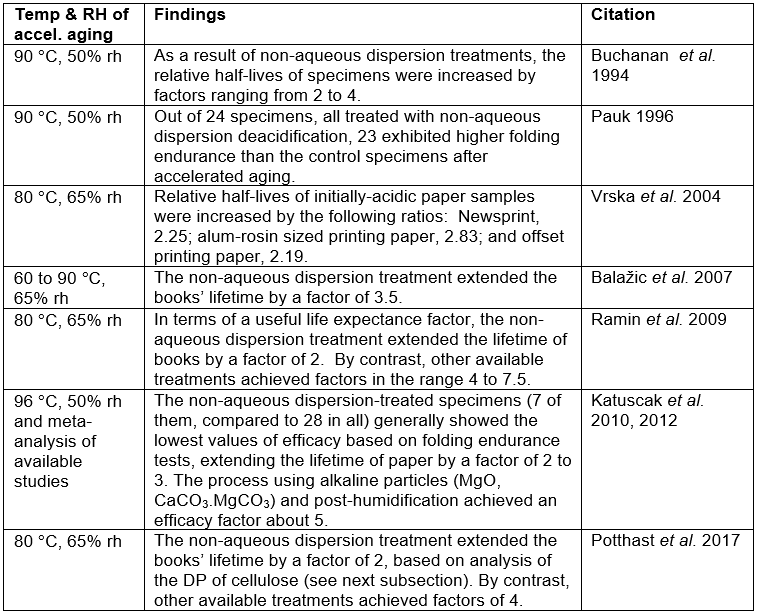
* Efficacy factors included in the table generally compare the time of accelerated aging needed to reduce paper strength or DP of deacidified paper to a critical level; the base case consists of specimens from the same source but without the deacidification. Strength criteria (or DP), as well as other details, differ from study to study.
The efficacy of the conservation process can be quantified as a coefficient expressing by how many times the process prolongs longevity, in terms of an evaluated property such as folding endurance, tensile strength, or degree or polymerization. Published evaluations of the effectiveness of non-aqueous dispersion deacidification have shown mixed results, especially with respect to the evaluation of paper strength after accelerated aging. Results of different studies dealing with the efficacy of the non-aqueous dispersion deacidification program are summarized in Table 2.
Evidence of efficacy based on cellulose degree of polymerization
A recently established standard (ISO/TS 183444:2016) can be expected to be adopted in various future studies related to those listed in Table 2. The ISO/TS provides test methods, minimum requirements, and levels of replication for accelerated aging tests. In addition, one of the key elements of the new standard is its reliance on evaluation of changes in the degree of polymerization of paper specimens exposed to accelerated aging. Potthast and Ahn (2017) used such an approach when conducting parallel evaluations of two kinds of deacidification treatments – non-aqueous dispersion and solution-based treatments. The tests were carried out with typical acidic paper from selected books. Accelerated aging was performed under conditions of 80 °C, 65% relative humidity, and 40 days of exposure. At the end of that period, the paper that had been deacidified by the non-aqueous dispersion process showed an effectiveness factor of about 1.8, based on a lower rate of the degree of chain scissions of the cellulose molecule when comparing the control specimens to those that had been deacidified. The corresponding factor was about 4.0 when comparing a control specimen to paper that had been deacidified by a non-aqueous solution-based process. In other words, the solution-based treatment was much more effective. In the described experiments, the effectiveness factor can be defined by the following equation,
Fe = [(DPo-DP)/DP]control / [(DPo-DP)/DP]deacidified
where DPo is the degree of polymerization of the cellulose in the paper prior to accelerated aging, DP is the degree of polymerization of the cellulose at the end of the exposure.
POST-DEACIDIFICATION CONCERNS
Evidence of Continued Acidity in some Deacidified Books and Papers
Various evidence can be found within the published literature relative to whether or not non-aqueous dispersion deacidification treatment has been successful, in various cases, in neutralizing acidic groups within the treated documents. Such evidence, to be discussed in this section, involves such factors as mechanical strength after accelerated aging, the continued evolution of gaseous products from the paper, the color of pH-sensitive dyes in the paper, and spectroscopic information that sheds light on the degree of neutralization of carboxylic acid groups within the paper.
Acidic species within a dry sheet of paper or book may be present in various forms, and they may be either at the surface or within the nanostructure of fiber cell walls. They can include weak acids, such as acetic acid and the resin acids of wood, and they may include strong acids, such as sulfuric acid (Katz et al. 1984). Protonated carboxyl groups, in particular, are known to be very common in cellulose-based materials (Scallan et al. 1989; Middleton et al. 1996).
Efficacy factors
If one refers back to Table 2, although the non-aqueous dispersion system was successful in some of the evaluations in extending the projected useful life of treated acidic paper (Buchanan et al. 1994; Hanus et al. 2008; Ramin et al. 2009; Banik 2005; Banik et al. 2006; Katuscak et al. 2010, 2012; Ahn 2013), not all such tests met the Library of Congress requirement of achieving an efficacy factor of at least 3 (Buchanan et al. 1994; Vrska et al. 2004; Ramin et al. 2009; Potthast and Ahn 2017). Based on some recent tests, the non-aqueous dispersion systems were judged to be less effective than several different deacidification programs that were considered (Katuscak et al. 2010, 2012). It should be noted that an addendum to the publication by Buchanan et al. (1994) indicated higher lifetimes for items treated with a non-aqueous dispersion system. The originally presented results were obtained with a 1 kg mass during the folding endurance tests, and the follow-up evaluations were with a 0.5 kg mass. However, in general the non-aqueous dispersion deacidification processes (e.g. BookkeeperÒ, ZfB:2, and formerly LibertecÒ) have been judged to be significantly less effective in achieving the desired neutralization (Blüher et al. 2006).
Table 3 shows results based on collected data from a consortium project (Katuscak et al. 2012, see Fig. 6 of that work).
Table 3. Mechanical Permanence Factors Reported for Documents Deacidified by Different Programs *
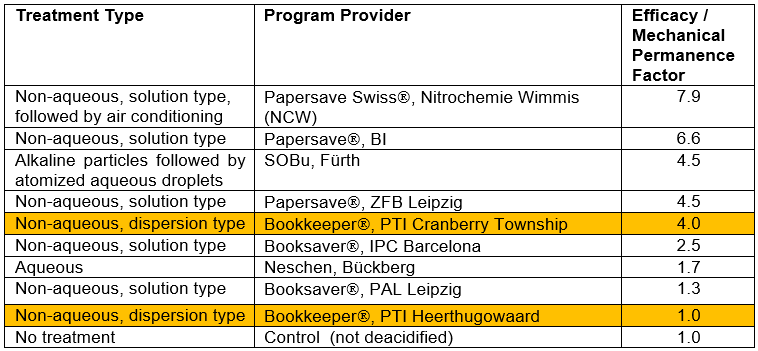
* Katuscak et al. 2012, see Fig. 6; the efficacy / mechanical performance factor was determined by comparing the time of accelerated aging (96 °C, 50% relative humidity) for the logarithm of folding endurance to reach zero in the case of modified vs. control samples, Stw = tlog w = 0,m / tlog w = 0,n .
As shown, two specimens that had been deacidified by the non-aqueous dispersion program exhibited efficacy factors that were, respectively, 1 (not significantly different from the untreated acidic paper control) and 4, whereas the most successful deacidification method increased the life expectancy factor to 7.9. Results corresponding to deacidification by means of non-aqueous dispersion treatment are shown in Table 3 with highlighting. It should be noted that data collected in the table had been combined from different sources; future studies are needed in which such comparisons are done under controlled conditions in an independent laboratory.
A larger study, reported in the same article (Katuscak et al. 2012), gave a multi-factor assessment rating of deacidified documents, included nine non-aqueous dispersion deacidified specimens among a total of 31 cases considered. The nine specimens deacidified by the non-aqueous dispersion program were among the 15 lowest-rated cases reported in the meta-analysis, the synthetic comparative analysis of all available objective measurements and studies. Such findings cannot be completely attributed to differences in deacidification, since some of the other systems involved application of strengthening agents.
Gas evolution
Banik (2005) reported that specimens of books treated by a non-aqueous dispersion program still contained a high concentration of acids. The acid levels were comparable with those of the untreated control acid paper. This was demonstrated by evaluating the volatile organic compounds (VOCs) in the headspace. The acid VOC concentration was approximately the same as for untreated books, under matched conditions of testing. Papers deacidified by non-aqueous dispersion processes, with two commercial programs (Bookkeeper® and Libertec®) judged to be relatively ineffective in bringing about the desired neutralization of acids in the paper. In contrast to the non-aqueous dispersion processes, the concentration of the volatile organic acids above the paper surface deacidified by aqueous-based treatments were decreased substantially in comparison with the acidic paper.
pH-sensitive dyes and litmus paper
It is well known that certain dyes can be employed in order to sense the pH in wetted paper (Walpole 1913). In principle, if a deacidification agent is able to neutralize acidic groups in the paper, then one would expect there to be a corresponding change in color of the dye. To demonstrate this, Middleton et al. (1996) placed blue litmus paper strips adjacent to acidic and alkaline paper sheets, all previously equilibrated to laboratory conditions (50% relative humidity). They found that the litmus paper strips adjacent to the acidic paper would turn red over the course of a day or so. Then, if the litmus paper strips were placed adjacent to an alkaline paper sheet, the original blue color would return after another day or two. However, Whitmore and Bogaard (1994) observed paper sheets in which the litmus dye color indicated the persistence of acidic conditions even after non-aqueous dispersion deacidification. The cited researchers concluded that the tested papers had not been successfully deacidified in those specimens, possibly due to a higher initial level of acidity compared to some other specimens that they tested. As noted earlier (Hubbe 2015), subsequent conversion of the litmus paper to a blue color, suggesting successful deacidification, could be achieved by exposure of the deacidified samples to high humidity.
Water, when it is used for pH measurement by a water extraction method, or even when a small drop of water is used for the traditional or standardized pH measurements, can cause redistribution of acidic or alkali ions or compounds determining the pH from one place to another. For example, such water can redistribute soluble acidic or alkaline species from the measured paper surface into the inner structure, pores, cell lumens, or inner surface of cellulosic fibers, as well as into cellulose macromolecules (Katuscak et al. 2016). A pH-sensitive dye was used in the cited work. As can be seen from part P1 of Fig. 4, based on the color change of the surface layers, there was an alkaline boundary that penetrated only a short distance from the outside of the paper thickness. Part P2 of the figure shows the same specimen during the measurement of pH, immediately after the drop of water needed for the measurement had been applied to the paper surface. Despite the fact that most of the paper thickness of the sample in Fig. 4 microstructure had been acidic, the paper after the pH measurement appears to be neutralized, deacidified, and alkaline, with a pH value of about 8 to 9. This result is misleading, caused by an improper method of pH measurement, which is blind to the presence of acidic species or locations inside the paper or books.
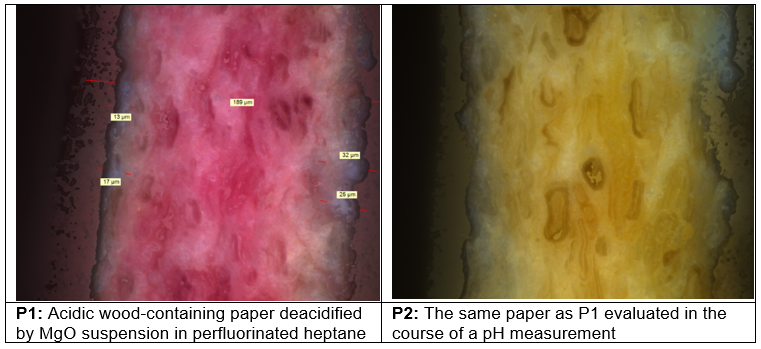
Fig. 4. Acid wood paper impregnated with pH indicator and deacidified (P1) and after superposing the prescribed drop of water during the surface pH measurement (P2).
P1 – The sample was acid wood (CTMP) paper (NOVO, KLUG Conservation), surface pH 4.5, impregnated by pH indicator methyl red, then deacidified by the immersion in suspension of MgO in perfluoroheptane, 10 seconds.
P2 – Image obtained at a later stage of the same experiment during the pH measurement sequence. The image was made immediately after the pH drop of water needed for the measurement had been applied to the measured surface.
Paper characteristics: weight 90 g/m2; pulp 55% CTMP with lignin content 17%, 45% bleached cellulose; filler 12-15% kaolin; sizing Cobb60 < 20 (rosin and alum); headbox pH 4-5, adjusted with alum.
The underlying cause of the findings just described can be referred to as secondary migration or secondary diffusion, in which the test procedure itself causes the movements of acidic or basic species from one position to another in the specimen. As a result of the secondary diffusion by the drop of water used for analysis, an artifact arises: the whole paper appears to be alkaline. This is the case even if the acids had been present inside the cell microstructure. Such acidic places can be dangerous because they cause rapid degradation at random locations along cellulose chains, increasing brittleness, and rapidly decreasing longevity of the material. On the other hand, this experiment clearly shows the potential of bringing about a beneficial effect by post-deacidification moisturizing or humidification. The pH test results demonstrated that the pH can be rendered homogeneously alkaline by a small amount of water. This transformation takes place despite the insolubility of the MgO. Similarly, it can be effective with other insoluble particles such as CaCO3 or MgCO3.
Spectroscopic evidence
Infrared absorbance maxima can be used not only as evidence of the presence of functional groups, but also in some cases to detect the chemical form in which they are present (Strlič et al. 2008; Lichtblau et al. 2009). In particular, the protonated and carboxylate forms of carboxylic acid groups can be distinguished (Pappas et al. 1999; Ayora-Cañada and Lendl 2000; Dioumaev 2001; Tannenbaum et al. 2004).
It is worth reconsidering here the work of Polovka et al. (2006), who used FTIR measurements to detect changes in acidic paper when treated by a non-aqueous dispersion deacidification method. Accelerated aging of the control and deacidified specimens was carried out at selected temperatures (35 and 80 °C) and relative humidities (81 and 98%), generally for a period of 5 days. Significant changes were found only at the higher temperature. From an analytical chemistry standpoint, the FTIR measurements had the advantage of providing information about changes in both the inorganic and organic species involved in the intended deacidification reactions (see Eqs. 1 through 3). A pronounced peak at 3700 cm-1, in the deacidified samples, was attributed to OH groups associated with metal ions (presumably Mg2+). Changes also were detected in the 3000 and 1600 cm-1 regions, which were attributed to water and hydrogen-bonded structures. Some changes also were detected in the 1720 to 1740 and 1630 to 1650 cm-1 wavenumber regions corresponding to changes in carbonyl/carboxylate groups. A known limitation of analyses based on infrared absorption is the fact that the intensities at different wavelengths often cannot be used precisely to compare the amounts of two different functional groups (Sakamoto and Tasumi 2014). Thus, only in the case of a complete disappearance of absorbance intensity related to the carboxylic acid group and a new appearance of a band corresponding to the carboxylate form can one make a highly precise statement about the extent of neutralization of carboxylic acids present in the paper. Nevertheless, given the high relevance of the information, it is hoped that future studies employ FTIR observations to evaluate the effects of various “equilibration” or “conditioning” conditions at specified temperature and relative humidity following non-aqueous deacidification treatments.
The visual color of paper also has been considered as evidence of whether a deacidification treatment has been successful. Stauderman et al. (1996) evaluated the color change when individual sheets were optionally deacidified for non-aqueous dispersion treatment, using a manual spraying operation. After the exposure to accelerated aging with 90 °C, 50% relative humidity treatment, all of the sheets had decreased in L* (lightness) value from an initial value in the range 83.l3 to 84.3 to a final value in the range 72.2 to 77.4. The loss in lightness was slightly larger in the case of the non-aqueous dispersion deacidified sheets, compared to the control specimens, whereas sheets that had been washed exhibited less decrease in L* value.
Evidence that Acid and Bases Can Exist Micro-meters Apart
There are several situations, described in published literature, which provide evidence that acidic species and alkaline species can exist in close proximity within dry materials at ordinary levels of humidity. Some examples to be considered are baking powders, archival papers that contain both acidic inks and alkaline particles, and archival papers that contain both acidic degraded linseed oil and alkaline particles.
Baking powder studies
Commonly available baking powders consist of sodium bicarbonate together with a dry acidic material such as tartaric acid, monocalcium phosphate, or sodium aluminum phosphate (Ciullo 1994). Cream of tartar also can be used as a source of acidity, but it tends to give immediate gassing, so it is less suitable for preparation of a dry powder that will need to be stored. Baking powders often contain about 30% starch, one of the roles of which is to absorb moisture and keep other components of the mixture dry. According to Wolke (2002), baking powder can lose its potency during several months of storage, especially if the conditions become humid.
Ishii (1950) evaluated the storage stability of baking powder. After two months of storage the amount of CO2 generated by the powder had declined to 62% of its initial value. After six months the CO2 production had declined to 20%, and after a year it had declined to 17%. By contrast, very little change could be detected if the sodium bicarbonate and the acidulent were stored separately under similar conditions.
Paper archival research with acidic ink and alkaline particles
Sequeira et al. (2006) studied the possibility of distributing Ca(OH)2 particles in an isopropanol medium as a means of deacidifying paper that had been printed with iron gall ink, which is highly acidic. The non-aqueous treatment promoted the stability of the paper, as measured by accelerated aging tests. However, greater stability could be achieved when using an aqueous deacidification treatment. Such results are consistent with a hypothesis that the neutralization of acidity was not complete when using the non-aqueous dispersion treatment. On the other hand, the observed effects may have been attributable to oxidation, and it is possible that the iron ions are effectively washed away by aqueous solutions.
Paper archival studies with degraded linseed oil and CaCO3 particles
Hansen (1937) reported observations of handmade paper that had been attacked by the acidity from degraded linseed oil. What was interesting about the paper was that the hydrolytic attack had been prevented in the immediate vicinity of some large CaCO3 particles, which were randomly distributed in the paper. The paper had been exposed to wide ranges of humidity, due to many years of storage and a lack of air conditioning. Thus, even though the example provides some indication that alkaline particles may bring about some deacidification, it was also shown that such neutralization may be limited to the immediate vicinity of such a particle.
Conditions Needed for Effective Neutralization within Paper
Conditions reflected by aqueous treatments
It has been well established that aqueous conditions can bring about neutralization reactions between acidic species and alkaline particles. Water as the mobile phase provides hydronium and hydroxyl ions, which act as transport agents and bring about neutralization (de Grotthuss 1806). By contrast, under dry conditions the acidic and basic species may be isolated from each other, in which the Grotthuss conductive mechanism would not function (Agmon 1995; Cukierman 2006; Potthast and Ahn 2017).
Time and concentration are key variables that appear to be important when using aqueous media to neutralize the acidic groups present in a sheet of paper. The reason for considering such information here is that it may help set a reasonable expectation of what is required to bring about completion of deacidification in a book that still contains acidic sites despite having been treated with alkaline particles. Bogaard et al. (2005) observed that short immersion times in concentrated pH-neutral buffer solutions were sufficient to bring about complete deacidification of acidic paper sheets. Some materials may require longer times or more severe conditions to wet the paper in order to bring about neutralization of acidic groups. It has been found that heavily sized or coated paper sheets may take a relatively long time to become become completely wetted with aqueous solutions.
Even after full wetting of a sheet of paper, some time may be required for acidic and alkaline chemical species present in the material to diffuse sufficiently in order to neutralize each other. Typical paper that has been prepared under acidic conditions contains a much higher amount of acidity than might be expected if one relied just on measurements of pH. As already has been mentioned, a key contribution to this acidity is the aluminum sulfate used by papermakers to anchor rosin-based hydrophobic agents in the paper (rosin sizing) (Strazdins 1989). As was noted earlier (Hubbe 2015), one of the authors of this review ran a test that revealed the alum-rosin system to be highly tenacious. The experiment was carried out by treating the rosin-containing papermaking furnish with aluminum sulfate. The fiber suspension was formed into a wet sheet of paper, using handsheet-forming equipment (TAPPI Method T205). Then, before draining all the water through the device, a specialized baffle was placed above the wet sheet so that a full “deckle” of water having a pH of 10 (having a depth of about 30 cm) was drained through the wet sheet of paper. Remarkably, when the paper was then dried, it developed hydrophobicity, just as if it had been prepared under acidic conditions. Hargrove and Thomas (1982) reported related findings when they attempted to neutralize the acidity in aluminum-containing soil. The acidity in the wet specimens did not immediately express itself upon addition of base; instead, the pH tended to revert to lower values after rapid addition of NaOH.
Humid air treatments
As mentioned earlier in this article, bound books represent a particular challenge because aqueous treatments would tend to swell the pages, likely exceeding the capacity of the bindings and possibly leading to breakage (Kellerman 1999). Also there is a concern that immersion of whole books into water would cause the paper to become wavy and to be difficult to dry in a flat condition. These circumstances have led some investigators to consider what can be achieved by treating the paper with humid air.
Middleton et al. (1996) carried out a remarkable study in which highly acidic paper sheets were successfully deacidified by placing them between alkaline sheets of paper and then subjecting the stacks of paper to high humidity, high mechanical pressure, and long exposure times. The system described corresponds to a patent by Page et al. (1995). Before considering the details, two points deserve emphasis: First, the experiments showed that the presence of alkaline particles can result in neutralization of acidity in an adjacent sheet. But second, the required conditions of treatment were severe and way outside of the range that is even possible in a hypothetical library. Sheets employed in the initial study were prepared from unbleached kraft pulp. The acidic sheets were prepared such that they had a pH of 4 (measured in the presence of salt). Because there was no alum used to provide buffering, one can regard the sheets as representing a relatively “easy to deacidify” condition. The corresponding alkaline sheets were prepared with 22% calcium carbonate content and a pH of 9.8, which can be regarded as a high but not unreasonable value of pH compared to conventional alkaline papermaking. The atmospheric conditions considered were 75, 85, 92, and 97% relative humidity. During treatment, the stacks of alternating acidic and alkaline sheets were exposed to 350 kPa of mechanical pressure, which is equivalent to 3.5 atmospheres, while the gas pressure was regulated to 7 kPa (1 psi.).
The high mechanical pressure applied to the stacks of paper had a remarkable effect of accelerating the deacidification process. After demonstrating the importance of all three variables – humidity, mechanical pressure, and time – the researchers settled on 92% relative humidity when they applied their method to a representative collection of different acidic papers. Depending on the type of paper, 92% relative humidity resulted in moisture contents between 8% and 18% within the paper specimens. A period of 85 days was sufficient to bring about neutralization of the acidic paper, while the sheets were being strongly compressed. These experiments showed that deacidification could be completed after a sufficient time period under appropriate conditions (high RH, the presence of alkaline particles in paper, e.g. added through deacidification). A gradually increasing effectiveness with increases in humidity, pressure, and time is consistent with the presence of a diversity of pore sizes within the irregular structures of fibers within paper.
To explain their results, the cited authors proposed that the water from the humid air would partly adsorb onto fiber surfaces and that part of it would condense in small capillary spaces between and among the fibers and calcium carbonate particles. They proposed further that ions of either acidic nature or alkaline nature (or both) would diffuse or flow within the narrow channels of capillary-condensed water, bringing about neutralization.
Conditions Required for Capillary Condensation
Kelvin equation and fundamental studies
The fact that, in the study by Middleton et al. (1996), it was necessary to apply high mechanical pressure to the stacks of alkaline and acidic sheets of paper in order to bring about deacidification, even under highly humid conditions, is consistent with what is known about the mechanism of capillary condensation. The fundamental relationship governing capillary condensation was derived by Kelvin (Mitropoulos 2008). Equation 7 shows the relationship in terms of relative pressure, where po represents the partial pressure at which the liquid would condense at an equivalent flat surface and p represents the partial pressure at which the same liquid would condense in an ideal capillary of radius rc.
![]() (7)
(7)
Other terms in the equation include the liquid-vapor surface tension (g), the molar volume of the bulk liquid (VL), the gas constant (R), the absolute temperature (T), and the limiting capillary radius rc, below which condensation will take place. Under ideal conditions, if one equates the relative humidity to the ratio p/po, one can estimate the size of a capillary (modeled as a cylinder) in which capillary condensation can be expected. If one enters the information for water into Eq. 7 and solves for the value of rc, one can estimate what size of idealized capillaries will fill at different levels of relative humidity. Some results of such a calculation are shown in Fig. 5.
Fig. 5. Predictions of the Kelvin equation for water at room temperature and ideal cylindrical pores
Based on Fig. 5, one can conclude that only extremely tiny pores will become or remain filled with condensed water at moderate levels of relative humidity. One needs to raise the relative humidity above about 80% to expect to fill ideal cylindrical mesopores having a radius of 10 nm. And one would need to raise the relative humidity to 98.95% (according to the calculation) to fill pores having a radius as large as 100 nm. To put things into perspective, such a pore is about one-fifth the size of a typical MgO particle employed in widely used forms of non-aqueous dispersion deacidification.
Figures 6A and 6B provide schematic representations, comparing the respective sizes of features such as a fiber in a sheet of printing paper, an alkaline particle such as MgO deposited on the fibers during a non-aqueous dispersion deacidification treatment, and a few idealized hypothetical pores possibly present in the solid material.
Reported findings related to capillary condensation in other circumstances can be helpful in judging how and to what extent the phenomenon is likely to affect events related to the completion of deacidification. Dourdain and Gibaud (2005) used X-ray reflectivity to detect the capillary condensation of water in thin films of mesoporous silica. Strong hysteresis of filling and emptying of pores was found in the relative humidity range of 30 to 55%, which corresponded to a preponderance of pores having diameters in the range 0.5 to 2 nm.
Bocquet et al. (1998) found that capillary condensation at points of contact was able to account for effects of time on the static frictional properties of granular media. It has been known for many years that piles of finely-divided dry particles under ambient conditions tend to “age”, such that they don’t pour quite as easily as when first spilled out into the pile. Such effects are apparently due to the development of nano-scale menisci at the points of contact, i.e. ultra-miniature “water bridges”. Note that such an effect is suggested in Fig. 6B. Related effects also have been observed when carrying out atomic force microscopy (AFM) in the presence of humid air (Charlaix and Ciccotti 2010). The development of menisci at nano-scale points of contact can have such a profound effect on AFM imaging that many researchers either use a completely dry atmosphere or carry out the work in completely immersed systems.
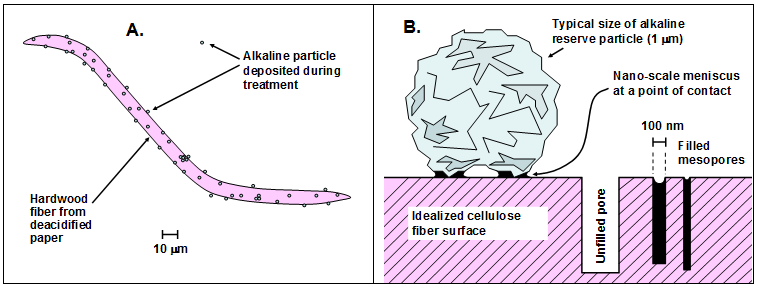
Fig. 6 A. Relative sizes of alkaline particles typically deposited onto a hardwood fiber during non-aqueous dispersion deacidification. B. Schematic illustration of situations in which capillary condensation is or is not expected to occur on a highly local basis within non-aqueous dispersion deacidified paper at a very high relative humidity (98.95%) below the saturation point
Localized and limited nature of capillary condensation
When typical cellulosic materials are exposed to sub-saturation levels of humidity, even in the 90% range, they still do not tend to take up very much water. For instance, Engelund et al. (2010) found that even at a relative humidity of 99.9%, samples of wood took up only about 0.35% of capillary condensed water in comparison to the dry mass of the wood. Likewise, Thygesen et al. (2010) found that capillary condensation does not play a significant role in water sorption by wood in the relative humidity range up to 99.9%. Xie et al. (2011) reported similar results for sorption of water by three types of cellulose – cotton linter, alpha-cellulose, and microcrystalline cellulose. Though the moisture uptake at 95% relative humidity was 14.2%, 20.6%, and 16.9% for the three materials, respectively, the authors concluded that capillary condensation did not play a significant role relative to the amounts of sorbed water.
To account for why typical cellulose structures do not tend to open themselves up more than they do, resulting in only modest uptake of water even at very high relative humidity, it is important to consider the highly organized and dense hydrogen bonding that takes place between adjacent cellulose macromolecular chains (Pönni et al. 2012). Even if there are submicroscopic spaces produced within cellulose fibers as a consequence of lignin removal and bleaching (Berthold and Salmén 1997), such spaces tend to close up when the material is dried, due to the action of capillary forces, which become very strong as the last film of water is being evaporated (Stone and Scallan 1966; Page 1993; Hubbe et al. 2007). Many of the closed-up spaces tend to remain closed thereafter due to the development of organized hydrogen bonding, i.e. a kind of “healing” between the adjacent cellulosic surfaces so that they act as a single hydrogen bonded solid (Pönni et al. 2012).
The capillary condensation model also can help to account for why Middleton et al. (1996) did not observe effective deacidification until the stacks of alternating acid and alkaline paper sheets had been pressed together strongly. In the absence of such pressure, the pores would generally have been too large to allow for contiguous capillary condensation so as to provide effective paths for diffusion of acidic or alkaline soluble species within the paper. Thus, materials would be in aqueous contact only at isolated nano-scale points, as was illustrated in Fig. 6B. The apparent density of typical paper sheets suitable for printing is typically within the range of 0.5 to 1 g/cm3 (Gorres and Luner 1992; Anjos et al. 2015). If one assumes that the solid phases within paper have an intrinsic density of 1.5 g/ cm3 (if there were not any pores), it follows that the fraction of void space within a typical paper sheet is in the range of 33 to 67%. However, most of that space involves dimensions that would be too large for capillary condensation to take place. Only by squeezing the material together do the spaces between the solids making up the stack come close enough together to enable a contiguous network of capillary water to develop at relative humidity levels lower than 99%.
Evidence of the limited ability of capillary condensation to bring about complete reaction between all the adjacent acidic and alkaline species in a solid material also can be drawn from Ishii’s (1950) study of the storage stability of baking powder, as discussed earlier in this article. Those data are plotted for the first time in Fig. 7. As shown, it appears that the ability of the powder to generate gas upon wetting tended to reach a stable plateau. This might be explained in terms of an ability of capillary condensation to effectively bridge between certain isolated points of acidity and alkalinity in the microstructure. However, such isolated menisci would be expected to develop in the same locations during repeated cycles of humidity change. It follows that the capillary condensation mechanism is likely to fail to wet many pockets of acidity in a paper microstructure.
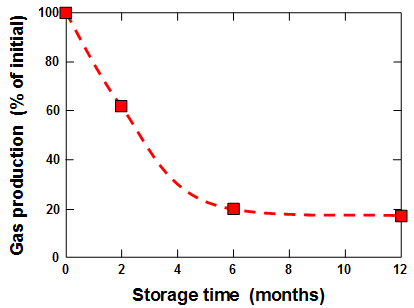
Fig. 7. First plotting of data from Ishii (1950) showing decline in gas-forming ability of baking powder when stored under typical conditions
Meniscus retraction and concentration of solutes
Noting that capillary condensation tends to be restricted to very small, localized features, it is meaningful to ask whether the phenomenon would be likely to adversely affect isolated microfibrils and other nano-scale features associated with the structure of paper. If capillary condensation causes a tiny droplet to repeatedly or persistently form in the same favored locations within a paper microstructure, then such water would be expected to promote water-dependent reactions, such as acid hydrolysis. Calvini et al. (2008) noted that acid hydrolysis tends to take place to a disproportionate degree on the non-crystalline parts of the paper’s polysaccharide structure. Kocherbitov et al. (2008) found that the amount of water sorption increased in the following order: cellulose I (as in native plants) < cellulose II (as in rayon) < amorphous cellulose. This is consistent with the much higher accessibility of amorphous cellulose in comparison to the crystalline domains of the cellulose (Hong et al. 2007; Park et al. 2010; Yu and Wu 2010). Stephens et al. (2008b) likewise showed that amorphous cellulose was more susceptible to acid hydrolysis. Such attack, when it occurs in within paper, has the potential to destroy connections between the crystalline regions within fibers (Whitmore 2011), in addition to some of the bonded areas between fibers in paper. Also, during changes in humidity it is likely that some of the menisci retract and even disappear, a process that can tend to concentrate any acidity present in the remaining water (Kohonen and Christenson 2000; Langlet et al. 2013). These findings suggest that highly localized phenomena may play an important role in the hydrolytic degradation of stored paper. Future research is needed to clarify such issues.
Concerns about Accelerated Aging of Deacidified Specimens
Standards
Several different sets of standard conditions have been prescribed for tests of accelerated aging (Zervos 2010). The general purpose of an accelerated aging test is to help predict the rate of deterioration of a selected paper-based item without having to wait for many years of natural aging to pass. This section will consider publications providing insight on which, if any, of the standard conditions are most suitable for tests of deacidified papers. Table 4 provides a brief summary of the available test methods, which are arranged in order of increasing relative humidity.
Table 4. Conditions of Temperature and Relative Humidity during Standard Accelerated Aging Tests
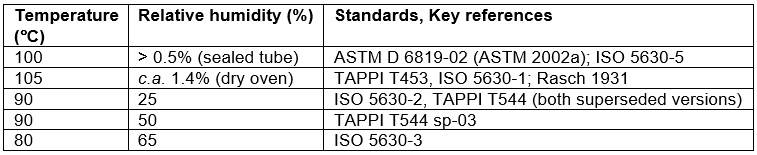
It should be noted that the ASTM test with sealed tubes is based on initial equilibration at a relative humidity of 55% at room temperature, followed by sealing the tube and heating the system to 100 °C. The corresponding relative humidity within the heated tube, based on the quantities of molecules in the gas phase, is about 0.5%. Because additional water molecules would have been present within the cellulosic material, the relative humidity prevailing at higher temperatures (with expected evaporation of water molecules) would be higher than 0.5% by an amount that would depend on the nature of the paper. What becomes apparent from Table 4 is that a very wide range of humidity values have been employed by different researchers.
Will dry accelerated aging represent natural aging?
When paper documents are subjected to accelerated aging, the usual goal is to estimate that rate of decomposition that would have occurred over a longer period of time but at the specified conditions of storage. If one were able to assume that paper’s decomposition was governed by a single, uniform mechanism, then one would in theory be able to apply an Arrhenius relationship of the type shown in Eq. 8,
![]() (8)
(8)
where k is the rate of the reaction, A is a coefficient, Ea is the activation energy, R is the universal gas constant, and T is the absolute temperature. The equation is often solved for Ea (Eq. 9), making it clear that the activation energy can be obtained by plotting ln k versus the reciprocal of T to determine the slope of a line through the data:
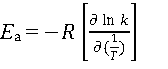 (9)
(9)
Some uncertainty will continue to exist, since it is never certain that the reactions taking place under the high-temperature conditions are representative of what would happen on a library shelf. Some factors that might lead to misleading results of accelerated aging tests are as follows:
- There may be more than one important mechanism governing decomposition, and the activation energies governing these mechanisms may be quite different from each other. A contributing mechanism with a different activation energy may become relatively more or less important at different temperature of exposure. As a consequence, the degradation at high temperature might take a different predominant reaction path that is unrepresentative of natural aging.
- Differences in the amount of moisture present at sites on and within the paper structure at the high-temperature conditions might favor different reaction paths. Again, this could make the results of the accelerated tests unrepresentative of natural aging.
- Decomposition products that tend to remain in the paper structure during storage under typical library conditions might become sufficiently volatile to escape into the atmosphere under the conditions of accelerated aging (especially if air is being refreshed during in oven aging treatment), thus changing the net amount of acidity present in the paper when it is heated under conditions of accelerated aging.
The validity of point (a) is supported by the fact that some previous studies have found different slopes of the Arrhenius relationship when some of the specifics of testing are changed (Zervos 2010). However, the activation energy also depends on which paper properties are considered. The relationship between paper strength and the chemical aspects of the fiber, such as DP, may be complex. The validity of point (b) is supported by tests showing that different proportions of byproducts are created during accelerated aging under dry (TAPPI T453) and moist (TAPPI T544) accelerated aging conditions (Ehrardt et al. 1987). Also, as reported by Gray (1969), the presence of even a small amount of moisture (dry or 5% based on solids) can markedly decrease the activation energy associated with paper’s aging. Such observations have caused several researchers to recommend that a completely dry accelerated aging test will not be a good choice to predict the (longer) process of natural aging (Graminski et al. 1979; Wilson and Parks 1983; Zou et al. 1996a).
Because acidic paper tends to age most rapidly under humid conditions (Zou et al. 1996a; Baty et al. 2010; Zervos 2010), it can be argued that an ideal accelerated aging test for acidic paper ought to involve humid conditions – possibly representing the highest relative humidity that the books are likely to encounter on a frequent basis. Indeed, some very important tests of the aging of deacidified books have been carried out recently at a relative humidity of 65% (Ramin et al. 2009) and others were done at 50% (Katuscak et al. 2012). Notably, both of these cited studies reported relatively poor aging results for non-aqueous dispersion deacidified specimens; the best results were reported for aqueous and homogeneous deacidification processes.
Will moist accelerated aging inadvertently complete the treatment?
A different conclusion might be reached if one places the highest priority on avoiding the inadvertent completion of deacidification in the course of evaluating the aging of papers that have experienced a non-aqueous dispersion deacidification treatment. Suppose that a book to be tested includes isolated points of both acidity and alkalinity. Introduction of liquid water might inadvertently bring about mutual neutralization, thus unintentionally completing a process of deacidification (Smith 2011). Evidence supporting the ability of humid, hot conditions to convert carboxylic acid groups to their conjugate base (carboxylate) form was provided by Polovka et al. (2006). FTIR results by these researchers showed that such reactions became important when the paper was heated to 80 °C at 81% relative humidity.
Since the relative humidity conditions employed were higher than those commonly used in accelerated aging studies (see Table 4), further studies are needed. Ideally, rates of neutralization of carboxylic acid groups in paper should be determined at the relevant conditions of temperature and humidity.
As the temperature is increased, air is able to hold increasing amounts of water. Thus, the hot atmosphere employed during implementation of TAPPI Method T544 (90 °C, 50% relative humidity) will contain about 37.7 times as much water (per constant amount of dry air) compared to air having the same relative humidity at 23 °C. With so much water present, it makes sense to ask whether or not some of that water would behave as a liquid in its interactions with the paper. Several possible mechanisms can be envisioned, as summarized in Table 5, to explain a possible wetting action resulting from high humidity exposure. Evidence for and against each of the listed hypothetical mechanisms is discussed in subsequent paragraphs.
Table 5. Hypothetical Ways in Which Hot, Humid Air Might Inadvertently Bring about Deacidification in the Course of Accelerated Aging

Evidence regarding capillary condensation
Until recently, the best evidence concerning conditions under which capillary condensation is able to bring about completion of deacidification consisted of the work reported by Middleton et al. (1996). The fact that 350 kPa of mechanical pressure had to be applied to each stack of paper in order for the treatment to be effective is best explained by capillary condensation. But at the same time, those results suggest that in the absence of strong compression and very high humidity, the acidic paper remained acidic.
Results presented by Potthast and Ahn (2017) provide evidence that accelerated aging under humid conditions (i.e. ISO 5630-3) may lead to a biased favorable evaluation of the effectiveness of non-aqueous dispersion deacidification treatments. The cited work, which focused on the changes in degree of polymerization during accelerated aging, compared results obtained under hot humid conditions (80 °C, 65% relative humidity, 40 days, ISO 5630-3) with results obtained under hot dry conditions (105 °C, heated ambient air with no additional moisture added, 100 days). Based on the median values of the degree of polymerization results, the humid-aging conditions yielded an effectiveness ratio of about 1.8 when comparing non-aqueous dispersion deacidified specimens with control specimens of the same source paper. By contrast, the dry accelerated aging tests failed to show any significant difference between the non-aqueous dispersion deacidified specimens and the control specimens. There was some scatter in the results, but the median values were essentially the same for the deacidified and control specimens. A possible interpretation of these findings, to the extent that they are predictive of natural aging under typical storage conditions, is that the high water content present in the air at 80 °C and 65% relative humidity played an important role in bringing about unintended completion of the deacidification reaction during the test procedure. On the other hand, since different reactions may be predominant under the dry accelerated aging conditions (Graminski et al. 1979; Wilson and Parks 1983; Zou et al. 1996a), some researchers will not regard results from such tests as being strongly predictive of natural aging.
Evidence regarding diffusion of ions through swollen cellulose
There is much evidence that ionic species can migrate through hydrogels (Scally et al. 2006). Such a mechanism is the basis for the widely-used gel electrophoresis methods, which make it possible to separate ionic polyelectrolytes on the basis of their rates of diffusion (Viovy 2000). But paper, even under rather high humidity conditions, generally contains much less water compared to the gels represented by the work cited above. There does not appear to be any clear evidence that ordinary paper is able to conduct ions appreciably at relative humidity levels below saturation. This is another area where future research is needed.
Evidence regarding unstable conditions and high humidity
The presence of large amounts of water in the vapor phase during certain accelerated aging tests implies that unintended condensation might occur due to unsteady conditions, especially during the starting up of the exposure regime. An unplanned dip in temperature could, in principle, cause inadvertent dampening of the paper. Hypothetically, this could come about due to the initially cooler temperature of the paper, compared to the hot and humid air. Condensation could then occur, until such point as the temperatures become equilibrated above the dew point. Future research appears to be needed in order to determine which of the following is true: On the one hand, the specific heat of the solid paper material might be enough to depress the local air temperature sufficiently to allow condensation. On the other hand, there may be enough equilibration of the air and enough difference between the set temperature and the dewpoint so that gross condensation leading to wetting of the paper is avoided during most practical deacidification treatments using humid air.
Absorption of water by paper as a function of relative humidity
Based on published results, it appears that, regardless of the temperature, the relative humidity can be used as a rough indication of how much water is likely to be present in adsorbed and/or condensed form within a paper specimen. These phenomena have been well studied at room temperature. For instance, Fig. 8 shows data replotted from Zervos (2010). As shown, when the relative humidity rises above about 80%, the water uptake starts to climb at a steeper and steeper slope, exceeding a moisture content of 10%.
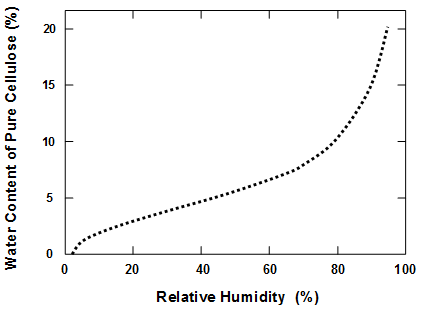
Fig. 8. Amount of water absorbed by pure cellulose as a function of relative humidity (replotted from data reported by Zervos 2010)
Katuscak et al. (2012) measured moisture contents in the range 4 to 5% when paper was equilibrated at 96 °C and 50% relative humidity. This is within the range, or possibly somewhat lower, than what is usually found for paper samples equilibrated under TAPPI standard conditions (23 °C and 50% relative humidity, TAPPI Method T 402). As was noted earlier, Middleton et al. (1996) observed moisture contents in the range 8 to 18% at room temperature and a relative humidity of 92%. These data suggest that, in terms of equilibrium conditions, the amount of water held within paper (either bound or condensed) might be estimated from the relative humidity alone over a wide temperature range. A study by Fellers (2007) showed that the moisture content of cotton is a strong function of relative humidity, and that increasing temperature slightly decreased the amount of water taken up by the cellulose at any specified relative humidity. It should be noted that water molecules at increased temperatures have increased average kinetic energy. Thus, even though there will be a high concentration of water molecules present in the air at the higher temperature (at constant relative humidity), any individual molecule will tend to spend most of its time in the gas phase.
Another challenge in predicting water uptake is that water is not expected to merely fill existing pores within cellulosic material; also it is likely that swelling will take place. Kocherbitov et al. (2008) found that specific surface areas of cellulose, based on adsorption of water, were much higher than corresponding values determined by nitrogen adsorption. The difference was attributed to capillary condensation in spaces that become wedged open between adjacent microfibrils. Their adsorption data was consistent with the presence (or “opening up”) of 3 nm spaces within cellulosic material. It was proposed that such spaces might be equivalent to missing chain segments of cellulose macromolecules within an incomplete or disturbed semi-crystalline structure.
Although capillary condensation is expected to account for a minor part of the water represented in Fig. 8, much of that water has been attributed to other mechanisms, such as hydration of the cellulosic material (Joubert et al. 1959), “bound water” (Park et al. 2006), or water participating in a type of hydrogel (Scally et al. 2006; Hubbe et al. 2013). Past research suggests that ionic species including H3O+ and OH– can readily diffuse within a hydrogel, though rates of diffusion may be slower in comparison to diffusion in bulk water (Bromberg 1991; Feng et al. 2011; Golmohamadi and Wilkinson 2013). Such mechanisms, since they can involve numerous hydrogen bonds of a small bunch of water molecules with cellulosic material, might account for a greater amount of energy preventing the evaporation of a given water molecule in comparison to evaporation of bulk water. Indeed, the energy required to remove the last water from cellulosic material during drying has been found to be greater than for the removal of bulk water (Park et al. 2006). In light of these findings, there is a need for more information about residual water present in paper and whether or not such water can participate in transport of acidic or alkaline species.
CHALLENGES AND OPTIONS FOR COMPLETION OF DEACIDIFICATION
This section of the article considers possible next steps in cases that books in a collection already have been treated using a non-aqueous dispersion-type deacidification program. Without pre-judging whether or not such a treatment program had achieved neutralization of the acidic compounds within the paper, published evidence will be considered regarding options for further treatment of the same items.
The next question may be “what kind of additional treatment?” Any type of intervention will require effort. Also, it is well known that any treatment that involves moistening the paper is likely to change the paper structure, and such effects are sometimes harmful (Banik and Brückle 2011). So, to gain greater benefits from that effort, some other treatments may be combined with deacidification. In particular, the material can be subjected to washing, the strengthening of already-brittle paper, treatment to resist mold organisms or other biological attack, and treatments to counter oxidative attack on the polysaccharide (cellulose and hemicellulose) content of paper.
Ways to Moisten the Paper
There are three main means of adding controlled amounts of moisture to a bound volume. As a summary, Table 6 lists some of the primary issues to consider in the case of treatments involving high relative humidity, fine sprays of aqueous solutions (which might include suspended particles), and systems involving full wetting or immersion of paper in aqueous media. The listed advantages and disadvantages of each of these options are discussed after the table.
Table 6. Advantages and Disadvantages of Different Ways of Introducing Enough Water to Complete the Deacidification Process within Paper
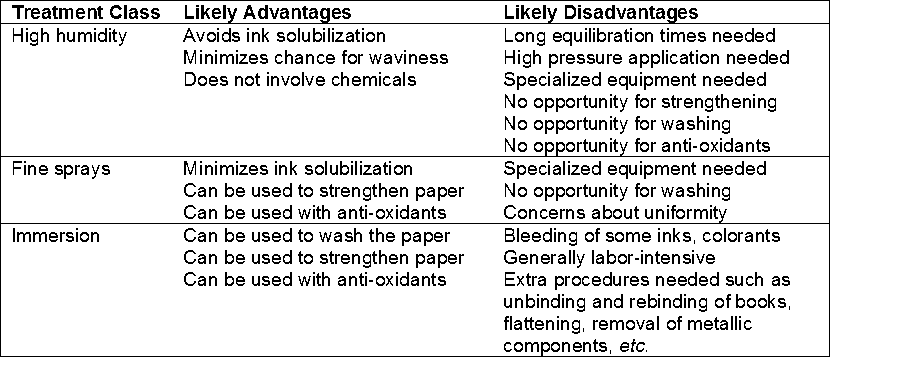
Humid air
As demonstrated by the results obtained by Page et al. (1995) and reported by Middleton et al. (1996) as well as by Hanus et al. (2002), highly humid air, in combination with mechanical pressure can be effective in bringing about the completion of deacidification. As has been noted, these researchers used inter-leaved alkaline sheets, very high humidity, application of mechanical pressure, and many hours of treatment. A key lesson from the study of Middleton et al. (1996) was that it is indeed possible to bring about deacidification by means of alkaline solid material, i.e. the calcium carbonate particles in the alkaline sheets of paper, but non-typical conditions will be required.
The use of the same type of humidification system would be expected to work at least as well for the treatment of paper that already contains alkaline particles, especially if those particles have permeated to the surfaces of fibers within the interior of a paper sheet. An individual sheet of typical printing paper is about 0.1 mm in thickness. By contrast, an individual hardwood fiber within such paper might be roughly 0.02 mm in thickness (or diameter). Thus there is a possibility that a non-aqueous dispersion deacidification program can get at least some of the particles about five times closer, on average, to the acidic sites to be neutralized in comparison to inter-leaving the document with alkaline paper sheets. In principle, some of the acidic groups may be bound to or located within cellulosic fibers, whereas others may be capable of also diffusing. However, due to the closure of pores in the cell walls during the drying of paper (Stone and Scallan 1966), it is not expected that alkaline particles in the micrometer size range will be able to get within the cell walls of the fibers.
An important consideration is whether or not the amounts of alkaline particles present within a sheet of paper deacidified by a non-aqueous dispersion system are sufficient to neutralize the total of the acidity that is present. If so, then it is reasonable to expect that either alkaline species, or acidic species, if they were to diffuse within the structure, would bring about neutralization upon wetting of the sheet by capillary condensation. In view of the fact that even full aqueous treatments are known to require some time of equilibration (see earlier discussion), it may be important to determine optimum treatment times.
The process described in the sources cited above can be logically extended to include intentional condensation of water in the paper. For example, after equilibration of the documents with heated, highly humid air, the system could be cooled below the dew point. Since research involving such a strategy was not found in the search of the literature, this would appear to be a promising field of study.
Sprays
It has been claimed in the patent of Zitzmann et al. (2002) that increased effectiveness of alkaline particles, used for deacidification of paper, can be achieved by applying a mist of moisture to the paper, preferably in advance of air-borne delivery of alkaline particles. The atomized moisture addition was claimed to give higher efficacy. The particles used by this process (e.g. 14 µm) can be 10-fold bigger than those used by BookkeeperÒ (about 1 µm). Research work is suggested to substantiate the patent claims and provide quantification.
Spray, for the purpose of completing a deacidification reaction, can potentially take advantage of technology that already had been developed for other aspects of paper conservation (Jablonsky et al. 2009b; Brückle and Banik 2011a). In particular, a process called safe aqueous technology (SAT) applies a controlled, uniform amount of fine aqueous spray (Katuscakova et al. 2008; Jablonsky et al. 2009b; Katuscak et al. 2009b; Tino et al. 2016; Vizarova 2016a), making possible the distribution of agents to adjust the paper’s pH and agents to provide functionality, such as strength or pest-resistance. The process entails cyclic spraying of atomized water, solutions, or colloid systems, followed by drying, while keeping the amount of applied atomized moisture in each cycle below a certain critical value (Katuscakova et al. 2008; Katuscak et al. 2009b). The maximum water retention in one impregnating cycle is controlled to be within a subdeformation range (e.g. 10 to 15 wt %) to avoid deformation, migration, color differences, optical shifts, and mechanical changes. The air used for drying may also contain dry or hygroscopic substances, such as MgO, polymers, strengthening, antioxidant, deacidifying, and other conserving substances. The SAT process can be controlled visually or by using characteristic parameters of deformation, surface roughness, waviness, migration parameters, color differences, optical, and mechanical changes or damage.
The SAT process, as a stand-alone deacidification measure, has been demonstrated to be effective in the neutralization of residual acidic sites within paper. As an example, laboratory measurements using a new method of measurement of pH distribution in paper cross-sections, paper core, and paper microstructure (Katuscak et al. 2016) had shown that SAT atomized water can penetrate through paper samples in the order of minutes; the paper samples that had been deacidified incompletely by MgO particles and containing 60 to 90% of acidic paper core were completely alkalized in minutes, providing homogeneous pH throughout the paper. The SAT process has been subjected to preliminary testing using lab-scale equipment (Katuscakova et al. 2008; Jablonsky et al. 2009b; Fekete et al. 2011; Jablonsky et al. 2016; Tino et al. 2016). The SAT procedures also have been subjected to preliminary tests in industrial equipment to apply vacuum and pressure conditions using alkaline particles and employing atomized moisturizing conditions in an air conditioning chamber, matching the conditions employed for ordinary usage in deacidification (Zitzman et al. 2002, see “Klimakammer – item no. 14”).
Strengthening Agents Applied while Completing Deacidification
As discussed in the previous section, remoistening of the paper has been suggested as a potential way to bring about completion of neutralization reactions if the paper material happens to contain both acidic and alkaline species. The goal of such a treatment would be, at least in part, to be able to benefit from the presence of alkaline particles that already had been distributed in the paper; in principle there could be an advantage of not having to add any more alkaline particles to complete the neutralization of acidic compounds in the paper. However, because of the costs and complexities of any proposed treatment for books – including sub-deformation moistening or condensation of moisture from humid air within the paper – conservators will be concerned about what other effects the moistening may have. On the negative side, moistening has the potential to make the paper somewhat bulkier and weaker (Brückle 2011). But it is important to consider some potential positive outcomes, depending on what materials are dissolved or dispersed in the water. Thus, the subsections that follow will consider publications indicating certain paper conservation treatments that might logically be carried out simultaneously with a moistening step, one of the purposes of which is to bring about completion of reactions between acidic and alkaline materials in the paper.
As has been pointed out, a large proportion of books in a typical library collection are likely to be already brittle (Smith 1969; Darragh 1978; Hanus 1994; Zou et al. 1994). Thus, a treatment that merely slows down future loss of strength does not fully address the problem. It has been recommended to combine deacidification with addition of a bonding agent, helping to restore such strength attributes as folding endurance and tensile strength (Katuscak et al. 2012). This needs to be done with care and moderation, such as to avoid adhering adjacent sheets together. Also, it is known that strengthening treatments tend to work well when applied to paper sheets that have not yet become excessively degraded.
Aqueous solutions and mists, when used in the deacidification of paper, already have been used to transport water-soluble strengthening agents to the paper (Jablonsky et al. 2009b; Brückle and Banik 2011a). Such agents can include starch (Katuscak et al. 2007; Havlinova et al. 2011), methyl cellulose (Wächter et al. 1998; Sundholm and Tahvanainen 2003a,b), and methyl hydroxyethyl cellulose (Katuscak et al. 2007). In addition, based on analysis of ancient papers that have retained their strength (Stephens et al. 2008a), it appears that gelatin would be a good candidate for aqueous strengthening of paper in the course of its deacidification. Figure 9 provides a schematic view of how strengthening agents are likely to function in the treatment of acid-embrittled paper. This figure emphasizes likely effects of moistening the paper with a solution of a strengthening polymer agent, followed by drying. Here it is assumed that such a solution, perhaps applied as a mist, is able to wet the surfaces of fibers within the paper, to fill cracks and eroded areas of the fiber, and to reinforce inter-fiber bonding.
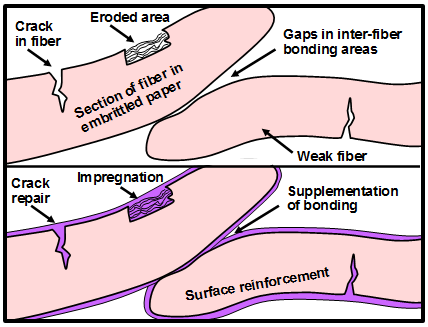
Fig. 9. Schematic illustration of some ways in which application of a solution of a bonding agent such as methylcellulose or starch, followed by drying, has potential to repair or reinforce various weak or damaged aspects of fibers in paper or the bonds between them. Upper figure: representation of fibers in an embrittled paper document. Lower figure: strengthened paper.
Other Conservation Treatments while Completing Deacidification
Chemical reduction step while completing deacidification
It is well known that paper documents can be adversely affected by airborne oxidants such as ozone and nitrogen oxides (Arney et al. 1979; Daniels 1996; Bogaard and Whitmore 2001; Stephens et al. 2009). In fact, such oxidation appears to make the paper more susceptible to acid-catalyzed hydrolysis in some cases (Whitmore and Bogaard 1995; Stephens et al. 2009). It has been suggested that the consequences of oxidation of paper documents can be addressed by chemical reduction (Katuscak et al. 2012). This can be achieved by treatment with reducing agents such as sodium borohydride (Tang 1986; Kolar et al. 1998; Bogaard et al. 2005) and potassium iodide (Kolar et al. 1998; Stephens et al. 2009; Havlinova et al. 2011). Such a treatment has potential to be incorporated into post-deacidification treatments involving aqueous droplets.
Nanoparticles added while completing deacidification
In addition to adding strengthening agents and reducing agents, a moisturizing step employing a spray to complete deacidification can provide an opportunity to apply additional alkaline reserve material. To enhance the opportunities for subsequent interaction of alkaline reserve particles with acidic sites in the paper, it makes sense to use very finely dispersed material, i.e. nanoparticles. Thus, it has been proposed to deacidify paper by addition of nano-sized Ca(OH)2 (Giorgi et al. 2002; Sequeira et al. 2006; Stefanis and Panayiotou 2007), or Mg(OH)2 (Giorgi et al. 2005; Stefanis and Panayiotou 2007; Giorgi 2015; Amornkitbamrung et al. 2015; Wojciak 2015). More research is needed to follow up in this area.
Pressing in the course of completing deacidification
Since the application of mechanical pressure between the front and back sides of books already has been demonstrated to greatly accelerate the completion of deacidification in books under very high humidity conditions (Page et al. 1995; Middleton et al. 1996), it makes sense to consider whether or not to take advantage of such pressing as a means to enhance paper strength and/or to combat a tendency of paper sheets to become thicker when exposed to moisture. This is just one aspect to consider regarding the wisdom of including such a step in the treatment of books, keeping in mind the possibility of mechanical damage. Mechanical pressing has been proposed to be used as a step in deacidification (Page et al. 1995; Middleton et al. 1996; Jablonsky et al. 2009b). Increased pressing of damp paper, during ordinary papermaking, is well known to increase the strength of the resulting paper (Howard and Jowsey 1989). However, such increases in strength have not been reported during pressing of completely dry paper. Research appears to be needed to determine the range of moisture content within which pressing can yield important improvements in strength or apparent density of paper.
Drying in the course of completing deacidification
Certain strategies for adding sufficient water to bring about completion of deacidification reactions within paper may make it necessary later to remove such water, and it is important that such drying be carried out in such a way that the paper articles are not damaged. Fortunately, the drying of books and paper articles has been much studied (Brückle and Banik 2011b). Freeze drying has become accepted as a routine method in paper conservation (Kelly and Fowler 1978; Liénardy 1994; Wächter et al. 1998; Kaplan and Ludwig 2005; Silverman et al. 2007; Jablonsky et al. 2009a,b; Neuvirt 2010; Hubacek et al. 2011). The method also can be used for the drying of books that have been wetted during a flood (Milevski and Nainis 1994). In practice, this could be achieved by applying vacuum to remove both air and water vapor from the vicinity of the treated materials, using procedures that have been established for pre-drying of books before non-aqueous solution-based deacidification (Eggersdorfer et al. 1990). Low pressure conditions allow evaporation to take place at a moderate rate even at temperatures below the melting point of water. Since evaporation removes heat from the system, controlled heat may be applied during freeze drying in order to promote the process of water release (Franks 1998; Nail et al. 2002). All of these options add to the cost of treating books, and costs need to be taken into consideration when selecting an overall treatment strategy.
RESEARCH NEEDS
Quantification of the Completion of Deacidification
Based on the information considered in this review, one can expect there to be continuing interest in the quantification of completion and hence the success of deacidification. In addition to quantifying the amounts of any remaining acid species in the material, there is value in quantifying the byproducts of successful deacidification reactions. There is also a need for more understanding of changes in the degree of polymerization and crystalline nature of cellulose, which may be affected by deacidification or subsequent accelerated aging. Literature addressing these issues is discussed in this section.
Quantifying the degree to which neutralization has been achieved
Whitmore (1994) was perhaps the first to raise concerns about the completion of deacidification reactions when a non-aqueous liquid is used to distribute alkaline particles within a paper document. He pointed out that although neutralization is favored by thermodynamics, it is not certain whether the rate of neutralization will exceed the rate of acid-catalyzed hydrolysis. It follows that methods need to be developed and improved for quantification of acidic species that may exist in relatively close proximity to alkaline particles. Such acidity cannot be detected by adding water, since such procedures will generally be related to the net acidity or alkalinity rather than nano-scale local conditions.
Spectroscopic techniques for presence of acidic groups
As was noted already, certain spectroscopic methods have been used to reveal the presence of carboxylic acids, in their protonated form, in samples of acidic paper (Polovka et al. 2006). In principle, the intensities of absorption bands detectable by infra-red analysis can be used to estimate the relative amounts of protonated vs. dissociated carboxylic acid groups in cellulosic material (Pappas et al. 1999; Ayora-Cañada and Lendl 2000; Dioumaev 2001; Tannenbaum et al. 2004). Because infrared analysis can involve overlapping peaks, especially in the case of unknown samples, there will be a need for further research of this type when applied to practical samples of paper.
Some of the carboxyl content of paper subjected to accelerated aging is possibly attributable to oxidation. Rychly et al. (2004, 2006) showed that the reaction to form such carboxylic groups gives rise to chemiluminescence, which can be detected. Notably, such oxidation was promoted by the presence of Mg species and humidity. Acidic species tended to make the material less thermostable, such that the oxidation reaction occurred at a somewhat lower temperature. The chemiluminescence method (Rychly et al. 2004, 2006; Katuscak et al. 2010, 2012) can be used for measuring the kinetics of cellulose degradation, as an alternative method. It can be therefore used for critical validation of the traditional heat-induced accelerated aging methods widely used for the testing of relative longevity of acid paper and paper deacidified by the different technologies. The chemiluminescence measurement method can also be used for research and testing of completion of deacidification by water, using either humification or SAT technologies and apparatuses, or their combinations (Vizarova et al. 2016). Chemiluminescence can be also used for comparative evaluation of longevity and efficacy of technologies for cellulose materials stabilization, deacidification, and conservation and their continuing development (Katuscak et al. 2012; Rychly et al. 2004, 2006).
Dye indicator color
As was noted earlier, color changes in dyes have been used to gain key insights into deacidification processes (Whitmore and Bogaard 1994; Middleton et al. 1996). In particular, researchers have found that the dye in litmus paper tends to become equilibrated with the pH conditions of paper that it is adjacent to (Middleton et al. 1996). Notably, the same has been shown for samples of litmus paper that were stored in the presence of powdered ammonium carbonate (Myers and Crane 1999). Such observations tend to confirm that at least one ionic species – either acidic or alkaline – is able to migrate and interact and become neutralized under typical laboratory conditions of relative humidity. In a response to the cited findings (Myers and Crane 1999), it was noted that the odor suggested the presence of gaseous ammonia. Thus, in at least that case, there is reason to expect that it was the alkaline species that was able to migrate to the paper, and not vice versa. Further research may be needed to fully explain why Middleton et al. (1996) were able to convert the color of litmus paper first one way and then the other by placing it adjacent to acidic paper, and then by placing a sheet of alkaline paper on the other side of it, forming a sandwich. One promising explanation is that species such as acetic acid and sodium acetate are both sufficiently mobile, if present, to act as a kind of messenger between the paper specimens and the litmus paper. The volatility and mobility of a conjugate base such as sodium acetate would be contrary to the findings of Barsanti et al. (2009). Since such interactions could be important in bringing about deacidification, there is a critical need for research to clarify the mechanisms in such cases.
Byproduct evolution and headspace analysis
Headspace analysis is yet another technique that has been mentioned as a promising way to provide evidence of the persistence of acidic domains within paper, including paper that may have been subjected to a deacidification treatment (Banik 2005; Hrivnak et al. 2009a,b; Area and Cheradame 2011; Becker et al. 2016). Either during routine storage or during accelerated aging the nature of the volatile organic compounds released from the paper material will provide evidence of the types of reactions that are occurring. Wenzl and Lankmayr (2000) have used similar methodology to quantify volatile aldehydes and aromatic compounds released from paperboard packaging materials. Nerin et al. (1998) showed that such methodology can be used to reveal the migration of volatile species associated with paper packages – again supporting the view that species commonly present in paper may be able to act as agents to transfer acidity of basicity from one site to another, given sufficient time. Based on these examples, it would appear that headspace analysis is a promising and relatively nonintrusive way to learn more about what is happening on a molecular level during the process of aging.
Extraction of paper specimens can provide another attractive approach for analysis of possible reactions occurring within paper during storage (Shahani 1995; Dupont et al. 2007; Krascenits 2007; Lehotay and Hrobonova 2007; Bogolitsyna et al. 2011). As noted earlier, Ehrardt et al. (1987) compared extracts from filter paper obtained before and after accelerated aging carried out under either dry or moist conditions and noted profound differences.
However, not much work of this type has been carried out. A great advantage of extraction-based analysis is that, in typical cases, one is able to collect a much larger amount of organic compounds, compared to what can be collected in a typical headspace analysis.
Cellulose degree of polymerization
Because hydrolytic cleavage of cellulose macromolecular chains appears to be a central mechanism in acid-catalyzed aging of paper documents, evaluation of the degree of polymerization can be expected to play a continuing important role in future studies, especially in studies that involve accelerated aging (Ahn et al. 2013; Katuscak et al. 2012; ISO/TS 18344 2016). Stephens et al. (2008a) observed striking differences in molecular mass when comparing naturally aged documents, and they were able to correlate the differences with pH and other indicators of acidity of the papers. Methods of detecting molecular mass, in studies of paper aging, have included viscometry (Kolar and Novak 1996; Selli et al. 1998; Ramin et al. 2009) and gel permeation chromatography (GPC) (Potthast et al. 2008; Kacik et al. 2009; Lojewski 2010; Łojewski et al. 2011; Ahn et al. 2012c, 2013; Becker et al. 2016; Potthast and Ahn 2017). An advantage of GPC is that it provides information about the distribution of molecular masses.
Examination of Candidate Mechanisms
Capillary condensation
Of the many concepts considered in this review article, one that appears to have received relatively little experimental attention is the mechanism of capillary condensation, as a possible means to deacidify paper. In fact, only Page et al. (1995) and Middleton et al. (1996) have reported extensive results in this area. Although it was possible in this work to support mechanistic explanations based on literature sources, the cited studies generally involved materials other than paper. Some possible areas for future experimental investigation are listed in Table 7.
The test strategies outlined in Table 7 are aimed at further clarification of capillary condensation as a possible contributing mechanism of completion of deacidification. However, they should not be taken as an excuse to neglect other mechanisms that have potential to be more important under the typical conditions of temperature, relative humidity, and long storage times on a typical library shelf. The present review of the literature suggests that capillary condensation will most often involve very small, isolated menisci of water, which, in the absence of compression of the books, will generally fail to allow effective diffusion of acidic or alkaline species to other points in the structure.
Vapor-phase migration
Figure 10 provides a schematic picture of some of the main mechanisms that could be considered in such future work, involving various molecular diffusion modes for acidic or alkaline compounds within paper.
Table 7. Areas of Experimental Investigation Related to the Role of Capillary Condensation in Completion of Deacidification
As mentioned already in this review, some published work can be viewed as evidence of air-borne migration of volatile materials associated with paper (Nerin et al. 1998; Wenzl and Lankmayr 2000; Banik 2005; Area and Cheradame 2011). For instance, Middleton et al. (1996) reported that litmus paper would turn color when placed in contact with paper of contrasting pH.
Table 8 lists some possible experiments involving vapor-phase migration and completion of deacidification that do not seem to have been reported in the literature up to this point.
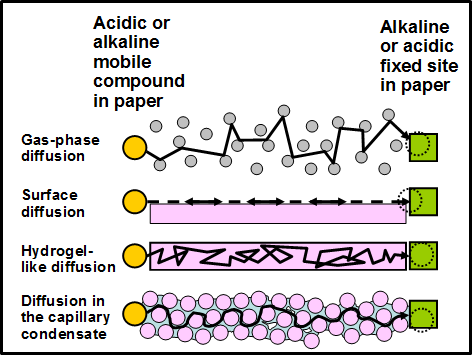
Fig. 10. Four hypothetical mechanisms by which a mobile acidic or alkaline compound within the microstructure of paper might be able to migrate toward another site in the paper where neutralization can take place
Table 8. Areas of Experimental Investigation Related to the Role of Vapor-phase Migration in Completion of Deacidification

Additional Questions for Future Research
Questions can be as important as answers for any successful research involving analytical and process technology development. Answers provided by science and continuing improvements then can lead us to new questions (Katuscak 2006a; Katuscak and Gfeller 2006). I
The pH at isolated sites within a dry sheet of paper
What are the pH values inside the incompletely deacidified pages? Is it possible to define and equivalent pH value corresponding to an isolated area that is too small for a conventional pH measurement to be carried out? What percentage of the mass and volume of the paper and books stays acidic after incomplete deacidification? How are local pH values affected by the amounts, types, and distribution of buffering agents and/or alkaline reserve compounds? These are challenging questions in light of the fact that conventional pH measurements require wetting of the paper, and wetting can be expected to allow completion of deacidification in many cases of interest.
Changes in pH or acidity as a function of time
What are the pH dynamics within a paper document? What are the kinetics of the pH changes – decrease or increase – in acidic parts of books? Is it true that the acidic pH in the acidic parts, acid paper core, and acid microstructures further decreases to even more dangerous values? For how long?
Relative rates of migration of acidic species vs. their generation
What are the relative rates of migration of acidic species in paper, compared to the rates of their in-situ generation or their arrival as a result of air pollution? Is the rate of migration of acidic species sufficiently high to decrease the acidity at point in the paper that were acidic prior to application of non-aqueous dispersion treatment?
Rates of reaction of acidic species with alkaline particle material
Given the fact that migration of acidic species present within paper has been demonstrated (Barrow 1953; Nerin et al. 1998; Wenzl and Lankmayr 2000; Banik 2005; Area and Cheradame 2011), it can be hypothesized that there might be a different rate-limiting step that can account for the relatively low effectiveness that some researchers have shown for non-aqueous dispersion-based deacidification processes, as discussed in this article. One such hypothesis, which may merit research attention, is whether, upon reaching the surface of an alkaline particle within paper, there fails to be a neutralization reaction with the acidic compound that has migrated. It has been proposed that some combination of migration and neutralization of acidic species does not take place fast enough to be effective in such instances (Banik 2005; Hanus et al. 2008; Katuscak et al. 2009b, 2012; Kacik et al. 2009; Jablonsky et al. 2011a,b, 2012b, 2013).
Rates of migration of alkaline species
It also can be proposed that the main mechanism of deacidification by means of non-aqueous dispersion treatment might depend on the migration of alkaline species to acidic sites, and not vice-versa. This view seems reasonable in light of the fact that migration of acids to the paper surface would still leave the paper core and fiber microstructure acidic. With continuous acid hydrolysis of cellulose macromolecules, more acidity would continue to develop. To examine the possibility of migration of alkaline species, one can envision future tests in which acidic paper is exposed to adjacent materials that contain such compounds as sodium acetate or calcium acetate, etc.
CLOSING THOUGHTS
The weight of the evidence presented in this review article suggests that treatment of books and paper with non-aqueous dispersions of alkaline particles such as MgO does not necessarily bring about the desired neutralization of acidic species located within the fibers that comprise paper. In particular, such a conclusion is strongly supported by recent research (Potthast and Ahn 2017). Findings reviewed in this article are consistent with the continuing existence of acidity remaining within fibers, within the core of paper sheets, or more generally within the paper even after treatment with non-aqueous dispersions.
The authors hope that the information collected in this article will be helpful to those responsible for books that may still contain acidic content after incomplete deacidification. Options that might be considered, as outlined in earlier sections of this article, include treatment under high humidity and pressure, intentional condensation of humid air, or the application of fine aqueous sprays. The latter might be combined with other conservation treatments. Those responsible for the care of book collections will need to weigh such options against other possibilities, such as storage of incompletely deacidified books under relatively cool and dry conditions, digitization of the content, or application of another type of deacidification. In each case it is possible to evaluate different treatment options and to decide which one of them is most cost-effective and best suited for the volumes under consideration.
Further increasing of the longevity and usability of the books as well as answering questions and challenges important for conservators and world memory institutions could be best brought about by an integrated effort. Many new methods and results have become available since non-aqueous dispersion-type treatments started to be implemented in 1994 (Buchanan et al. 1994). It is proposed that the key question of completeness can be answered by multidimensional testing, using traditional and new methods and knowledge, by comparative evaluation of longevity and efficacy. Objective testing of the most important parameters is needed of each process. Information generated by independent research will be of benefit to all libraries and memory institutions. Conservators on all continents need solid data to support the best means of caring for the world’s collections (Drewes and France 2012; Ahn et al. 2012). Over many years there has been a gathering of experience, drawing on practices in North America, Europe, Asia, and all countries caring for the world’s collections (Blüher et al. 2006; Hanus et al. 2006, 2008; Drewes and France 2012; Johnson et al. 2012; Baty et al. 2010; Katuscak et al. 2012; Ahn et al. 2012c; Georgi 2015; Katuscak et al. 2016; Vizarova et al. 2016a). The authors hope that this review article can provide a stimulus and also a platform from which to embark on some of the needed efforts on behalf of the world’s cultural heritage that has been recorded on paper.
ACKNOWLEDGEMENTS
Thanks are extended to the Ministry of Education and Science SR, EU for allowing of publishing results and images from their national grant KnihaSK.eu as well as to the Library of Congress for providing the testing machines necessary for the comparative testing. The authors are grateful for input from nine specialists who participated in reviewing the article.
LITERATURE CITED
Agmon, N. (1995). “The Grotthuss mechanism,” Chem. Phys. Lett. 244(5-6), 456-462.
Ahn, K. (2013). “Sustainability of mass deacidification of library objects,” PhD Thesis, Universität für Bodenkultur, Wien.
Ahn, K., Banik, B., Henniges, U., and Potthast, A. (2012a). “Nachhaltigkeit in der Massenentsäuerung von Biblioteksgut,” in: Eine Zukunft für Saures Papier (A Future for Acid Paper), R. Altenhöner, A. Blüher, A. Mälck, E. Niggemann, A. Potthast, and B. Schneider-Kemf (eds.), Vittorio Klostermann, Frankfurt am Main, pp. 29-81.
Ahn, K., Banik, G., and Potthast, A. (2012b). “Sustainability of mass deacidification. Part II. Evaluation of alkaline reserve,” Restaurator 33(1), 48-75. DOI: 10.1515/res-2012-0003
Ahn, K., Henniges, U., Banik, G., and Potthast, A. (2012c). “Is cellulose degradation due to β-elimination processes a threat in mass deacidification of library books?,” Cellulose 19, 1149-1159.
Ahn, K., Henniges, U., Blüher, A., Banik, G., and Potthast, A. (2011). “Sustainability of mass-deacidification – Part I: Concept, selection of sample books, and pH-determination,” Restaurator 32, 193-222.
Ahn, K., Rosenau, T., and Potthast, A. (2013). “The influence of the alkaline reserve on the aging behavior of book papers,” Cellulose 20, 1989-2001. DOI: 10.1007/s10570-013-9978-3
Amornkitbamrung, L., Mohan, T., Hribernik, S., Reichel, V., Fairvre, D., Gregorova, A., Engel, P., Kargl, R., and Ribitsch, V. (2015). “Polysaccharide stabilized nanoparticles for deacidification and strengthening of paper,” RSC Advan. 5, 32950-32961. DOI: 10.1039/C4RA15153D, Paper
Andres, H., Blüher, A., Grossenbacher, G., Reist, M., Vogelsanger, B., and Wälchli, M. (2008). “The Papersave Swiss – Process quality control and efficacy,” Restaurator 28(1), 3-28. DOI: 10.1515/rest.2008.002
Andretta, M., Coppola, F., and Seccia, L. (2016). “Investigation on the interaction between the outdoor environment and the indoor microclimate of a historical library,” J. Cultural Heritage 17, 75-86. DOI: 10.1016/j.culher.2015.07.002
Anjos, O., García-Gonzalo, E., Santos, A. J. A., Simões, R., Martínez-Torres, J., Pereira, H., and García-Nieto, P. J. (2015). “Using apparent density of paper from hardwood kraft pulps to predict sheet properties, based on unsupervised classification and multivariable regression techniques,” BioResources 10(3), 5920-5931. DOI: 10.15376/biores.10.3.5920-5931
ANSI (1984). “Permanence of paper for printed library materials,” American National Standards Institute, Z 39.48.
Area, M. C., and Cheradame, H. (2011). “Paper aging and degradation: Recent findings and research methods,” BioResources 6(4), 5307-5337. DOI: 10.15376/biores.6.4.5307-5337
Arney, J. S., and Chapdelaine, A. H. (1981). “A kinetic study of the influence of acidity on the accelerated aging of paper,” in: Preservation of Paper and Textiles of Historic and Artistic Value. II., Williams, J. C. (ed.), Advances in Chemistry Ser. 193, American Chemical Society, Washington, DC, Ch. 14, 189-204. DOI: 10.1021/ba-1981-0193.ch014
Arney, J. S., Jacobs, A. J., and Newman, R. (1979). “Influence of deacidification on the deterioration of paper,” J. Am. Inst. Conserv. 19(1), 34-41. DOI: 10.2307/3179571
Arnson, T. R., and Stratton, R. A. (1983). “The adsorption of complex aluminum species by cellulose fibers,” Tappi J. 66(12), 72-75.
Aspler, J. (1989). “Slow fires: The paper preservation symposium,” Tappi J. 72(2), 192-194.
ASTM D (2002a): “Standard test method for accelerated temperature aging of printing and writing paper by dry oven exposure apparatus,” Method 6819-02.
ASTM D (2002b): “Test method for accelerated pollutant aging of printing and writing paper by pollution chamber exposure apparatus,” Method 6833-02.
Ayora-Cañada, M. J., and Lendl, B. (2000). “Study of acid-base titration of succinic and malic acid in aqueous solution by two-dimensional FTIR correlation spectroscopy,” Vibrational Spectroscopy 24(2), 297-306. DOI: 10.1016/S0924-2031(00)00098-9
Back, E., and Allen, L. (2000). Pitch Control, Wood Resin and Deresination, TAPPI Press, Atlanta, 392 pp.
Balažic, A., Habicht, Š., Smodiš, M., Kolar, J., and Strlić, M. (2007). “Extending the useful life of paper – Evaluation of the effect of various preservation actions,” in: Museum Microclimates, T. Padfield and K. Borchersen (eds.), National Museum of Denmark, ISBN 978-87-7602-080-4
Banik, G. (2004). “Evaluation of the efficacy of CSC-Booksaver process for the deacidification for archives and library materials, based on test treatments at the preservation academy Leipzig (PAL),” 2004, s. 1-20.
Banik, G. (2005). “Mass deacidification technology in Germany and its quality control,” Restaurator 26(1), 63-75. DOI: 10.1515/rest.2005.63
Banik, G., and Brückle, I. (eds.). (2011). Paper and Water: A Guide for Conservators, Elsevier, Butterworth-Heinemann, Amsterdam, Boston, Oxford.
Banik, G., Doering, T., and Hähner, U. (2006). “Current efforts to establish an effective quality management for mass deacidification,” in: Save Paper! Mass Deacidification, Today’s Experiences, Tomorrow’s Perspectives, A. Blüher, G. Grossenbacher, and G. Banik (eds.), Papers given at the international conference, 15-17 February, 2006, pp. 94-110.; http://www.helveticat.ch/lib/item?id=chamo:1479183
Bansa, H. (1990). “Meßmethoden zur Bestimmung der Alterungsbeständigkeit von Papier,” (“Methods for measuring the aging resistance of paper”) Papier 44(9), 481-492.
Barrow, W. J. (1953). “Migration of impurities in paper,” Archivum 3, 105-108.
Barrow, W. J. (1965). “Deacidification and lamination of deteriorated documents 1938-63,” Amer. Archivist 28, 285-290.
Barrow, W. J. (laboratory of) (1974). Permanence/Durability of the Book – VII. Physical and Chemical Properties of Book Papers 1507-1949, Barrow Research Laboratory, Inc., Richmond, VA.
Barsanti, K. C., McMurry, P. H., and Smith, J. N. (2009). “The potential contribution of organic salts to new particle growth,” Atmos. Chem. Phys. 9(9), 2949-2957. DOI: 10.5194/acp-9-2949-2009
Baty, J. W., Maitland, C. L., Minter, W., Hubbe, M. A., and Jordan-Mowery, S. K. (2010). “Deacidification for the conservation and preservation of paper-based works: A review,” BioResources 5(3), 1955-2023. DOI: 10.15376/biores.5.3.1955-2023
Baumann, S. O., Schneider, J., Sternig, A., Thomele, D., Stankic, S., Berger, T., Grönbeck, H., and Diwald, O. (2015). “Size effects in MgO cube dissolution,” Langmuir 31(9), 2770-2776. DOI: 10.1021/la504651y
Baynes-Cope, A. D. (1969). “Non-aqueous deacidification of documents,” Restaurator 1(1), 2-9.
Becker, M., Meyer, F., Jeong, M. J., Ahn, K., Henniges, U., and Potthast, A. (2016). “The museum in a test tube – Adding a third dimension to the evaluation of the impact of volatile organic acids on paper,” Polym. Degrad. Stabil. 130, 109-117. DOI: 10.1016/j.polymdegradstab.2016.05.026
Berthold, J., and Salmén, L., (1997). “Effects of mechanical and chemical treatments on the pore size distribution in wood pulps examined by inverse size-exclusion chromatography,” J. Pulp Paper Sci. 23(6), J245-J254.
Bielikova, L. (2005). “Study of the distribution of substances in lignocellulosics materials on the development of multicomponent conservation substances,” Master Thesis STU, Slovak University of Technology in Bratislava, Slovakia, 2005, pp. 107.
Bielikova, L., and Katuscak, S. (2009). “Study of the distribution of elements in the lignocellulosic material in the development of multicomponent conservation agents,” (Štúdium distribúcie prvkov v lignocelulózových materiáloch pri vývoji multikomponentných modifikačných látok)”, Annex P.6.15, in: Katuscak, S. et al. (2009): “Preservation, stabilization and conservation of traditional carriers of information in Slovak Republic – KnihaSK,” STU Bratislava, Ministry of Education SR Report No. 09/533, Archival Report No. 09533/2009. pp. 140-147.
Blüher, A., Grossenbacher, G., and Banik, G. (editors), (2006). Save Paper! Mass Deacidification, Today’s Experiences, Tomorrow’s Perspectives: Papers given at the international conference, 15-17 February. 2006, Swiss National Library, ISBN 3-9523188-1-7, ISBN 978-3-9523188-1-2, online: www.snl.admin.ch.; http://www.helveticat.ch/lib/item?id=chamo:1479183
Blüher, A., and Vogelsanger, B. (2001). “Mass deacidification of paper,” Chimia 55, 981-989.
Bocquet, L., Charlaix, E., Ciliberto, S., and Crassous, J. (1998). “Moisture-induced ageing in granular media and the kinetics of capillary condensation,” Nature 396(6713), 735-737. DOI: 10.1038/25492
Bogaard, J., Morris, H. R., and Whitmore, P. M. (2005). “A method for the aqueous deacidification of oxidized paper,” J. Amer. Inst. Conservat. 44(2), 63-74. DOI: 10.1179/019713605806082347
Bogaard, J., and Whitmore, P. M. (2001). “Effects of dilute calcium washing treatments on paper,” J. Amer. Inst. Conservation 40(2), 105-123. DOI: 10.2307/3180025
Bogolitsyna, A., Becker, M., Dupont, A.-L., Borgards, A., Rosenau, T., and Potthast, A. (2011). “Determination of carbohydrate- and lignin-derived components in complex effluents from cellulose processing by capillary electrophoresis with electospray ionization-mass spectrometric detection,” Journal of Chromatography A 1218, 8561-8566. DOI: 10.1016/j.chroma.2011.09.063
Bredereck, K., Haberditzl, A., and Blüher, A. (1990). “Paper deacidification in large workshops: Effectiveness and practicability,” Restaurator 11(3), 165-178. DOI: 10.1515/rest.1990.11.3.165
Bristow, J. A. (1971). “Swelling of paper during sorption of aqueous liquids,” Svensk Papperstidn. 74(20), 645.
Bromberg, L. E. (1991). “Transport of monocharged ions through environment-sensitive composite membranes based on polyelectrolyte complexes,” J. Membrane Sci. 62(2), 117-130. DOI: 10.1016/0376-7388(91)80056-C
Brückle, I. (2011). “Aqueous treatment in context,” in: Paper and Water: A Guide for Conservators, G. Banik and I. Brückle (eds.), Elsevier, Butterworth-Heinemann, Amsterdam, Boston, Oxford, pp. 419-436.
Brückle, I., and Banik, G. (2011a). “The introduction of water into paper,” in: Paper and Water: A Guide for Conservators, G. Banik and I. Brückle (eds.), Elsevier, Butterworth-Heinemann, Amsterdam, Boston, Oxford, pp. 255-288.
Brückle, I., and Banik, G. (2011b). “Drying paper in conservation practice,” in: Paper and Water: A Guide for Conservators, G. Banik and I. Brückle (eds.), Elsevier, Butterworth-Heinemann, Amsterdam, Boston, Oxford, pp. 389-418.
Buchanan, S., Bennet, W., Domach, M. M., Melnick, S. M., Tancin, C., Whitmore, P. M., Harris, K. E., and Shahani, C. (1994). “An evaluation of the Bookkeeper mass deacidification process: Technical evaluation team report for the Preservation Directorate,” Library of Congress, September 22, 1994 (1994) https://www.loc.gov/preservation/resources/rt/bookkeeper.pdf; also at: http://cool.conservation-us.org/byorg/lc/massdeac/bookkeep.html
Bukovský, V. (1999). “Is deacidification a step to the rescue of historic newspapers?” Restaurator 20(2), 77-96. DOI: 10.1515/rest.1999.20.2.77
Bukovský, V. (2005). “The analysis of alkaline reserve in paper after deacidification,” Restaurator 26(4), 265-275. DOI: 10.1515/REST.2005.265
Burd, J. (2012). “Bookkeeper deacidification: The chemistry behind the process,” The American Institute for Conservation of Historic and Artistic Works, The Book and Paper Group Annual 31, pp. 101-102.
Calvini, P., Gorassini, A., and Merlani, A. L. (2007). “Autocatalytic degradation of cellulose paper in sealed vessels,” Restaurator 28(1), 47-54. DOI: 10.1515/rest.2007.47
Calvini, P., Gorassini, A., and Merlani, A. L. (2008). “On the kinetics of cellulose degradation: Looking beyond the pseudo zero order rate equation,” Cellulose 15, 193-203. DOI: 10.1007/s10570-007-9162-8
Cedzova, M., Gallova, I., and Katuscak, S. (2006). “Patents for paper deacidification,” Restaurator 27(1), 35-45. DOI: 10.1515/REST.2006.35
Charlaix, E., and Ciccotti, M. (2010). “Capillary condensation in confined media,” in: Handbook of Nanophysics – Vol. 1, K. Sattler (ed.), CRC Press.
Cheradame, H., Ipert, S., and Rousset, E. (2003). “Mass deacidification of paper and books. I: Study of the limitations of the gas phase processes,” Restaurator 24(4), 227-239. DOI: 10.1515/rest.2003.227
Ciullo, P. A. (1994). Saleratus: The Curious History & Complete Uses of Baking Soda, Maradia Press, Naugatuck, CT, 182 pp.
Clark, R. J. H., Gibbs, P. J., and Jarjis, R. A. (1998). “An investigation into the deacidification of paper by ethoxymagnesium ethylcarbonate,” J. Mater. Chem. 8(12), 2685-2690. DOI: 10.1039/a804453h
Cox, B. G. (2013). Acids and Bases: Solvent Effects on Acid-Base Strength, Oxford Univ. Press, Oxford. DOI: 10.1093/acprof:oso/9780199670512.001.0001
Cukierman, S. (2006). “Et tu Grotthuss! and other unfinished stories,” Biocheim Biophys. Acta – Bioenergetics 1757(8), 876-878. DOI: 10.1016/j.bbabio.2005.12.001
Cunha, G. M. (1987). “Mass deacidification for libraries,” Library Technology Reports 23(3), 361-472.
Cunha, G. M. (1989). “Mass deacidification for libraries, 1989 Update,” Library Technology Reports 25(1), 5-81.
Daniels, V. D. (1996). “The chemistry of paper conservation,” Chem. Soc. Rev. 25(3), 179-186. DOI: 10.1039/cs9962500179
Darragh, D. W. (1978). “Deacidification of brittle manuscripts and documents,” Restaurator 2(3/4), 179-184. DOI: 10.1515/rest.1978.2.3.179
de Grotthuss, C. J. T. (1806). “Sur la décomposition de l’eau et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique,” Ann. Chim. 58, 54-73.
Derr, H. R., Lewis, T., Derr, B. J. (2000). “Gas me up, or, A baking powder diver,” J. Chem. Ed. 77(2), 171-172. DOI: 10.1021/ed077p171
Dioumaev, A. K. (2001). “Infrared methods for monitoring the protonation state of carboxylic amino acids in the photocycle of bacteriorhodopsin,” Biochemistry – Moscow 66(11), 1269-1276. DOI: 10.1023/A:1013135419529
Drewes, J., and France, F. (2012). “Taking the measure: Treatment and testing in mass deacidification,” a subtopic within the compiled discussion, “Joint discussion session 2012: Mass deacidification today,” J. Johnson, M. Lee, and C. Schneider (eds.), The American Institute for Conservation of Historic and Artistic Works, The Book and Paper Group Annual 31, 104-105.
Dourdain, S., and Gibaud, A. (2005). “On the capillary condensation of water in mesoporous silica films measured by x-ray reflectivity,” Appl. Phys. Lett. 87(22), article no. 223105. DOI: 10.1063/1.2136412
Dufour, J., and Havermans, J. B. G. A. (2001). “Study of the photo-oxidation of mass-deacidified papers,” Restaurator 22(1), 20-40. DOI: 10.1515/rest.2001.20
Dupont, A.-L., Barthez, J., Jerosch, H., and Lavédrine, B. (2002). “Testing CSC Book SaverÒ, a commercial deacidification spray,” Restaurator 23(1), 39-47. DOI: 10.1515/REST.2002.39
Dupont, A.-L., Egasse, C., Morin, A., and Vasseur, F. (2007). “Comprehensive characterisation of cellulose- and lignocelluloses- degradation products in aged papers: Capillary zone electrophoresis of low-molar mass organic acids, carbohydrates, and aromatic lignin derivatives,” Carbohydrate Polymers 68, 1-16. DOI: 10.1016/j.carbpol.2006.07.005
Earle, M. J., and Seddon, K. R. (2000). “Ionic liquids. Green solvents for the future,” Pure Appl. Chem. 72(7), 1392-1398. DOI: 10.1351/pac200072071391
Eggersdorfer, R., Schwerdt, P., and Wittekind, J. (1990). “Process and device for nonpolluting mass deacidification of books and other paper products,” US Patent 5,120,500, assigned to the Battelle Institute.
Ehrardt, D., von Endt, E., and Hopwood, W. (1987). “The comparison of accelerated aging conditions through the analysis of artificially aged paper,” in: Preprints of Papers Presented at the Fifteenth Annual Meeting, Vancouver, British Columbia, Canata, May 20-24, 1987, American Inst. for Conservation, Washington, DC, pp. 43-55.
Engelund, E. T., Thygesen, L. G., and Hoffmeyer, P. (2010). “Water sorption in wood and modified wood at high values of relative humidity. Part 2: Appendix. Theoretical assessment of the amount of capillary water in wood microvoids,” Holzforschung 64(3), 325-330. 10.1515/HF.2010.061
Enomae, T., and Lepoutre, P (1998). “Observation of the swelling behavior of kraft fibers and sheets in the environmental scanning electron microscope,” Nordic Pulp Paper Res. J. 13(4), 280-284. DOI: 10.3183/NPPRJ-1998-13-04-p280-284
Fekete, R., Peciar, M., Kazíková, J., Jablonský, M., and Katuščák, S. (2011). “Simulation and visualization of flow between the sheets of book” (“Simulácia a vizualizácia prúdenia medzi jednotlivými listami knihy”), in: CHISA 2011 [elektronický zdroj] : Sborník 58. konference chemického a procesního inženýrství. Srní, Česká republika, 24.-27. 10. 2011. Praha : ČSCHI, 2011, s.[11]. ISBN 978-80-905035-0-2
Fellers, C. (2007). “The interaction of paper with water vapour,” in: Paper Products – Physics and Technology, Book series: Pulp and Paper Chemistry and Technology, Book 4, M. Ek, G. Gellerstedt, and G. Henriksson (eds.), Royal Institute for Technology, Fibre and Polymer Technology, Stockholm, pp. 109-144.
Feng, Y., Lee, M., Taraban, M., and Yu, Y. B. (2011). “Diffusion of small molecules inside a peptide hydrogel,” Chem. Commun. 47(37), 10455-10457. DOI: 10.1039/c1cc13943f
Franks, F. (1998). “Freeze-drying of bioproducts: Putting principles into practice,” Eur. J. Pharm. Biopharm. 45(3), 221-229. DOI: 10.1016/S0939-6411(98)00004-6
Giorgi, R. (2015). “Innovative nanotechnologies for cleaning and deacidification of books and manuscripts,” NanoItaly 21-24 September 2015, Sapienza University of Rome. http://www.nanoitaly.it/nanoitaly/en/home-eng/12-abstract-ita/264-ts-iii-2-3.
Giorgi, R., Bozzi, C., Dei, L., Gabbiani, C., Ninham, B. W., and Baglioni, P. (2005). “Nanoparticles of Mg(OH)2: Synthesis and application to paper conservation,” Langmuir 21(18), 8495-8501. DOI: 10.1021/la050564m
Giorgi, R., Dei, L., Ceccato, M., Schettino, C., and Baglioni, P. (2002). “Nanotechnologies for conservation of cultural heritage: Paper and canvas deacidification,” Langmuir 18(21), 8198-8203. DOI: 10.1021/la025964d
Golmohamadi, M., and Wilkinson, K. J. (2013). “Diffusion of ions in a calcium alginate hydrogel-structure is the primary factor controlling diffusion,” Carbohydrate Polymers 94(1), 82-87. DOI: 10.1016/j.carbpol.2013.01.046
Gorres, J., and Luner, P. (1992). “An apparent density model of paper,” J. Pulp Paper Sci. 18(4), J127-J130.
Graminski, E. L., Parks, E. J., and Toth, E. E. (1979). “The effect of temperature and moisture on the accelerated aging of paper,” in: Durability of Macromolecular Materials, Eby, R. K. (ed.), ACS Symp. Ser. 95, American Chemical Society, Washington, DC, Ch. 24, pp. 341-355. DOI: 10.1021/bk-1979-0095.ch024
Gray, G. G. (1969). “An accelerated aging study comparing kinetic rates vs. TAPPI Standard 453,” TAPPI J. 52(2), 325-334.
Gray, G. G. (1977). “Determination and significance of activation energy in permanence tests,” In: Preservation of Paper and Textiles of Historic and Artistic Value, Advances in Chemistry Ser. 164, Williams, J. C. (ed.), American Chemical Soc., Washington, DC., 286-313.
Green, J. W., Pearl, I. A., Hardacker, K. W., Andrews, B. D., and Haigh, F. C. (1977). “Peeling reaction in alkaline pulping,” Tappi 60(10), 120-125.
Green, L. R., and Leese, M. (1991). “Nonaqueous deacidification of paper with methyl magnesium carbonate,” Restaurator 12(3), 147-162.
Gryglewicz, S. (1999). “Rapeseed oil methyl esters preparation using heterogenous catal-ysts,” Bioresour. Technol. 70(3), 249-253. DOI: 10.1016/S0960-8524(99)00042-5
Gurnagul, N., Page, D. H., and Paice, M. G. (1992). “The effect of cellulose degradation on the strength of wood pulp fibers,” Nordic Pulp Paper Res. J. 7(3), 152-154. DOI: 10.3183/NPPRJ-1992-07-03-p152-154
Hansen, F. (1937). “A study of some of the factors which affect the resistance of paper to natural aging,” M.A. Thesis, Institute of Paper Chemistry, Appleton, WI.
Hanus, J. (1994). “Changes in brittle paper during conservation treatment,” Restaurator 15(1), 46-54. DOI: 10.1515/rest.1994.15.1.46
Hanus, J., Bakos, D., Vrska, M., Jablonsky, M., Katuscak, S., Holubkova, S., Bajzikova, M., Bukovsky, V., and Rychly, J. (2008). “The Kniha Project in Slovakia,” in: 2nd International Symposium and Workshops, Ljubljana, Slovenia, Durability of Paper and Writing 2 2008, ISBN 978-961-6286-97-8, p. 17-19
Hanus, J., Katuscak, S., Ceppan, M., Bukovsky, V., Rychly, J., Hanusova, E., Minarikova, J., and Szaboova, Z. (2006). “Research on paper deacidification in Slovakia,” in: Save Paper! Mass Deacidification, Today’s Experiences, Tomorrow’s Perspectives, Blüher A., Grossenbacher G., and Banik G. (eds), Papers given at the international conference, 15-17 February, 2006; http://www.helveticat.ch/lib/item?id=chamo:1479183
Hanus, J., Minarikova, J., and Hanusova, E. (2002). “Deacidification without equipment and money: Dream or reality?” Triennial meeting (13th), ICOM, Rio de Janeiro, 22-27 September 2002, preprints, pp. 603-608.
Hargrove, W. L., and Thomas, G. W. (1982). “Titration properties of Al-organic matter,” Soil Sci. 134(4), 216-225. DOI: 10.1097/00010694-198210000-00002
Havermans, J. (2002). “The impact of European research on paper aging and preservative conservation strategies,” Resturator 23(2), 68-76. DOI: 10.1515/REST.2002.68
Havlinova, B., Makova, A., Minarikova, J., and Katuscak, S. (2011). “Mixture, useful for modification of acidic papers, comprises combination of deacidification solution consisting of solution of magnesium hydrogen carbonate and solution of calcium hydrogen carbonate, potato starch and potassium iodide,” Patent Number(s): WO2011053262-A2; SK200905068-A3; WO2011053262-A3.
Henniges, U., Banik, G., and Potthast, A. (2004). “CSC Book Saver spraying system in single item conservation,” In: ICOM-CC Graphic Documents Meeting, National and University Library, Ljubljana, pp. 53-54.
Henniges, U., Schiehsser, S., Ahn, K., Hofinger, A., Geschke, A., Potthast, A., and Rosenau, T. (2012). “On the structure of the active compound in mass deacidification of paper,” Holzforschung 66(4), 447-450. DOI: 10.1515/hf.2011.174
Hong, J., Ye, X. H., and Zhang, Y. H. P. (2007). “Quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications,” Langmuir 23(25), 12535-12540. DOI: 10.1021/la7025686
Howard, R. C., and Jowsey, C. J. (1989). “The effect of cationic starch on the tensile strength of paper,” Journal of Pulp and Paper Science 15(6), J225-J229.
Hrivnak, J. (2007). “Evaluation of organic acids and furfuralaldehyde in paper,” Technical Report, pp.2. DOI: 10.13140/RG.2.2.10191.69289 https://www.researchgate.net/publication/307976349_Evaluation_of_organic_acids_and_furfuralaldehyde_in_paper
Hrivnak, J., Tolgyessy, P., Figedyova, S., and Katuscak, S. (2009a). “A solid-phase microcolumn extraction method for the analysis of acetic acid and furfural emitted from paper,” Chromatographia 70(3-4), 619-622. DOI: 10.1365/s10337-009-1193-x
Hrivnak, J., Tolgyessy, P., Figedyova, S., and Katuscak, S. (2009b). “Solid phase microcolumn extraction and gas chromatography–mass spectrometry identification of volatile organic compounds emitted by paper,” Talanta Journal 80(2), 400-402.
Hubacek, Z., Jablonsky, M., and Katuscak, S. (2011). “Cellulosic materials modifying device, has vacuum pump provided with freezer unit and blower, another vacuum pump connected to liquid and substance mixing tank, and liquid modifier substance container,” Patent Number(s): SK201050017-A3 ; SK288231-B6, CERTEX AS (CERT-Non-standard), Derwent Primary Accession Number: 2011-Q55854 [77]
Hubbe, M. A. (2005). “Acidic and alkaline sizings for printing, writing, and drawing papers,” The Book and Paper Group Annual 23, 139-151.
Hubbe, M. A. (2015). “Oops, I thought that those books had been deacidified,” BioResources 10(4), 6305-6309. DOI: 10.15376/biores.10.4.6305-6309
Hubbe, M. A., Ayoub, A., Daystar, J. S., Venditti, R. A, and Pawlak, J. J. (2013). “Enhanced absorbent products incorporating cellulose and its derivatives: A review,” BioResources 8(4), 6556-6629. DOI: 10.15376/biores.8.4.6556-6629
Hubbe, M. A., and Bowden, C. (2009). “Handmade paper: A review of its history, craft, and science,” BioResources 4(4), 1736-1792. DOI: 10.15376/biores.4.4.1736-1792
Hubbe, M. A., Gardner, D. J., and Shen, W. (2015). “Contact angles and wettability of cellulosic surfaces: A review of proposed mechanisms and test strategies,” BioResources 10(4), 8657-8749. DOI: 10.15376/biores.10.4.Hubbe_Gardner_Shen
Hubbe, M. A., Venditti, R. A., and Rojas, O. J. (2007). “What happens to cellulosic fibers during papermaking and recycling? A review,” BioResources 2(4), 739-788. DOI: 10.15376/biores.2.4.739-788
Ishii, R. (1950). “On the quality and stability of baking powder during the storage,” J. Ferment. Tech. 28(4), 154-159.
ISO 5630-1 (1991). “Paper and board – Accelerated ageing – Part 1: Dry heat treatment at 105 degrees C,” https://www.iso.org/standard/11709.html
ISO 5630-2 (1985). “Paper and board – Accelerated ageing – Part 2: Moist heat treatment at 90 degrees C and 25% relative humidity,” (status: withdrawn), https://www.iso.org/standard/11710.html
ISO 5630-3 (1996). “Paper and board – Accelerated ageing – Part 3: Moist heat treatment at 80 degrees C and 65% relative humidity,” https://www.iso.org/standard/23402.html
ISO 6588-2 (2012). “Paper, board and pulps – Determination of pH of aqueous extracts – Part 2: Hot extraction,” https://www.iso.org/standard/59784.html
ISO 9706 (1994a). “Information and documentation – Paper for documents – Requirements for permanence,” https://www.iso.org/standard/17562.html
ISO 10716 (1994b). “Paper and board – Determination of alkali reserve,” https://www.iso.org/standard/18809.html
ISO/TS 18344 (2016). “Effectiveness of paper deacidification processes,” https://www.iso.org/obp/ui/#iso:std:iso:ts:18344:ed-1:v1:en
Jablonsky, M., and Katuscak, S. (2009a). “Mehrzweckvorrichtung zur Modifizierung von Zellulosematerialien und Art der Modifizierung von Zellulosematerialien” Patent DE 102008034100 A1. http://google.com/patents/DE102008034100A1?hl=sk&cl=ar
Jablonsky, M., Katuscak, S., and Katuscakova, G. (2009b). “Verfahren zur chemischen Modifizierung von Papierdokumenten,” Patent DE 102008032639 B4.
Jablonsky, M., Katuscak, S., Kacik, F., and Kacikova, D. (2011a). “Changes in newsprint paper during accelerated ageing,” Cell Chem. Technol. 45(5-6), 405-411.
Jablonsky, M., Katuscak, S., Holubkova, S., Hrobonova, K., and Lehotay, J. (2011b). “The effect of acetic and formic acid formation during accelerated ageing on embrittlement of newsprint paper,” Restaurator 32, 318-347. DOI: 10.1515/rest.2011.015
Jablonsky, M., Botkova, M., and Hrobonova, K. (2012a). “Accelerated ageing of wood-containing papers: Formation of weak acids and deterioration of tensile strength,” Wood Research 57(3), 419-434.
Jablonsky, M., Hrobonova, K., Katuscak, S., Lehotay, J., and Botkova, M. (2012b). “Formation of acetic and formic acid in unmodified and modified papers during accelerated ageing,” Cellulose Chem. Technol. 46(5-6), 331-340.
Jablonsky, M., Kazikova, J., Botkova, M., and Holubkova, S. (2012c). “Kinetic dependences for the decrease of polymerization of paper undergoing accelerated ageing,” Cellulose Chemistry and Technology 46, 9-10, 625-630.
Jablonsky, M., Holubkova, S., Kazikova, J., Botkova, M., Haz, A., and Bajzikova M. (2013). “The treatment of acid newsprint paper: Evaluation of treatment by MgO or by a mixture of MgO and methyl methoxy magnesium carbonate,” Wood Research 58(2), 151-164, ISSN 1336-4561
Jablonsky, M., Vizarova, K., Kazikova, J., Fekete, R., Kaskoto, M., Tino, R., and Katuscak, R. (2016). “Is it really possible to deacidify books by aqueous processes?” (Dajú sa knihy deacidifikovať vodnými procesmi?) Proc. CSTI 2015. Conservation Science, Technology and Industry. Bratislava 7 to 9 October 2015. SNM Bratislava 2016, pp. 87-95, ISBN 978-80-8060-377-9.
Jeong, M.-J., Kang, K.-Y., Bacher, M., Kim, H.-J., Jo., B.-M., and Potthast, A. (2014). “Deterioration of ancient cellulose paper, Hanji: Evaluation of paper permanence,” Cellulose 21, 4621-4632. DOI 10.1007/s10570-014-0455-4
Johnson, J., Lee, M., and Schneider, C. (2012). “Research and technical studies–Book and Paper Group Joint Discussion, Session 2012: Mass Deacidification Today, May 11th, 2012, during the 40th AIC Annual Meeting in Albuquerque, New Mexico, The Book and Paper Group Annual 31, 101-112.
Johnson, R. (1891). “Inferior paper a menace to the permanency of literature,” Library Journal 16(August), 241-242.
Joubert, J. M., Kirge, G. J. R., and Borgin, K. (1959). “Evidence for a hydrate of cellulose from studies of its surface properties,” Nature 184(4698), 1561-1562. DOI: 10.1038/1841561b0
Kacik, F., Kacikova, D., Jablonsky, M., and Katuscak, S. (2009). “Cellulose degradation in newsprint paper ageing,” Polym. Degrad. Stabil. 94(9), 1509-1514. DOI: 10.1016/j.polymdegradstab.2009.04.033
Kaminska, E. M. (1995). “Book review: Mass deacidification of paper – A comparative study of existing processes – Brandt, A. C.,” J. Amer. Inst. Conservation 34(2), 153-154. DOI: 10.2307/3179737
Kaminska, E. M., and Burgess, H. D. (1994). “Evaluation of commercial mass deacidification processes, AKZO-DEZ, Wei T’o and FMC-MG3, Phase II: New and artificially aged modern papers,” Canadian Conservation Institute, Conservation Processes Research Division, Ottawa, 51 pp.
Kaplan, H. A., and Ludwig, K. A. (2005). “Comparison of drying methods,” in: Preparing for the Worst, Planning for the Best: Protecting our Cultural Heritage from Disaster, Proceedings of a Conference, IFLA publication No. 111, J. G. Wellheiser and N. E. Gwinn (eds.), München: Saur., pp. 149-162.
Katuscak, S. (2006). EQ. Environmental Quality of Materials and Products (in Slovak)”, 2nd Ed., E< Bratislava, Slovak University of Technology (STU), Bratislava. ISBN 80- 227-2458-0.
Katuscak S., Bakos D., Bukovsky, V., Katuscak, D., Hanus, J., Rychly, J., Rehakova, M., Vizarova, K., Tino, R., and Kirschnerova, S. (2006): “Preservation, stabilization and conservation of traditional carriers of information of the Slovak Republic (KnihaSK.eu)”. STU Bratislava, Ministry of education SR, Report No. 06/533, Archival Report No. 06533/2006.
Katuscak, S., and Gfeller, B. (2006). Biocompatibility and Habitability of Materials and Products, 3rd Ed., Ekorex SK, Bratislava, ISBN 80-88812-17-8.
Katuscak, S., Vizarova, K., Bakos, D., Vrska, M., and Cedzova, M. (2007). “Multicomponent mixture for stabilization and strengthening of paper (Multikomponentná zmes určená na stabilizáciu a spevňovanie papierových hárkov). SK Patent 286410.
Katuscakova, G., Katuscak, S., and Jablonsky, M. (2008). “Method of chemical modification of paper documents,” SK Patent 287 856.
Katuscak, D., Bukovsky, V., Katuscak, S., Sabov, P., Bajzikova, M., Trnková, M., Kristofova, K., and Katuscak M. (2009a). “Complex protection of library written heritage program” (Štúdia k návrhu programu komplexnej ochrany knižničného písomného dedičstva). In: Knižnica, 10, 2009, 1, pp. 7-14. Available at: http://knihask.eu/novinky_2009/Studia%20navrhu%20programu%20komplexnej%20ochrany%20kniznicneho%20pisomneho%20dedicstva%20Slovenska.pdf. ISSN 1336-0965
Katuscak, S., Bakos, D., Bukovsky, V., Katuscak, D., Hanus, J., Rychly, J., Rehakova, M., Vizarova, K., Fikar, M., Kacik, F., Lehotay, J., Vodny, S., Urlandova, A., Sima, J., Fellner, P., Tino, R., Kirschnerova, S., Jablonsky, M., et al. (2009b). “Preservation, stabilization and conservation of traditional carriers of information in Slovak Republic – KnihaSK,” STU Bratislava, Ministry of education SR Report No. 09/533, Archival Report No. 09533/2009.
Katuscak, S., Jablonsky, M., Vizarova K., Rehakova, M., Holubkova, S., Hanus, J., Bajzikova, M., Rychly, J., and Vodny S. (2010). “Comparative evaluation of deacidification processes,” In: Ergebnisse des Projekts »Nachhaltigkeit der Massenentsäuerung von Bibliotheksgut«. Abschlusskonferenz am Dienstag, 26. Oktober 2010. Deutsche Nationalbibliothek, Frankfurt am Main.
Katuscak, D. (2011). National project Digital library and digital archive (Národný projekt Digitálna knižnica a digitálny archív). In: Bulletin Slovenskej asociácie knižníc, 19, 2011, 4, pp 5 – 45. ISSN 1335-7905. Online: http://www.sakba.sk/bulletin/2011/SAK_2011_4.pdf; ISSN 1335-7905.
http://www.uniza.sk/komunikacie/archiv/2013/2/2_2013en.pdf. ISSN 1335-4205.
Katuscak, S., Jablonsky, M., and Holubkova, S. (2012). “Comparative evaluation of deacidification processes,” in: Eine Zukunft für Saures Papier, Altenhöner, R., Blüher, A., Mälck, A., Niggemann, E., Potthast, A., and Schneider-Kempf, B. (eds.), Vittorio Klostermann, Frankfurt am Main, pp. 149-176.
Katuscak, D., and Konvit, M. (2013). “Optimisation of the processes of written heritage preservation and digitisation,” in: Communications: Scientific Letters of the University of Žilina. 15, 2, 112-117.
Katuscak, S., Vodny, S., and Vizarova, K. (2016). “Method and apparatus for pH distribution measurement in porous material microstructure,” US Patent Pending 62/392,456.
Katz, S., Beatson, R., and Scallan, A. M. (1984). “The determination of strong and weak acid groups in sulfite pulps,” Svensk Papperstidn. 87(6), R48-R53.
Kellerman, L. S. (1999). “Combating whole-book deterioration: The rebinding & mass deacidification program at the Penn State University Libraries,” Library Resources Tech. Serv. 43(3), 170-177. DOI: 10.5860/lrts.43n3.170
Kelly, G. B., Jr., and Fowler, S. (1978). “Penetration and placement of alkaline compounds in solution-deacidified paper,” J. Am. Inst. Conserv. 17(2), 33-43. DOI: 10.2307/3179754
Kelly, G. B., Jr., Tang, L. C., and Krasnow, M. K. (1977). “Methylmagnesium carbonate – An improved nonaqueous deacidification agent,” Adv. in Chem. Ser. (ACS) 164 (Preserv. of Paper & Textiles Symp., San Francisco, Aug. 1976), Ch. 4, 62-71.
Kocherbitov, V., Ulvenlund, S., Kober, M., Jarring, K., and Arnebrant, T. (2008). “Hydration of microcrystalline cellulose and milled cellulose studied by sorption calorimetry,” J. Phys. Chem. B 112(12), 3728-3734. DOI: 10.1021/jp711554c
Kohler, S., and Hall, G. (1925). “Acidity in paper,” Paper Industry 7(7), 1059.
Kohonen, M. M., and Christenson, H. K. (2000). “Capillary condensation of water between rinsed mica surfaces,” Langmuir 16(18), 7285-7288. DOI: 10.1021/la991404b
Kolar, J., and Novak, G. (1996). “Effect of various deacidification solutions on the stability of cellulose pulps,” Restaurator 17(1), 25-31. DOI: 10.1515/rest.1996.17.1.25
Kolar, J., Strlič, M., Novak, G., and Philar, B. (1998). “Aging and stabilization of alkaline paper,” J. Pulp Paper Sci. 24(3), 89-94.
Koura, A., and Krause, T. (1978). “The aging of paper. Part 3. Influence of the pH value of the pulp suspension and of counter-ions associated with the carboxyl groups,” Das Papier 32(12), 558-563.
Koura, A., and Krause, T. (1980). “Increase of paper permanence by treatment with liquid ammonia solutions: Part I, Fundamental basis and influence on fibre and paper structure and properties,” International Conference on the Conservation of Library and Archive Materials and the Graphic Arts. London: Institute of Paper Conservation, 20-24.
Krascenits, Z. (2007). “Determination of organic acids and furfuralaldehyde in paper. HPLC determination with UV-DAD detection,” Technical Report, pp. 6. DOI: 10.13140/RG.2.2.13547.13606; https://www.researchgate.net/publication/307976532_Determination_of_organic_acids_and_furfuralaldehyde_in_paper_HPLC_determination_with_UV-DAD_detection
Kruth, L. M. (1988). “A survey of recent scientific research which has caused a re-evaluation of commonly used practices in book and paper conservation,” The Book and Paper Group Annual 7, 30-39.
Kundrot, R. A. (1985). “Deacidification of library materials,” US Pat. 4,522,843
Langlet, M., Benali, M., Pezron, I., Saleh, K., Guigon, P., and Metlas-Komunjer, L. (2013). “Caking of sodium chloride: Role of ambient relative humidity in dissolution and recrystallization process,” Chem. Eng. Sci. 86, 78-86. DOI: 10.1016/j.ces.2012.05.014
Lehotay, J., and Hrobonova, K. (2007). “Materials for evaluation of organic acids and furfuralaldehyde in paper. HPLC determination,” Technical Report, pp. 7. https://www.researchgate.net/publication/307976527_Materials_for_evaluation_of_organic_acids_and_furfuralaldehyde_in_paper_HPLC_determination DOI: 10.13140/RG.2.2.23613.46560
Leiner, L. H., and Burd, J. E. (1998). “Method and apparatus for the deacidification of library materials,” US Patent 5770148 A
Leiner, L. H., and Kifer, E. W. (1995). “Deacidification of cellulose based materials using perfluorinated carriers,” WO 1995006779 A1.
Li, Q., Xi, S., and Zhang, X. (2014). “Deacidification of paper relics by plasma technology,” J. Cultural Heritage 15, 159-164. DOI: 10.1016/j.culher.2013.03.004
Library of Congress (2004). “Library of Congress technical specifications for mass deacidification,” Preservation Directorate, Washington, DC.
Lichtblau, D., Dobrusskin, S., and Blüher, A. (2009). “Das SurveNIR – System zer Bewertung des Zustands and des Alterungsverhaltens von Papier,” Bericht über das Forschungsseminar und den Workshop 8-10 Sept. 2009, Bern, and der Hochschule de Künste Bern, Forschungsschwerpunkt Materialität in Kunst und Kultur und an der Schweizerischen Nationalbibliothek Bern.
Lide, D. R., and Keniaian, H. V. (1994). CRC Handbook of Thermophysical and Thermochemical Data, CRC Press, Boca Raton, FL.
Liénardy, A. (1994). “Evaluation of seven mass deacidification treatments,” Restaurator 15(1), 1-25. DOI: 10.1515/rest.1994.15.1.1
Liers, J. (1999). “Determination of the content of alkalis and acids in paper,” Restaurator 20(3-4), 126-136. DOI: 10.1515/rest.1999.20.3-4.126
Lojewski, T. (2010). “Five years of mass-scale deacidification in Poland,” Die Massenentsäuerung auf dem Prüfstand, Ergebnisse des Projekts »Nachhaltigkeit der Massenentsäuerung von Bibliotheksgut«, Abschlusskonferenz, 26. Oktober 2010, Deutsche Nationalbibliothek, Frankfurt am Main. http://www.dnb.de/SharedDocs/Downloads/DE/DNB/wir/vortragLojewskiMassScalePoland2010.pdf?__blob=publicationFile
Łojewski, T., Zięba, K., Kołodziej, A., and Łojewska, J. (2011). “Following cellulose depolymerization in paper: Comparison of size exclusion chromatography techniques,” Cellulose 18, 1349-1363. DOI 10.1007/s10570-011-9562-7
Luner, P. (1969). “Paper permanence,” Tappi 52(5), 796-805.
MacAlister, J. Y. W. (1898). “The durability of modern book papers,” Libri 10, 295-304. DOI: 10.1093/library/s1-X.1.295
MacInnes, A. N., and Barron, A. R. (1992). “Spectroscopic evaluation of the efficacy of two mass deacidification processes for paper,” J. Mater. Chem. 2(10), 1049-1056. DOI: 10.1039/JM9920201049
McCrady, E. (1990). “Deacidification vs. microfilming,” Abbey Newsl. 14(6), 112-113.
Middleton, S. R., Scallan, A. M., Zou, X., and Page, D. H. (1996). “A method for the deacidification of paper and books,” Tappi J. 79(11), 187-195.
Milevski, R. J., and Nainis, L. (1994). “Water-soaked book salvage method – involves forming book-receiving, laminated, corrugated carbon which is transported to vacuum or freeze drying installation,” US Patent US5325602-A, assigned to: PROTEXT INC, Derwent Primary Accession Number: 1994-216446 [64]
Mitropoulos, A. C. (2008). “The Kelvin equation,” J. Colloid Interface Sci. 317(2), 643-648. DOI: 10.1016/j.jcis.2007.10.001
Moll, W. (1965). “Permanence durability of the book, Vol. 3, Spray deacidification – W. J. Barrow Res. Lab.,” Bull Medical Libr. Assoc. 53(1), 131-132.
Morrow, G. (1988). “Mass deacidification: Operational experience at the National Archives and the National Library of Canada,” Paper Conservator Conf. 12, 40-46. DOI: 10.1080/03094227.1988.9638561
Mukhopadhyay, S., and Fangueiro, R. (2009). “Physical modification of natural fibers and thermoplastic films for composites – A review,” J. Thermoplastic Compos. Mater. 22(2), 135-162. DOI: 10.1177/0892705708091860
Murray, J. (1824). Observations and Experiments on the Bad Composition of Modern Paper. Whittaker, London; Remarks on Modern Paper, Blackwood, Edinburgh; Cadell, London.
Myers, D. R., and Crane, J. (1999). “Re-blue-ing blue litmus paper,” J. Chem. Ed. 76(3), 319-319. DOI: 10.1021/ed076p319.2
Nail, S. L., Jiang, S., Chongprasert, S., and Knopp, S. A. (2002). “Fundamentals of freeze-drying,” in: Development and Manufacture of Protein Pharmaceuticals, S. Nail and M. Akers (eds.), Pharm. Biotech. 14, 281-360. DOI: 10.1007/978-1-4615-0549-5_6
Nerin, C., Batlle, R., and Cacho, J. (1998). “Design of a test for migration studies in the vapour phase,” Food Additives Contaminants 15(1), 84-92. DOI: 10.1080/02652039809374602
Neuvirt, J. (2010). “Universal drying chamber for flood-damaged paper objects,” Restaurator 31, 222-345. DOI: 10.1515/rest.2010.017
Newberg, J. T., Starr, D. E., Yamamoto, S., Kaya, S., Kendelewicz, T., Mysak, E. R., Porsgaard, S., Salmeron, M. B., Brown, G. E., Nilsson, A., and Bluhm, H. (2011). “Formation of hydroxyl and water layers on MgO films studied with ambient pressure XPS,” Surface Sci. 605, 89-94. DOI: 10.1016/j.susc.2010.10.004
Page, D. (1993). “A quantitative theory of the strength of wet webs,” J. Pulp Paper Sci. 19(4), J175-J176.
Page, D. H., Scallan, A. M., Middleton, S. R., and Zou, X. (1995). “Method for the deacidification of papers and books,” U.S. Pat. 5,433,827.
Pappas, C., Rodis, P., Tarantilis, P. A., and Polissiou, M. (1999). “Prediction of the pH in wood by diffuse reflectance infrared Fourier transform spectroscopy,” Appl. Spectrosc. 53(7), 805-809. DOI: 10.1366/0003702991947360
Park, S., Baker, J. O., Himmel, M. E., Parilla, P. A., and Johnson, D. K. (2010). “Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance,” Biotech. Biofuels 3(1), article 10. DOI: 10.1186/1754-6834-3-10
Park, S., Venditti, R. A., Jameel, H., and Pawlak, J. J. (2006). “Changes in pore size distribution during the drying of cellulose fibers as measured by differential scanning calorimetry,” Carbohydrate Polymers 66(1), 97-103. DOI: 10.1016/j.carbpol.2006.02.026
Pauk, S. (1996). “The Bookkeeper mass deacidification process – Some effects on 20th-century library material,” Abbey Newsl. 20(4/5), 50-53.
Pearson, R. G. (1969). Modern Theory of Acids and Bases, Amer. Chem. Soc., Washington DC.
Piltonen, P., Stoor, T., and Niinimäki, J. (2013). “The effect of rewetting on the adhesion tendency of styrene-butadiene latices on steel surfaces,” Intl. J. Adhes. Adhesives 40, 108-111. DOI: 10.1016/j.ijadhadh.2012.09.004
Polovka, M., Polovková, J., Vizárová, K., Kirschnerová, S., Bieliková, L., and Vrška, M. (2006). “The application of FTIR spectroscopy on characterization of paper samples, modified by Bookkeeper process,” Vibrational Spectroscopy 41(1), 112-117. DOI: 10.1016/j.vibspec.2006.01.010
Pönni, R., Vuorinen, T., and Kontturi, E. (2012). “Proposed nano-scale coalescence of cellulose in chemical pulp fibers during technical treatments,” BioResources 7(4), 6077-6108. DOI: 10.15376/biores.7.4.6077-6108
Porck, H. J. (1996). “Mass deacidification. An update of possibilities and limitations,” European Commission on Preservation and Access, Royal Netherlands Academy of Arts and Sciences.
Porck, H. J. (2000). “Rate of paper degradation. The predictive value of artificial aging tests,” European Commission on Preservation and Access, Royal Netherlands Academy of Arts and Sciences.
Potthast, A., and Ahn, K. (2017). “Critical evaluation of approaches toward mass deacidification of paper by dispersed particles,” Cellulose 24, 323-332. DOI: 10.1007/s10570-016-1112-x
Potthast, A., Henniges, U., and Banik, G. (2008). “Iron gall ink-induced corrosion of cellulose: Aging, degradation and stabilization. Part 1: Model paper studies,” Cellulose 15(6), 849-859. DOI: 10.1007/s10570-008-9237-1
Preservation Technologies. (2017). “Bookkeeper®,” http://ptlp.com/en/ptlp/overview/about-us/
Ramin, M., Andres, H., Blüher, A., Reist, M., and Wälchli, M. (2009). “Paper de-acidification – A comparative study,” J. Paper Conservation 10(3), 17-25.
Rasch, R. H. (1931). “Accelerated aging test for paper,” Bureau of Standards J. Res. 7(1/3), 465-475. DOI: 10.6028/jres.007.026
Rasch, R. H., and Scribner, B. W. (1933). “Comparison of natural aging of paper with accelerated aging by heating,” Bureau of Standards J. Res. 11(4/6), 729-732. DOI: 10.6028/jres.011.050
Rychly, J., Matisova-Rychla, L., Lazar, M., Slovak, K., Strlic, M., Kocar, D., Kolar, J. (2004). “Thermal oxidation of cellulose investigated by chemiluminescence. The effect of water at temperatures above 100°C,” Carbohydrate Polymers 58, 301-309. DOI:10.1016/j.carbpol.2004.07.006
Rychly, J., Matisova-Rychla, L., Lazar, M., Janigova, I., Strlic, M., Kocar, D., Hanus, J., Minarikova, J., and Katuscak, S. (2006). “Thermal oxidation of cellulose investigated by chemiluminescence. The effect of magnesium and calcium carbonates and of different pHs,” Comptes Rendus Chemie 9(11-12), 1425-1432. DOI: 10.1016/j.crci.2006.07.004
Scallan, A. M. (1990). “The pH inside the fiber wall,” in: Cellulose Sources and Exploitation, J. F. Kennedy, G. O. Phillips, and P. A. Williams (eds.), Eric Horwood, London, p. 211.
Scallan, A. M., Katz, S., and Argyropoulos, D. S. (1989). “The conductometric titration of cellulose fibers,” in: Cellulose and Wood, C. Schuerch (ed.), John Wiley, New York, p. 1457.
Scallan, A. M., and Tigerström, A. C. (1992). “Swelling and elasticity of the cell walls of pulp fibers,” J. Pulp Paper Sci. 18(5), J188-J193.
Scally, S., Davison, W., and Zhang, H. (2006). “Diffusion coefficients of metals and metal complexes in hydrogels used in diffusive gradients in thin films,” Anal. Chim. Acta 558(1-2), 222-229. DOI: 10.1016/j.aca.2005.11.020
Sclawy, A. C., and Williams, J. C. (1981). “Alkalinity – The key to paper ‘permanence’,” Tappi 64(5), 49-50.
Scott, M. (1987). “Mass deacidification at the National Library of Canada,” Restaurator 8(2/3), 94-99. DOI: 10.1515/rest.1987.8.2-3.94
Selli, E., Beltrame, P. L., Testa, G., Bonfatti, A. M., Rossi, E., and Seves, A. (1998). “Kinetic studies on the accelerated aging of cellulosic materials,” Angew. Makromol. Chemie 257, 63-69. DOI: 10.1002/(SICI)1522-9505(19980601)257:1<63::AID-APMC63>3.0.CO;2-I
Sequeira, S., Casanova, C., and Cabrita, E. J. (2006). “Deacidification of paper using dispersions of Ca(OH)2 nanoparticles in isopropanol. Study of efficiency,” J. Cultural Heritage 7(4), 264-272. DOI: 10.1016/j.culher.2006.04.004
Shahani, C. (1995). “Accelerated aging of paper: Can it really foretell the permanence of paper,” in: Preservation Research and Testing Series, 9503, Proceedings from the ASTM/ISR Workshop on the Effects of Aging on Printing and Writing Papers, Philadelphia, PA July 1994http://lcweb.loc.gov/preserv/rt/age/.
Shahani, C. J., Hengemihle, F. H., and Weberg, N. (1989). “The effect of variations in relative humidity on the accelerated aging of paper,” ACS Symp. Ser. 410, Ch. 5, pp. 63-80. DOI: 10.1021/bk-1989-0410.ch005
Shahani, C., Lee, S. B., Hengemihle, F. H., Harrison, G., Song, P., Sierra, M. L., Ryan, C. C., and Weberg, N. (2001). “Accelerated aging of paper: I. Chemical analysis of degradation products. II. Application of Arrhenius relationship. III. Proposal for a new accelerated aging test: ASTM research program into the effect of aging on printing and writing papers,” Washington, DC, Library of Congress.
Silverman, R., Bliss, M., Erickson, H., Fidopiastis, N., Franci, J., Knight, B., Lively, K., Neuwirt, J., Novotny, D., and Yeager, N. (2007). “Comparing mass drying and sterilization protocols for water-damaged books,” International Preservation News, No. 42, October, 22-27; The Book and Paper Group Annual 26, 85-97.
Smith, R. D. (1969). “Paper impermanence as a consequence of pH and storage conditions,” The Library Quarterly 39(2), 153-195. DOI: 10.1086/619740
Smith, R. D. (1970). The Nonaqueous Deacidification of Paper and Books, PhD. Dissertation, Univ. Chicago, Chicago, IL, 295 pp.
Smith, R. D. (1977). “Design of a liquefied gas mass deacidification system for paper and books,” in: Preservation of Paper and Textiles of Historic and Artistic Value, J. C. Williams (ed.), Adv. Chem. Ser. (ACS) 164, 149-158. DOI: 10.1021/ba-1977-0164.ch011
Smith, R. D. (1987). “Deacidifying library collections: Myths and realities,” Restaurator 8(2/3), 69-93. DOI: 10.1515/rest.1987.8.2-3.69
Smith, R. D. (1988). “Nonaqueous deacidification: Philosophies, origin, development, and status,” Paper Conservator Conf. 12, 31-34.
Smith, R. D. (1992). “Disaster recovery – Problems and procedures,” Internat. Fed. Lib. Assoc. J. 18(1), 13-24. DOI: 10.1177/034003529201800106
Smith, R. D. (2004). “Paper stability and collection risk: A perspective and choices,” Restaurator 25(3), 199-210. DOI: 10.1515/rest.2004.199
Smith, A. W. (2011). “Aqueous deacidification of paper,” in: Paper and Water: A Guide for Conservators, G. Banik and I. Brückle (eds.), Elsevier, Butterworth-Heinemann, Amsterdam, Boston, Oxford, pp. 341-388.
Smith, R. D. (2011). “Dry and moist heat and alkaline particle size: Their effect on paper stability,” personal communication.
Sparks, P. G. (1987). “Mass deacidification at the library of congress,” Restaurator 8(2/3), 106-110. DOI: 10.1515/rest.1987.8.2-3.106
Stauderman, S. D., Brückle, I., and Bischoff, J. J. (1996). “Observations on the use of BookkeeperÒ deacidification spray for the treatment of individual objects,” The Book and Paper Group Annual 15, retrieved from the following site on July 13, 2016: http://cool.conservation-us.org/coolaic/sg/bpg/annual/v15/bp15-17.html
Stefanis, E., and Panayiotou, C. (2007). “Protection of lignocellulosic and cellulosic paper by deacidification with dispersions of micro- and nano-particles of Ca(OH)2 and Mg(OH)2 in alcohols,” Restaurator 28(3), 185-200. DOI: 10.1515/rest.2007.185
Stephens, C. H., Barrett, T., Whitmore, P. M., Wade, J. A., Mazurek, J., and Schilling, M. (2008a). “Composition and condition of naturally aged papers,” J. Amer. Inst. Conservat. 47(3), 201-215. DOI: 10.1179/019713608804539583
Stephens, C. H., Whitmore, P. M., Morris, H. R., and Bier, M. E. (2008b). “Hydrolysis of the amorphous cellulose in cotton-based paper,” Biomacromol. 9(4), 1093-1099. DOI: 10.1021/bm800049w
Stephens, C. H., Whitmore, P. M., Morris, H. R., and Smith, T. (2009). “Assessing the risks of alkaline damage during deacidification treatments of oxidized paper,” J. Amer. Inst. Conservat. 48(3), 235-249. DOI: 10.1179/019713612804514251
Stone, J. E., and Scallen, A. M. (1966). “Influence of drying on the pore structures of the cell wall,” in: Consolidation of the Paper Web, Trans. Symp., Cambridge, F. Bolam (ed.), Tech. Section British Paper and Board Makers Assoc., London, Vol. 1, pp. 145-174.
Strazdins, E. (1989). “Theoretical and practical aspects of alum use in papermaking,” Nordic Pulp Paper Res. J. 4(2), 128-134. DOI: 10.3183/NPPRJ-1989-04-02-p128-134
Strlič, M., Kolar, J., and Lichtblau, D. A. (2008). “SurveNIR product: Non-destructive characterization of historical paper,” Conservation J. 56, not numbered, http://www.vam.ac.uk/content/journals/conservation-journal/issue-56/survenir-project-non-destructive-characterisation-of-historical-paper/, accessed Feb. 2017.
Sundholm, F., and Tahvanainen, M. (2003a). “Paper conservation using aqueous solutions of calcium hydroxide/methyl cellulose – 1. Preparation of the solution,” Restaurator 24(1), 1-17. DOI: 10.1515/REST.2003.1
Sundholm, F., and Tahvanainen, M. (2003b). “Paper conservation using aqueous solutions of calcium hydroxide/methyl cellulose – 2. The influence of accelerated ageing temperature on properties of treated paper,” Restaurator 24(3), 178-188. DOI: 10.1515/REST.2003.178
Sundholm, F., and Tahvanainen, M. (2004). “Paper conservation using aqueous solutions of calcium hydroxide/methyl cellulose – 3. The influence on the degradation of papers,” Restaurator 25(1), 15-25. DOI: 10.1515/REST.2004.15
Sutcu, M., Akkurt, S., and Okur, S. (2009). “Influence of crystallographic orientation on hydration of MgO single crystals,” Ceramics Intl. 35(7), 2571-2576. DOI: 10.1016/j.ceramint.2009.02.012
Swanson, J. W., and Cordingly, S. (1959). “Surface chemical studies of pitch. II. The mechanism of the loss of absorbency and development of self-sizing in papers made from wood pulps,” Tappi 42(10), 812-819.
Tang, L. C. (1981). “Washing and deacidifying paper in the same operation,” Adv. in Chem. Ser. 193(Preserv. Paper & Textiles Symp., Sept. 1979), 63-86.
Tang, L. C. (1986). “Stabilisation of paper through sodium borohydride treatment,” in: Historic Textile and Paper Materials: Conservation, Degradation and Characterization of Paper, Needles, H. L., and Zeronian, S. H. (eds.), Advances in Chemistry Series, American Chemical Society, Washington D.C., 427-441.
Tannenbaum, R., King, S., Lecy, J., Tirrell, M., and Potts, L. (2004). “Infrared study of the kinetics and mechanism of adsorption of acrylic polymers on alumina surfaces,” Langmuir 20(11), 4507-4514. DOI: 10.1021/la036137v
TAPPI (Technical Association of the Pulp and Paper Industry). T509 om-02: “Hydrogen ion concentration (pH) of paper extracts (cold extraction method),” 2002-2003 TAPPI Test Methods [CD-ROM]; TAPPI Press: Atlanta, GA, USA, 2002a.
TAPPI (Technical Association of the Pulp and Paper Industry). T435 om-02: “Hydrogen ion concentration (pH) of paper extracts (hot extraction method),” 2002-2003 TAPPI Test Methods [CD-ROM]; TAPPI Press: Atlanta, GA, USA, 2002b.
TAPPI (Technical Association of the Pulp and Paper Industry). T529 om-99: “Surface pH measurement of paper,” 2002-2003 TAPPI Test Methods [CD-ROM]; TAPPI Press: Atlanta, GA, USA, 2002c.
TAPPI (Technical Association of the Pulp and Paper Industry). T453 sp-08. “Effect of dry heat on properties of paper and board,”
TAPPI (Technical Association of the Pulp and Paper Industry). T544 pm-85 (1985). “Effect of moist heat on properties of paper and board.”
Testova, L., Nieminen, K., Penttila, P. A., Serimaa, R., Potthast, A., and Sixta, H. (2014). “Cellulose degradation in alkaline media upon acidic pretreatment and stabilization,” Carbohydrate Polymers 100, 185-194. DOI: 10.1016/j.carbpol.2013.01.093
Thygesen, L. G., Engelund, E. T., and Hoffmeyer, P. (2010). “Water sorption in wood and modified wood at high values of relative humidity. Part I: Results for untreated, acetylated, and furfurylated Norway spruce,” Holzforschung 64(3), 315-323. DOI: 10.1515/HF.2010.044
Tino, R., Katusščák, S., Jablonský, M., Kazíková, J., Vizárová, K., and Fikar, M. (2016). “The new method and multifunctional device for jet application of conservation substances and mixtures based on water onto paper sheets, sheet files and books,” (Slovak), in: Peer reviewed proceedings. CSTI 2015 – Integration of conservation science and technologies into interdisciplinary protection of materials and objects of heritage. Bratislava, October 7.-9, 2015, Slovak Republic. Pp.95-102, ISBN 978-80-8060-377-9, DOI: 10.13140/RG.2.2.10571.80167
Vandeperre, L. J., and Al-Tabbaa, A. (2007). “Accelerated carbonation of reactive MgO cements,” Advan. Cement Res. 19(2), 67-79. DOI: 10.1680/adcr.2007.19.2.67
Viovy, J. L. (2000). “Electrophoresis of DNA and other polyelectrolytes: Physical mechanisms,” Rev. Modern Phys. 72(3), 813-872. DOI: 10.1103/RevModPhys.72.813
Vizarova, K., Kazikova, J., Povazanec, F., Kirschnerova, S., and Gallova, I. (2008). “Development of antioxidants for conservation of books,” (in Slovak) Amendments P4.5-P4.7 in Research report: Preservation, stabilization and conservation of traditional carriers of information in Slovak Republic – KnihaSK., State program R&D of Ministry of education SR, project no.: 2003SP200280301,2003-2008, DOI: 10.13140/RG.2.2.15178.98241
Vizarova, K., Fekete, R., Jablonsky, M., Tino, R., and Katuscák, S. (2016a). “Apparatus and method for conservation objects,” US PP 62/389,106 Filing date: 2016-02-17;
Vizarova, K., Tino, R., Jablonsky, M., Rehakova, M., Hanus, J., and Katuscak, S. (2016b). “Development of multifunctional conservation technologies for the preservation of cultural heritage objects and materials,” Slovak University of Technology in Bratislava 2016, ISBN 978-80-89597-41-3.
Vrska, M., Katuscak, S., Polovka, M., Vizarova, K., Cedzova, M., Hanus, J., Minarikova, J., and Bukovsky, V. (2004). “Lignocellulosics stabilization efficacy measurement and evaluation,” Wood Research 49(4), 53-56.
Wächter, O., Ruhm, W., and Banik, G. (1998). “Verfarhren and Vorrichtung zur konservatorischen Behandlung von Papier,” EP 0273902 A2.
Wagner, B., Bulska, E., and Sobucki, W. (2008). “Magnesium distribution in paper subjected to deacidification investigated by means of laser ablation inductively coupled plasma mass spectroscopy,” J. Cultural Heritage 9(1), 60-65. DOI: 10.1016/j.culher.2007.11.001
Walker, B. F. (1977). “Morpholine deacidification of whole books,” Adv. in Chem. Ser. (ACS) 164 (Preserv. of Paper & Textiles Symp., San Francisco, Aug. 1976), 72-87.
Walpole, G. S. (1913). “The use of litmus paper as a quantitative indicator of reaction,” Biochem. J. 7(3), 260-267. DOI: 10.1042/bj0070260
Wenzl, T., and Lankmayr, E. P. (2000). “Reduction of adsorption phenomena of volatile aldehydes and aromatic compounds for static headspace analysis of cellulose based packaging materials,” J. Chromatography A 897, 269-277. DOI: 10.1016/S0021-9673(00)00790-1
Whitmore, P. M. (1994). “Evaluation of Bookkeeper process chemistry,” in: An Evaluation of the Bookkeeper Mass Deacidification Process, Buchanan S. (Chair, evaluation team), Domach, M. M., Tancin, C., Bennet, W., Melnick, S. M., and Whitmore, P. M., Library of Congress, Preservation Directorate, pp. 12-20. http://www.loc.gov/preservation/; http://www.loc.gov/preserv/deacid/bkkphome.html.
Whitmore, P. M., and Bogaard, J. (1994). “Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper,” Restaurator 15, 26-45. DOI: 10.1515/rest.1994.15.1.26
Whitmore, P. M., and Bogaard, J. (1995). “The effect of oxidation on the subsequent oven aging of filter paper,” Restaurator 16(1), 10-30. DOI: 10.1515/rest.1995.16.1.10
Whitmore, P. M. (2011). “Paper ageing and the influence of water,” in: Paper and Water: A Guide for Conservators, G. Banik and I. Brückle (eds.), Elsevier, Butterworth-Heinemann, Amsterdam, Boston, Oxford, pp. 219-254.
Williams, J. C. (1971). “Chemistry of deacidification of paper,” Bull. Am. Group IIC 12(1), 16-32. DOI: 10.2307/3178991
Wilson, W. K., and Parks, E. J. (1979). “An analysis of the aging of paper: Possible reactions and their effects on measurable properties,” Restaurator 3, 37-61. DOI: 10.1515/rest.1979.3.1-2.37
Wilson, W. K., and Parks, E. J. (1980). “Comparison of accelerated aging of book papers in 1937 with 36 years natural aging,” Restaurator 4, 1-55. DOI: 10.1515/rest.1980.4.1.1
Wilson, W. K., and Parks, E. J. (1983). “Historical survey of research at the National Bureau of Standards on materials for archival records,” Restaurator 5, 191-241. DOI: 10.1515/rest.1983.5.3-4.191
Wittekind, J. (1994). “The Battelle mass deacidification process: A new method for deacidifying books and archival materials,” Restaurator 15(4), 189-207. DOI: 10.1515/rest.1994.15.4.189
Wittekind, J., Eggersdorfer, R., Schwerdt, P., Scherer, K.-H., and Schmitt, R.-E. (1994). “Neutralizing agent for paper products,” US Pat. 5322558.
Wojciak, A. (2015). “Washing, spraying, and brushing. A comparison of paper deacidification by magnesium hydroxide nanoparticles,” Restaurator 36(1), 3-23. DOI: 10.1515/res-2014-0010
Wolke, R. L. (2002). What Einstein Told His Cook, W. W. Norton & Co., New York, pp. 350.
Xie, Y. J., Hill, C. A. S., Jalaludin, Z., and Sun, D. Y. (2011). “The water vapour sorption behaviour of three celluloses: Analysis using parallel exponential kinetics and interpretation using the Kelvin-Voigt viscoelastic model,” Cellulose 18(3), 517-530. DOI: 10.1007/s10570-011-9512-4
Yu, Y., and Wu, H. W. (2010). “Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water,” Indust. Eng. Chem. Res. 49(8), 3902-3909. DOI: 10.1021/ie901925g
Zentrum für Bucherhaltung (2017). “Gentle deacidification with the ZfB:2 process,” http://www.zfb.com/en/mass-deacidification/zfb2-method. Viewed March 2017.
Zervos, S., and Moropoulou, A. (2005). “Cotton cellulose ageing in sealed vessels. Kinetic model of autocatalytic depolymerization,” Cellulose 12(5), 485-496. DOI: 10.1007/s10570-005-7131-7
Zervos, S., and Moropoulou, A. (2006). “Methodology and criteria for the evaluation of paper conservation interventions – A literature review,” Restaurator 27(4), 219-274. DOI: 10.1515/rest.2006.219
Zervos, S. (2007). “Accelerated ageing kinetics of pure cellulose paper after washing, alkalization and impregnation with methylcellulose,” Restaurator 28(1), 55-69. DOI: 10.1515/rest.2007.55
Zervos, S. (2010). “Natural and accelerated ageing of cellulose and paper: A literature review,” in Cellulose: Structure and Properties, Derivatives and Industrial Uses, Lejeune, A., and Deprez, T. (eds.), Nova Science Publ., Inc., New York.
Zervos, S., and Alexopoulou, I. (2015). “Paper conservation methods: A literature review,” Cellulose 22(5), 2859-5897. DOI: 10.1007/s10570-015-0699-7
Zitzmann, P., Leisch, N., and Kratzer, A. (2002). “Verfahren und Vorrichtung für Entsäuerung Büchern,” European Patent 1 283 300 A2; DE 10139517 A1.
Zou, X., Gurnagul, N., Uesaka, T., and Bouchard, J. (1994). “Accelerated aging of papers of pure cellulose – Mechanism of cellulose degradation of paper embrittlement,” Polymer Degrad. Stabil. 43(3), 393-402. DOI: 10.1016/0141-3910(94)90011-6
Zou, X., Uesaka, T., and Gurnagul, N. (1996a). “Prediction of paper permanence by accelerated aging. 1. Kinetic analysis of the aging process,” Cellulose 3(4), 243-267. DOI: 10.1007/BF02228805
Zou, X., Uesaka, T., and Gurnagul, N. (1996b). “Prediction of paper permanence by accelerated aging. 2. Comparison of the predictions with natural aging results,” Cellulose 3(4), 269-279. DOI: 10.1007/BF02228806
Zumbühl, S., and Wuelfert, S. (2001). “Chemical aspects of the Bookkeeper deacidification of cellulosic materials: The influence of surfactants,” Studies in Conservation 46(3), 169-180.
DOI: 10.15376/biores.12.2.Hubbe
Erratum: On April 3, 2017, a mistake was corrected in the subsection “Conditions reflected by aqueous treatments.” A sentence about aqueous treatments had been mistakenly attributed to Kelly and Fowler. The words “deacidified using an aqueous treatment” were replaced by “become completely wetted with aqueous solutions”.
